Abstract
The abnormal accumulation of amyloid-β (Aβ) and neurofibrillary tangles (NFTs) containing phosphorylated tau proteins are the main histopathological feature of Alzheimer’s disease (AD). Synaptic damage and loss are earlier events than amyloid plaques and NFTs in AD progress and best correlate with cognitive deficits in AD patients. Soluble oligomeric Aβ initiates the progression of AD and tau mediates the subsequent synaptic impairments at an early stage of AD. In this review we discuss how Aβ or/and tau causes synaptic dysfunction. Aβ oligomers gather at synapses and give rise to synaptic death in a variety of ways such as regulating receptors and receptor tyrosine kinases, unbalancing calcium homeostasis, and activating caspases and calcineurin. A large amount of hyperphosphorylated tau exists in the synapse of the AD brain. Aβ-triggered synaptic deficits are dependent on tau. Soluble, hyperphosphorylated tau is much more correlated to cognitive decline in AD patients. Tau-targeted therapies have received more attention because the treatments targeting Aβ failed in AD. Here, we also review the therapy strategies used to intervene in the very early stages of AD. Soluble hyperphosphorylated tau forms a complex with cell surface receptors, scaffold proteins, or intracellular signaling molecules to damage synaptic function. Therefore, therapeutic strategies targeting synaptic tau at the early stage of AD may ameliorating pathology in AD. This review aims to provide an update on the role of oligomeric Aβ and soluble hyperphosphorylated tau in the early pathogenesis of Alzheimer’s disease and to develop a new treatment strategy based on this.
Keywords: Alzheimer Disease; Amyloid beta-Peptides; MAPT Protein, Human; Neurofibrillary Tangles
Background
Alzheimer’s disease (AD) is characterized by two hallmark pathological lesions in the brain: the extracellular amyloid plaques deposition of amyloid-β (Aβ) peptides and intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau [1,2]. The internal relations between these two hallmarks and the cognitive dysfunction occurred in AD patients remain elusive [3]. Accumulation of toxic Aβ is proposed as one of the important early events in AD, but unsuccessful clinical trials based on Aβ-targeting drugs have triggered researchers to investigate new therapeutic strategies targeting alternative disease mechanisms [4]. Aβ oligomers but not amyloid plaques target the postsynaptic compartment of excitatory synapses with high affinity, changing the structure and function of synapses [5]. More and more research indicates that the pathology of tau correlates with cognitive defects in AD [6]. However, soluble forms of tau oligomers but not NFTs appear to be an important factor inducing neuronal dysfunction and cognitive impairment [7,8]. Memory deficits highly correlate with synaptic decline in the hippocampus of AD patient’s brains [9–11]. Synaptic loss is the first indicator of AD progression, even in the earliest of stage, called mild cognitive impairment (MCI), and has the strongest biological correlation with cognitive deficits found in AD patients [12–14]. Mislocalization of tau to dendrites, which is an early event in AD pathogenesis prior to tau aggregation, is a neuropathological feature of AD brains [15,16]. Synaptic tau correlates with the onset of cognitive decline in AD [17,18]. This review aims to provide an update on what is currently known about the role of Aβ and tau in the early pathogenesis of Alzheimer’s disease.
Oligomeric Aβ and Synaptic Dysfunction
Long-term potentiation (LTP) and long-term depression (LTD), which are forms of activity-dependent synaptic plasticity, and the formation of dendritic spines are considered to underlie learning and memory [19]. LTP increases in synaptic strength depend on activating NMDA receptors by recurring synaptic activity. In contrast, LTD reduces synaptic activity through phosphorylation of AMPA receptors [20].
Synaptic loss can be induced by oligomeric forms of Aβ [1,21,22], suggesting that Aβ is a driver of synaptic dysfunction in AD [23]. The senile plaques were considered to be the major pathogenic substance in AD, but clinical investigations did not reveal a strong association between extracellular amyloid plaque and cognitive defects [24]. During AD progress, Aβ oligomers begin to be enriched at synapses, which is earlier than the formation of amyloid plaques or accumulation of phosphorylated tau at synapses [25,26]. Extracellular Aβ accumulates around the postsynaptic compartment more abundantly than at presynaptic terminals [27].
