Abstract
BACKGROUND
Clostridioides (formerly Clostridium) difficile infection (CDI) is an increasingly frequent cause of morbidity and mortality in hospitalized patients. Multiple risk factors are documented in the literature that includes, but are not limited to, antibiotics use, advanced age, and gastric acid suppression. Several epidemiological studies have reported an increased incidence of CDI in advanced liver disease patients. Some have also demonstrated a higher prevalence of nosocomial infections in cirrhotic patients.
AIM
To use a large nationwide database, we sought to determine CDI’s risk among liver cirrhosis patients in the United States.
METHODS
We queried a commercial database (Explorys IncTM, Cleveland, OH, United States), and obtained an aggregate of electronic health record data from 26 major integrated United States healthcare systems comprising 360 hospitals in the United States from 2018 to 2021. Diagnoses were organized into the Systematized Nomenclature of Medicine Clinical Terms (SNOMED–CT) hierarchy. Statistical analysis for the multivariable model was performed using Statistical Package for Social Sciences (SPSS version 25, IBM CorpTM). For all analyses, a two-sided P value of < 0.05 was considered statistically significant.
RESULTS
There were a total of 19387760 patients in the database who were above 20 years of age between the years 2018-2021. Of those, 133400 were diagnosed with liver cirrhosis. The prevalence of CDI amongst the liver cirrhosis population was 134.93 per 100.000 vs 19.06 per 100.000 in non-cirrhotic patients (P < 0.0001). The multivariate analysis model uncovered that cirrhotic patients were more likely to develop CDI (OR: 1.857; 95%CI: 1.665-2.113, P < 0.0001) compared to those without any prior history of liver cirrhosis.
CONCLUSION
In this large database study, we uncovered that cirrhotic patients have a significantly higher CDI prevalence than those without cirrhosis. Liver cirrhosis may be an independent risk factor for CDI. Further prospective studies are needed to clarify this possible risk association that may lead to the implementation of screening methods in this high-risk population.
Keywords: Clostridioides difficile, Chronic liver disease, Liver cirrhosis, Liver transplant
Core Tip: Clostridium difficile infections (CDI) are a leading cause of hospital morbidity and mortality. The risk factors for CDI in liver cirrhosis patients are studied in the national data base. CDIs in liver transplantation is a life-threatening situation as these patients are malnourished and immunocompromised. Therefore, special emphasis was given to the cohort with history of liver transplantation and relevant literature was reviewed.
INTRODUCTION
Clostridiodes difficile is a gram-positive anaerobic bacillus. It is widespread in the surrounding environment and a significant contributor to inpatient mortality in vulnerable subgroups[1]. Risk factors for being predisposed to CDI include advanced age, enteral feeding, smoking, alcohol abuse, and use of antibiotics and acid-suppressive therapy. It is particularly predominant in elderly patients who reside in nursing homes and long-term acute care facilities and have a history of recurrent hospitalizations. CDI carries a significant economic burden on the USA health care system. A recent study by Desai et al[2] uncovered that CDI's economic cost was roughly $5.4 billion, with $4.7 billion in the healthcare settings and $725 million in the community.
CDI has a spectrum of clinical symptoms, including nausea, vomiting, abdominal pain, watery diarrhea with the formation of pseudomembranous, progression to fulminant colitis, and even toxic mega colon[3-8]. CDI can culminate in the possible rupture of the large colon, septic shock, and death. Reactive arthritis is also seen as one of the complications of CDI[9].
Broad-spectrum antibiotic use (penicillin, cephalosporins, clindamycin, fluoroquinolones) predispose individuals to selective elimination of healthy gut microbiota and overgrowth of Clostridium difficile (C. difficile) in the gastrointestinal flora[10,11] with the highest risk of CDI within the first three months of antibiotic exposure[12]. As the environment and normal human gastrointestinal tract are heavily colonized with C. difficile[5,13-15], it is just a matter of loss of balance where C. difficile invades the protective gastrointestinal barriers through the production of toxins (enterotoxin A, cytotoxin B, binary toxin/CDT) and enzymes (collagenase, chondroitin sulfatase, hyaluronidase) which promote inflammation[16-18]. The virulence and pathogenicity are compounded by new hypervirulent strains and the potential ability of C. difficile to create biofilms in vivo (after an in vitro demonstration)[19,20]. For instance, C. difficile, especially the new hypervirulent strain, NAP1/BI/027 that was uncovered in the year 2000, was responsible for a significant CDI-related mortality increase 5.7 deaths per million in 1999 to 23.7 deaths per million in 2004[21]. CDI is currently considered the most common cause of nosocomial diarrhea in the western world.
CDI's have been classified based on the severity of infection, utilizing the markers of inflammation and organ function, including white blood cell count (WBC), creatinine and albumin levels. Prognostic markers in patients with C. difficile colitis included low serum albumin (< 2.5 mg/dL) or a 1.1 mg/dL reduction in serum albumin from baseline, use of multiple antibiotics, and a positive CD cytotoxin in stool after completion of treatment (after seven or more days of treatment)[22].
The poor outcomes with CDI are not uncommon. They are particularly pronounced in patients with underlying chronic comorbidities (congestive heart failure, chronic obstructive pulmonary disease, and chronic kidney disease), history of solid organ transplants and immunosuppressive therapy, and chronic inflammatory diseases, including Crohn's disease and Ulcerative colitis[23-29]. The morbidity and mortality from liver cirrhosis is on the rise[30]. A prospective study by Bouza et al[31] that focused on the recent outbreak of C. difficile PCR ribotype 027 in Spain uncovered that this strain was most evident in patients with age > 75 years, the male gender, and comorbidities such hypertension, chronic cardiovascular disease, type 2 diabetes, and liver cirrhosis. Interestingly, liver cirrhosis was associated with an increased CDI recurrence risk of 44.4% vs 14.8%[31]. The increased prevalence of CDI in patients with advanced liver disease is being investigated as they are already immunocompromised[32,33].
Poor outcomes in cirrhotic patients who acquired CDI are reported in a recent study by Abdalla et al[34]. Liver cirrhosis itself can predispose the individuals to nosocomial infections, the deadliest of them being CDI. For instance, several studies have reported that CLD patients with CDI have a higher mortality rate, prolonged length of stay, and higher hospital cost[35-37]. We performed this large database study to re-evaluate the risk and severity of CDI in patients with cirrhosis. Prevalence of C. difficile associated disease (CDAD) was determined in the subgroups with established risk factors and comorbidities and prior history of liver disease and liver transplant.
MATERIALS AND METHODS
Database
Our study is a retrospective cohort analysis of a large, multicenter database (Explorys, Cleveland, OH, United States). Explorys aggregates healthcare data of more than 50 million unique patient records. Diagnoses, findings, and procedures are arranged into the Systematized Nomenclature of Medicine–Clinical Terms (SNOMED-CT) hierarchy, whereas prescription drug orders are mapped into RxNorm. Explorys provides an interactive search engine to generate multiple cohorts based on medical diagnoses. Medical data are de-identified, and therefore, it is a Health Insurance Portability and Accountability Act-compliant platform.
