Abstract
Mesenchymal stem cells (MSCs) have received significant attention in recent years due to their large potential for cell therapy. Indeed, they secrete a wide variety of immunomodulatory factors of interest for the treatment of immune-related disorders and inflammatory diseases. MSCs can be extracted from multiple tissues of the human body. However, several factors may restrict their use for clinical applications: the requirement of invasive procedures for their isolation, their limited numbers, and their heterogeneity according to the tissue of origin or donor. In addition, MSCs often present early signs of replicative senescence limiting their expansion in vitro, and their therapeutic capacity in vivo. Due to the clinical potential of MSCs, a considerable number of methods to differentiate induced pluripotent stem cells (iPSCs) into MSCs have emerged. iPSCs represent a new reliable, unlimited source to generate MSCs (MSCs derived from iPSC, iMSCs) from homogeneous and well-characterized cell lines, which would relieve many of the above mentioned technical and biological limitations. Additionally, the use of iPSCs prevents some of the ethical concerns surrounding the use of human embryonic stem cells. In this review, we analyze the main current protocols used to differentiate human iPSCs into MSCs, which we classify into five different categories: MSC Switch, Embryoid Body Formation, Specific Differentiation, Pathway Inhibitor, and Platelet Lysate. We also evaluate common and method-specific culture components and provide a list of positive and negative markers for MSC characterization. Further guidance on material requirements to produce iMSCs with these methods and on the phenotypic features of the iMSCs obtained is added. The information may help researchers identify protocol options to design and/or refine standardized procedures for large-scale production of iMSCs fitting clinical demands.
Keywords: Mesenchymal stem cells, Induced pluripotent stem cells, Mesenchymal stem cells derived from induced pluripotent stem cells, Differentiation methods, Culture components, Mesenchymal stem cell markers
Core Tip: Heterogeneity of mesenchymal stem cell (MSC) quality might have hampered the robust success of stem cell clinical trials (CTs). The production of MSCs from a single homogeneous source (i.e. induced pluripotent stem cells, iPSCs) could elevate stem cell therapeutics standardization to unprecedented levels. However, a unique optimized procedure for large-scale production of MSCs, of homogenous quality, from iPSCs (iMSCs) is missing. Main methods, culture components, and common MSC markers to produce iMSCs are provided here as reference resources for the establishment of harmonized Good Manufacturing Procedures towards obtaining clinical-grade iMSCs with improved CT performance.
INTRODUCTION
Stem cells (SCs) are unspecialized cells capable of self-replication and of generating specific cell types through differentiation. They are necessary for the regular renewal of our tissues and organs such as the skin or gut´s lining, but also for the regeneration of damaged tissues upon injury; and, thus, for human homeostasis and survival. Most of the time SCs are found in a “dormant” state and become activated by signals received from tissues needing to be repaired. SCs, however, lose their potential for repair over their lifetime[1,2]. During embryonic development, SCs are very active, they can differentiate into a wide range of cell types and can migrate easily throughout the embryonic structure, whereas in the adult they decrease in number, restrict themselves to specific tissue locations and become more specialized.
In particular, mesenchymal SCs (MSCs) are adult pluripotent SCs that can be found in various tissues at low numbers. They were initially (mid 60s) identified by Friedenstein in the bone marrow of mice[3], but later have been found in many additional human tissues[4] including adipose tissue[5], umbilical cord[6], neural crest cells[7], and dental tissues[8-11]. Basically, all vascularized human tissues seem to harbor MSCs[12]. MSCs are consensually described by fulfilling the minimal criterion established by the International Society for Cellular Therapy (ISCT) including the presence of specific cell surface markers, their capacity for tri-lineage differentiation, their fibroblast-morphology, and adherent ability[13].
MSCs can inhibit pathological immune responses and suppress inflammation, partly due to secretion of soluble factors and cell-cell contact mechanisms. Indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), transforming growth factor beta (TGF-β), soluble human leukocyte antigen-G5 (HLA-G5), interleukin-10 (IL-10) and IL-6 are a few of these soluble factors allowing MSCs and immune cell cross-talk. The release of cytokines by immune cells is thus regulated by MSCs, by virtue of modifying their proliferation, survival, and cytotoxicity[14].
Cultured MSCs can be safely used without major potential risks for immune rejection because of their low expression levels of major histocompatibility complex (MHC) class I molecules, and the lack of MHC class II and co-stimulatory molecules (cluster of differentiation 80 [CD80], CD86, CD40)[15].
MSC cell therapy can be applied to a wide-range of disease types such as inflammatory diseases (i.e. arthritis)[16], auto-immune diseases (i.e. lupus erythematosus)[17], but also increases the success of organ transplants[18], improve cell engraftment[19], bone[20,21] or cartilage regeneration[22], and wound healing[23]. However, several disadvantages restrain their use in the clinic such as the requirement of invasive techniques for their isolation, the limited numbers that can be obtained from a single donor, their limited capacity to proliferate and engraft in vivo, the diversity of isolation and expansion protocols, and their heterogeneous quality depending on donor´s features and tissue´s source[24-28]. In addition, it was shown that during in vitro culture expansion MSC cells rapidly senesce[29-31], limiting the amounts obtained from donors. Culture conditions might impact their epigenetic profiles or induce genomic changes[32]. Still, their use for the treatment of inflammatory and autoimmune diseases is becoming recognized[33,34]. Readers are referred to recent systematic reviews summarizing the results of MSC clinical trials (CTs) for the treatment of inflammatory disorders and for regenerative therapies[35,36].
Induced pluripotent stem cells (iPSCs) represent an inexhaustible source for MSC production because they can be grown indefinitely in pluripotent state without signs of replicative senescence. Importantly, their phenotype and functions are better defined, and contrary to ESCs, they do not require destruction of embryos[37,38]. The differentiation of iPSCs into MSCs to produce iMSCs of homogeneous quality, therefore, holds great promise to overcome the actual limitations that adult MSC present for clinical applications.
Various methods to produce iMSCs with potential for industrial scale-up have already emerged[39]. They are particularly mediated via various culture components and growth factor complementation, such as the use of coating materials and the addition of pathway inhibitors. Ectopic expression of MSC-related genes may also be used to further refine these protocols[40]. As iPSCs can spontaneously differentiate, a need to evaluate iMSC (MSCs from iPSCs) appearance in large-scale production protocols to support reproducible iMSC quality production results obvious.
This review gathers information on the main current non-commercial protocols used to differentiate iPSC into iMSC, describes cell sources of the iPSCs assayed, and lists the characterization procedures applied to the obtained iMSCs based on cell surface markers. This information can guide future scale-up protocols, and required refinements, towards optimization of iMSC production of homogeneous quality fitting therapeutic demands.
IPSC CULTURE CONDITIONS TOWARDS IMSC PRODUCTION
Main approaches to generate iMSCs
We have reviewed 32 selected studies describing original methods to generate iMSCs, and have classified the identified procedures according to the following five main strategies: MSC Switch (15/32), Embryoid Bodies (EBs) (13/32), Specific Differentiation (5/32), Pathway Inhibitor (5/32), and Platelet Lysate (3/32). They will be presented by order of their frequency of use in the selected literature (Table 1-5). Some of the studies used more than one of these methods, as detailed in Supplementary Table 1. To define the method variants used by different groups, an “iPSC to iMSC Protocol” column has been incorporated in each table (Table 1-5). Additional features incorporated in the study summary tables as potential relevant for iMSC production are: the minimal required time to obtain iMSCs from iPSCs in days, the type of adult cell from which iPSCs were obtained (“iPSC origin”), and the application explored in the mentioned study.
Table 1.
Protocols to produce induced pluripotent stem cell-derived mesenchymal stem cells by the mesenchymal stem cell Switch methods
|
Ref.
