Abstract
Since the adoption of the model for end-stage liver disease (MELD) score for organ allocation in 2002, numerous changes to the system of liver allocation and distribution have been made with the goal of decreasing waitlist mortality and minimizing geographic variability in median MELD score at time of transplant without worsening post-transplant outcomes. These changes include the creation and adoption of the MELD-Na score for allocation, Regional Share 15, Regional Share for Status 1, Regional Share 35/National Share 15, and, most recently, the Acuity Circles Distribution Model. However, geographic differences in median MELD at time of transplant remain as well as limits to the MELD score for allocation, as etiology of liver disease and need for transplant changes. Acute-on-chronic liver failure (ACLF) is a subset of liver failure where prevalence is rising and has been shown to have an increased mortality rate and need for transplantation that is under-demonstrated by the MELD score. This underscores the limitations of the MELD score and raises the question of whether MELD is the most accurate, objective allocation system. Alternatives to the MELD score have been proposed and studied, however MELD score remains as the current system used for allocation. This review highlights policy changes since the adoption of the MELD score, addresses limitations of the MELD score, reviews proposed alternatives to MELD, and examines the specific implications of these changes and alternatives for ACLF.
Keywords: Model for end-stage liver disease score, Acute-on-chronic liver failure, Regional sharing
Core Tip: Since the adoption of the model for end-stage liver disease (MELD) score for organ allocation in 2002, there have been numerous changes to policy in an effort to make organ allocation and distribution more fair and equitable. This review highlights policy changes since the adoption of the MELD score, addresses limitations of the MELD score, reviews proposed alternatives to MELD, and examines the specific implications of these changes and alternatives for acute-on-chronic liver failure.
INTRODUCTION
Organ allocation for liver transplantation was revolutionized in 2002 by wide adoption of the model for end-stage liver disease (MELD) scoring system, which utilized objective criteria to facilitate equitable organ allocation. Although this system has improved fairness in prioritizing patients for transplantation, important disparities remain. In this review, we discuss current organ allocation policy and future directions through a historical lens, from the pre-MELD era through the development of MELD exception points, regional sharing, and implementation of the MELD-Na score. We conclude with an examination of limitations of the MELD scoring system in assessing mortality in certain patient groups and areas for improvement in current organ allocation policy.
OVERVIEW AND HISTORY OF MELD
Pre-MELD era
Prior to 1997, liver transplant priority was determined by hospitalization status and time on the waiting list. For example, a patient in the intensive care units (ICU) was given priority over a non-ICU hospitalized patient who was given priority over an outpatient. This system was based on subjective criteria that could be manipulated by hospitalizing patients or admitting to the ICU when there was no medical indication, thereby fraudulently giving a patient an advantage over others.
In 1998, United Network for Organ Sharing (UNOS) adopted the Child-Turcotte-Pugh (CTP) scoring system to stratify patients as Status 2A, 2B, or 3 for patients at high risk of death without transplantation, with Status 1 reserved for patients with acute liver failure. The CTP score incorporated objective data into waiting list priority, but still included subjective grading of encephalopathy and ascites which allowed for wide variability and the potential for inappropriately scoring the severity of a patient’s condition. The CTP score was originally proposed in 1964 by surgeons Child et al[1] as a way to assess operative risk in patients undergoing surgical portosystemic shunt for variceal bleeding—patients were given a subclass score of A-C depending on bilirubin, albumin, ascites, hepatic encephalopathy, and nutritional status[1]. In 1973, Pugh et al[2] modified the scoring system by adding prothrombin time and removing nutritional status which became known as the CTP score[2]. In 2000, the United States Department of Health and Human Services released the Final Rule, which mandated that organ allocation should be based upon medical urgency that is determined by objective and reproducible data and that access to transplant should not be affected by geography[3].
Adoption of MELD score and donation service areas
The Mayo transjugular intrahepatic portosystemic shunt (TIPS) model was originally developed in 2000 as a scoring system to predict three-month mortality in patients with cirrhosis who underwent a TIPS procedure[4]. A year later this scoring system was shown to also be a reliable predictor of three-month mortality in patients with cirrhosis and became known as the MELD score[5]. The MELD score incorporated serum bilirubin, serum creatinine, international normalized ratio (INR) for prothrombin time, and etiology of liver disease. However, etiology of liver disease was shown to have minimal impact on outcomes and was later removed from the scoring system[6].