Patient selection
Using the Explorys platform, we identified cohorts of patients diagnosed with Liver cirrhosis between the period of March 2018 and March 2021. The study cohorts (liver cirrhosis) were identified by searching the database for a SNOMED-CT diagnosis of "Cirrhosis of Liver" after excluding patients younger than 20 years old. The control group was then identified for those who have no liver cirrhosis. Subsequently, a cohort of patients with "clostridioides difficle infection" diagnosis was identified between the period of March 2018 to March 2021 to calculate the prevalence of CDI in both study groups. Risk factors and predisposing medical conditions associated with CDI, in addition to demographic information, were collected. Possible risk factors included comorbid medical conditions, antibiotics, acid-suppressive therapy, liver transplant, and inpatient/skilled nursing facility settings were investigated using SNOMED-CT diagnostic codes.
Statistical analysis
The prevalence was calculated by dividing the total number of individuals with CDI in each cohort (liver cirrhosis and non-cirrhotics) by the total number of individuals in each cohort as identified by Explorys [2018-2021], thus making sure that all patients in the denominator had an equal opportunity of being diagnosed with CDI. We calculated the prevalence in subgroups based on sex, race, and age by dividing the number of individuals with CDI in each subgroup by a total number of patients in the same subgroup. A multivariate regression model was constructed using binary logistic regression, with CDI being the outcome to adjust for possible confounding from the covariates listed previously. We used SPSS version 25 (IBM Corp) to perform the multivariate regression analysis. A 2-sided P value of < 0.05 was considered statistically significant.
RESULTS
Descriptive epidemiology
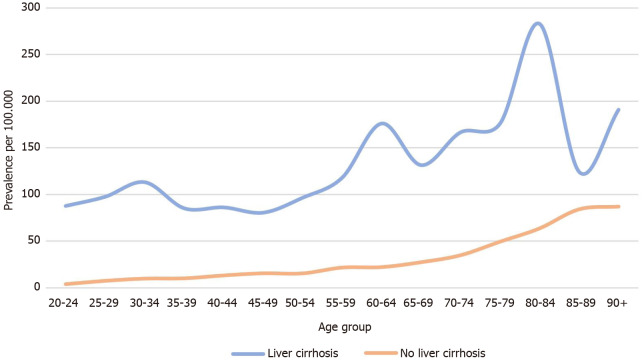
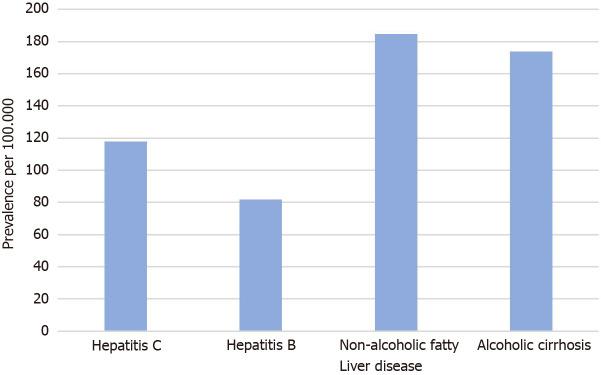
There were a total of 19387760 patients in the database who were above 20 years of age. Of those, 133400 were diagnosed with liver cirrhosis. The baseline characteristics of the study population are presented in Table 1. The prevalence of CDI amongst the liver cirrhosis population was 134.93 per 100.000 vs 19.06 per 100.000 in non-cirrhotic patients (P < 0.0001). Figure 1 represents the prevalence of CDI in different age groups among cirrhotics. Females and Caucasian patients had a higher CDI prevalence than males and non-caucasian among both study groups (Table 2). Patients with nonalcoholic liver disease (NAFLD) as well as an alcoholic liver disease were found to have a higher prevalence of CDI when compared to cirrhotic patients with viral hepatitis (184.9/100.000 in NAFLD vs 174.0/100.000 in alcoholic liver disease vs 117.9/100.000 in hepatitis C vs 81.7/100.000 in hepatitis B) (Figure 2).
Table 1.
Baseline characteristics of study population, n (%)
|
|
Patients with history of liver cirrhosis (n = 133400)
|
Patients with no history of liver cirrhosis (n = 19254360)
|
| Age groups (yr) | ||
| 20-64 | 73620 (55) | 14934590 (78) |
| > 65 | 59780 (45) | 4319770 (22) |
| Gender | ||
| Male | 71230 (53) | 8702330 (45) |
| Female | 62170 (47) | 10552030 (55) |
| Race | ||
| Caucasian | 101680 (76) | 11165060 (58) |
| Non-Caucasian | 31720 (24) | 8089300 (42) |
| Comorbidities | ||
| Smoking | 54400 (41) | 2985460 (16) |
| Alcohol abuse | 30040 (23) | 359900 (2) |
| HTN | 100830 (76) | 5307920 (28) |
| DM | 63770 (48) | 2211420 (11) |
| Obesity | 24400 (18) | 1148460 (6) |
| Chronic kidney disease | 33640 (25) | 853630 (4) |
| Coronary artery disease | 6280 (5) | 229410 (1) |
| Heart failure | 39960 (30) | 761710 (4) |
| Chronic obstructive pulmonary disease | 42740 (32) | 986400 (5) |
DM: Diabetes mellitus.
Figure 1.
Prevalence of Clostridioidesdifficile infection in patients with liver cirrhosis vs no cirrhosis.
Table 2.
Prevalence of Clostridioides difficile infection in different age and race groups in patients with liver cirrhosis vs no cirrhosis (per 100000)
|
|
Liver cirrhosis
|
No cirrhosis
|
| Female | 176.93 | 23.31 |
| Male | 112.31 | 14.34 |
| Caucasian | 147.52 | 26.87 |
| African American | 54.59 | 7.34 |
| Hispanic/Latino | 257.73 | 10.96 |
Figure 2.
Prevalence of Clostridioidesdifficile infection in cirrhotic patients based on the etiology of cirrhosis.
Multivariate analysis
The multivariate analysis model uncovered that cirrhotic patients were more likely to develop CDI (OR 1.857; 95%CI: 1.665-2.113, P < 0.0001) compared to those without any prior history of liver cirrhosis. The characteristics of the liver cirrhosis patients who developed CDI revealed that they were more likely to be of advanced age (age > 65) as opposed to being young (age < 65) with an OR 2.307, 95%CI: 2.179-2.442 (P < 0.0001); had prior use of antibiotics (OR 19.749, 95%CI: 17.3-22.545, P < 0.0001 ); had used acid-suppressive therapy (OR 2.243, 95%CI: 2.122-2.371, P < 0.0001); and were mostly inpatients/skilled nursing facility occupants vs the community (OR 2.02, 95%CI: 1.911-2.134, P < 0.0001 ). Among cirrhotic patients, those with a history of liver transplant (OR 2.737, 95%CI: 2.087-3.589) were highly likely to develop CDI. The multivariate analysis model with CDI being the outcome is presented in Table 3.