|
iPSC origin
|
iPSC to iMSC protocol
|
Time
|
Application
|
Citations
|
| Lian et al[41], 2010 | Lung fibroblast | (1) iPSC cultured on a gelatinized dish + KO DMEM + 10% SRM + bFGF + PDGFAB + EGF; and (2) FACS SORTING: CD24- and CD105+ and single cell clones plating | 7 | Limb ischemia in mice | 419 |
| Giuliani et al[42], 2011 | Amniocytes | iPSC cultured 4 wk in DMEM/F12 + 10% hiFBS + b-FGF + NEAA + L-Glutamine + β-ME + p/s | 28 | Immunomodulatory properties of iMSC on NK cytolytic activity | 110 |
| Liu et al[43], 2012 | Dermal fibroblast | (1) iPSC cultured on collagen-coated dishes with α-MEM + 10% FBS + dexamethasone + magnesium L-ascorbic acid phosphate + p/s; and (2) Cells cultured on collagen-coated dishes with α-MEM + 10% FBS + L-Glutamine + NEAA + p/s | 10 | iMSC generation w/ Fibrillar Collagen Coating | 118 |
| Zou et al[44], 2013 | Dermal fibroblasts | iPSC medium switched for MSC medium: DMEM-low glucose + 10% FBS + L-Glutamine | 14 | Generation of osteogenesis 3D scaffolds | 98 |
| Hynes et al[45], 2014 | Gingival fibroblast periodontal ligaments | (1) iPSC cultured with MSC medium: α-MEM + FCS + sodium pyruvate + l-ascorbate-2-phosphate + L-Glutamine + NEAA + HEPES + p/s; and (2) Cells cultured on gelatin-coated-flasks then switch to non-coated flasks | 14 | Generation of iMSC | 102 |
| Jeong et al[46], 20141 | NA | (1) iPSCs cultured in iMSC-inducing medium: DMEM/F12 + 20% KOSR + SB431542 (TGFβ inhibitor); (2) EB grown on matrigel + DMEM/F12 + 0.5% BSA + 10% ITS + SB431542 (TGFβ inhibitor); and (3) Outgrowth grown with DMEM/F12 + 10% FBS + p/s | 17 | Duchene muscular dystrophy | 17 |
| Hu et al[47], 2015 | iPS-S-01, C1P33, PCKDSF001C1 | iPSC medium switched for MSC medium: DMEM-low glucose + 10% FBS + L-Glutamine, then cultured in gelatin-coated dishes | 14 | Limb ischemia | 177 |
| Kang et al[48], 2015 | Dermal fibroblasts | iPSC cultured with MSC medium: DMEM low glucose + FBS 10% + L-Glutamine + p/s then cultured on gelatin-coated dishes | 14 | iMSC plasticity (less adipogenesis) | 65 |
| Zhang et al[23], 2015 | PBMCs | (1) iPSC medium switched for MSC medium: DMEM-low glucose + 10% FBS + L-Glutamine + NEAA + p/s; and (2) Cells cultured on gelatin-coated dishes | 17 | Cutaneous wound healing | 262 |
| Lian et al[49], 2016 | NA | (1) iPSC cultured on gelatin-coated plates with MSC differentiation medium: KO DMEM + KOSR + bFGF + PDGFAB + EGF; and (2) FACS: CD24- CD105+ cells cultured on gelatin-coated plates with DMEM + 10% FBS + bFGF + PDGFAB + EGF | 20 | Directed differentiation of iPSC to MSC | 15 |
| Gao et al[50], 2017 | Urine cell; Amniocytes | (1) iPSC cultured with MSC-inducing culture media: α-MEM + SRM + sodium pyruvate + l-ascorbate-2-phosphate + L-Glutamine + NEAA + p/s on gelatin-coated plates; and (2) Cells cultured with MSC maintenance medium = high-glucose DMEM + 10% FBS + bFGF + EGF | 17 | iMSC effect on dendritic cells | 27 |
| Nachlas et al[51], 20181 | NA | (1) iPSC cultured in suspension (to promote cell aggregate) with differentiation media: KO-DMEM + β-ME+ L-Glutamine + 20% FBS + NEAA + p/s, then, cells were cultured on gelatin coated plates; and (2) Cells cultured with iMSC media: KO-DMEM + L-Glutamine + 10% FBS + NEAA + p/s | 12 | Generation of valve interstitial-like cells from iMSC | 14 |
| Wang et al[52], 2018 | Amniocytes | (1) iPSC cultured with induction medium: α-MEM + 10% FBS + p/s + L-Glutamine + NEAA + sodium pyruvate + l-ascorbate-2- phosphate; (2) Cells plated on gelatin-coated plates; and (3) Cells plated on uncoated plates with iPSC-MSC medium = High-Glucose DMEM + FBS + bFGF + EGF + p/s | 14 | Effect of Dexamethasone on iMSC | 3 |
| Wang et al[53], 2018 | PBMCs | iPSC cultured with MSC medium: Low-Glucose DMEM + 10% FBS + p/s + L-glutamine | NA | Immunomodulatory properties of MSC, transcriptome analysis | 8 |
| McGrath et al[54], 20191 | Dermal fibroblast | (1) iPSC-MP thawed and expanded in KO DMEM + bFGF + L-Glutamine + MEM + NEAA + 20% FBS + Antibiotic-Antimycotic + β-ME; and (2) Cell are plated into gelatin coated-plates with KO DMEM + heparin + hPL + bFGF + L-Glutamine + MEM NEAA + Antibiotic-Antimycotic + β-ME | NA | iMSC differentiation: GMP-compatible and xeno-free cultivation | 6 |
iPSC origin refers to the cell type used for reprograming; Time is indicated as the minimum number of days required to obtain iMSCs; Citations show numbers on March 2020.
In references indicate the study includes methods in more than one protocol category. bFGF: Basic fibroblast growth factor; β-ME: β-Mercaptoethanol; BSA: Bovine serum albumin; CDM: Chemical defined medium; DMEM/F12: Dulbecco's modified Eagle’s medium F12; EB: Embryoid body; EGF: Epidermal growth factor; FBS: Fetal bovine serum; FCS: Fetal calf serum; HEPES: 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; hiFBS: Heat inactivated fetal bovine serum; hPL: human platelet lysate; IMDM: Iscove's modified Dulbecco's media; iMSCs: Induced pluripotent stem cell-derived mesenchymal stem cells; iPSC: Induced pluripotent stem cell; ITS: Insulin-transferrin-selenium; KO-DMEM: Knockout Dulbecco's modified Eagle's medium; KOSR: Knock-out serum replacement; α-MEM: Minimum essential medium Eagle; NA: Not available; NEAA: Non-essential amino acid; p/s: Penicillin-streptomycin; SRM: Serum replacement medium; TGF: Transforming growth factor.
Table 5.
Protocols to produce induced pluripotent stem cell-derived mesenchymal stem cells by approaches that use Platelet Lysate
|
Ref.
|
iPSC origin
|
iPSC to iMSC protocol
|
Time
|
Application
|
Citations
|
| Frobel et al[71], 2014 | BM-MSCs | (1) EB formation on ultra-low attachment plates; and (2) Cells cultured with standard medium for MSC: DMEM + L-Glutamine + p/s + hPL + heparin on matrigel-coated wells then passaged on gelatin-coated wells | 35 | Epigenetic study of iMSC | 116 |
| Luzzani et al[72], 2015 | Foreskin fibroblasts | (1) iPSC cultured in matrigel/geltrex-coated dishes with a-MEM + 10% PL + p/s + B7 or DMEM + 10% FBS; and (2) Cells cultured with no-coated dishes | 20 | MSC differentiation using platelet lysate | 26 |
| McGrath et al[54], 20191 | Dermal fibroblasts | (1) iPSC-MP thawed and expanded in KO DMEM + bFGF + L-Glutamine + MEM NEAA + FBS (20%) + Antibiotic-Antimycotic+ β-ME; and (2) Cell are plated on gelatin coated-plates with DMEM KO + heparin + hPL+ bFGF + L-Glutamine + MEM NEAA + Antibiotic-Antimycotic+ β-ME | NA | iMSC differentiation: GMP-compatible and xeno-free cultivation | 6 |
iPSC origin refers to the cell type used for reprograming; Time is indicated as the minimum number of days required to obtain iMSCs; Citations show numbers on March 2020.
In references indicate the study includes methods in more than one protocol category. bFGF: Basic fibroblast growth factor; BM: Bone marrow; β-ME: β-Mercaptoethanol; EB: Embryoid body; DMEM: Dulbecco's modified Eagle's medium; FBS: Fetal bovine serum; GMP: Good manufacturing procedures; hPL: human platelet lysate; hEGF: Human epidermal growth factor; iMSCs: Induced pluripotent stem cell-derived mesenchymal stem cells; iPSC: Induced pluripotent stem cell; KO DMEM: Knockout Dulbecco's modified Eagle's medium; α-MEM: Minimum essential medium Eagle; MSC: Mesenchymal stem cell; NA: Not available; NEAA: Non-essential amino acid; p/s: Penicillin-streptomycin.
As shown in Table 1, the MSC Switch strategy basically consists of replacing or “switching” the iPSC culture media by MSCs to induce spontaneous MSC differentiation. Some authors describe simple details, such as the addition of certain compounds or coatings, while other apply more sophisticated methods. The latter is the case of Lian et al[41] who use cell sorting (flow cytometry cell sorting (FACS) to select a subpopulation of cells positive for the CD105 marker and negative for CD24 before the iMSC differentiation step[41].
Another set of procedures are grouped under the modality denominated EBs as they are present in producing clusters of cells or EBs to later be seeded into MSC-specific media to induce differentiation. Again, a considerable number of options have been described by different research groups (Table 2). It is interesting that the variant method used by Eto et al[55] also applies FACS to select a subpopulation prior to its differentiation. In this case, however, the markers used for cell selection were the receptors platelet derived growth factor receptor alpha (PDGFRα) and vascular endothelial growth factor receptor 2 (VEGFR2), both of which are mediators of cell proliferation but neither are on the list of minimal MSC criteria[13].
Table 2.