Accumulated research results indicate that Aβ causes synaptic death, LTD and LTP via modulating excitatory receptors and receptor tyrosine kinases, unbalancing calcium homeostasis, and activating caspases and calcineurin. Aβ binds to AMPA receptors and causes their internalization, leading to increased LTD. Aβ binds to 7α-nicotinic acetylcholine receptors, leading to an internalization of NMDA receptors and LTD [28]. Oligomeric Aβ stimulates NMDA receptors to upregulate calcium and redox reactions, leading to synaptic dysfunction and neuronal loss [29]. Calcineurin, activated by Aβ elevated calcium, dephosphorylates actin filaments to cause dendritic spine loss. Calcineurin activation can also reduce NMDA receptor expression on the surface and lead to greater AMPA receptor internalization. Soluble Aβ dephosphorylates AMPA receptors and increases receptor internalization. Oligomeric Aβ also binds with tyrosine kinases to modulate NMDA receptor trafficking and reduce LTP [30].
Oligomeric Aβ can impair LTP while increasing LTD in a concentration-dependent manner. Low levels of oligomeric Aβ facilitate LTP, but high levels of Aβ impair it [29] by affecting calcium channel activity and glutamate receptor-dependent signaling pathways [31].
The increased NMDA activity stimulated by oligomeric Aβ can lead to increased tau accumulation. Increased phosphorylated tau associates with, and further amplifies, the dendritic spine loss [32]. Age-dependent accumulations of Aβ and tau and their interactions at synapses largely impair synaptic activity via altering LTP and LTD levels [30].
Tau and Synaptic Deficits
Tau normally exists in synapses of both healthy and AD brains, while there is a greater level of hyperphosphorylated tau present in the AD synapse [33]. Soluble, hyperphosphorylated tau is much more closely related to synaptic dysfunction and cognitive decline in AD patients compared to Aβ and aggregated tangles of tau [34]. In vivo experiments showed the role of tau in synaptic plasticity. Reduction or depletion of tau blocked the induction of LTD but not LTP. Replacement of endogenous tau with human tau restored LTD. These data support the essential role of tau in a NMDAR-dependent LTD in the hippocampus [35]. Tau’s role in LTD depends on its phosphorylation at serine 396, which internalizes the AMPA receptor [36]. Reducing the phosphorylation of tau by inhibiting tau kinases rescues tau-dependent LTP deficits and alleviates synaptic loss in tau transgenic mice [37,38]. Aβ may need tau to impair LTP, since tau-null mice showed no impairment in LTP when Aβ was applied [29]. Synaptic deficits induced by Aβ were closely related with tau, since reducing endogenous tau levels in Aβ-forming AD mouse models prevented dysfunctions [39–42]. In contrast, overexpression of human tau in amyloidosis mouse models increased synaptic loss and memory impairment [40,43].
Transgenic mice with human P301S or P301L mutant tau had impaired LTP in the hippocampus [18,44]. Mice expressing human V337M mutant tau had reduced excitatory synaptic transmission, associated with decreased synaptic glutamate receptors levels in both the ventral striatum and the insular cortex [45]. In cortical neurons, expression of human P301L mutant tau resulted in mushroom spine loss [46].
Mitochondria and Synaptic Degeneration
Mitochondria serves as energy supplier for synaptic functions such as synaptic transmission, synaptic outgrowth, and synaptic vesicle formation [47,48]. Aβ or phosphorylated tau triggers damage and transportation of mitochondria to synaptic terminals, which may provide low levels of ATP to synapses, leading to synaptic degeneration [30]. Mitochondrial dysfunction occurs early in AD progression [49]. Synapse loss is an early event of AD, which is attributed to soluble Aβ, phosphorylated tau, and increased free radicals generated by mitochondria at synapses [30]. More abnormal mitochondria are found in synapses of AD brains compared to healthy brains [50].