Table 3.
Multivariable model with Clostridioides difficile infection being the outcome
|
Multivariable model
|
Odds ratio
|
95%CI
|
P
value
|
| Age (> 65 yr vs < 65 yr) | 2.307 | 2.179-2.442 | < 0.0001 |
| Gender (female vs male) | 1.29 | 1.221-1.363 | < 0.0001 |
| Race (non-Caucasian vs Caucasian) | 1.16 | 1.088-1.237 | < 0.0001 |
| Antibiotics | 19.749 | 17.3-22.545 | < 0.0001 |
| Skilled nursing facility or inpatients | 2.02 | 1.911-2.134 | < 0.0001 |
| Acid suppressive therapy (Proton pump inhibitors or H2 blockers) | 2.243 | 2.122-2.371 | < 0.0001 |
| Comorbidities 1 | 1.258 | 1.192-1.328 | < 0.0001 |
| Liver transplant | 2.737 | 2.087-3.589 | < 0.0001 |
| Liver cirrhosis | 1.875 | 1.665-2.113 | < 0.0001 |
Comorbidities: One or more of the following (heart failure, coronary artery disease, chronic kidney disease, inflammatory bowel disease, chronic obstructive pulmonary disease, diabetes mellitus, obesity, hypertension or metabolic syndrome).
DISCUSSION
The multivariate analysis of this database study holds true for the high prevalence of CDI in cirrhotic patients with all the established risk factors (advanced age, use of antibiotics and acid suppression therapy, enteral feeding, residence at long term care facilities, and frequent hospitalizations) and comorbidities (obesity, hypertension, Diabetes Mellitus, chronic obstructive pulmonary disease, congestive heart failure)[38-45]. The highest prevalence of CDI was reported in patients with a history of antibiotic use. CDI’s were also encountered in traditionally low-risk population groups in hospitalized patients with recent antibiotic exposure[46].
The colonization of C. difficile has been higher in cirrhotic patients with simultaneous hepatic encephalopathy and advanced stage (Child-Pugh C)[47]. Risk factors of CDI in cirrhotic patients have been determined by Yan et al[48] in their latest study (advanced age, antibiotics, and proton pump inhibitors, prolonged and recurrent hospitalizations, hyponatremia, C. difficile colonization, hepatic encephalopathy).
The bacterial infections, which generally would have been countered with immunoregulatory mechanisms (chemotaxis, phagocytosis, oxidation, interferon cascade, complement system, inflammatory response) in an immunocompetent individual, go rampant[49-52]. High ammonia levels alter these neutrophilic responses[53]. The inflammation response is also dampened from poor nutrition status and alcoholism, which come with cirrhosis. The mechanisms responsible include reticuloendothelial system dysfunction, portosystemic shunting, hyperdynamic circulation, increased permeability of gut, and bacterial translocation. The systemic inflammatory response syndrome (SIRS) is amplified by the increased nitric oxide (NO) and the cytokine storm.
The rate of CDI was significantly high in patients who underwent hepatic transplantation. CDI risk is increased in immunocompromising health conditions involving any solid organ transplant[54,55], including liver transplant recipients[56]. The timeline of CDI in post-transplant patients has been established based on the underlying severity of cirrhosis dictated by model for end-stage liver disease (MELD) scoring, concurrent intra-abdominal hemorrhage, repeat grafting and transplant, vascular complications, infections, and the need for endoscopy with sicker patients developing CDI earlier with higher mortality[57,58]. Musa et al[59] researched CDI and chronic liver disease with an additional focus on liver transplant patients. Male sex and high pre-op creatinine levels (> 1 g/L) are considered predisposing risk factors for CDI in the subgroup who received a living donor hepatic transplant[60]. Advanced cirrhosis (High MELD score), impaired renal function in the donor, and postoperative complications (infection, bleeding, wound) leading to prolonged hospital stay were concluded predisposing factors for CDI after a deceased liver transplant[57]. Recurrence of pseudomembranous colitis up to five times after living donor liver transplantation has been reported in the literature[61].
The CDI rate was higher in patients with autoimmune hepatitis, prolonged hospital stay, and antibiotic exposure in a study performed by Vanjak et al[62]. Hepatitis C is increasingly identified as an underlying viral infection responsible for cirrhosis in patients who developed CDI later in life[63]. Comparing CDI incidence in cirrhosis due to hepatitis B and hepatitis C has not been explored yet. Our study explores this comparison and demonstrates that the prevalence of CDI is higher in inpatient subgroups with hepatitis C than hepatitis B. Sundaram et al[36] reported higher inpatient mortality secondary to CDI in patient subgroups with alcohol abuse-related hepatic cirrhosis. Additionally, NAFLD has been identified as a risk factor for CDI by Papić et al[64]; after adjusting for other comorbidities, hospitalization rates, and antibiotic exposure (Sundaram et al[36]).
Acid suppressive therapy has been implicated with CDI in the general population. A study reported increased 30-d mortality in cirrhotic patients with proton pump inhibitor (PPI) use[35]. The association is being attributed to their excessive unindicated use. The majority of people presenting with variceal bleed get discharged with PPIs renewed on each visit[33,65]. Chronic use of PPIs causes altered gut flora and motility and decreased neutrophilic function[66]. Long-term PPIs use has been attributed to CDI’s by suppressing gastric acid, although the evidence[67-71]. PPIs are said to have worse outcomes in cirrhotic patients than H2 blockers in one study[8]. Hence, the proper need for PPIs should be assessed at each visit and discharge.
Generally, women are more likely to get CDI regardless of their liver function, which was also reflected in our study[72]. The incidence rate of CDI is higher in Caucasians with cirrhosis. A higher incidence and mortality rate from CDI in the caucasian population has been reported in the literature[73-75]. An even higher prevalence of CDI was seen in the African American and Hispanic/Latin subgroups, which could be due to regional data differences[76]. The hospitalization patterns have been fluctuating, and long-term mortality from CDI has been counterintuitively low, as concluded by recent studies[59,76,77].