Protocols to produce induced pluripotent stem cell-derived mesenchymal stem cells by embryoid bodies approaches
|
Ref.
|
iPSC origin
|
iPSC to iMSC protocol
|
Time
|
Application
|
Citations
|
| Ahfeldt et al[56], 2012 | Foreskin fibroblast | (1) iPSC cultured into low-adhesion dishes for EB formation with DMEM + 15% FBS + L-Glutamine; (2) EB plated into gelatin-coated dishes with DMEM + 15% FBS + L-Glutamine; and (3) Cells plated with Mensenchymal Progenitor Cell (MCP) medium: DMEM + 15% FBS + L-Glutamine + bFGF | 12 | Producing white and brown adipocytes from hPSCs | 194 |
| Chen et al[57], 20121 | Lung fibroblast | SB431542 inhibitor differentiation method (feeder free); iPSC cultured in inhibitor differentiation medium: KOSR medium + SB431542 (TGFβ inhibitor)Without bFGF to enhance differentiation. Embryoid body differentiation method: (1) EB formation in KOSR medium; and (2) EB cultured with MSC medium: DMEM + 10% FCS + L-Glutamine + gentamicin + p/s | 17 | Generation of iMSC with TGF-beta inhibitor | 136 |
| Villa-Diaz et al[58], 2012 | Dermal fibroblasts | (1) EB formation in suspension cultured into ultra-low-attachment plates; and (2) EB plated on gelatin-coated dishes with MSC medium: α-MEM + 10% FBS + L-Glutamine + NEAA + FGF2 | 21 | iMSC from iPSC cultured on synthetic substrate (PMEDSAH) | 262 |
| Wei et al[59], 20121 | Dermal fibroblasts | (1) EB formation through cardiac differentiation protocol involving cardiomyogenic medium CARM: High-Glucose DMEM + L-Glutamine + NEAA + Selenium Transferrin + β-ME + SB 203580 (p38-MAPK inhibitor); and (2) EB plating on gelatin-coated plates with DMEM + 2% FBS | 21 | Generation of iMSC | 64 |
| Shao et al[60], 2013 | MSC | (1) iPSC cultivated in suspension in the differentiation medium: KO DMEM + 20% FBS+ 1% NEAA + β-ME+ L-Glutamine for EB formation; and (2) Embryoid bodies plated on gelatin-coated dishes | 19 | iMSC DNA methylation profiles | 48 |
| Jeong et al[46], 20141 | NA | (1) iPSCs cultured in iMSC-inducing medium: DMEM/F12 + 20% KOSR + SB431542 (TGFβ inhibitor); (2) EB grown on matrigel + DMEM/F12 + 0.5% BSA + 10% ITS + SB431542 (TGFβ inhibitor); and (3) Outgrowth grown with DMEM/F12 + FBS (10%) + p/s | 17 | Duchene muscular dystrophy | 17 |
| Miao et al[61], 2014 | Dermal fibroblasts | EB cultured with DMEM + 10% FBS | NA | Myocardial infarctus | 38 |
| Tang et al[21], 2014 | bone marrow | (1) iPSC cultured in ultra-low attachment plate to form EB with differentiation medium: DMEM/F12 + 20% KSR + MEF medium + NEAA + L-Glutamine + β-ME; and (2) EB plated into gelatin-coated plates + MSC growth medium: DMEM + 10% FBS + L-Glutamine + p/s | 20 | iMSC and calcium phosphate scaffold for bone regeneration | 88 |
| Sheyn et al[20], 2016 | Dermal fibroblasts | (1) iPSC plated into PCR plates to form EB with IMDM medium: MDM media + KOSR + NEAA + β-ME + PSA antifungal-antibacterial solution; (2) EB transferred to poly-HEMA-coated flasks; (3) Attached EB (aiMSCs) and Transferred EB (tiMSCs) cultured into gelatin-coated flask with medium + TGF-β1; and (4) Medium switched for DMEM + 10% FBS + L-Glutamine + p/s | 10 | Generation of iMSC and repair bone defect | 60 |
| Eto et al[55], 20181 | Skin fibroblast | (1) iPSCs treated with CTK (collagenase type IV + 0.25% trypsin + KSR) and transferred to petri dishes to form EB with: DMEM/F12 + 20% KOSR + glutamine + NEAA + BMP4 + p/s; (2) Specific Differentiation or Mesodermal Differentiation: EB cultured on collagen-coated plates + αMEM + 10% FBS + bFGF + BMP4 + Activin A + LiCl + p/s; or Neuroepithelial differentiation: αMEM + 10% FBS + β-ME + RA; and (3) FACS: PDGFR-α+ and VEGFR2+ cells resuspended on collagen-coated plates with αMEM + 10% FBS + 20% KOSR | 10 | iMSC from mesoderm or neuroepithelium differentiation | 7 |
| Nachlas et al[51], 20181 | NA | (1) iPSC cultured in suspension (to promote cell aggregate) with differentiation media: KO-DMEM + β-ME + L-Glutamine + 20% FBS + NEAA + p/s, then cells were cultured on gelatin coated plates; and (2) Cells cultured with iMSC media: KO-DMEM + L-Glutamine + 10% FBS + NEAA + p/s | 12 | Generation of valve interstitial-like cells from iMSC | 14 |
| Karam et al[62], 2020 | PBMC | (1) EB cultured into ultra-low attachment plates with differentiation medium: Low-Glucose DMEM + 15% FBS + p/s; (2) Later, RA is added to enhance EB formation; (3) EB plating into gelatin/matrigel coated plates + differentiation medium; and (4) Later bFGF is added | 14 | Generation of iMSC and adipocytes | NA |
| Huang et al[63], 2020 | PBMC | (1) iPSC cultured in suspension to form EB; and (2) Cells plated into gelatin-coated plates with α-MEM + FGF2 | NA | Repair of acute kidney injury | NA |
iPSC origin refers to the cell type used for reprograming; Time is indicated as the minimum number of days required to obtain iMSCs; Citations show numbers on March 2020.
In references indicate the study includes methods in more than one protocol category. bFGF: Basic fibroblast growth factor; β-ME: β-Mercaptoethanol; BMP4: Bone morphogenetic protein 4; BSA: Bovine serum albumin; CDM: Chemical defined medium; DMEM/F12: Dulbecco's modified Eagle’s medium F12; EB: Embryoid body; EGF: Epidermal growth factor; FBS: Fetal bovine serum; FCS: Fetal calf serum; FGF2: Fibroblast growth factor 2; HEPES: 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; hiFBS: Heat inactivated fetal bovine serum; hPL: human platelet lysate; IMDM: Iscove's modified Dulbecco's media; iMSCs: Induced pluripotent stem cell-derived mesenchymal stem cells; iPSC: Induced pluripotent stem cell; ITS: Insulin-transferrin-selenium; KO-DMEM: Knockout Dulbecco's modified Eagle’s medium; KOSR: Knock-out serum replacement; KSR: Knockout serum replacement; α-MEM: Minimum essential medium Eagle; NA: Not available; PBMC: Peripheral blood mononuclear cell; p/s: Penicillin-streptomycin; SRM: Serum replacement medium; TGF: Transforming growth factor; VEGFR: Vascular endothelial growth factor.
As mentioned, three other less popular methods to induce iMSC production have been described. In the Specific Differentiation method, specific progenitors are obtained before culturing the cells in MSC media to induce differentiation to mesenchymal cells (Table 3). For example, iPSCs are pre-differentiated into neural or cardiac cell progenitors (priming of iPSCs), before inducing their differentiation to MSCs. The rationale behind this procedure resides in the observation that these progenitor-like cells are more closely related to MSCs than the multipotent iPSCs. Mitsuzawa et al[64], Eto et al[55], Ouchi et al[65], and Fukuta et al[66] first generated neural progenitors as a previous step towards iMSC production. It must be noted that Fukuta et al[66] used SB431542 (a TGF-β inhibitor) to support the differentiation of iPSCs, and CHIR (a glycogen synthase kinase-3 [GSK3] inhibitor, inducer of Wnt/β-catenin signaling pathway) to promote differentiation to neural cells. Mitsuzawa et al[64] modified Fukuta et al[66]´s protocol by using Dulbecco's modified Eagle's medium (DMEM) instead of α-MEM, and added fibroblast growth factor (FGF) to the media to promote MSC differentiation. Eto et al[55] initially treated iPSCs with CTK (dissociation solution made of collagenase type IV, trypsin and KSR, or Knockout Serum Replacement), reducing cell oxidative stress; and the EBs with bone morphogenetic protein 4 + activin A + lithium chloride for pre-mesodermal differentiation or retinoic acid to enhance EB differentiation, plus β-mercaptoethanol to encourage neural differentiation before culturing the cells in MSC media. Ouchi et al[65] generated neural progenitors by simply culturing iPSCs with specific neural media and proved that the cells grown under these conditions had the ability to turn into MSC afterwards. On another side, Wei et al[59] generated EBs through a cardiac differentiation protocol “cardiomyogenic medium,” promoted by the addition of SB 203580 (a p38 mitogen-activated protein kinase [MAPK] inhibitor), followed by cell plating on classic MSC media.
Table 3.