The Final Rule led to the Organ Procurement and Transplant Network to implement the MELD score to prioritize patients awaiting deceased donor liver transplantation using only three objective lab values in its calculation—serum bilirubin, serum creatinine, and INR. In February 2002, donor liver allocation based on MELD score was implemented in the United States. The use of the MELD score led to more transplants for sicker patients and reduced waitlist mortality without reducing post-transplant survival[7]. However, distribution of donor livers prioritized patients within the local donation service area (DSA), followed by the UNOS region, and finally the nation. For example, if an organ became available, it was prioritized to the patient with the highest MELD score within that DSA. If the liver was not accepted by a transplant center within that DSA, it would be offered within the UNOS region, and then nationally. However, the differences in population size and demographics within DSAs and UNOS regions gradually led to significant geographic disparities in the MELD score at time of transplant, and therefore access to liver transplantation[7].
Policy changes to liver allocation and distribution since 2002
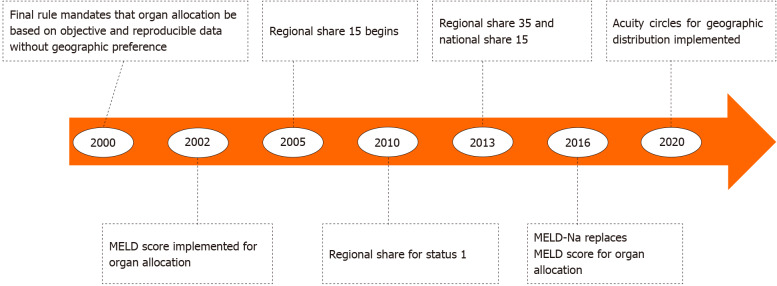
Since 2002, numerous changes to the system of liver allocation and distribution have been made with the goal of decreasing waitlist mortality and minimizing geographic variability in median MELD score at time of transplant without worsening post-transplant outcomes (Figure 1). Liver allocation refers to how waitlisted patients are prioritized by medical urgency based on the MELD score while liver distribution refers to the system by which donor livers are matched to patients on the waitlist based on geographic units. Each of these will be discussed below.
Figure 1.
History of changes in organ allocation policy in the United States. MELD: Model for end-stage liver disease.
CHANGES IN LIVER ALLOCATION AND DISTRIBUTION IN THE UNITED STATES
Incorporation of serum sodium level (MELD-Na)
Multiple studies have shown that hyponatremia is an independent predictor of mortality in patients with cirrhosis[8-10]. Hyponatremia has also been shown to be a predictor of hepatorenal syndrome occurrence which is also associated with increased mortality[11]. In 2008, Kim et al[12] showed that adding serum sodium to the MELD score was a better predictor of mortality than MELD alone, making the argument that serum sodium should be added to the MELD score model[12]. The incorporation of serum sodium into the MELD score calculation was eventually adopted by UNOS in 2016. Studies evaluating the effectiveness of the MELD-Na score have shown the MELD-Na to be a more accurate predictor of 90-d mortality and that using the MELD-Na for liver allocation leads to a decrease in waitlist mortality[12-14].
Regional share 15
In 2005, Merion et al[15] showed mortality risk reduction in patients transplanted with a MELD score of 18 or greater with an increasing mortality reduction as the MELD score increased. But they also showed increased mortality in patients transplanted with a MELD score less than 14 compared to candidates who remained on the waitlist[15]. Due to these findings, the Regional Share 15 policy was implemented, which called for an organ to first be offered within the local DSA to patients with a MELD greater than 15 and then regionally before being offered locally to patients with a MELD less than 15.
Regional share for Status 1
Patients listed as Status 1 for liver transplantation are critically ill with acute liver failure and have a life expectancy of 7 d or less without transplantation. Under Regional Share for Status 1, patients listed as Status 1 would receive priority for transplant ahead of all other patients listed within an entire UNOS region. This policy change was implemented in December 2010 and was found to significantly increase the probability of transplantation within 7 d of listing as status 1 without negatively impacting waitlist mortality for non-status 1 patients in the same region[16].
Regional share 35 and national share 15
In 2012, it was shown that patients with a MELD score ³35 had a waitlist mortality similar to patients listed with acute liver failure status 1, but only status 1 patients were eligible for regional sharing[17]. This lead to the Regional Share 35 and the National Share 15 policy change in 2013, which called for donor livers to be offered first to patients with a MELD score ³35 Listed within a region. If the liver was not accepted by a center then the distribution sequence was as follows: offered to patients with a MELD score ³15 within the DSA, offered to patients with a MELD score ³15 within the region, offered nationally to patients with MELD score ³15, before finally being offered locally to patients with MELD scores < 15. One year later, the Regional Share 35 policy was found to have the following effects: An increase in total transplants, 30% lower waitlist mortality for patients with MELD greater than 30, a decrease in in the number of unused organs, and no worsening of early post-transplant outcomes[18]. No difference was seen nationally when comparing post-transplant survival before and after implementation of Regional Share 35, however two regions did show significantly worse post-transplant outcomes after the policy was enacted[19].
