Abstract
Understanding the timing of vitellogenesis is essential for identifying threats to the reproductive success of endangered oviparous vertebrate species, such as sea turtles. We measured concentrations of testosterone (T) and vitellogenin (VTG) in green sea turtles (Chelonia mydas) nesting at Tortuguero, Costa Rica, as biomarkers of ovarian development. Testosterone concentration increased from the first to second month and VTG concentration increased at the third week of sampling. These results show that Tortuguero green sea turtles were still producing both biomarkers early into the nesting season. VTG concentration was negatively correlated with female weight, suggesting that larger females start nesting earlier at Tortuguero and that we may have sampled larger females further into their reproductive cycle.
Keywords: Conservationendocrinologyreproductive biologysea turtlesTortuguero
Testosterone concentration increased on the second month and vitellogenin (VTG) concentration increased on the third week of the green sea turtle nesting season at Tortuguero, Costa Rica, presumably as both biomarkers were being produced post-nuptially. Heavier female green sea turtles showed lower VTG concentration, likely because those individuals started nesting earlier.
Introduction
Vitellogenesis is a similar process in most oviparous reptiles, during which lipids and proteins are mobilized from the body’s stores and progressively added to developing ovarian follicles, forming the egg yolk that nurtures growing embryos (Ho et al. 1980, 1982; Hamann et al. 2003; Polzonetti-Magni et al. 2004). The series of physiological processes occurring during vitellogenesis are regulated by specific gonadal and non-gonadal hormones (Urist and Schjeide 1961; Callard and Klotz 1973; Licht et al. 1980; Ho et al. 1980, 1982; Ho 1987; Wibbels et al. 1990; Heck et al. 1997; Mosconi et al. 1998; Hamann et al. 2003; Polzonetti-Magni et al. 2004).
Oestrogens induce growth and structural modifications in the liver of snakes and lizards that are associated with the production and secretion of proteins (reviewed by Ho 1987). Evidence indicates that estradiol 17β (E2) induces the production of vitellogenin (VTG), which is the main protein sequestered into growing ovarian follicles during vitellogenesis and cleaved into the main proteinaceous components of egg yolk (Bergink and Wallace 1974; Ho et al. 1981; Ho 1987; Hamann et al. 2003; Polzonetti-Magni et al. 2004).
Because yolk deposition into ovarian follicles is crucial to reproductive success of oviparous vertebrates (Polzonetti-Magni et al. 2004), the importance of studying endocrine mechanisms that control vitellogenesis has been highlighted by several studies, many of which focused on threatened and endangered sea turtle species (Licht et al. 1980; Owens and Morris 1985; Wibbels et al. 1990; Rostal et al. 1998; Hamann et al. 2003; Polzonetti-Magni et al. 2004; Sifuentes-Romero et al. 2006; Smelker et al. 2014; Myre et al. 2016). However, there are significant gaps regarding the hormonal regulation of the onset and completion of vitellogenesis in reptiles (Hamann et al. 2003; Polzonetti-Magni et al. 2004). Research is lacking on the variation of such hormones, associated proteins and requisite body condition during the reproductive cycle of free-ranging migratory reptiles, such as sea turtles (Hamman et al. 2003).
Sea turtles start depositing yolk into follicles 8–12 months prior to the nesting season (Wibbels et al. 1990; Miller 1997; Hamann et al. 2003). A pre-migratory surge of E2 4–6 weeks prior to migration is followed by a significant and gradual decline in the concentration of this hormone 1–2 weeks before migration, declining gradually towards the end of the nesting season (Wibbels et al. 1990; Smelker et al. 2014; Myre et al. 2016). Although E2 induces the production of VTG, these two biomarkers of reproduction are usually not correlated, given the long-lasting inductive effects of E2 (Heck et al. 1997). Thus, E2 peaks in advance of VTG, and VTG concentration then decreases significantly but gradually throughout the nesting season of female loggerhead sea turtles (Caretta caretta) (Smelker et al. 2014; Myre et al. 2016). In this way, pre-ovulatory follicles may act as a sink for VTG.