Protocols to produce induced pluripotent stem cell-derived mesenchymal stem cells by Specific Differentiation approaches
|
Ref.
|
iPSC origin
|
iPSC to iMSC protocol
|
Time
|
Application
|
Citations
|
| Wei et al[59], 20121 | Dermal fibroblasts | (1) EB formation through cardiac differentiation protocol involving cardiomyogenic medium CARM: High-Glucose DMEM + L-Glutamine + NEAA + Selenium Transferrin + β-ME + SB 203580 (p38-MAPK inhibitor); and (2) EB plating on gelatin-coated plates with DMEM + 2% FBS | 21 | Generation of iMSC | 64 |
| Fukuta et al[66], 20141 | Dermal fibroblast | (1) Induction of hNCC from iPSC; (2) Cells cultured on fibronectin-coated dishes with STK2 medium + CDM (IMDM/Ham's F-12 + lipid concentrate + apo-transferrin + monothioglycerol + BSA + insulin + p/s) + SB431542 (TGFβ inhibitor) + CHIR (Wnt Agonist); and (3) Cells cultured with αMEM + 10% FBS | 15 | iMSC differentiation through neural crest lineage | 80 |
| Ouchi et al[65], 2016 | Dermal fibroblast | (1) Generation of NCL (neural crest like-cells); and (2) NCL cultured into DMEM/F12 + Neurobasal medium + L-Glutamine + Gem21 Neuroplex + N2 Supplement + hbFGF + hEGF + insulin + p/s | 10 | iNCC can develop into iMSC | 8 |
| Eto et al[55], 20181 | skin fibroblast | (1) iPSCs treated with CTK (collagenase type IV + 0.25% trypsin + KSR) and transferred to petri dishes to form EB with: DMEM/F12 + 20% KOSR + glutamine + NEAA + BMP4 + p/s; (2) Specific Differentiation or Mesodermal Differentiation: EB cultured on collagen-coated plates + αMEM + 10% FBS + bFGF + BMP4 + Activin A + LiCl + p/s or Neuroepithelial differentiation: αMEM + 10% FBS + β-ME + RA; (3) FACS: PDGFR-α+ and VEGFR2+ cells resuspended on collagen-coated plates with αMEM + 10% FBS + 20% KOSR | NA | iMSC from mesoderm or neuroepithelium differentiation | 7 |
| Mitsuzawa et al[64], 2019 | NA | (1) Induction of hNCC: CDM (IMDM/Ham's F-12 + lipid concentrate + apo-transferrin+ monothioglycerol + BSA + insulin + p/s) + SB431542 (TGFβ inhibitor) + CHIR (Wnt Agonist)Maintenance in DMEM + 10% FBS + FGF2; and (2) Induction of iMSC with DMEM + 10% FBS + FGF2 on fibronectin coated plates | 25 | Hind limb in rat allotransplantation | 1 |
iPSC origin refers to the cell type used for reprograming; Time is indicated as the minimum number of days required to obtain iMSCs; Citations show numbers on March 2020.
In references indicate the study includes methods in more than one protocol category. bFGF: Basic fibroblast growth factor; β-ME: β-Mercaptoethanol; BMP4: Bone morphogenetic protein 4; BSA: Bovine serum albumin; CARM: Cardiomyogenic medium; CDM: Chemical defined medium; CHIR: Wnt agonist, potent GSK3 inhibitor; DMEM/F12: Dulbecco's modified Eagle's medium EB: Embryoid body; FBS: Fetal bovine serum; FGF2: Fibroblast growth factor 2; Ham's F12: Medium formulated for single-cell plating of near-diploid Chinese hamster ovary cells; hbFGF: Human basic fibroblast growth factor; hEGF: Human epidermal growth factor; IMDM: Iscove's modified Dulbecco's media; iMSCs: Induced pluripotent stem cell-derived mesenchymal stem cells; iPSC: Induced pluripotent stem cell; ITS: Insulin-transferrin-selenium; KOSR: Knock-out serum replacement; KSR: Knockout serum replacement; NA: Not available; NEAA: Non-essential amino acid; α-MEM: Minimum essential medium Eagle; PDGFR: Platelet-derived growth factor receptor A; p/s: Penicillin-streptomycin; RA: Retinoic acid; TGF: Transforming growth factor; VEGFR: Vascular endothelial growth factor.
The pathway inhibitor method consists of the addition of chemical inhibitors of specific pathways in the culture media to induce differentiation of the iPSCs into MSCs (Table 4). Examples of these supplements are again, SB-431542, which is a TGF-β inhibitor, the GSK-3 inhibitor CHIR, or the p38 MAPK inhibitor SB-203580. CHIR and SB-431542 had been used by Fukuta et al[66], as mentioned earlier, to induce neural progenitor-like cells before inducing MSC differentiation. In fact, some of the methods merge two of the five strategies here described for the production of iMSCs. Zhao et al[67], Jeong et al[46], and Chen et al[57] confirmed that this treatment worked with iPSCs. Indeed, SB431542 leads to the downregulation of pluripotency genes by inhibiting the TGF-β signaling pathway, promoting differentiation into MSCs. These results are encouraging because they show that MSC differentiation methods used earlier for the differentiation of hESCs[68-70] could be applied to produce MSCs from iPSCs.
Table 4.
Protocols to produce induced pluripotent stem cell-derived mesenchymal stem cells by Pathway Inhibitor approaches
|
Ref.
|
iPSC origin
|
iPSC to iMSC protocol
|
Time
|
Application
|
Citations
|
| Chen YS et al[57], 20121 | Lung fibroblast | SB431542 Inhibitor Differentiation Method (feeder free); iPSC cultured in inhibitor differentiation medium: KOSR medium + SB431542 (TGFβ inhibitor)Without bFGF to enhance differentiation; Embryoid body differentiation method: (1) EB formation in KOSR medium; and (2) EB cultured with MSC medium: DMEM + 10% FCS + L-Glutamine + gentamicin + p/s | 17 | Generation of iMSC with TGF-beta inhibitor | 136 |
| Wei et al[59], 20121 | Dermal fibroblasts | (1) EB formation through cardiac differentiation protocol involving cardiomyogenic medium CARM: High-Glucose DMEM + L-Glutamine + NEAA + Selenium Transferrin + β-ME + SB 203580 (p38-MAPK inhibitor); and (2) EB plating on gelatin-coated plates with DMEM + 2% FBS | 21 | Generation of iMSC | 64 |
| Fukuta et al[66], 20141 | Dermal fibroblast | (1) Induction of hNCC from iPSC; (2) Cells cultured on fibronectin-coated dishes with STK2 medium + CDM (IMDM/Ham's F-12 + lipid concentrate + apo-transferrin+ monothioglycerol +BSA + insulin + p/s)+ SB431542 (TGFβ inhibitor) + CHIR (Wnt Agonist); and (3) Cells cultured with αMEM + 10% FBS | 15 | iMSC differentiation through neural crest lineage | 80 |
| Jeong et al[46], 20141 | NA | (1) iPSCs cultured in iMSC-inducing medium: DMEM/F12 + 20% KOSR + SB431542 (TGFβ inhibitor); and (2) EB grown on matrigel + DMEM/F12 + 0.5% BSA + 10% ITS + SB431542. 3. Outgrowth grown with DMEM/F12 + 10% FBS + p/s | 17 | Duchene muscular dystrophy | 17 |
| Zhao et al[67], 2015 | Blood cells | (1) iPSC cultured with mTeSR1 + SB431542 (TGFβ inhibitor) on matrigel-coated plates (7.5% CO2 atmosphere); and (2) Cells cultured with ESC–MSC medium: KO DMEM + KOSR + NEAA + p/s + L-Glutamine + β-ME + bFGF + EGF + SB431542 | 45 | Tumor tropism of iMSC | 79 |
iPSC origin refers to the cell type used for reprograming; Time is indicated as the minimum number of days required to obtain iMSCs; Citations show numbers on March 2020.
In references indicate the study includes methods in more than one protocol category. bFGF: Basic fibroblast growth factor; β-ME: β-Mercaptoethanol; BSA: Bovine serum albumin; CARM: Cardiomyogenic medium; CDM: Chemical defined medium; DMEM/F12: Dulbecco's modified Eagle's medium F12; CHIR: Wnt agonist, potent GSK3 inhibitor; EB: Embryoid body; FBS: Fetal bovine serum; FCS: Fetal calf serum; Ham's F12: Medium formulated for single-cell plating of near-diploid Chinese hamster ovary cells; iMSCs: Induced pluripotent stem cell-derived mesenchymal stem cells; iPSC: Induced pluripotent stem cell; ITS: Insulin-transferrin-selenium; KO DMEM: Knockout Dulbecco's modified Eagle's medium; KOSR: Knock-out serum replacement; α-MEM: Minimum essential medium Eagle; MSC: Mesenchymal stem cell; NA: Not available; NEAA: Non-essential amino acid; p/s: Penicillin-streptomycin; TGF: Transforming growth factor.
Only 3 of the 32 studies selected used media supplemented with human platelet lysate (hPL), in replacement of fetal bovine serum (FBS), to produce iMSCs. As the name suggests, it uses a lysate of platelets obtained from human peripheral blood to supplement iPSC´s growing media, replacing the FBS component, which by its animal origin raised ethical and safety concerns. This method, therefore, could improve the safety of the final iMSCs produced.
McGrath et al[54], Frobel et al[71], and Luzzani et al[72] decided to use this method to grant a procedure free of animal components. The differentiation protocol is similar to the already described for the MSC Switch category, with the exception that the anticoagulant heparin is used in the media to prevent clotting. It should be highlighted that Frobel et al[71] revealed an incomplete reacquisition of the immunomodulatory properties of iMSCs obtained with this variant of the protocol. In addition, McGrath et al[54] also noticed an alteration in the release of trophic factors, necessary for immunomodulatory properties being attributed to MSCs. This may impose limitations for certain therapeutic uses to the iMSCs produced with this set of protocols.
Basic components to obtain iMSCs
As per the basic components of the culture medium used in these 32 studies, we evaluated the composition of the basal media, culture medium supplements other than chemical inhibitors, and coatings used for cell culture while producing iMSCs. All this with the intention to identify the most commonly used components within each of the five studied protocol categories. The analysis allows identification of the elements required to produce iMSCs regardless of the method of choice.