Vancomycin and metronidazole have been used historically in the treatment of initial and recurrent CDI. Several meta-analyses have been performed, emphasizing the non-inferiority of metronidazole, and thereby, guidelines have been revised. Vancomycin and fidaxomicin are considered the mainstay of antibiotic treatment now, along with fecal microbiota transplantation (FMT). Surgery is pursued when there is a suspicion of toxic megacolon or colon perforation[5,17,78-82]. Lactulose was also evaluated in liver cirrhosis patients carrying C. difficile in a study done by Ito et al[83] with promising results. Lactulose may increase fecal acidity by decreasing short-chain fatty acids and increasing lactate and acetate, leading to possible suppression of C. difficile growth. FMT has been used in patient subgroups with cirrhosis to help with recurrent CDI colonization[78,84]. Additionally, lactulose as a prebiotic may play a prominent role in restoring the hosts' indigenous microbiota and conferring resistance against CDI[85]. Recently, the benefit of preventing CDI by using maintenance rifaximin[86].
The benefit of screening hospitalized cirrhotic patients for C. difficile might be purely theoretical, as screening in the absence of symptoms would lead to over-reporting[87,88]. Meltzer et al[89] did a 10-wk surveillance study after screening asymptomatic patients on admission. They demonstrated a higher incidence of CDI during hospitalization in patients who tested positive for C. difficile on admission rectal swabs. Whether clinicians should treat a prior CDI carrier state still remains unclear, as most of the positive patients in that particular study had the classical risk factors for CDI (prolonged hospital and rehabilitation stays, exposure to infections, and antibiotics). Third-generation cephalosporins are the treatment of choice for subacute bacterial peritonitis (SBP), which are counterintuitively associated with increased risk of CDI. Both SBP and CDI translate into poor outcomes for the patient[21]. Bactrim and fluoroquinolones (ciprofloxacin, norfloxacin) are recommended as SBP prophylaxis in high-risk patients, but their long-term benefit is questionable for now[90].
C. difficile toxins in stool sample or visualization of pseudomembrane formation on endoscopic or histological examination are diagnostic for CDI. Due to its ability to spread by spore formation[91,92], poor hygiene contributes to its rapid spread via the fecal-oral route and can result in outbreaks in health care facilities. Hand hygiene, therefore, has been the cornerstone in the control of CDI spread along with isolation of symptomatic patients and implementation of environmental sanitation protocols[93-97].
The results obtained from this database are significant due to the large sample size, appropriate gender and racial representation, and inclusion of patients above the age of twenty years. Recent studies have confirmed poor outcomes with concurrent CDI and CLD[37]. All data prior to 2018 has been excluded to determine the persistence of historically established risk factors for CDI based on point prevalence. Relevant comorbidities have been included along with a subgroup of patients with liver transplants. The underlying cause of cirrhosis has also been delineated (Table 2).
The study is at a disadvantage as it is retrospective. The sample size is subjected to selection bias which was attempted to be minimized by relevant inclusion and exclusion criterion. The prevalence of liver cirrhosis in the population database is lower than the general population (0.69 %)[98]. While this may reduce the effective sample size, it has no bearing on the conclusions drawn regarding the risk factors associated with CDI in cirrhotic patients. The inclusion of patient classification based on their MELD score would have indicated the severity of CDI at different cirrhosis stages. The multivariate analysis by Hong et al[99] had suggested that the patients with higher MELD scoring are at increased risk of mortality from CDI (1.06 ± 0.02, P-value < 0.022 with an increase of 21.5% mortality rate with every five-unit increase of MELD score), and MELD scoring should be used to triage them and monitor their outcomes. However, the application of MELD score in SNOMED-CT would be scrupulous as the routine discharge diagnoses are not updated based on the patient's current MELD scores. Results from future perspective studies with patient cohorts stratified into liver, solid organ transplants and MELD classes can vindicate the yield of C. difficile screening in asymptomatic patients.
CONCLUSION
The prevalence of CDI is seven times higher in cirrhotic patients than those without liver cirrhosis. In the multivariate analysis, cirrhotic patients with advanced age, frequent hospitalizations, residence in a nursing home and long-term facilities, along with the use of antibiotics, acid-suppressive therapy, chronic comorbidities, and history of hepatic transplantation, were more likely to develop CDI. Further studies are needed to explore this risk, and precautionary measures are needed to be implemented to prevent CDI in this group of patients.
ARTICLE HIGHLIGHTS
Research background
Clostridium difficile (C. difficile) is one of the major causes of nosocomial diarrhea and associated morbidity and mortality. The risk factors of C. difficile are historically established. Cirrhosis is a major disease burden in the United States health care system. The risk of morbidity and mortality is higher in cirrhotic patients who acquire C. difficile infection.
Research motivation
This research was motivated by the lack of recent large population study describing the risk factors of C. difficile in liver cirrhotic patients. We also wanted to study the association in patient cohorts who underwent liver transplant as it was not done previously with such higher sample size.
Research objectives
To determine the prevalence of C. difficile infection in patients with liver cirrhosis and to establish the risk factors of C. difficile infection in patients with liver cirrhosis with special emphasis on liver transplantation cohort.
Research methods
The authors used the Explorys database to obtain data that was classified using SNOMED diagnostic codes. Prevalence and association were calculated using multi-variate regression and SPS Software. Details are in the main manuscript.
Research results
The prevalence of C. difficile infection (CDI) amongst the liver cirrhosis population was 134.93 per 100.000 vs 19.06 per 100.000 in non-cirrhotic patients. The multivariate analysis model showed that cirrhotic patients were more likely to develop CDI.
Research conclusions
This research study concluded that cirrhotic patients have a significantly higher CDI prevalence, and liver cirrhosis may be an independent risk factor for CDI.
Research perspectives
There is a possibility of reducing the CDI mortality in cirrhotic patients by screening them for CDI. Future prospective studies are needed in this regard.
Footnotes
Institutional review board statement: Study protocol was reviewed with Research Department. It was deemed as a population-based study with no patient identifiers and did not need IRB approval.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: All authors report no conflicts of interests (personal or financial).
STROBE statement: The authors have read the STROBE Statement, and the manuscript was prepared and revised according to the STROBE Statement.
Manuscript source: Unsolicited manuscript
Peer-review started: May 8, 2021
First decision: June 7, 2021
Article in press: July 22, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta MK S-Editor: Ma YJ L-Editor: A P-Editor: Liu JH
Contributor Information
Syeda Sahra, Department of Internal Medicine, Staten Island University Hospital, Staten Island, NY 10305, United States. ssahra@northwell.edu.
Mohammad Abureesh, Department of Internal Medicine, Staten Island University Hospital, Staten Island, NY 10305, United States.
Shivantha Amarnath, Department of Internal Medicine, Staten Island University Hospital, Staten Island, NY 10305, United States.
Motasem Alkhayyat, Department of Internal Medicine, Cleveland Clinic Foundation, Cleveland, OH 44195, United States.
Rawan Badran, Department of Internal Medicine, Staten Island University Hospital, Staten Island, NY 10305, United States.
Abdullah Jahangir, Department of Internal Medicine, Staten Island University Hospital, Staten Island, NY 10305, United States.