Synaptic Proteins and Synaptic Function
Extensive studies on synaptic proteins in a large number of healthy controls and AD patients reveal that postsynaptic and presynaptic proteins are important for synaptic function and may be related to cognitive impairments in AD [51]. Synaptophysin, a presynaptic protein, was decreased by around 25% in MCI patients, which can occur before Aβ plaque formation. Loss of synaptophysin correlates with cognitive decline and is also a marker for disease progression in AD patients [11]. The postsynaptic density-95 (PSD-95) protein is the most abundant scaffold proteins inside the postsynaptic density (PSD), a densely packed multi-protein structure for synaptic formation and function at the distal tip of dendritic spine heads [52–54]. PSD-95 is crucial for trafficking and anchoring of synaptic glutamate receptors [55–58]. It is predicted that tau, Fyn, PSD-95, and NMDARs form a protein complex at the synapse [59]. Loss of PSD-95 results in decrease of synapses containing glutamate receptors and impairments in AMPAR and NMDAR transmissions [60]. It has been reported that the expression of PSD-95 is aberrant in several human disorders, including AD [61–65]. Researchers identified postmortem synapse and synaptic marker loss from AD patients in a meta-analysis [66].
Other Factors Related to Aβ and Tau in AD
Infection
The “inflammation hypothesis” [67], “cholinergic hypothesis” [68], and “amyloid cascade hypothesis” [69] are three important hypotheses on the etiopathogenesis of AD. Several microbes, such as human herpesviruses, spirochetes, Chlamydia pneumoniae, and Borrelia burgdorferi, have been proposed as triggers of AD [70]. Infections may induce the generation of Aβ in the brain [71]. The antiviral property of Aβ could protect the brain from infection [72].
Oxidative Stress
Oxidative stress (OS) plays a critical role in AD pathogenesis [73,74]. Oxidative stress promotes both tau hyperphosphorylation and Aβ deposition and then the loss of synapses and neurons [75].
Prions of Aβ and Tau
Prions are defined as host-encoded proteins that adopt alternative conformations and are self-propagating [76]. Both Aβ and tau are found to have prion features in AD [77]. Tau prions has been shown to spread throughout the brain along known neuroanatomical pathways over the course of AD [78].
The Gut Microbiota
Activated proinflammatory cytokines by the altering of gut microbiota increase intestinal permeability and lead to insulin resistance that is associated with AD [79]. Aβ oligomers translocating from the gut to the brain contribute to the onset of AD and neuroinflammation [80]. Eubacterium rectale, Porphyromonas gingivalis, and Lactobacillus rhamnosus are important in the origination of AD [81–85].
Astroglia
Astrocytes are key components of the neurovascular unit (NVU) [86]. In pathological situations, astrocytes turn into reactive astrocytes undergoing a series of morphological and functional alterations. Reactive astrocytes are typically found in the region with high Aβ or tau pathology in postmortem AD brains [87–89]. Reactive astrocytes release cytokines, inflammatory factors, and reactive oxygen species (ROS), thus contributing to neuroinflammatory changes in AD [90].
TREM2
Genome-wide association studies (GWAS) identified the gene triggering receptor expressed on myeloid cells 2 (TREM2) that are associated with a high risk of AD [91]. TREM2, a microglia surface receptor, is especially highly expressed in microglial cells [92,93]. The R47H and D87N TREM2 mutation confer significant risk of AD in humans [91,94]. Soluble TREM2 levels are related to levels of total tau and phospho-tau in cerebrospinal fluid (CSF), but not to Aβ1–42 levels in AD brains [95–98].
Synapse-Based Therapy in Alzheimer’s Disease
Synaptic deficiency is an early sign of AD pathogenesis and is closely correlated with the cognitive decline in AD. Oligomeric Aβ or soluble hyperphosphorylated tau interacts with cell surface receptors, scaffold proteins, or intracellular signaling molecules to destroy synaptic structure and function. Therefore, therapeutic strategies targeting synaptopathy at the early stage of AD may ameliorate pathology in AD [99]. To date, no effective intervention alleviating Aβ load has been developed, although oligomeric Aβ is the main factor involved in the synaptotoxicity in AD. It is more important to find Aβ species-specific interventions [100]. For example, Aβ*56 identified in the brains and CSF of people with normal cognitive function triggers a specific intracellular response to hyperphosphorylated tau through CaMKIIα but not GSK-3β or Cdk5. Aβ*56 or its downstream signaling cascades may be promising targets for early-stage AD interventions [101]. Moreover, because APP processing is regulated by neuronal activity, enhancing synaptic function, which prevents synaptic dysfunction and reduces Aβ production, could have a dual beneficial effect in reversing AD progression [3]. Since tau is more closely associated with cognitive decline than Aβ in the AD brain, great efforts have been made to explore tau-focused therapeutic interventions [102], and approaches focusing on pathological tau removal have been developed. Antibody-mediated [103–106] or anti-sense oligonucleotide (ASO)-mediated tau reduction [107] successfully reduced synaptic dysfunction and cognitive deficits in AD animal models. Drugs which aim at new molecular targets related to dendritic and postsynaptic tau need more exploration. Since the synaptotoxicity of Aβ is mediated by receptor complexes, multiple strategies have been developed to target these cell surface proteins and their downstream signaling pathways. Extracellular regulation of the activity of these receptors makes them more accessible targets for AD treatment.