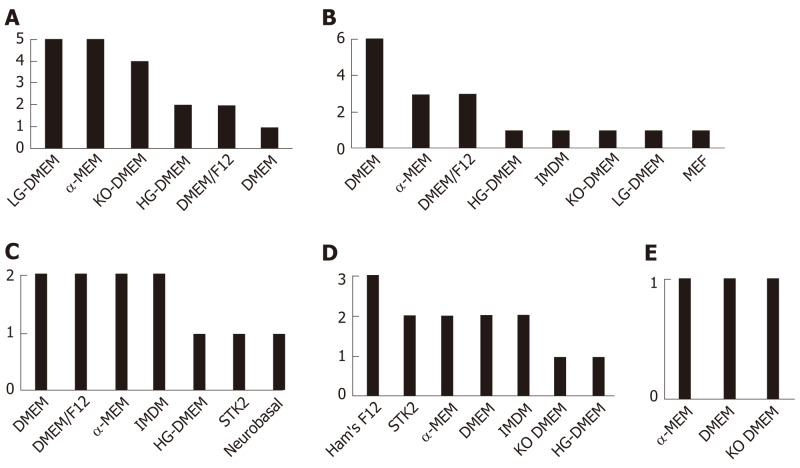
Basal medium composition: The main basal commercial media used to differentiate iPSC into iMSC were either DMEM, or α-MEM, or other DMEM derivatives such as DMEM knockout (KO) (a serum-free media), or DMEM F-12, which slightly vary in salt composition and glucose concentration (Figure 1). It should be noted that some studies use more than one medium type (Supplementary Table 2).
Figure 1.
Basal commercial media used to produce induced pluripotent stem cell-derived mesenchymal stem cells. The relative frequencies of media used by the 32 studies are displayed as number of events within each of the five categories of protocols being described: Mesenchymal stem cell Switch (A), Embryoid Bodies (B), Specific Differentiation (C), Pathway Inhibitor (D), and Platelet Lysate (E). DMEM: Dulbecco's modified Eagle's medium; DMEM/F12: Dulbecco's modified Eagle's medium F12; Ham's F12: Medium formulated for single-cell plating of near-diploid Chinese hamster ovary cells; HG-DMEM: High glucose Dulbecco's modified Eagle's medium; IMDM: Iscove's modified Dulbecco's media; KO-DMEM: Knockout Dulbecco's modified Eagle's medium; LG-DMEM: Low glucose Dulbecco's modified Eagle's medium; MEF: Mouse embryonic fibroblast media; αMEM/MEM: Minimum essential medium Eagle.
Culture medium supplements: The main supplements commonly used, as an overall, in all five described iMSC protocol categories are: FBS, L-Glutamine and antibiotics (penicillin-streptomycin or P/S), followed by non-essential amino acids, and FGF (Supplementary Table 2).
In the category of MSC Switch, an important supplement (4/15 studies) used is epidermal growth factor. The studies by Hynes et al[45], Gao et al[50], and Wang et al[52], commonly used l-ascorbate-2-phosphate and sodium pyruvate. KO Serum Replacement (KOSR) was used in an additional 3/15 studies.
For EB, however, a prevalent supplement is β-mercaptoethanol, representing 6/13 studies, followed by KOSR (3/13 studies), Insulin-Transferrin-Selenium (ITS) and SB431542 (2/13 studies). All three components were used in the study by Jeong et al[46].
For Specific Differentiation methods, the most common supplement seems to be insulin (3/5 studies), followed by β-mercaptoethanol and SB431542, which were present in more than one study. The components being used together with apo-transferrin, bovine serum albumin (BSA), CHR, lipid concentrate, and monothioglycerol were included only in the studies by Fukuta et al[66] and Mitsuzawa et al[64].
The SB431542 supplement, which is a TGF-β inhibitor, is used by practically all (4/5 studies) the studies reviewed fitting in the Pathway Inhibitor category. Only β-mercaptoethanol, BSA, and ITS were used in more than one study of this category. The low number of studies for this category (n = 5) should be noticed.
Finally, the specific supplements of the last described category present in all three studies are hPL, per definition, and heparin (Supplementary Table 2, the latter due to the requirement to prevent media clotting, as mentioned.
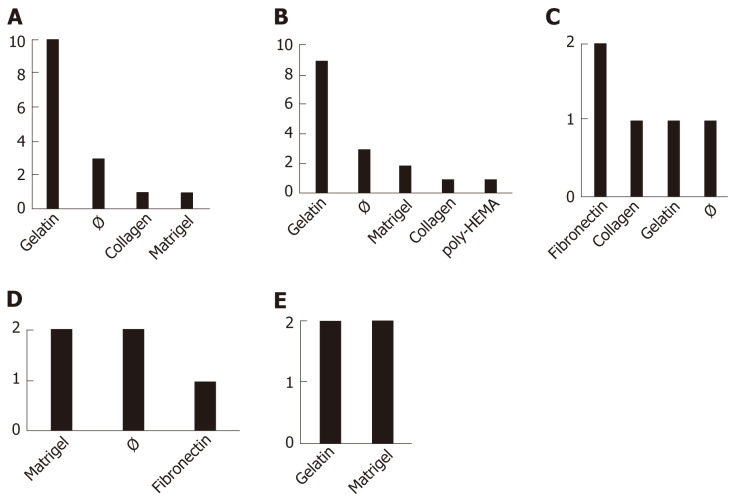
Coatings: Coatings seem to be decisive for cell differentiation. Indeed, it has been shown that collagen type-I can activate the nuclear factor kappa B pathway and drives the epithelial to mesenchymal transition[73]. Polymer coatings such as poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide] helps maintain the self-renewal and pluripotency features of SCs[58]. The most common coating for iMSC production in the protocols presented here, overall, appeared to be gelatin, with the exception of the Specific Differentiation set of protocols (Figure 2). Collagen, Matrigel, or absence of coating (Ø) follow in the most commonly used coating materials list. Fibronectin is only used in Specific Differentiation and Pathway Inhibitor procedures. The most uncommon coating was found to be poly-HEMA (polymer forming hydrogel in water)[20], which was used only in 1 of the 13 EB protocols reviewed (Figure 2). It should be noted that some studies use more than one type of coating (Supplementary Table 2).
Figure 2.
Coatings used to produce induced pluripotent stem cell-derived mesenchymal stem cells. The relative frequencies of coatings used by the 32 studies are displayed as number of events within each of the 5 categories of protocols being described: Mesenchymal stem cell Switch (A), Embryoid Bodies (B), Specific Differentiation (C), Pathway Inhibitor (D), and Platelet Lysate (E). Note that some studies use more than one type of coating. The symbol “Ø” indicates (absence of coating). poly HEMA: Polymer forming hydrogel in water.
iPSC cell origin
As iPSCs have epigenetic memory of their tissue of origin[74], it might be relevant to document what are the most common cell types used to produce iMSCs across the 32 original studies being reviewed here. This feature, however, could only be tracked in 25 of the studies. Among the different cell types found, dermal fibroblast represents the most frequent tissue of origin, with 12 of 25 studies using these cells, followed by peripheral blood mononuclear cells (4/25 of the studies), amniocytes and bone marrow (3/25 of the studies for each mentioned cell type), and foreskin fibroblast (2/25 of the studies). Lastly, fetal endothelial cells, lung fibroblasts, MSCs, periodontal ligaments, peripheral gingival fibroblasts, and urine-contained cells were each reported by single studies. The studies corresponding to each cell type can be consulted in Supplementary Table 3.
CHARACTERIZATION OF IMSCS
Cell surface markers and cytokines
In addition to the 32 studies selected as the main representatives for the five categories to produce iMSCs by non-commercial methods, 12 additional studies were included in this section. The reason for their inclusion was that they describe the characterization of iMSCs obtained by one of the five protocol categories studied in this review, despite not being original descriptors of the method (Supplementary Tables 1 and 4).
Top cell surface markers selected to characterize iMSCs in the reviewed literature (n = 44) include the common markers defined for the MSC minimal criteria, as described by the ISCT: CD73, CD105, and CD90 must be expressed (positive); and cells should lack expression of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface molecules (negative). As can be appreciated on Table 6, common markers used to characterize iMSCs additional to those defined by the ISCT for MSCs, are markers CD44, CD29, and CD166, as positive markers (they have been found on adult MSCs[75], although their significance remains unknown); and CD133 and TRA-1-81 as negative (controls to ensure absence of pluripotent SCs or cancer SCs[76]) (Table 6). Supplementary Table 4 can be consulted to identify particular studies assessing the presence or absence of these markers on iMSCs.
Table 6.