Vivek Gumaste, Department of Gastroenterology, Staten Island University Hospital, Staten Island, NY 10305, United States.
Data sharing statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request
References
- 1.Kuipers EJ, Surawicz CM. Clostridium difficile infection. Lancet . 2008;371:1486–1488. doi: 10.1016/S0140-6736(08)60635-2. [DOI] [PubMed] [Google Scholar]
- 2.Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis . 2016;16:303. doi: 10.1186/s12879-016-1610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simor AE. Diagnosis, management, and prevention of Clostridium difficile infection in long-term care facilities: a review. J Am Geriatr Soc . 2010;58:1556–1564. doi: 10.1111/j.1532-5415.2010.02958.x. [DOI] [PubMed] [Google Scholar]
- 4.Ergen EK, Akalin H, Yilmaz E, Sinirtaş M, Alver O, Heper Y, Ozakin C, Bakker D, Ener B, Mistik R, Helvaci S, Kuijper EJ. Nosocomial diarrhea and Clostridium Difficile associated diarrhea in a Turkish University Hospital. Med Mal Infect . 2009;39:382–387. doi: 10.1016/j.medmal.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 5.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis . 2018;66:e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clabots CR, Johnson S, Olson MM, Peterson LR, Gerding DN. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J Infect Dis . 1992;166:561–567. doi: 10.1093/infdis/166.3.561. [DOI] [PubMed] [Google Scholar]
- 7.Johnson S, Clabots CR, Linn FV, Olson MM, Peterson LR, Gerding DN. Nosocomial Clostridium difficile colonisation and disease. Lancet . 1990;336:97–100. doi: 10.1016/0140-6736(90)91605-a. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj JS, Ratliff SM, Heuman DM, Lapane KL. Proton pump inhibitors are associated with a high rate of serious infections in veterans with decompensated cirrhosis. Aliment Pharmacol Ther . 2012;36:866–874. doi: 10.1111/apt.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaishnavi C. Clinical spectrum & pathogenesis of Clostridium difficile associated diseases. Indian J Med Res . 2010;131:487–499. [PubMed] [Google Scholar]
- 10.Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, Goldman S, Wultańska D, Garlicki A, Biesiada G. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis . 2019;38:1211–1221. doi: 10.1007/s10096-019-03539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol . 2008;3:563–578. doi: 10.2217/17460913.3.5.563. [DOI] [PubMed] [Google Scholar]
- 12.Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother . 2012;67:742–748. doi: 10.1093/jac/dkr508. [DOI] [PubMed] [Google Scholar]
- 13.Hensgens MP, Keessen EC, Squire MM, Riley TV, Koene MG, de Boer E, Lipman LJ, Kuijper EJ European Society of Clinical Microbiology and Infectious Diseases Study Group for Clostridium difficile (ESGCD) Clostridium difficile infection in the community: a zoonotic disease? Clin Microbiol Infect . 2012;18:635–645. doi: 10.1111/j.1469-0691.2012.03853.x. [DOI] [PubMed] [Google Scholar]
- 14.Hung YP, Lee JC, Lin HJ, Liu HC, Wu YH, Tsai PJ, Ko WC. Clinical impact of Clostridium difficile colonization. J Microbiol Immunol Infect . 2015;48:241–248. doi: 10.1016/j.jmii.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Crobach MJT, Vernon JJ, Loo VG, Kong LY, Péchiné S, Wilcox MH, Kuijper EJ. Understanding Clostridium difficile Colonization. Clin Microbiol Rev . 2018;31 doi: 10.1128/CMR.00021-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Androga GO, Hart J, Foster NF, Charles A, Forbes D, Riley TV. Infection with Toxin A-Negative, Toxin B-Negative, Binary Toxin-Positive Clostridium difficile in a Young Patient with Ulcerative Colitis. J Clin Microbiol . 2015;53:3702–3704. doi: 10.1128/JCM.01810-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baktash A, Terveer EM, Zwittink RD, Hornung BVH, Corver J, Kuijper EJ, Smits WK. Mechanistic Insights in the Success of Fecal Microbiota Transplants for the Treatment of Clostridium difficile Infections. Front Microbiol . 2018;9:1242. doi: 10.3389/fmicb.2018.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers . 2016;2:16020. doi: 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vuotto C, Donelli G, Buckley A, Chilton C. Clostridium difficile Biofilm. Adv Exp Med Biol . 2018;1050:97–115. doi: 10.1007/978-3-319-72799-8_7. [DOI] [PubMed] [Google Scholar]
- 20.Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol . 2010;4:409–416. doi: 10.1586/egh.10.48. [DOI] [PubMed] [Google Scholar]
- 21.Smith EZ, Northup PG, Argo CK. Predictors of Mortality in Cirrhosis Inpatients With Clostridium difficile Infection. J Clin Gastroenterol . 2018;52:747–751. doi: 10.1097/MCG.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 22.Bloomfield MG, Sherwin JC, Gkrania-Klotsas E. Risk factors for mortality in Clostridium difficile infection in the general hospital population: a systematic review. J Hosp Infect . 2012;82:1–12. doi: 10.1016/j.jhin.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA . 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 24.Sidler JA, Battegay M, Tschudin-Sutter S, Widmer AF, Weisser M. Enterococci, Clostridium difficile and ESBL-producing bacteria: epidemiology, clinical impact and prevention in ICU patients. Swiss Med Wkly . 2014;144:w14009. doi: 10.4414/smw.2014.14009. [DOI] [PubMed] [Google Scholar]
- 25.Czepiel J, Kędzierska J, Biesiada G, Birczyńska M, Perucki W, Nowak P, Garlicki A. Epidemiology of Clostridium difficile infection: results of a hospital-based study in Krakow, Poland. Epidemiol Infect . 2015;143:3235–3243. doi: 10.1017/S0950268815000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Negrón ME, Rezaie A, Barkema HW, Rioux K, De Buck J, Checkley S, Beck PL, Carroll M, Fedorak RN, Dieleman L, Panaccione R, Ghosh S, Kaplan GG. Ulcerative Colitis Patients With Clostridium difficile are at Increased Risk of Death, Colectomy, and Postoperative Complications: A Population-Based Inception Cohort Study. Am J Gastroenterol . 2016;111:691–704. doi: 10.1038/ajg.2016.106. [DOI] [PubMed] [Google Scholar]