Conclusions
Synaptic defects closely associated with cognitive decline are considered the early events in AD pathogenesis. Based on a large number of studies, soluble Aβ oligomers appear to be the primary cause of synaptic dysfunction, and synaptic tau seems to be the indispensable mediator in the process (Figure 1). Aβ oligomers affect LTP, LTD, and synaptic death by acting on receptors, unbalancing calcium homeostasis, and activating caspases and calcineurin. Soluble hyperphosphorylated tau impairs synaptic function by interacting with scaffold proteins, cell surface receptors, or intracellular signaling molecules. Aβ or phosphorylated tau also triggers synaptic degeneration via damaging and transportation of mitochondria.
Figure 1.
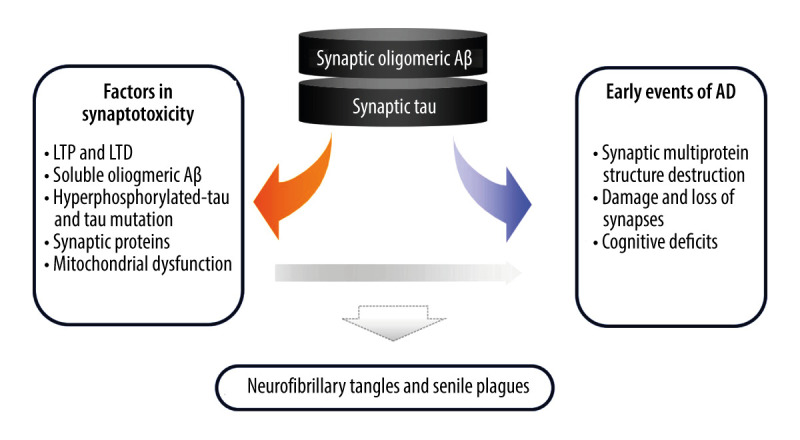
Feedback mechanisms of synaptic Aβ and tau in the progress of Alzheimer’s disease. Soluble oligomeric Aβ is the primary cause of synaptic dysfunction, whereas synaptic tau is the mediator in the progress. They locate in the synapse and work with receptors, synaptic proteins, and mitochondria to cause synaptic dysfunction, and ultimately lead to cognitive deficits. During the pathogenesis of AD, this is the early event, which occurs long before the formation of neurofibrillary tangles and senile plaques.
However, further investigations are still needed to answer key questions: 1) Why have therapeutic strategies targeting Aβ all failed if Aβ oligomers are the initiator of AD’s early pathology? 2) Why is synaptic tau required in the synaptotoxicity attributed to Aβ? In the future, a combination therapy targeting synaptic tau and Aβ together may be a potential therapeutic approach for AD patients at the very early stage.