Testosterone (T) concentration in marine turtles significantly increases 1–2 weeks prior to the reproductive migration (Wibbels et al. 1990), and a general decreasing trend in the concentration of T has been shown for loggerhead sea turtles towards the end of the nesting season, following a trend akin that of VTG (Wibbels et al. 1990; Rostal et al. 1998; Smelker et al. 2014; Myre et al. 2016). Concentration of T is also thought to be responsible for mating behaviour (Wibbels et al. 1990; Hamann et al. 2003; Polzonetti-Magni et al. 2004) and for termination of the reproductive cycle (Ho et al. 1981; Wibbels et al. 1990; Myre et al. 2016).
The fluctuations of these biomarkers—which control ovarian recrudescence, as well as trigger sea turtle reproductive migration—are poorly understood (Hamann et al. 2003; Polzonetti-Magni et al. 2004). The objective of this study was to investigate concentration of T and VTG in green sea turtles (Chelonia mydas) nesting at Tortuguero, Costa Rica. We also investigated the effects of body size and condition on concentrations of reproductive biomarkers in female green sea turtles nesting at Tortuguero.
Materials and methods
Tortuguero National Park (TNP), on the Caribbean coast of Costa Rica, was created in 1970 to protect the 29 km of what is now the most important mating and nesting ground for green sea turtles in the Atlantic basin (Troëng and Rankin 2005). From 1986 to 2019, an estimated yearly average of 98 137 (± 44 633) green sea turtle nests were laid along the 29 km of Tortuguero’s nesting beach (Bruno et al. 2020).
Green sea turtles arrive in Tortuguero in late April and early May to reproduce (Carr 1954). Nesting significantly increases in July, peaks in September and occurs through late October (Garcia-Varela et al. 2014). Our study was conducted in July and August 2018 on the northernmost 8 km of Tortuguero Beach, near the Sea Turtle Conservancy (STC) station.
Under Southeastern Louisiana University’s IACUC and the Costa Rican Ministry of the Environment’s permits (001/2018 and 002/2018, respectively), we used 18-gauge, 2.5-inch heparinized needles to collect up to 15 ml of blood from the cervical sinus of adult female green sea turtles nesting at Tortuguero. All blood sampling was conducted following Owens and Ruiz (1980) protocols. We sampled blood from nesting females during oviposition and placed the samples on ice until centrifugation, and blood plasma was stored in liquid nitrogen prior to analysis.
We exported the samples to a −80o C freezer at Southeastern Louisiana University, Hammond, LA, USA (CITES 18US75808C/9). Following procedures by Myre et al. 2016, we extracted steroid hormones twice from 10 to 30 μl of plasma with 5 ml of diethyl ether in each extraction. After steroid extraction, we reconstituted samples with 300 μl of assay buffer and plated it in duplicate in a 96-well microtiter plate coated with Anti-T antibodies of a commercial high sensitivity enzyme-linked immunosorbent assay (ELISA) kit (ENZO Life Sciences, Farmingdale). This assay was fully validated to measure T in green turtles (Allen et al., 2015). Subsequently, we performed ELISAs according to manufacturer’s specifications and read the plate in a microplate reader at 405 nm. To find the concentrations of T in each sample, ELISA results were analysed using the four-parameter logistic equation in SigmaPlot v14.0.
For the VTG assay, we followed the same protocol that was described by Smelker et al. (2014). For this, a solution of purified sea turtle VTG was used to create a standard curve for the indirect ELISA. We diluted the samples 1:30000 to ensure that optical density fit into the linear portion of the standard curve (between 20% and 80% of absorbance). We plated the samples in duplicate into a 96-well flat bottom polysterene microtiter plate (Thermo Fisher Scientific, Pittsburg, PA, USA), which were sealed and incubated overnight at 4oC. The next day, we washed the plates three times with 150 μl of a phosphate buffered saline (PBS) solution and a non-ionic detergent (Tween-20). To maximize specificity, we pre-incubated rabbit anti-VTG antibody with 5 μl of male sea turtle plasma in a solution of PBS with 5% non-fat dry milk (blotto) for 1 hour. Pre-adsorbed anti-VTG antibody (1:40000) was then added to the plates, which were sealed and incubated for 1 hour at room temperature on a shaking platform. After three more washes, we added 100 μl of goat anti-rabbit antibody coupled with horseradish peroxidase diluted in blotto to the plates (BIORAD, Hercules, CA, USA) and incubated for 2 hours at room temperature on a shaking platform. After another washing cycle, we added 100 μl of tetramethybenzidine peroxidase for enzyme-immunoassay to each well of the plate and incubated for 10 minutes at room temperature to promote colour development. To stop the colorimetric reaction, we added 100 μl of sulphuric acid (1 N H2SO4) to each well. The plates were then read in a Bio-Rad Model 680 Microplate Reader with a 450-nm wavelength filter.