Acuity circles distribution system
Despite the adoption of policy changes for donor liver distribution in the United States such as Regional Share for Status 1, Regional Share 35, and National Share 15, significant geographic variability in access to liver transplantation remained within the local-regional-national system of organ distribution with the median MELD score at transplant varying as much as 12 points in high vs low MELD score regions[20]. Spurred by lawsuits involving the lung transplant allocation system which prompted calls to eliminate the use of DSAs and UNOS regions as units of organ distribution, a new liver distribution system, known as Acuity Circles, based on concentric geographic circles around the donor site hospital was accepted in 2018 and implemented in 2020[21]. Acuity circles calls for a donor liver to first be offered to patients listed Status 1 within 500 nautical miles (nm) of the donor hospital. The organ is then offered to patients with a MELD score of at least 37 within 150 miles of the donor hospital, then to patients with a MELD score of at least 37 within 250 miles, and finally to patients with a MELD score of at least 37 within 500 miles. If the organ is not accepted for any of these patients, then it is allocated to patients with decreasing MELD score thresholds of 33, then 29, then 15 in expanding geographic circles at each MELD score tier as above before being allocated nationally, until finally being offered to patients with a MELD score under 15. As with prior policy changes, the new system was implemented to further minimize geographic disparities in access to liver transplantation.
WORLDWIDE ORGAN ALLOCATION
The MELD score is still used by many countries worldwide that perform a high volume of liver transplantations yearly. The MELD score was implemented for liver allocation in the United States in 2002 by UNOS. It was followed by North Italian Transplant (2006), Eurotransplant (2006), Canada (2006) and many others[22]. In Asia, South Korea became the first country to used MELD score for organ allocation in 2016[23]. Some countries allow for a center-specific allocation policy, although that can only be applied in areas with high organ donation rates such as Scandinavia, Spain and Portugal[22,24].
Other countries have tried to combine recipient needs with donor availability. In 2007, France began using the French Liver Allocation Score which uses objective data of the recipient like MELD score, but additionally uses other data points such as donor-recipient distance and waiting time[24,25]. The United Kingdom began using a new allocation model in 2018 that aims to give urgent cases priority—the transplant benefit score uses donor and recipient parameters to determine optimal match[24].
LIMITATIONS OF THE MELD AND MELD-NA SCORE
The system of awarding MELD exceptions as described in the preceding section is helpful to account for conditions not addressed by the MELD calculation, however there are inherent limitations to the MELD model itself which will be discussed below.
Renal function assessment
The MELD score incorporates renal function into its calculation by using the serum creatinine value. However, patients with advanced cirrhosis often have significant muscle wasting which can lead to a “normal” creatinine level that underestimates the severity of their renal dysfunction[26,27]. Differences in muscle mass between men and women also leads to a disadvantage in organ allocation for women--their lower muscle mass leads to a lower creatinine level for equivalent renal function, leading to a lower MELD score[28,29]. Serum creatinine levels can also vary day-to-day in patients with ascites undergoing diuresis or paracentesis, and this variance is unlikely to actually reflect a true change in mortality risk[27]. Differences in the calculation of serum creatinine have also been shown to depend on the assay used by each laboratory[30].
The serum creatinine value in the MELD calculation also has a lower limit of 1 mg/dL and upper limit of 4 mg/dL, both of which have been called into question. The lower limit is in place to avoid negative values after logarithmic transformation in the MELD calculation[31], but this would assume that mortality risk is constant for all values below 1 mg/dL. The upper limit boundary was created so as to not raise the MELD score due to intrinsic kidney disease, however there is evidence that patients with a creatinine level greater than 4 mg/dL have a significantly higher mortality than those with a lower creatinine level[32].
Acute-on-chronic liver failure
Acute-on-chronic liver failure (ACLF) has been identified as a separate clinical entity from acute liver failure and acute decompensated cirrhosis and defined as “a syndrome in patients with chronic liver disease with or without cirrhosis, which is characterized by acute hepatic decompensation, organ failures, and a 28-d mortality greater than 15%[33,34].” The prevalence of ACLF is rising in the United States, particularly in the elderly[35,36]. ACLF is graded according to concurrent organ failures—ACLF grade 1 (ACLF-1) is single organ failure, ACLF grade 2 (ACLF-2) includes patients with two organ failures, and ACLF grade 3 (ACLF-3) includes patients with 3 organ failures or more[34]. ACLF-3 has a mortality without liver transplantation of 80% at 28 d and greater than 90% at one year[37].