Footnotes
Declaration of Figure Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This work was supported in part by Nantong University and grants from the National Natural Science Foundation of China (81872875, 81170317 and 81473218 to Wei Qian; 81503077 to Xiaomin Yin) and the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD)
References
- 1.Ittner LM, Gotz J. Amyloid-beta and tau – a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12(2):65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 2.Scheltens P, Blennow K, Breteler MM, et al. Alzheimer’s disease. Lancet. 2016;388(10043):505–17. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Fu AKY, Ip NY. Synaptic dysfunction in Alzheimer’s disease: Mechanisms and therapeutic strategies. Pharmacol Ther. 2019;195:186–98. doi: 10.1016/j.pharmthera.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Naseri NN, Wang H, Guo J, et al. The complexity of tau in Alzheimer’s disease. Neurosci Lett. 2019;705:183–94. doi: 10.1016/j.neulet.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koffie RM, Meyer-Luehmann M, Hashimoto T, et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA. 2009;106(10):4012–17. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serrano-Pozo A, Frosch MP, Masliah E, et al. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–81. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sydow A, Van der Jeugd A, Zheng F, et al. Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic Tau mutant. J Neurosci. 2011;31(7):2511–25. doi: 10.1523/JNEUROSCI.5245-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagy Z, Jobst KA, Esiri MM, et al. Hippocampal pathology reflects memory deficit and brain imaging measurements in Alzheimer’s disease: Clinicopathologic correlations using three sets of pathologic diagnostic criteria. Dementia. 1996;7(2):76–81. doi: 10.1159/000106857. [DOI] [PubMed] [Google Scholar]
- 10.Hyman BT, Van Hoesen GW, Damasio AR, et al. Alzheimer’s disease: Cell-specific pathology isolates the hippocampal formation. Science. 1984;225(4667):1168–70. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 11.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 12.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–80. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 13.Masliah E, Hansen L, Albright T, et al. Immunoelectron microscopic study of synaptic pathology in Alzheimer’s disease. Acta Neuropathol. 1991;81(4):428–33. doi: 10.1007/BF00293464. [DOI] [PubMed] [Google Scholar]
- 14.Scheff SW, Price DA, Schmitt FA, et al. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27(10):1372–84. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121(2):171–81. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 16.DeVos SL, Corjuc BT, Oakley DH, et al. Synaptic tau seeding precedes tau pathology in human Alzheimer’s disease brain. Front Neurosci. 2018;12:267. doi: 10.3389/fnins.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann Neurol. 1990;27(5):457–64. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 18.Yoshiyama Y, Higuchi M, Zhang B, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53(3):337–51. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157(1):163–86. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Tatavarty V, Sun Q, Turrigiano GG. How to scale down postsynaptic strength. J Neurosci. 2013;33(32):13179–89. doi: 10.1523/JNEUROSCI.1676-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blurton-Jones M, Laferla FM. Pathways by which Abeta facilitates tau pathology. Curr Alzheimer Res. 2006;3(5):437–48. doi: 10.2174/156720506779025242. [DOI] [PubMed] [Google Scholar]
- 22.Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2(7):a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ittner A, Ittner LM. Dendritic tau in Alzheimer’s dfisease. Neuron. 2018;99(1):13–27. doi: 10.1016/j.neuron.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 25.Bilousova T, Miller CA, Poon WW, et al. Synaptic amyloid-beta oligomers precede P-tau and differentiate high pathology control cases. Am J Pathol. 2016;186(1):185–98. doi: 10.1016/j.ajpath.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klementieva O, Willen K, Martinsson I, et al. Pre-plaque conformational changes in Alzheimer’s disease-linked Abeta and APP. Nat Commun. 2017;8:14726. doi: 10.1038/ncomms14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forner S, Baglietto-Vargas D, Martini AC, et al. Synaptic impairment in Alzheimer’s disease: A dysregulated symphony. Trends Neurosci. 2017;40(6):347–357. doi: 10.1016/j.tins.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer’s disease: Synapses gone cold. Mol Neurodegener. 2011;6(1):63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pozueta J, Lefort R, Shelanski ML. Synaptic changes in Alzheimer’s disease and its models. Neuroscience. 2013;251:51–65. doi: 10.1016/j.neuroscience.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 30.John A, Reddy PH. Synaptic basis of Alzheimer’s disease: Focus on synaptic amyloid beta, P-tau and mitochondria. Ageing Res Rev. 2021;65:101208. doi: 10.1016/j.arr.2020.101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajmohan R, Reddy PH. Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J Alzheimers Dis. 2017;57(4):975–99. doi: 10.3233/JAD-160612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu X, Chong WP, Zhai Y, et al. Functional polymorphisms of the CCL2 and MBL genes cumulatively increase susceptibility to severe acute respiratory syndrome coronavirus infection. J Infect. 2015;71(1):101–9. doi: 10.1016/j.jinf.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fein JA, Sokolow S, Miller CA, et al. Co-localization of amyloid beta and tau pathology in Alzheimer’s disease synaptosomes. Am J Pathol. 2008;172(6):1683–92. doi: 10.2353/ajpath.2008.070829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura T, Whitcomb DJ, Jo J, et al. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2014;369(1633):20130144. doi: 10.1098/rstb.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regan P, Piers T, Yi JH, et al. Tau phosphorylation at serine 396 residue is required for hippocampal LTD. J Neurosci. 2015;35(12):4804–12. doi: 10.1523/JNEUROSCI.2842-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Jeugd A, Hochgrafe K, Ahmed T, et al. Cognitive defects are reversible in inducible mice expressing pro-aggregant full-length human Tau. Acta Neuropathol. 2012;123(6):787–805. doi: 10.1007/s00401-012-0987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seo J, Kritskiy O, Watson LA, et al. Inhibition of p25/Cdk5 attenuates tauopathy in mouse and iPSC models of frontotemporal dementia. J Neurosci. 2017;37(41):9917–24. doi: 10.1523/JNEUROSCI.0621-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bi M, Gladbach A, van Eersel J, et al. Tau exacerbates excitotoxic brain damage in an animal model of stroke. Nat Commun. 2017;8(1):473. doi: 10.1038/s41467-017-00618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ittner LM, Ke YD, Delerue F, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142(3):387–97. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 41.Roberson ED, Scearce-Levie K, Palop JJ, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316(5825):750–54. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 42.Roberson ED, Halabisky B, Yoo JW, et al. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci. 2011;31(2):700–11. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chabrier MA, Cheng D, Castello NA, et al. Synergistic effects of amyloid-beta and wild-type human tau on dendritic spine loss in a floxed double transgenic model of Alzheimer’s disease. Neurobiol Dis. 2014;64:107–17. doi: 10.1016/j.nbd.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoover BR, Reed MN, Su J, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68(6):1067–81. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warmus BA, Sekar DR, McCutchen E, et al. Tau-mediated NMDA receptor impairment underlies dysfunction of a selectively vulnerable network in a mouse model of frontotemporal dementia. J Neurosci. 2014;34(49):16482–95. doi: 10.1523/JNEUROSCI.3418-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crimins JL, Rocher AB, Luebke JI. Electrophysiological changes precede morphological changes to frontal cortical pyramidal neurons in the rTg4510 mouse model of progressive tauopathy. Acta Neuropathol. 2012;124(6):777–95. doi: 10.1007/s00401-012-1038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han S, Jeong YY, Sheshadri P, et al. Mitophagy regulates integrity of mitochondria at synapses and is critical for synaptic maintenance. EMBO Rep. 2020;21(9):e49801. doi: 10.15252/embr.201949801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14(2):45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reddy PH, Tripathi R, Troung Q, et al. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: Implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2012;1822(5):639–49. doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pickett EK, Rose J, McCrory C, et al. Region-specific depletion of synaptic mitochondria in the brains of patients with Alzheimer’s disease. Acta Neuropathol. 2018;136(5):747–57. doi: 10.1007/s00401-018-1903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddy PH, Mani G, Park BS, et al. Differential loss of synaptic proteins in Alzheimer’s disease: Implications for synaptic dysfunction. J Alzheimers Dis. 2005;7(2):103–17. doi: 10.3233/jad-2005-7203. discussion 173–80. [DOI] [PubMed] [Google Scholar]
- 52.Carlin RK, Grab DJ, Cohen RS, et al. Isolation and characterization of postsynaptic densities from various brain regions: Enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86(3):831–45. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Winters C, Azzam R, et al. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci USA. 2008;105(11):4453–58. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheng M, Kim E. The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol. 2011;3(12):a005678. doi: 10.1101/cshperspect.a005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X, Levy JM, Hou A, et al. PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proc Natl Acad Sci USA. 2015;112(50):E6983–92. doi: 10.1073/pnas.1517045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17(7):343–52. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Opazo P, Sainlos M, Choquet D. Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr Opin Neurobiol. 2012;22(3):453–60. doi: 10.1016/j.conb.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 58.Zhu J, Zhou Q, Shang Y, et al. Synaptic targeting and function of SAPAPs mediated by phosphorylation-dependent binding to PSD-95 MAGUKs. Cell Rep. 2017;21(13):3781–93. doi: 10.1016/j.celrep.2017.11.107. [DOI] [PubMed] [Google Scholar]