Frequency of use of iMSC surface markers
|
Positive CSM
|
%
|
Negative CSM
|
%
|
| CD73 | 18.1 | CD45 | 24.5 |
| CD105 | 17.1 | CD34 | 23.0 |
| CD90 | 15.7 | CD14 | 8.6 |
| CD44 | 12.5 | CD31 | 7.2 |
| CD29 | 9.3 | HLA-DR | 5.8 |
| CD166 | 6.9 | CD11b | 5.0 |
| CD146 | 3.7 | CD133 | 2.9 |
| CD49(a) | 2.8 | TRA181 | 2.9 |
| HLA-ABC | 2.3 | CD19 | 2.2 |
| CD49(e) | 1.9 | CD24 | 2.2 |
| CD106 | 1.4 | CD3 | 1.4 |
| CD271 | 1.4 | CD40 | 1.4 |
| CD49(d) | 0.9 | CD56 | 1.4 |
| CD140alpha | 0.9 | CD80 | 1.4 |
| Sca1 | 0.9 | CD86 | 1.4 |
| CD33 | 0.5 | Oct3/4 | 1.4 |
| CD49(f) | 0.5 | CD4 | 0.7 |
| CD54 | 0.5 | CD20 | 0.7 |
| CD71 | 0.5 | CD79a | 0.7 |
| CD140(b) | 0.5 | CD117 | 0.7 |
| CD144 | 0.5 | CD309 | 0.7 |
| CD172alpha | 0.5 | Sox2 | 0.7 |
| αSMA+ | 0.5 | TRA-160 | 0.7 |
| Stro1 | 0.5 | TRA-161 | 0.7 |
| TRA180 | 0.7 | ||
| SSEA-4 | 0.7 |
Positive and negative surface expression markers found in mesenchymal stem cells are listed together with the frequency of findings (%) in the reviewed studies (n = 44). CD: Cluster of differentiation; CSM: Cell surface marker; HLA: Human leukocyte antigen; SSEA: Stage specific embryonic antigen; TRA: Teratocarcinoma surface antigen.
DISCUSSION
The therapeutic potential of MSCs holds great promise for the handling of many diseases. The lack of consistency in the outcomes of adult MSC trials, sometimes leading to contradictory results and classically attributed to the heterogeneous nature of these cells, will not be overcome unless a unique standardized method of MSC production is implemented. Establishing a standard protocol for producing large-scale MSCs is therefore clearly necessary if an efficient treatment for clinical application is pursued.
Production of iMSCs seems to constitute an ideal option as these cells could be obtained in unlimited numbers from genetically homogeneous iPSC cell lines. However, the review of the literature shows that the current methods for producing iMSCs are far too different to produce homogeneous populations of iMSCs.
By reviewing 32 studies describing original methods for producing MSCs from iPSCs, we distinguished five different modalities: MSC Switch, EBs, Specific Differentiation, Pathway Inhibitor, and Platelet Lysate methods. These methods presented their own advantages, depending on the application pursued.
MSC Switch methods appears to be the most popular method (6 method variants have been cited over 100 times) (Table 1), and seems to be the least complex of the protocols, at the expense of, perhaps, increased variability of the obtained iMSCs[24]. It presents itself as a fast (from 7 d, for the Lian et al[41] variant) and technically simple approach as it only requires to directly switch the growth medium. FACS sorting allows the selection of cell subpopulations possibly leading to more consistent results.
Feeder free conditions simplify the process, and the use of chemically defined media allows control over animal-derived products and reduces batch-to-batch variability.
EB methods seem to be extensively cited as well (4 variants have been cited more than 100 times), however it presents itself with some technical difficulties, such as EB average size optimization.
Specific Differentiation methods present several disadvantages: they are laborious, costly, time-consuming, and require complex media. However, they present the advantage to eliminate, to a further extent, the remaining iPSCs from the final iMSC population. By adding a pre-differentiation step before the generation of iMSC, the risk of tumorigenicity is reduced, increasing iMSC safety, an aspect of particular relevance when cells are infused into immunocompromised patients.
Pathway inhibitor protocols, focusing on controlling cellular signaling pathways to generate iMSCs, might become helpful in combination with the other described methods, perhaps allowing the development of faster or more robust protocols.
Finally, Platelet Lysate Methods are aimed at avoiding the use of FBS, to prevent transmission of animal prions and reduce the risk of rejection or undesired immune reactions upon cell transplants[77].
Despite the fact that all the studies report the production of well-characterized MSCs, according to the ISCT[13], the methods found are excessively different to produce MSCs of similar quality. To encourage the clinical use of iMSCs use, a standardized protocol should be established. We found that many of the protocols belonging to the same category show small differences in media composition for cell differentiation, suggesting that a general protocol could emerge, following empirical testing of minority factors.
Fast methods and simplicity of the technique to generate iMSC can be considered as advantages to generate important quantities. A limitation found, however, is the absolute lack of information detected with respect to yields obtained by each method. Although the most important point that should be taken into account is the safety of the therapy, the robustness of the method to generate homogeneous and reproducible cell populations is undoubtfully relevant as well.
MSCs have received significant attention due to their strong potential for cell-based and tissue regenerative therapies[20-23]. Indeed, they have been shown to secrete a wide variety of immunomodulatory factors. A fact that has attracted interest for the treatment of immune-related disorders or diseases with an inflammatory component. According to Frobel et al[71] and McGrath et al[54], iMSCs obtained with safe platelet lysate methods, however, might limit their use as immunomodulators.
Despite the great potential for the use of iMSCs as immunomodulatory entities, the investigation of potential avenues in this direction still remains at its infancy, with few studies evaluating only some of the secreted molecules. Omic-based analysis of the obtained iMSCs should lead to a more complete phenotyping and thus a deeper understanding of iMSC potential therapeutic benefits.
As per iMSC use in the Clinic, the only CT using iMSCs we are aware of is the CT registered with the title: “Safety and Efficacy of MSC for the Treatment of Adults with Steroid-Resistant Acute Graft Versus Host Disease (GvHD)” (trial number NCT02923375). The study included 16 patients who developed refractory GvHD from Australia and the United Kingdom, who received 1-2 million cells/kg on Day 0 and Day 7 and visits on Days 0, 3, 7, 14, 21, 28, 60 and 100. Cynata Therapeutics, a company directed by Dr. Kilian Kelly, started this CT in March 1 2017. The results seem very encouraging, what should be supportive of the progress of this therapeutic use of iMSCs towards Phase 2 trials.
However, the use of iMSCs in the Clinic still presents with some handicaps to be overcome, starting by the high cost of iPSC cell culture and maintenance. Although the risk of rejection is very low, as MSC do not display MHC, autologous rather than allogeneic therapies are preferred[78,79], and iMSCs should be generated without animal components under xenofree conditions for safety compliance.
There are ethical issues concerning the use of iPSCs and iMSCs themselves that should be taken into account[80]. Importantly, iPSC donors consenting should be ensured, as well as the protection of donor data[81]. On another side, iPSCs used for iMSC generation should be generated excluding viral-based methods, even being the most efficient. Alternative methods may include micro-RNAs, plasmids or chemicals[82].
The use of iMSCs for cell therapy is not free of risks, since potential remaining iPSC or iMSC can directly generate tumor or metastasis in immunocompromised patients[83]. Indeed, the c-Myc factor used to reprogram iPSCs is an oncogenic factor in immunocompromised mice[84].
An alternative for risk reduction could be the use of cell-free iMSC derived fractions, presently being explored. For example, extracellular vesicles (EVs) might result safer while still holding healing properties of extended value[85-87].
On another end, although the differentiated cell-type used for reprogramming could translate into different iMSC products, an avenue worth of future explorations, the possibility of iMSC standardization production by far surpasses that of adult MSCs.
In summary, this review provides valuable information about the current methodological options to differentiate iPSC into iMSC to obtain MSCs of more homogeneous features than those isolated from adult tissues. It evaluates main culture media components, supplements and requirements of coatings based on a considerable number of original protocols (n = 32). In addition, the study of main surface markers identified on iMSCs (n = 44) show that in addition to fulfilling the ISCT minimal criteria, additional common iMSC markers are getting defined. Relationships between iMSCs surface markers and cell properties is yet another underexplored, but high priority issue towards the development of protocols meeting therapeutic demands[88]. For example, despite the availability of commercial kits to produce iMSCs already in the market, such as StemDiff Mesenchymal Progenitors kit (STEMCELL technologies, cat #05240), the specific immunomodulation properties harboring the cells differentiated and expanded under these conditions remain to be defined. Systematic combinatorial evaluations of minor components and their impact on iMSC features awaits toward settling large-manufacturing systems that give support to truly standardized MSC therapeutics.
CONCLUSION
Different culture conditions have been described to produce iMSCs, which can be classified in the following five categories: MSC Switch, EBs, Specific Differentiation, Pathway Inhibitor, and Platelet Lysate methods, according to their strategy and components. Each approach presents with some advantages and limitations, as described. Some have been more widely explored than others, but there is still no consensus Good Manufacturing Procedures improved method for a scale-up production of iMSCs. This review details these methods and lists required components and cell phenotyping markers, to serve as a reference guide for future improvements on iMSC manufacturing.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing interests.
Manuscript source: Invited manuscript
Peer-review started: February 28, 2021
First decision: April 19, 2021
Article in press: July 14, 2021
Specialty type: Cell and tissue engineering
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Hashmi S, Saleh F, Scalise S S-Editor: Gao CC L-Editor: Filipodia P-Editor: Zhang YL
Contributor Information
Victoria Dupuis, Faculté des Sciences et d’Ingénierie, Sorbonne Université, Paris 75252, France.
Elisa Oltra, Department of Pathology, Universidad Católica de Valencia San Vicente Mártir, Valencia 46001, Spain; Centro de Investigación Traslacional San Alberto Magno, Universidad Católica de Valencia San Vicente Mártir, Valencia 46001, Spain. elisa.oltra@ucv.es.