The MELD score has been shown to be accurate for assessing mortality risk in decompensated cirrhosis, but ACLF presents a distinct entity with increased systemic inflammation and development of organ failures[37] and so the mortality risk of these patients is not completely demonstrated within their calculated MELD score. A study of the UNOS database showed that patients with ACLF-3 and MELD-Na score less than 25 had greater waitlist mortality than those without ACLF and a MELD-Na score greater than 35[38]. A recent study from the same group showed that ACLF-3 has a higher risk of waitlist mortality or delisting within 14 d compared to patients listed as status 1a, independent of their MELD score, however status 1a patients with acute liver failure have the highest chance of obtaining a liver transplant under the current organ allocation system[39]. The same study also found a rising 21-d mortality rate in patients with ACLF-3 compared to an unchanged mortality rate among status 1a listed patients[39]. A separate study from the UNOS database further demonstrated that utilization of MELD based regional sharing did not improve waitlist mortality among patients with ACLF-3[40].
Changing epidemiology of liver disease
MELD score was adopted as an accurate, objective, and reproducible tool to assess 90-d mortality risk in patients listed for liver transplant. Godfrey et al[41] looked to assess the predictive power of MELD score in assessing mortality risk since its adoption for organ allocation, finding that the MELD score’s concordance with 90-d mortality was decreasing from 0.80 in 2003 to 0.70 in 2015[41]. The authors also found that the concordance of MELD score with mortality was lower in alcohol-related liver disease and non-alcoholic fatty liver disease while higher in patients with hepatitis C virus (HCV) related cirrhosis[41]. Given the shift from HCV-related cirrhosis to alcohol and nonalcoholic steato hepatitis-related cirrhosis as the leading indications for liver transplantation in the United States, these changes may be magnified in the years ahead. In addition to the changing epidemiology of liver disease, the emergence of ACLF as a distinct clinical entity, and the increasing reliance on MELD score exceptions, further studies are needed to determine if a MELD-based system can continue to be the most accurate, objective system for liver allocation.
ALTERNATIVES TO MELD SCORE ALLOCATION
Alternative scoring models have been proposed to the MELD score, as well as alterations to the calculation of the MELD score itself (Table 1). These alternative scoring systems attempt to address some of the issues with the MELD score that were addressed in the preceding section.
Table 1.
Alternatives to the model for end-stage liver disease and model for end-stage liver disease-Na score
|
Test
|
Description
|
Comparison to MELD score
|
Ref.
|
| MELD-GRAIL | Creatinine replaced with GRAIL | Improved 90-d mortality predictor in patients with severe disease (MELD-Na > 32), however similar to MELD-Na in patient with lesser disease severity | Asrani et al[42,43], 2019 |
| MELD-Lactate | Addition of lactate | Better predictor of in-hospital mortality when MELD < 15 or when infection is cause of hospitalization. Similar to MELD-Na in non-infectious admissions | Sarmast et al[44], 2020 |
| Mahmud et al[45], 2021 | |||
| MELD-Plus | Addition of albumin, total cholesterol, WBC count, age, and length of stay | Improved 90-d mortality predictor compared to MELD-Na, however can only be used after a hospital admission | Kartoun et al[46], 2017 |
| CLIF-C ACLF | Score determined by six different organ systems failures, age and WBC count | Improved predictor of 28-d mortality compared to MELD-Na in patients with ACLF. However, only applicable for ACLF and not generalizable for decompensated cirrhosis | Jalan et al[51], 2014 |
| Engelmann et al[52], 2018 | |||
| Ramzan et al[53], 2020 |
GRAIL: Glomerular filtration rate assessment in liver disease; WBC: White blood cell; MELD: Model for end-stage liver disease; ACLF: Acute-on-chronic liver failure; CLIF: Chronic liver failure.
MELD-glomerular filtration rate assessment in liver disease
This scoring system aims to replace serum creatinine as a measure of renal function with a new calculation for glomerular filtration rate (GFR). The GFR assessment in liver disease (GRAIL) uses objective variables (creatinine, blood urea nitrogen, age, gender, race, and albumin) to better estimate renal function in patients awaiting liver transplantation[42]. GRAIL was developed by examining all adult patients with liver disease that underwent admission measurements of GFR using iothalamate clearance from 1985 to 2015[42]. Retrospective analysis showed that MELD-GRAIL-Na had the greatest difference compared to MELD-Na at increased disease severity—for a score ³32 (observed 90 d mortality of 0.68), MELD-GRAIL-Na predicted mortality was 0.67 compared to MELD-Na predicted mortality of 0.51[43]. This scoring system would have resulted in a reclassified status for 16% of patients on the waitlist in 2015[43].