- 59.Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: Randomised controlled trial. Br J Psychiatry. 2011;198(5):351–56. doi: 10.1192/bjp.bp.110.080044. [DOI] [PubMed] [Google Scholar]
- 60.Levy JM, Chen X, Reese TS, et al. Synaptic consolidation normalizes AMPAR quantal size following MAGUK loss. Neuron. 2015;87(3):534–48. doi: 10.1016/j.neuron.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arbuckle MI, Komiyama NH, Delaney A, et al. The SH3 domain of postsynaptic density 95 mediates inflammatory pain through phosphatidylinositol-3-kinase recruitment. EMBO Rep. 2010;11(6):473–78. doi: 10.1038/embor.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Bartolomeis A, Latte G, Tomasetti C, et al. Glutamatergic postsynaptic density protein dysfunctions in synaptic plasticity and dendritic spines morphology: Relevance to schizophrenia and other behavioral disorders pathophysiology, and implications for novel therapeutic approaches. Mol Neurobiol. 2014;49(1):484–511. doi: 10.1007/s12035-013-8534-3. [DOI] [PubMed] [Google Scholar]
- 63.Savioz A, Leuba G, Vallet PG. A framework to understand the variations of PSD-95 expression in brain aging and in Alzheimer’s disease. Ageing Res Rev. 2014;18:86–94. doi: 10.1016/j.arr.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J, Saur T, Duke AN, et al. Motor impairments, striatal degeneration, and altered dopamine-glutamate interplay in mice lacking PSD-95. J Neurogenet. 2014;28(1–2):98–111. doi: 10.3109/01677063.2014.892486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong S, Beja-Glasser VF, Nfonoyim BM, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–16. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Wilde MC, Overk CR, Sijben JW, et al. Meta-analysis of synaptic pathology in Alzheimer’s disease reveals selective molecular vesicular machinery vulnerability. Alzheimers Dement. 2016;12(6):633–44. doi: 10.1016/j.jalz.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2013;9(1):25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- 68.Bartus RT, Dean RL, 3rd, Beer B, et al. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 69.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12(10):383–88. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 70.Abbott A. Are infections seeding some cases of Alzheimer’s disease? Nature. 2020;587(7832):22–25. doi: 10.1038/d41586-020-03084-9. [DOI] [PubMed] [Google Scholar]
- 71.Bourgade K, Le Page A, Bocti C, et al. Protective effect of amyloid-beta peptides against herpes simplex virus-1 infection in a neuronal cell culture model. J Alzheimers Dis. 2016;50(4):1227–41. doi: 10.3233/JAD-150652. [DOI] [PubMed] [Google Scholar]
- 72.White MR, Kandel R, Tripathi S, et al. Alzheimer’s associated beta-amyloid protein inhibits influenza A virus and modulates viral interactions with phagocytes. PLoS One. 2014;9(7):e101364. doi: 10.1371/journal.pone.0101364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butterfield DA, Boyd-Kimball D. Oxidative stress, amyloid-beta peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer’s disease. J Alzheimers Dis. 2018;62(3):1345–67. doi: 10.3233/JAD-170543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen X, Guo C, Kong J. Oxidative stress in neurodegenerative diseases. Neural Regen Res. 2012;7(5):376–85. doi: 10.3969/j.issn.1673-5374.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Z, Zhong C. Oxidative stress in Alzheimer’s disease. Neurosci Bull. 2014;30(2):271–81. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ayers JI, Paras NA, Prusiner SB. Expanding spectrum of prion diseases. Emerg Top Life Sci. 2020;4(2):155–67. doi: 10.1042/ETLS20200037. [DOI] [PubMed] [Google Scholar]
- 77.Fan L, Mao C, Hu X, et al. New insights into the pathogenesis of Alzheimer’s disease. Front Neurol. 2019;10:1312. doi: 10.3389/fneur.2019.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 79.Ma Q, Xing C, Long W, et al. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J Neuroinflammation. 2019;16(1):53. doi: 10.1186/s12974-019-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun Y, Sommerville NR, Liu JYH, et al. Intra-gastrointestinal amyloid-beta1–42 oligomers perturb enteric function and induce Alzheimer’s disease pathology. J Physiol. 2020;598(19):4209–23. doi: 10.1113/JP279919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mehrabadi S, Sadr SS. Assessment of probiotics mixture on memory function, inflammation markers, and oxidative stress in an Alzheimer’s disease model of rats. Iran Biomed J. 2020;24(4):220–28. doi: 10.29252/ibj.24.