References
- 1.Li Y, Wu Q, Wang Y, Li L, Bu H, Bao J. Senescence of mesenchymal stem cells (Review) Int J Mol Med. 2017;39:775–782. doi: 10.3892/ijmm.2017.2912. [DOI] [PubMed] [Google Scholar]
- 2.Yan W, Diao S, Fan Z. The role and mechanism of mitochondrial functions and energy metabolism in the function regulation of the mesenchymal stem cells. Stem Cell Res Ther. 2021;12:140. doi: 10.1186/s13287-021-02194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 4.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 7.Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- 8.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Salazar M, Gonzalez-Galofre ZN, Casamitjana J, Crisan M, James AW, Péault B. Five Decades Later, Are Mesenchymal Stem Cells Still Relevant? Front Bioeng Biotechnol. 2020;8:148. doi: 10.3389/fbioe.2020.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 14.El Haddad N. Mesenchymal Stem Cells: Immunology and Therapeutics Benefits. Intech Open . 2010 [Google Scholar]
- 15.Wang Y, Tian M, Wang F, Heng BC, Zhou J, Cai Z, Liu H. Understanding the Immunological Mechanisms of Mesenchymal Stem Cells in Allogeneic Transplantation: From the Aspect of Major Histocompatibility Complex Class I. Stem Cells Dev. 2019;28:1141–1150. doi: 10.1089/scd.2018.0256. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalo-Gil E, Pérez-Lorenzo MJ, Galindo M, Díaz de la Guardia R, López-Millán B, Bueno C, Menéndez P, Pablos JL, Criado G. Human embryonic stem cell-derived mesenchymal stromal cells ameliorate collagen-induced arthritis by inducing host-derived indoleamine 2,3 dioxygenase. Arthritis Res Ther. 2016;18:77. doi: 10.1186/s13075-016-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Niu L, Feng X, Yuan X, Zhao S, Zhang H, Liang J, Zhao C, Wang H, Hua B, Sun L. Long-term safety of umbilical cord mesenchymal stem cells transplantation for systemic lupus erythematosus: a 6-year follow-up study. Clin Exp Med. 2017;17:333–340. doi: 10.1007/s10238-016-0427-0. [DOI] [PubMed] [Google Scholar]
- 18.Moravej A, Geramizadeh B, Azarpira N, Zarnani AH, Yaghobi R, Kalani M, Khosravi M, Kouhpayeh A, Karimi MH. Mesenchymal stem cells increase skin graft survival time and up-regulate PD-L1 expression in splenocytes of mice. Immunol Lett. 2017;182:39–49. doi: 10.1016/j.imlet.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Khan MA, Alanazi F, Ahmed HA, Shamma T, Kelly K, Hammad MA, Alawad AO, Assiri AM, Broering DC. iPSC-derived MSC therapy induces immune tolerance and supports long-term graft survival in mouse orthotopic tracheal transplants. Stem Cell Res Ther. 2019;10:290. doi: 10.1186/s13287-019-1397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheyn D, Ben-David S, Shapiro G, De Mel S, Bez M, Ornelas L, Sahabian A, Sareen D, Da X, Pelled G, Tawackoli W, Liu Z, Gazit D, Gazit Z. Human Induced Pluripotent Stem Cells Differentiate Into Functional Mesenchymal Stem Cells and Repair Bone Defects. Stem Cells Transl Med. 2016;5:1447–1460. doi: 10.5966/sctm.2015-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang M, Chen W, Liu J, Weir MD, Cheng L, Xu HH. Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration. Tissue Eng Part A. 2014;20:1295–1305. doi: 10.1089/ten.tea.2013.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta PK, Das AK, Chullikana A, Majumdar AS. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther. 2012;3:25. doi: 10.1186/scrt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi: 10.1186/s12967-015-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner W, Ho AD. Mesenchymal stem cell preparations--comparing apples and oranges. Stem Cell Rev. 2007;3:239–248. doi: 10.1007/s12015-007-9001-1. [DOI] [PubMed] [Google Scholar]
- 25.Elahi KC, Klein G, Avci-Adali M, Sievert KD, MacNeil S, Aicher WK. Human Mesenchymal Stromal Cells from Different Sources Diverge in Their Expression of Cell Surface Proteins and Display Distinct Differentiation Patterns. Stem Cells Int. 2016;2016:5646384. doi: 10.1155/2016/5646384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa LA, Eiro N, Fraile M, Gonzalez LO, Saá J, Garcia-Portabella P, Vega B, Schneider J, Vizoso FJ. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell Mol Life Sci. 2021;78:447–467. doi: 10.1007/s00018-020-03600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. doi: 10.1186/1741-7015-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu L, Liu Y, Sun Y, Wang B, Xiong Y, Lin W, Wei Q, Wang H, He W, Li G. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther. 2017;8:275. doi: 10.1186/s13287-017-0716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Neri S, Borzì RM. Molecular Mechanisms Contributing to Mesenchymal Stromal Cell Aging. Biomolecules. 2020;10 doi: 10.3390/biom10020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Ding Y, Liu Z, Liang X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front Cell Dev Biol. 2020;8:258. doi: 10.3389/fcell.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neri S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al Jumah MA, Abumaree MH. The immunomodulatory and neuroprotective effects of mesenchymal stem cells (MSCs) in experimental autoimmune encephalomyelitis (EAE): a model of multiple sclerosis (MS) Int J Mol Sci. 2012;13:9298–9331. doi: 10.3390/ijms13079298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Figueroa FE, Carrión F, Villanueva S, Khoury M. Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biol Res. 2012;45:269–277. doi: 10.4067/S0716-97602012000300008. [DOI] [PubMed] [Google Scholar]
- 35.Ko JZ, Johnson S, Dave M. Efficacy and Safety of Mesenchymal Stem/Stromal Cell Therapy for Inflammatory Bowel Diseases: An Up-to-Date Systematic Review. Biomolecules. 2021;11 doi: 10.3390/biom11010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Zacharias DG, Nelson TJ, Mueller PS, Hook CC. The science and ethics of induced pluripotency: what will become of embryonic stem cells? Mayo Clin Proc. 2011;86:634–640. doi: 10.4065/mcp.2011.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdal Dayem A, Lee SB, Kim K, Lim KM, Jeon TI, Seok J, Cho AS. Production of Mesenchymal Stem Cells Through Stem Cell Reprogramming. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20081922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steens J, Zuk M, Benchellal M, Bornemann L, Teichweyde N, Hess J, Unger K, Görgens A, Klump H, Klein D. In Vitro Generation of Vascular Wall-Resident Multipotent Stem Cells of Mesenchymal Nature from Murine Induced Pluripotent Stem Cells. Stem Cell Reports. 2017;8:919–932. doi: 10.1016/j.stemcr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 42.Giuliani M, Oudrhiri N, Noman ZM, Vernochet A, Chouaib S, Azzarone B, Durrbach A, Bennaceur-Griscelli A. Human mesenchymal stem cells derived from induced pluripotent stem cells down-regulate NK-cell cytolytic machinery. Blood. 2011;118:3254–3262. doi: 10.1182/blood-2010-12-325324. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Goldberg AJ, Dennis JE, Gronowicz GA, Kuhn LT. One-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coating. PLoS One. 2012;7:e33225. doi: 10.1371/journal.pone.0033225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou L, Luo Y, Chen M, Wang G, Ding M, Petersen CC, Kang R, Dagnaes-Hansen F, Zeng Y, Lv N, Ma Q, Le DQ, Besenbacher F, Bolund L, Jensen TG, Kjems J, Pu WT, Bünger C. A simple method for deriving functional MSCs and applied for osteogenesis in 3D scaffolds. Sci Rep. 2013;3:2243. doi: 10.1038/srep02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hynes K, Menicanin D, Mrozik K, Gronthos S, Bartold PM. Generation of functional mesenchymal stem cells from different induced pluripotent stem cell lines. Stem Cells Dev. 2014;23:1084–1096. doi: 10.1089/scd.2013.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeong J, Shin K, Lee SB, Lee DR, Kwon H. Patient-tailored application for Duchene muscular dystrophy on mdx mice based induced mesenchymal stem cells. Exp Mol Pathol. 2014;97:253–258. doi: 10.1016/j.yexmp.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, Deng ZF. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6:10. doi: 10.1186/scrt546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang R, Zhou Y, Tan S, Zhou G, Aagaard L, Xie L, Bünger C, Bolund L, Luo Y. Mesenchymal stem cells derived from human induced pluripotent stem cells retain adequate osteogenicity and chondrogenicity but less adipogenicity. Stem Cell Res Ther. 2015;6:144. doi: 10.1186/s13287-015-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lian Q, Zhang Y, Liang X, Gao F, Tse HF. Directed Differentiation of Human-Induced Pluripotent Stem Cells to Mesenchymal Stem Cells. Methods Mol Biol. 2016;1416:289–298. doi: 10.1007/978-1-4939-3584-0_17. [DOI] [PubMed] [Google Scholar]