- 27.Chitnis AS, Holzbauer SM, Belflower RM, Winston LG, Bamberg WM, Lyons C, Farley MM, Dumyati GK, Wilson LE, Beldavs ZG, Dunn JR, Gould LH, MacCannell DR, Gerding DN, McDonald LC, Lessa FC. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med . 2013;173:1359–1367. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PubMed] [Google Scholar]
- 28.Phatharacharukul P, Thongprayoon C, Cheungpasitporn W, Edmonds PJ, Mahaparn P, Bruminhent J. The Risks of Incident and Recurrent Clostridium difficile-Associated Diarrhea in Chronic Kidney Disease and End-Stage Kidney Disease Patients: A Systematic Review and Meta-Analysis. Dig Dis Sci . 2015;60:2913–2922. doi: 10.1007/s10620-015-3714-9. [DOI] [PubMed] [Google Scholar]
- 29.Thongprayoon C, Cheungpasitporn W, Phatharacharukul P, Edmonds PJ, Kaewpoowat Q, Mahaparn P, Bruminhent J, Erickson SB. Chronic kidney disease and end-stage renal disease are risk factors for poor outcomes of Clostridium difficile infection: a systematic review and meta-analysis. Int J Clin Pract . 2015;69:998–1006. doi: 10.1111/ijcp.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ . 2018;362:k2817. doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouza E, Alcalá L, Marín M, Valerio M, Reigadas E, Muñoz P, González-Del Vecchio M, de Egea V. An outbreak of Clostridium difficile PCR ribotype 027 in Spain: risk factors for recurrence and a novel treatment strategy. Eur J Clin Microbiol Infect Dis . 2017;36:1777–1786. doi: 10.1007/s10096-017-2991-y. [DOI] [PubMed] [Google Scholar]
- 32.Deschênes M, Villeneuve JP. Risk factors for the development of bacterial infections in hospitalized patients with cirrhosis. Am J Gastroenterol . 1999;94:2193–2197. doi: 10.1111/j.1572-0241.1999.01293.x. [DOI] [PubMed] [Google Scholar]
- 33.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol . 2011;9:727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 34.Abdalla AO, Pisipati S, Elnaggar M, Rishi M, Doshi R, Gullapalli N. Outcomes of Clostridioides difficile Infection in Patients With Liver Cirrhosis: A Nationwide Study. Gastroenterology Res . 2020;13:53–57. doi: 10.14740/gr1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bajaj JS, Ananthakrishnan AN, Hafeezullah M, Zadvornova Y, Dye A, McGinley EL, Saeian K, Heuman D, Sanyal AJ, Hoffmann RG. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: A national and tertiary center perspective. Am J Gastroenterol . 2010;105:106–113. doi: 10.1038/ajg.2009.615. [DOI] [PubMed] [Google Scholar]
- 36.Sundaram V, May FP, Manne V, Saab S. Effects of Clostridium difficile infection in patients with alcoholic hepatitis. Clin Gastroenterol Hepatol . 2014;12:1745–52.e2. doi: 10.1016/j.cgh.2014.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dotson KM, Aitken SL, Sofjan AK, Shah DN, Aparasu RR, Garey KW. Outcomes associated with Clostridium difficile infection in patients with chronic liver disease. Epidemiol Infect . 2018;146:1101–1105. doi: 10.1017/S0950268818001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomes AC, Hoffmann C, Mota JF. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes . 2018;9:308–325. doi: 10.1080/19490976.2018.1465157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madan R, Petri WA Jr. Role of obesity and adipose tissue-derived cytokine leptin during Clostridium difficile infection. Anaerobe . 2015;34:182–186. doi: 10.1016/j.anaerobe.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savin Z, Kivity S, Yonath H, Yehuda S. Smoking and the intestinal microbiome. Arch Microbiol . 2018;200:677–684. doi: 10.1007/s00203-018-1506-2. [DOI] [PubMed] [Google Scholar]
- 41.Trifan A, Girleanu I, Stanciu C, Miftode E, Cojocariu C, Singeap AM, Sfarti C, Chiriac S, Cuciureanu T, Stoica O. Clostridium difficile infection in hospitalized octogenarian patients. Geriatr Gerontol Int . 2018;18:315–320. doi: 10.1111/ggi.13186. [DOI] [PubMed] [Google Scholar]
- 42.Qu HQ, Jiang ZD. Clostridium difficile infection in diabetes. Diabetes Res Clin Pract . 2014;105:285–294. doi: 10.1016/j.diabres.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 43.de Miguel-Díez J, López-de-Andrés A, Esteban-Vasallo MD, Hernández-Barrera V, de Miguel-Yanes JM, Méndez-Bailón M, Jiménez-García R. Clostridium difficile infection in hospitalized patients with COPD in Spain (2001-2015) Eur J Intern Med . 2018;57:76–82. doi: 10.1016/j.ejim.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 44.Vlasov AA, Salikova SP, Grinevich VB, Bystrova OV, Osipov GA, Meshkova ME. [Gut microbiota and systemic inflammation in patients with chronic heart failure] Kardiologiia . 2020;60:859. doi: 10.18087/cardio.2020.5.n859. [DOI] [PubMed] [Google Scholar]
- 45.Méndez-Bailón M, Jiménez-García R, Hernández-Barrera V, Miguel-Díez J, Miguel-Yanes JM, Muñoz-Rivas N, Lorenzo-Villalba N, Carabantes-Alarcon D, Zamorano-León JJ, Astasio-Arbiza P, Ortega-Molina P, López-de-Andrés A. Heart Failure Is a Risk Factor for Suffering and Dying of Clostridium difficile Infection. Results of a 15-Year Nationwide Study in Spain. J Clin Med . 2020;9 doi: 10.3390/jcm9030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention (CDC) Severe Clostridium difficile-associated disease in populations previously at low risk--four states, 2005. MMWR Morb Mortal Wkly Rep . 2005;54:1201–1205. [PubMed] [Google Scholar]
- 47.Yan D, Chen Y, Lv T, Huang Y, Yang J, Li Y, Huang J, Li L. Clostridium difficile colonization and infection in patients with hepatic cirrhosis. J Med Microbiol . 2017;66:1483–1488. doi: 10.1099/jmm.0.000596. [DOI] [PubMed] [Google Scholar]
- 48.Yan D, Huang YD, Chen YB, Lv T, Gu SL, Li YT, Huang JR, Li LJ. Risk factors for Clostridium difficile infection in cirrhotic patients. Hepatobiliary Pancreat Dis Int . 2019;18:237–241. doi: 10.1016/j.hbpd.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J Hepatol . 2016;8:307–321. doi: 10.4254/wjh.v8.i6.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghassemi S, Garcia-Tsao G. Prevention and treatment of infections in patients with cirrhosis. Best Pract Res Clin Gastroenterol . 2007;21:77–93. doi: 10.1016/j.bpg.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis . 2000;182:526–533. doi: 10.1086/315742. [DOI] [PubMed] [Google Scholar]