MELD-lactate
The MELD-lactate score incorporates serum lactate into the MELD calculation. This scoring model was developed by examining all patients with chronic liver disease in two health care systems in Texas from 2010-2015[44]. MELD-Lactate was shown to be a better predictor of in-hospital mortality compared to MELD and MELD-Na [area under the curve (AUC) 0.789 vs 0.776 vs 0.760; P < 0.001], with a more pronounced change in patients with a MELD < 15 (MELD-Lactate AUC 0.763 vs 0.674 for MELD)[45]. The MELD-lactate was also a better in-hospital mortality predictor when infection was the reason for hospitalization, however its performance was no different from MELD-Na in other situations[45].
MELD-plus
The MELD-Plus score uses the MELD-Na score along with additional variables found within the electronic medical record. This was developed by examining all cirrhosis related admission from 1992-2010 at Massachusetts General Hospital and Brigham and Women’s Hospital and evaluating variables including demographic information, comorbidities using diagnosis codes, standard laboratory values, and current medication use[46]. Further analysis found that nine variables were the most effective predictors of 90 d mortality (bilirubin, INR, creatinine, Na, albumin, total cholesterol, white blood cell, age, and length of stay) and these were used to calculate the MELD-Plus score. A retrospective analysis showed the MELD-plus had improved 90 d mortality prediction compared to MELD-Na following a hospital admission [0.78 (95%CI: 0.75-0.81) vs 0.70 (95%CI: 0.66-0.73)][46].
ACLF
Patients with ACLF are defined by multi-organ failure and have increased mortality that is underestimated by the MELD score[47]. Scoring systems that may better predict the mortality rate of these patients compared to MELD are being studied. The chronic liver failure–sequential organ failure assessment (CLIF-SOFA) score is a modification to the SOFA score which is used to predict outcomes in ICU level patients[48]. CLIF-SOFA includes sub scores (0 to 4) for each of its six organ components (liver, renal, neurologic, coagulation, circulation, respiratory) with higher scores indicating increased organ disease severity[49]. However, a meta-analysis showed MELD-Na to have a superior AUC compared to CLIF-SOFA for three month mortality in patients with ACLF[50].
A simplified scoring system with the same six organ components became known as the CLIF organ failure (CLIF-OF) score[37]. Further analysis showed that in addition to the CLIF-OF score, age and white cell count were also independently associated with mortality and these were combined with the CLIF-OF score to create the CLIF-C ACLF score[51]. The CLIF-C ACLF score was shown to be the most accurate predictor of 28-d mortality compared to CLIF-OF and MELD for ACLF patients (AUC 0.8 vs 0.75 vs 0.68, respectively)[52]. Another recent study found CLIF-C ACLF score ³70 at 48 h predicted mortality more accurately than MELD score[53]. These scoring systems may be superior to MELD-Na for liver allocation in patients with ACLF.
FUTURE DIRECTIONS
The number of patients awaiting liver transplantation continues to grow and outpace the amount of available organs, necessitating a fair and equitable organ allocation system. Since the creation of the MELD score in 2002, there have been many policy changes and alternatives systems proposed, however there still remains regional disparities. The recent implementation of acuity circles to address geographic distribution will need to be studied and assessed in the coming years. The success of this model will guide policy decision makers in the coming years.
MELD remains the standard scoring system to define disease severity and determine priority for transplantation, however many alternative scoring options have been discussed in this review as well but none have improved enough on the current standard to necessitate a change. Some countries have begun to explore systems that match recipient factors with donor factors to increase utilization of available organs, but more analyzation and assessment of efficacy and improvement will be needed prior to global implementation.
CONCLUSION
Liver transplant organ allocation models and policy have been changing dynamically since the release of the Final Rule in 2000. These changes have led to improvements in liver organ utilization and making transplantation more equitable and fair for all patients, but many limitations and areas for improvement remain. Assessment of recent and past policy changes will be needed to continue to guide future direction for a more equitable liver allocation system.
Footnotes
Conflict-of-interest statement: The authors report no conflicts of interest in relation to this manuscript.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases.
Peer-review started: January 28, 2021
First decision: May 3, 2021
Article in press: July 22, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabbous H, karnam RS, Mrzljak A S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
Contributor Information
Alexander Polyak, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA 90048, United States.
Alexander Kuo, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA 90048, United States.
Vinay Sundaram, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA 90048, United States. vinay.sundaram@cshs.org.