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang CH, Lin CH, Lane HY. d-glutamate and gut microbiota in Alzheimer’s disease. Int J Mol Sci. 2020;21(8):2676. doi: 10.3390/ijms21082676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–17. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 84.Singhrao SK, Harding A, Poole S, et al. Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer’s disease. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/137357. 137357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cattaneo A, Cattane N, Galluzzi S, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 86.Iadecola C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Serrano-Pozo A, Mielke ML, Gomez-Isla T, et al. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am J Pathol. 2011;179(3):1373–84. doi: 10.1016/j.ajpath.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marutle A, Gillberg PG, Bergfors A, et al. (3)H-deprenyl and (3)H-PIB autoradiography show different laminar distributions of astroglia and fibrillar beta-amyloid in Alzheimer brain. J Neuroinflammation. 2013;10:90. doi: 10.1186/1742-2094-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lemoine L, Saint-Aubert L, Nennesmo I, et al. Cortical laminar tau deposits and activated astrocytes in Alzheimer’s disease visualised by (3)H-THK5117 and (3)H-deprenyl autoradiography. Sci Rep. 2017;7:45496. doi: 10.1038/srep45496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heneka MT, Rodriguez JJ, Verkhratsky A. Neuroglia in neurodegeneration. Brain Res Rev. 2010;63(1–2):189–211. doi: 10.1016/j.brainresrev.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 91.Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368(2):107–16. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lill CM, Rengmark A, Pihlstrom L, et al. The role of TREM2 R47H as a risk factor for Alzheimer’s disease, frontotemporal lobar degeneration, amyotrophic lateral sclerosis, and Parkinson’s disease. Alzheimers Dement. 2015;11(12):1407–16. doi: 10.1016/j.jalz.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin SC, Benitez BA, Karch CM, et al. Coding variants in TREM2 increase risk for Alzheimer’s disease. Hum Mol Genet. 2014;23(21):5838–46. doi: 10.1093/hmg/ddu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guerreiro R, Wojtas A, Bras J, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368(2):117–27. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Henjum K, Almdahl IS, Arskog V, et al. Cerebrospinal fluid soluble TREM2 in aging and Alzheimer’s disease. Alzheimers Res Ther. 2016;8(1):17. doi: 10.1186/s13195-016-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Piccio L, Deming Y, Del-Aguila JL, et al. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 2016;131(6):925–33. doi: 10.1007/s00401-016-1533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suarez-Calvet M, Kleinberger G, Araque Caballero MA, et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol Med. 2016;8(5):466–76. doi: 10.15252/emmm.201506123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heslegrave A, Heywood W, Paterson R, et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol Neurodegener. 2016;11:3. doi: 10.1186/s13024-016-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25(1):59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 100.Figueiredo CP, Clarke JR, Ledo JH, et al. Memantine rescues transient cognitive impairment caused by high-molecular-weight abeta oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J Neurosci. 2013;33(23):9626–34. doi: 10.1523/JNEUROSCI.0482-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amar F, Sherman MA, Rush T, et al. The amyloid-β oligomer Aβ*56 induces specific alterations in neuronal signaling that lead to tau phosphorylation and aggregation. Sci Signal. 2017;10(478) doi: 10.1126/scisignal.aal2021. eaal2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li C, Gotz J. Somatodendritic accumulation of Tau in Alzheimer’s disease is promoted by Fyn-mediated local protein translation. EMBO J. 2017;36(21):3120–38. doi: 10.15252/embj.201797724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010;30(49):16559–66. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Castillo-Carranza DL, Guerrero-Munoz MJ, Sengupta U, et al. Tau immunotherapy modulates both pathological tau and upstream amyloid pathology in an Alzheimer’s disease mouse model. J Neurosci. 2015;35(12):4857–68. doi: 10.1523/JNEUROSCI.4989-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ittner A, Bertz J, Suh LS, et al. Tau-targeting passive immunization modulates aspects of pathology in tau transgenic mice. J Neurochem. 2015;132(1):135–45. doi: 10.1111/jnc.12821. [DOI] [PubMed] [Google Scholar]
- 106.Yanamandra K, Kfoury N, Jiang H, et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80(2):402–14. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.DeVos SL, Miller RL, Schoch KM, et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med. 2017;9(374) doi: 10.1126/scitranslmed.aag0481. eaag0481. [DOI] [PMC free article] [PubMed] [Google Scholar]