- 52.Garfia C, García-Ruiz I, Solís-Herruzo JA. Deficient phospholipase C activity in blood polimorphonuclear neutrophils from patients with liver cirrhosis. J Hepatol . 2004;40:749–756. doi: 10.1016/j.jhep.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Shawcross DL, Wright GA, Stadlbauer V, Hodges SJ, Davies NA, Wheeler-Jones C, Pitsillides AA, Jalan R. Ammonia impairs neutrophil phagocytic function in liver disease. Hepatology . 2008;48:1202–1212. doi: 10.1002/hep.22474. [DOI] [PubMed] [Google Scholar]
- 54.Paudel S, Zacharioudakis IM, Zervou FN, Ziakas PD, Mylonakis E. Prevalence of Clostridium difficile infection among solid organ transplant recipients: a meta-analysis of published studies. PLoS One . 2015;10:e0124483. doi: 10.1371/journal.pone.0124483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West M, Pirenne J, Chavers B, Gillingham K, Sutherland DE, Dunn DL, Matas AJ. Clostridium difficile colitis after kidney and kidney-pancreas transplantation. Clin Transplant . 1999;13:318–323. doi: 10.1034/j.1399-0012.1999.130407.x. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan T, Weinberg A, Rana M, Patel G, Huprikar S. The Epidemiology and Clinical Features of Clostridium difficile Infection in Liver Transplant Recipients. Transplantation . 2016;100:1939–1943. doi: 10.1097/TP.0000000000001309. [DOI] [PubMed] [Google Scholar]
- 57.Albright JB, Bonatti H, Mendez J, Kramer D, Stauffer J, Hinder R, Michel JA, Dickson RC, Hughes C, Nguyen J, Chua H, Hellinger W. Early and late onset Clostridium difficile-associated colitis following liver transplantation. Transpl Int . 2007;20:856–866. doi: 10.1111/j.1432-2277.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 58.Mittal C, Hassan S, Arshad S, Jeepalyam S, Bruni S, Miceli M, Jacobsen G, Abouljoud M, Bajjoka I, Ramesh M, Alangaden G. Clostridium difficile infection in liver transplant recipients: a retrospective study of rates, risk factors and outcomes. Am J Transplant . 2014;14:1901–1907. doi: 10.1111/ajt.12798. [DOI] [PubMed] [Google Scholar]
- 59.Musa S, Moran C, Rahman T. Clostridium difficile infection and liver disease. J Gastrointestin Liver Dis . 2010;19:303–310. [PubMed] [Google Scholar]
- 60.Hashimoto M, Sugawara Y, Tamura S, Kaneko J, Matsui Y, Togashi J, Makuuch M. Clostridium difficile-associated diarrhea after living donor liver transplantation. World J Gastroenterol . 2007;13:2072–2076. doi: 10.3748/wjg.v13.i14.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abe K, Shiba H, Furukawa K, Sakamoto T, Ishida Y, Yanaga K. Repeated Clostridium difficile infection after living donor liver transplantation. Clin J Gastroenterol . 2018;11:309–311. doi: 10.1007/s12328-018-0840-x. [DOI] [PubMed] [Google Scholar]
- 62.Vanjak D, Girault G, Branger C, Rufat P, Valla DC, Fantin B. Risk factors for Clostridium difficile infection in a hepatology ward. Infect Control Hosp Epidemiol . 2007;28:202–204. doi: 10.1086/511790. [DOI] [PubMed] [Google Scholar]
- 63.Abraham G, Pratap B, Sankarasubbaiyan S, Govindan P, Nayak KS, Sheriff R, Naqvi SA. Chronic peritoneal dialysis in South Asia - challenges and future. Perit Dial Int . 2008;28:13–19. [PubMed] [Google Scholar]
- 64.Papić N, Jelovčić F, Karlović M, Marić LS, Vince A. Nonalcoholic fatty liver disease as a risk factor for Clostridioides difficile infection. Eur J Clin Microbiol Infect Dis . 2020;39:569–574. doi: 10.1007/s10096-019-03759-w. [DOI] [PubMed] [Google Scholar]
- 65.Kalaitzakis E, Björnsson E. Inadequate use of proton-pump inhibitors in patients with liver cirrhosis. Eur J Gastroenterol Hepatol . 2008;20:512–518. doi: 10.1097/MEG.0b013e3282f4aa01. [DOI] [PubMed] [Google Scholar]
- 66.Agastya G, West BC, Callahan JM. Omeprazole inhibits phagocytosis and acidification of phagolysosomes of normal human neutrophils in vitro. Immunopharmacol Immunotoxicol . 2000;22:357–372. doi: 10.3109/08923970009016425. [DOI] [PubMed] [Google Scholar]
- 67.Novack L, Kogan S, Gimpelevich L, Howell M, Borer A, Kelly CP, Leffler DA, Novack V. Acid suppression therapy does not predispose to Clostridium difficile infection: the case of the potential bias. PLoS One . 2014;9:e110790. doi: 10.1371/journal.pone.0110790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arriola V, Tischendorf J, Musuuza J, Barker A, Rozelle JW, Safdar N. Assessing the Risk of Hospital-Acquired Clostridium Difficile Infection With Proton Pump Inhibitor Use: A Meta-Analysis. Infect Control Hosp Epidemiol . 2016;37:1408–1417. doi: 10.1017/ice.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trifan A, Stanciu C, Girleanu I, Stoica OC, Singeap AM, Maxim R, Chiriac SA, Ciobica A, Boiculese L. Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J Gastroenterol . 2017;23:6500–6515. doi: 10.3748/wjg.v23.i35.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis . 2006;43:1272–1276. doi: 10.1086/508453. [DOI] [PubMed] [Google Scholar]
- 71.Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ . 2004;171:33–38. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Natarajan M, Rogers MA, Bundy J, Micic D, Walk ST, Santhosh K, Rao K, Winters S, Young VB, Aronoff DM. Gender Differences in Non-Toxigenic Clostridium difficile Colonization and Risk of Subsequent C. difficile Infection. Clin Res Infect Dis . 2015;2 [PMC free article] [PubMed] [Google Scholar]
- 73.Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999-2004. Emerg Infect Dis . 2007;13:1417–1419. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcia-Tsao G, Surawicz CM. Editorial: Clostridium difficile infection: Yet another predictor of poor outcome in cirrhosis. Am J Gastroenterol . 2010;105:114–116. doi: 10.1038/ajg.2009.604. [DOI] [PubMed] [Google Scholar]
- 75.Gravel D, Miller M, Simor A, Taylor G, Gardam M, McGeer A, Hutchinson J, Moore D, Kelly S, Boyd D, Mulvey M Canadian Nosocomial Infection Surveillance Program. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis . 2009;48:568–576. doi: 10.1086/596703. [DOI] [PubMed] [Google Scholar]