References
- 1.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 2.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 3.Procurement O, Network T. Final rule. Federal Register . 1999;64:56650–56661. [PubMed] [Google Scholar]
- 4.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 5.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 6.Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567–580. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 7.Trotter JF, Osgood MJ. MELD scores of liver transplant recipients according to size of waiting list: impact of organ allocation and patient outcomes. JAMA. 2004;291:1871–1874. doi: 10.1001/jama.291.15.1871. [DOI] [PubMed] [Google Scholar]
- 8.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R, Kamath P, Klintmalm G. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–343. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 10.Moini M, Hoseini-Asl MK, Taghavi SA, Sagheb MM, Nikeghbalian S, Salahi H, Bahador A, Motazedian M, Jafari P, Malek-Hosseini SA. Hyponatremia a valuable predictor of early mortality in patients with cirrhosis listed for liver transplantation. Clin Transplant. 2011;25:638–645. doi: 10.1111/j.1399-0012.2010.01350.x. [DOI] [PubMed] [Google Scholar]
- 11.Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 12.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagai S, Chau LC, Schilke RE, Safwan M, Rizzari M, Collins K, Yoshida A, Abouljoud MS, Moonka D. Effects of Allocating Livers for Transplantation Based on Model for End-Stage Liver Disease-Sodium Scores on Patient Outcomes. Gastroenterology. 2018;155:1451–1462.e3. doi: 10.1053/j.gastro.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Goudsmit BFJ, Putter H, Tushuizen ME, de Boer J, Vogelaar S, Alwayn IPJ, van Hoek B, Braat AE. Validation of the Model for End-stage Liver Disease sodium (MELD-Na) score in the Eurotransplant region. Am J Transplant. 2021;21:229–240. doi: 10.1111/ajt.16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 16.Washburn K, Harper A, Klintmalm G, Goss J, Halff G. Regional sharing for adult status 1 candidates: reduction in waitlist mortality. Liver Transpl. 2006;12:470–474. doi: 10.1002/lt.20768. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, Schaubel DE, Gong Q, Guidinger M, Merion RM. End-stage liver disease candidates at the highest model for end-stage liver disease scores have higher wait-list mortality than status-1A candidates. Hepatology. 2012;55:192–198. doi: 10.1002/hep.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massie AB, Chow EK, Wickliffe CE, Luo X, Gentry SE, Mulligan DC, Segev DL. Early changes in liver distribution following implementation of Share 35. Am J Transplant. 2015;15:659–667. doi: 10.1111/ajt.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halazun KJ, Mathur AK, Rana AA, Massie AB, Mohan S, Patzer RE, Wedd JP, Samstein B, Subramanian RM, Campos BD, Knechtle SJ. One Size Does Not Fit All--Regional Variation in the Impact of the Share 35 Liver Allocation Policy. Am J Transplant. 2016;16:137–142. doi: 10.1111/ajt.13500. [DOI] [PubMed] [Google Scholar]
- 20.Bertsimas D, Papalexopoulos T, Trichakis N, Wang Y, Hirose R, Vagefi PA. Balancing Efficiency and Fairness in Liver Transplant Access: Tradeoff Curves for the Assessment of Organ Distribution Policies. Transplantation. 2020;104:981–987. doi: 10.1097/TP.0000000000003017. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto S, Uchida H, Takeuchi I, Irie R, Shimizu S, Yanagi Y, Takeda M, Fukuda A, Yoshioka T, Arai K, Kasahara M. Sequential Deceased Donor Intestine Transplantation Followed by Living Donor Liver Transplantation, Also Known as Hybrid Intestine-liver Transplantation. Transplantation. 2020;104:e42–e43. doi: 10.1097/TP.0000000000002883. [DOI] [PubMed] [Google Scholar]
- 22.Tschuor C, Ferrarese A, Kuemmerli C, Dutkowski P, Burra P, Clavien PA Liver Allocation Study Group. Allocation of liver grafts worldwide - Is there a best system? J Hepatol. 2019;71:707–718. doi: 10.1016/j.jhep.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Kim DG, Lee JY, Lee JG, Joo DJ, Kim SI, Kim MS. Impact of Model for End-stage Liver Disease Score-based Allocation System in Korea: A Nationwide Study. Transplantation. 2019;103:2515–2522. doi: 10.1097/TP.0000000000002755. [DOI] [PubMed] [Google Scholar]