- 50.Gao WX, Sun YQ, Shi J, Li CL, Fang SB, Wang D, Deng XQ, Wen W, Fu QL. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res Ther. 2017;8:48. doi: 10.1186/s13287-017-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nachlas ALY, Li S, Jha R, Singh M, Xu C, Davis ME. Human iPSC-derived mesenchymal stem cells encapsulated in PEGDA hydrogels mature into valve interstitial-like cells. Acta Biomater. 2018;71:235–246. doi: 10.1016/j.actbio.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang D, Sun YQ, Gao WX, Fan XL, Shi JB, Fu QL. An in Vitro and in Vivo Study of the Effect of Dexamethasone on Immunoinhibitory Function of Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells. Cell Transplant. 2018;27:1340–1351. doi: 10.1177/0963689718780194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang LT, Jiang SS, Ting CH, Hsu PJ, Chang CC, Sytwu HK, Liu KJ, Yen BL. Differentiation of Mesenchymal Stem Cells from Human Induced Pluripotent Stem Cells Results in Downregulation of c-Myc and DNA Replication Pathways with Immunomodulation Toward CD4 and CD8 Cells. Stem Cells. 2018;36:903–914. doi: 10.1002/stem.2795. [DOI] [PubMed] [Google Scholar]
- 54.McGrath M, Tam E, Sladkova M, AlManaie A, Zimmer M, de Peppo GM. GMP-compatible and xeno-free cultivation of mesenchymal progenitors derived from human-induced pluripotent stem cells. Stem Cell Res Ther. 2019;10:11. doi: 10.1186/s13287-018-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eto S, Goto M, Soga M, Kaneko Y, Uehara Y, Mizuta H, Era T. Mesenchymal stem cells derived from human iPS cells via mesoderm and neuroepithelium have different features and therapeutic potentials. PLoS One. 2018;13:e0200790. doi: 10.1371/journal.pone.0200790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahfeldt T, Schinzel RT, Lee YK, Hendrickson D, Kaplan A, Lum DH, Camahort R, Xia F, Shay J, Rhee EP, Clish CB, Deo RC, Shen T, Lau FH, Cowley A, Mowrer G, Al-Siddiqi H, Nahrendorf M, Musunuru K, Gerszten RE, Rinn JL, Cowan CA. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol. 2012;14:209–219. doi: 10.1038/ncb2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen YS, Pelekanos RA, Ellis RL, Horne R, Wolvetang EJ, Fisk NM. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cells Transl Med. 2012;1:83–95. doi: 10.5966/sctm.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villa-Diaz LG, Brown SE, Liu Y, Ross AM, Lahann J, Parent JM, Krebsbach PH. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells. 2012;30:1174–1181. doi: 10.1002/stem.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei H, Tan G, Manasi , Qiu S, Kong G, Yong P, Koh C, Ooi TH, Lim SY, Wong P, Gan SU, Shim W. One-step derivation of cardiomyocytes and mesenchymal stem cells from human pluripotent stem cells. Stem Cell Res. 2012;9:87–100. doi: 10.1016/j.scr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Shao K, Koch C, Gupta MK, Lin Q, Lenz M, Laufs S, Denecke B, Schmidt M, Linke M, Hennies HC, Hescheler J, Zenke M, Zechner U, Šarić T, Wagner W. Induced pluripotent mesenchymal stromal cell clones retain donor-derived differences in DNA methylation profiles. Mol Ther. 2013;21:240–250. doi: 10.1038/mt.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miao Q, Shim W, Tee N, Lim SY, Chung YY, Ja KP, Ooi TH, Tan G, Kong G, Wei H, Lim CH, Sin YK, Wong P. iPSC-derived human mesenchymal stem cells improve myocardial strain of infarcted myocardium. J Cell Mol Med. 2014;18:1644–1654. doi: 10.1111/jcmm.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karam M, Younis I, Elareer NR, Nasser S, Abdelalim EM. Scalable Generation of Mesenchymal Stem Cells and Adipocytes from Human Pluripotent Stem Cells. Cells. 2020;9 doi: 10.3390/cells9030710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang X, Wang H, Xu Y. Induced Pluripotent Stem Cells (iPSC)-derived Mesenchymal Stem Cells (MSCs) Showed Comparable Effects in Repair of Acute Kidney Injury as Compared to Adult MSCs. Urol J. 2020;17:204–209. doi: 10.22037/uj.v0i0.5362. [DOI] [PubMed] [Google Scholar]
- 64.Mitsuzawa S, Ikeguchi R, Aoyama T, Ando M, Takeuchi H, Yurie H, Oda H, Noguchi T, Ohta S, Zhao C, Ikeya M, Matsuda S. Induced pluripotent stem cell-derived mesenchymal stem cells prolong hind limb survival in a rat vascularized composite allotransplantation model. Microsurgery. 2019;39:737–747. doi: 10.1002/micr.30507. [DOI] [PubMed] [Google Scholar]
- 65.Ouchi T, Morikawa S, Shibata S, Fukuda K, Okuno H, Fujimura T, Kuroda T, Ohyama M, Akamatsu W, Nakagawa T, Okano H. LNGFR+THY-1+ human pluripotent stem cell-derived neural crest-like cells have the potential to develop into mesenchymal stem cells. Differentiation. 2016;92:270–280. doi: 10.1016/j.diff.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Fukuta M, Nakai Y, Kirino K, Nakagawa M, Sekiguchi K, Nagata S, Matsumoto Y, Yamamoto T, Umeda K, Heike T, Okumura N, Koizumi N, Sato T, Nakahata T, Saito M, Otsuka T, Kinoshita S, Ueno M, Ikeya M, Toguchida J. Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS One. 2014;9:e112291. doi: 10.1371/journal.pone.0112291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao Q, Gregory CA, Lee RH, Reger RL, Qin L, Hai B, Park MS, Yoon N, Clough B, McNeill E, Prockop DJ, Liu F. MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs. Proc Natl Acad Sci U S A. 2015;112:530–535. doi: 10.1073/pnas.1423008112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sánchez L, Gutierrez-Aranda I, Ligero G, Rubio R, Muñoz-López M, García-Pérez JL, Ramos V, Real PJ, Bueno C, Rodríguez R, Delgado M, Menendez P. Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells. 2011;29:251–262. doi: 10.1002/stem.569. [DOI] [PubMed] [Google Scholar]
- 69.Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, Cheng L, Elisseeff J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci USA. 2008;105:20641–20646. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frobel J, Hemeda H, Lenz M, Abagnale G, Joussen S, Denecke B, Sarić T, Zenke M, Wagner W. Epigenetic rejuvenation of mesenchymal stromal cells derived from induced pluripotent stem cells. Stem Cell Reports. 2014;3:414–422. doi: 10.1016/j.stemcr.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luzzani C, Neiman G, Garate X, Questa M, Solari C, Fernandez Espinosa D, García M, Errecalde AL, Guberman A, Scassa ME, Sevlever GE, Romorini L, Miriuka SG. A therapy-grade protocol for differentiation of pluripotent stem cells into mesenchymal stem cells using platelet lysate as supplement. Stem Cell Res Ther. 2015;6:6. doi: 10.1186/scrt540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medici D, Nawshad A. Type I collagen promotes epithelial-mesenchymal transition through ILK-dependent activation of NF-kappaB and LEF-1. Matrix Biol. 2010;29:161–165. doi: 10.1016/j.matbio.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.P M, S H, R M, M G, W S K. Adult mesenchymal stem cells and cell surface characterization - a systematic review of the literature. Open Orthop J. 2011;5:253–260. doi: 10.2174/1874325001105010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matteucci C, Balestrieri E, Argaw-Denboba A, Sinibaldi-Vallebona P. Human endogenous retroviruses role in cancer cell stemness. Semin Cancer Biol. 2018;53:17–30. doi: 10.1016/j.semcancer.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E, Drexler C, Lanzer G, Linkesch W, Strunk D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436–1446. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 78.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Atoui R, Chiu RC. Concise review: immunomodulatory properties of mesenchymal stem cells in cellular transplantation: update, controversies, and unknowns. Stem Cells Transl Med. 2012;1:200–205. doi: 10.5966/sctm.2011-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng YL. Some Ethical Concerns About Human Induced Pluripotent Stem Cells. Sci Eng Ethics. 2016;22:1277–1284. doi: 10.1007/s11948-015-9693-6. [DOI] [PubMed] [Google Scholar]
- 81.Morrison M, Bell J, George C, Harmon S, Munsie M, Kaye J. The European General Data Protection Regulation: challenges and considerations for iPSC researchers and biobanks. Regen Med. 2017;12:693–703. doi: 10.2217/rme-2017-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deng XY, Wang H, Wang T, Fang XT, Zou LL, Li ZY, Liu CB. Non-viral methods for generating integration-free, induced pluripotent stem cells. Curr Stem Cell Res Ther. 2015;10:153–158. doi: 10.2174/1574888X09666140923101914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36–45. doi: 10.7150/ijms.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 85.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y, Cheng Q, Hu G, Deng T, Wang Q, Zhou J, Su X. Extracellular vesicles in mesenchymal stromal cells: A novel therapeutic strategy for stroke. Exp Ther Med. 2018;15:4067–4079. doi: 10.3892/etm.2018.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rahimzadeh A, Mirakabad FS, Movassaghpour A, Shamsasenjan K, Kariminekoo S, Talebi M, Shekari A, Zeighamian V, Ghalhar MG, Akbarzadeh A. Biotechnological and biomedical applications of mesenchymal stem cells as a therapeutic system. Artif Cells Nanomed Biotechnol. 2016;44:559–570. doi: 10.3109/21691401.2014.968823. [DOI] [PubMed] [Google Scholar]