- 76.Rosenblatt R, Mehta A, Cohen-Mekelburg S, Shen N, Snell D, Lucero C, Jesudian A, Fortune B, Crawford CV, Kumar S. The rise of Clostridioides difficile infections and fall of associated mortality in hospitalized advanced cirrhotics. Liver Int . 2019;39:1263–1270. doi: 10.1111/liv.14077. [DOI] [PubMed] [Google Scholar]
- 77.Kim D, Yoo ER, Li AA, Tighe SP, Cholankeril G, Ahmed A. Trends in Hospitalizations for Clostridioides difficile Infection in End-Stage Liver Disease, 2005-2014. Dig Dis Sci . 2021;66:296–307. doi: 10.1007/s10620-020-06162-0. [DOI] [PubMed] [Google Scholar]
- 78.Cheng YW, Alhaffar D, Saha S, Khanna S, Bohm M, Phelps E, Ghabril M, Orman E, Sashidhar S, Rogers N, Xu H, Khoruts A, Vaughn B, Kao D, Wong K, Cammarota G, Ianiro G, Dhere T, Kraft CS, Mehta N, Woodworth MH, Allegretti JR, Nativ L, Marcus J, El-Nachef N, Fischer M. Fecal Microbiota Transplantation Is Safe and Effective in Patients With Clostridioides difficile Infection and Cirrhosis. Clin Gastroenterol Hepatol . 2021;19:1627–1634. doi: 10.1016/j.cgh.2020.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nelson RL, Suda KJ, Evans CT. Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults. Cochrane Database Syst Rev . 2017;3:CD004610. doi: 10.1002/14651858.CD004610.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Debast SB, Bauer MP, Kuijper EJ European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect . 2014;20 Suppl 2:1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 81.Guery B, Menichetti F, Anttila VJ, Adomakoh N, Aguado JM, Bisnauthsing K, Georgopali A, Goldenberg SD, Karas A, Kazeem G, Longshaw C, Palacios-Fabrega JA, Cornely OA, Vehreschild MJGT EXTEND Clinical Study Group. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis . 2018;18:296–307. doi: 10.1016/S1473-3099(17)30751-X. [DOI] [PubMed] [Google Scholar]
- 82.Ooijevaar RE, van Beurden YH, Terveer EM, Goorhuis A, Bauer MP, Keller JJ, Mulder CJJ, Kuijper EJ. Update of treatment algorithms for Clostridium difficile infection. Clin Microbiol Infect . 2018;24:452–462. doi: 10.1016/j.cmi.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 83.Ito Y, Moriwaki H, Muto Y, Kato N, Watanabe K, Ueno K. Effect of lactulose on short-chain fatty acids and lactate production and on the growth of faecal flora, with special reference to Clostridium difficile. J Med Microbiol . 1997;46:80–84. doi: 10.1099/00222615-46-1-80. [DOI] [PubMed] [Google Scholar]
- 84.Pringle PL, Soto MT, Chung RT, Hohmann E. Patients With Cirrhosis Require More Fecal Microbiota Capsules to Cure Refractory and Recurrent Clostridium difficile Infections. Clin Gastroenterol Hepatol . 2019;17:791–793. doi: 10.1016/j.cgh.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 85.Agarwalla A, Weber A, Davey S, Hamilton K, Goldberg D, Rhim AD, Yang YX. Lactulose Is Associated With Decreased Risk of Clostridium difficile Infection in Decompensated Cirrhosis. Clin Gastroenterol Hepatol . 2017;15:953–954. doi: 10.1016/j.cgh.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 86.Feuerstadt P, Hong SJ, Brandt LJ. Chronic Rifaximin Use in Cirrhotic Patients Is Associated with Decreased Rate of C. difficile Infection. Dig Dis Sci . 2020;65:632–638. doi: 10.1007/s10620-019-05804-2. [DOI] [PubMed] [Google Scholar]
- 87.Pop A, Procopet B, Stefanescu H, Cavasi A, Tantau M, Andreica V. Clostridium Difficile Screening in Cirrhosis: One for All, or Some for One? Dig Dis Sci . 2015;60:3825–3826. doi: 10.1007/s10620-015-3913-4. [DOI] [PubMed] [Google Scholar]
- 88.Saab S, Alper T, Sernas E, Pruthi P, Alper MA, Sundaram V. Hospitalized Patients with Cirrhosis Should Be Screened for Clostridium difficile Colitis. Dig Dis Sci . 2015;60:3124–3129. doi: 10.1007/s10620-015-3707-8. [DOI] [PubMed] [Google Scholar]
- 89.Meltzer E, Smollan G, Huppert A, Fluss R, Tal I, Gilboa M, Zilberman-Daniels T, Keller N, Rahav G, Regev-Yochay G SHIC research group. Universal screening for Clostridioides difficile in a tertiary hospital: risk factors for carriage and clinical disease. Clin Microbiol Infect . 2019;25:1127–1132. doi: 10.1016/j.cmi.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez SA. Antibiotic Prophylaxis for Spontaneous Bacterial Peritonitis: Benefit or Risk? Am J Gastroenterol . 2019;114:553–555. doi: 10.14309/ajg.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 91.Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol . 2016;14:609–620. doi: 10.1038/nrmicro.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin JS, Monaghan TM, Wilcox MH. Clostridium difficile infection: epidemiology, diagnosis and understanding transmission. Nat Rev Gastroenterol Hepatol . 2016;13:206–216. doi: 10.1038/nrgastro.2016.25. [DOI] [PubMed] [Google Scholar]
- 93.Johnson S, Gerding DN, Olson MM, Weiler MD, Hughes RA, Clabots CR, Peterson LR. Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission. Am J Med . 1990;88:137–140. doi: 10.1016/0002-9343(90)90462-m. [DOI] [PubMed] [Google Scholar]
- 94.Bobulsky GS, Al-Nassir WN, Riggs MM, Sethi AK, Donskey CJ. Clostridium difficile skin contamination in patients with C. difficile-associated disease. Clin Infect Dis . 2008;46:447–450. doi: 10.1086/525267. [DOI] [PubMed] [Google Scholar]
- 95.Wilcox MH, Fawley WN, Wigglesworth N, Parnell P, Verity P, Freeman J. Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile infection. J Hosp Infect . 2003;54:109–114. doi: 10.1016/s0195-6701(02)00400-0. [DOI] [PubMed] [Google Scholar]
- 96.Mayfield JL, Leet T, Miller J, Mundy LM. Environmental control to reduce transmission of Clostridium difficile. Clin Infect Dis . 2000;31:995–1000. doi: 10.1086/318149. [DOI] [PubMed] [Google Scholar]
- 97.Eckstein BC, Adams DA, Eckstein EC, Rao A, Sethi AK, Yadavalli GK, Donskey CJ. Reduction of Clostridium Difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect Dis . 2007;7:61. doi: 10.1186/1471-2334-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol . 2020;18:2650–2666. doi: 10.1016/j.cgh.2019.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hong SJ, Feuerstadt P, Brandt LJ. MELD is the only predictor of short-term mortality in cirrhotic patients with C. difficile infection. Dig Liver Dis . 2019;51:275–280. doi: 10.1016/j.dld.2018.07.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request