- 24.Müller PC, Kabacam G, Vibert E, Germani G, Petrowsky H. Current status of liver transplantation in Europe. Int J Surg. 2020;82S:22–29. doi: 10.1016/j.ijsu.2020.05.062. [DOI] [PubMed] [Google Scholar]
- 25.Francoz C, Belghiti J, Castaing D, Chazouillères O, Duclos-Vallée JC, Duvoux C, Lerut J, Le Treut YP, Moreau R, Mandot A, Pageaux G, Samuel D, Thabut D, Valla D, Durand F. Model for end-stage liver disease exceptions in the context of the French model for end-stage liver disease score-based liver allocation system. Liver Transpl. 2011;17:1137–1151. doi: 10.1002/lt.22363. [DOI] [PubMed] [Google Scholar]
- 26.Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003;41:269–278. doi: 10.1053/ajkd.2003.50035. [DOI] [PubMed] [Google Scholar]
- 27.Francoz C, Prié D, Abdelrazek W, Moreau R, Mandot A, Belghiti J, Valla D, Durand F. Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: impact on the model for end-stage liver disease score. Liver Transpl. 2010;16:1169–1177. doi: 10.1002/lt.22128. [DOI] [PubMed] [Google Scholar]
- 28.Cholongitas E, Marelli L, Kerry A, Goodier DW, Nair D, Thomas M, Patch D, Burroughs AK. Female liver transplant recipients with the same GFR as male recipients have lower MELD scores--a systematic bias. Am J Transplant. 2007;7:685–692. doi: 10.1111/j.1600-6143.2007.01666.x. [DOI] [PubMed] [Google Scholar]
- 29.Allen AM, Heimbach JK, Larson JJ, Mara KC, Kim WR, Kamath PS, Therneau TM. Reduced Access to Liver Transplantation in Women: Role of Height, MELD Exception Scores, and Renal Function Underestimation. Transplantation. 2018;102:1710–1716. doi: 10.1097/TP.0000000000002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cholongitas E, Marelli L, Kerry A, Senzolo M, Goodier DW, Nair D, Thomas M, Patch D, Burroughs AK. Different methods of creatinine measurement significantly affect MELD scores. Liver Transpl. 2007;13:523–529. doi: 10.1002/lt.20994. [DOI] [PubMed] [Google Scholar]
- 31.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 32.Huo TI, Hsu CY, Lin HC, Lee PC, Lee JY, Lee FY, Hou MC, Lee SD. Selecting an optimal cutoff value for creatinine in the model for end-stage liver disease equation. Clin Transplant. 2010;24:157–163. doi: 10.1111/j.1399-0012.2009.01099.x. [DOI] [PubMed] [Google Scholar]
- 33.Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute-on-chronic liver failure. Lancet. 2015;386:1576–1587. doi: 10.1016/S0140-6736(15)00309-8. [DOI] [PubMed] [Google Scholar]
- 34.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437, 1437.e1. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 35.Sundaram V, Jalan R, Ahn JC, Charlton MR, Goldberg DS, Karvellas CJ, Noureddin M, Wong RJ. Class III obesity is a risk factor for the development of acute-on-chronic liver failure in patients with decompensated cirrhosis. J Hepatol. 2018;69:617–625. doi: 10.1016/j.jhep.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Sundaram V, Jalan R, Shah P, Singal AK, Patel AA, Wu T, Noureddin M, Mahmud N, Wong RJ. Acute on Chronic Liver Failure From Nonalcoholic Fatty Liver Disease: A Growing and Aging Cohort With Rising Mortality. Hepatology. 2021;73:1932–1944. doi: 10.1002/hep.31566. [DOI] [PubMed] [Google Scholar]
- 37.Arroyo V, Moreau R, Jalan R, Ginès P EASL-CLIF Consortium CANONIC Study. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J Hepatol. 2015;62:S131–S143. doi: 10.1016/j.jhep.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 38.Sundaram V, Jalan R, Wu T, Volk ML, Asrani SK, Klein AS, Wong RJ. Factors Associated with Survival of Patients With Severe Acute-On-Chronic Liver Failure Before and After Liver Transplantation. Gastroenterology. 2019;156:1381–1391.e3. doi: 10.1053/j.gastro.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Sundaram V, Shah P, Wong RJ, Karvellas CJ, Fortune BE, Mahmud N, Kuo A, Jalan R. Patients With Acute on Chronic Liver Failure Grade 3 Have Greater 14-Day Waitlist Mortality Than Status-1a Patients. Hepatology. 2019;70:334–345. doi: 10.1002/hep.30624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundaram V, Shah P, Mahmud N, Lindenmeyer CC, Klein AS, Wong RJ, Karvellas CJ, K Asrani S, Jalan R. Patients with severe acute-on-chronic liver failure are disadvantaged by model for end-stage liver disease-based organ allocation policy. Aliment Pharmacol Ther. 2020;52:1204–1213. doi: 10.1111/apt.15988. [DOI] [PubMed] [Google Scholar]
- 41.Godfrey EL, Malik TH, Lai JC, Mindikoglu AL, Galván NTN, Cotton RT, O'Mahony CA, Goss JA, Rana A. The decreasing predictive power of MELD in an era of changing etiology of liver disease. Am J Transplant. 2019;19:3299–3307. doi: 10.1111/ajt.15559. [DOI] [PubMed] [Google Scholar]
- 42.Asrani SK, Jennings LW, Trotter JF, Levitsky J, Nadim MK, Kim WR, Gonzalez SA, Fischbach B, Bahirwani R, Emmett M, Klintmalm G. A Model for Glomerular Filtration Rate Assessment in Liver Disease (GRAIL) in the Presence of Renal Dysfunction. Hepatology. 2019;69:1219–1230. doi: 10.1002/hep.30321. [DOI] [PubMed] [Google Scholar]
- 43.Asrani SK, Jennings LW, Kim WR, Kamath PS, Levitsky J, Nadim MK, Testa G, Leise MD, Trotter JF, Klintmalm G. MELD-GRAIL-Na: Glomerular Filtration Rate and Mortality on Liver-Transplant Waiting List. Hepatology. 2020;71:1766–1774. doi: 10.1002/hep.30932. [DOI] [PubMed] [Google Scholar]
- 44.Sarmast N, Ogola GO, Kouznetsova M, Leise MD, Bahirwani R, Maiwall R, Tapper E, Trotter J, Bajaj JS, Thacker LR, Tandon P, Wong F, Reddy KR, O'Leary JG, Masica A, Modrykamien AM, Kamath PS, Asrani SK. Model for End-Stage Liver Disease-Lactate and Prediction of Inpatient Mortality in Patients With Chronic Liver Disease. Hepatology. 2020;72:1747–1757. doi: 10.1002/hep.31199. [DOI] [PubMed] [Google Scholar]
- 45.Mahmud N, Asrani SK, Kaplan DE, Ogola GO, Taddei TH, Kamath PS, Serper M. The Predictive Role of Model for End-Stage Liver Disease-Lactate and Lactate Clearance for In-Hospital Mortality Among a National Cirrhosis Cohort. Liver Transpl. 2021;27:177–189. doi: 10.1002/lt.25913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kartoun U, Corey KE, Simon TG, Zheng H, Aggarwal R, Ng K, Shaw SY. The MELD-Plus: A generalizable prediction risk score in cirrhosis. PLoS One. 2017;12:e0186301. doi: 10.1371/journal.pone.0186301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernaez R, Liu Y, Kramer JR, Rana A, El-Serag HB, Kanwal F. Model for end-stage liver disease-sodium underestimates 90-day mortality risk in patients with acute-on-chronic liver failure. J Hepatol. 2020;73:1425–1433. doi: 10.1016/j.jhep.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 49.Pan HC, Jenq CC, Tsai MH, Fan PC, Chang CH, Chang MY, Tian YC, Hung CC, Fang JT, Yang CW, Chen YC. Scoring systems for 6-month mortality in critically ill cirrhotic patients: a prospective analysis of chronic liver failure - sequential organ failure assessment score (CLIF-SOFA) Aliment Pharmacol Ther. 2014;40:1056–1065. doi: 10.1111/apt.12953. [DOI] [PubMed] [Google Scholar]
- 50.Zheng YX, Zhong X, Li YJ, Fan XG. Performance of scoring systems to predict mortality of patients with acute-on-chronic liver failure: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:1668–1678. doi: 10.1111/jgh.13786. [DOI] [PubMed] [Google Scholar]
- 51.Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, Hopf C, Alessandria C, Rodriguez E, Solis-Muñoz P, Laleman W, Trebicka J, Zeuzem S, Gustot T, Mookerjee R, Elkrief L, Soriano G, Cordoba J, Morando F, Gerbes A, Agarwal B, Samuel D, Bernardi M, Arroyo V CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Engelmann C, Thomsen KL, Zakeri N, Sheikh M, Agarwal B, Jalan R, Mookerjee RP. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care. 2018;22:254. doi: 10.1186/s13054-018-2156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramzan M, Iqbal A, Murtaza HG, Javed N, Rasheed G, Bano K. Comparison of CLIF-C ACLF Score and MELD Score in Predicting ICU Mortality in Patients with Acute-On-Chronic Liver Failure. Cureus. 2020;12:e7087. doi: 10.7759/cureus.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]