In the field, we measured curved carapace length (CCL) with a flexible, 1.5-m measuring tape on the centre of the carapace from the top of the nuchal scale to the inner part of the notch between the supracaudal scales. We measured straight carapace length (SCL) with a metal calliper between the most anterior and the most posterior part of the turtle’s carapace (Wyneken 2001). Both CCL and SCL measurements were taken at least three times in cm, until three measurements did not differ by more than 1 cm. In addition to body measurements, we weighed (kg) and calculated the condition index (CI) of a sub-group of 16 female green sea turtles nesting at TNP using the following formula by Bjorndal et al. (2000)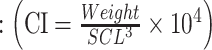 . Finally, we marked turtles with metal tags for individual identification.
. Finally, we marked turtles with metal tags for individual identification.
STC’s research personnel has monitored sea turtle nesting activity at TNP since 1955. This included the collection of morphometric data of nesting females at the northernmost 8 km of nesting beach (Bjorndal and Carr 1989, Troëng and Rankin 2005). Since 1986, to account for the total number of nests laid at TNP during one nesting season, STC staff has conducted a weekly survey of the entire 30 km of nesting beach (Troëng and Rankin 2005; Garcia-Varela et al. 2014). The estimation of green sea turtle clutches laid at TNP yearly was described in detail by Troëng and Rankin (2005). For this study, we accessed STC’s long-term database to extract biometric data of Tortuguero green sea turtles.
For all statistical analyses, we used SYSTAT 13.0 and we used ggplot2 package in R 3.6.3 to create the figures. To assess the normality of the data, we visually analysed the histogram of the studentized residuals and carried out One Sample KS tests. We used linear regression analyses to investigate the relationship between T and VTG and the morphometric parameters of individual female green sea turtles (such as SCL, CCL, weight and CI). To analyse temporal fluctuation of T and VTG early in the nesting season and to investigate differences in SCL and CI per month, we used analysis of variance (ANOVA).
Results
We measured circulating concentrations of T and VTG in 66 blood samples of green sea turtles nesting at Tortuguero. Two of these samples were collected from the same female and in consecutive nesting events (11 days apart). Mean T concentration was 1100 (± 1000 pg ml−1). Our average intra-assay coefficient of variation (CV) for the T assays was 8% and the inter-assay CV was 12.9%. Mean VTG concentration in the samples was 27.9 (± 6.6 mg ml−1). Validation and specificity testing of VTG ELISA was conducted by Smelker et al. (2014). Our intra-assay CV for these VTG assays was 5.1% and the inter-assay CV was 12.8%.
We found a positive relationship between T and VTG (linear regression, r2 = 0.315, F1,63 = 28.995, P < 0.005) (Fig. 1). VTG concentration was negatively correlated with green sea turtle weight (linear regression, r2 = 0.278, F1,13 = 5.017, P < 0.05) (Fig. 2). SCL of female green sea turtles nesting at TNP between 1955 and 2017 varied per month, and significantly larger females nested in June and July than in August and September (ANOVA, F1,5542 = 39.974, P > 0.001) (Fig. 3).
Figure 1. VTG concentration (n = 66) was positively correlated with testosterone concentration (r2 = 0.315). Scatter plots shown with the regression line and 95% confidence interval.

Figure 2. VTG concentration (n = 16) was negatively correlated with green sea turtle weight (r2 = 0.278). Scatter plots shown with the regression line and 95% confidence interval.

Figure 3. SCL of turtles nesting in June and July was significantly greater (asterisk) than that of turtles nesting in August and September. Boxplot shown with standard deviation and median. Whiskers showing highest and lowest observation (n = 5547).

Mean T concentration was significantly lower (ANOVA, F1,63 = 6.426, P = 0.014) in July than in August (Fig. 4). From 20 July to 22 August, mean VTG concentration (n = 56) was significantly higher during the third week of sampling (ANOVA, F1,52 = 7528, P = 0.008) (Fig. 5). The only nesting green sea turtle we recaptured in this study showed a similar pattern of increasing T and VTG circulating concentrations over 11 days. Both VTG and T were higher in the second nesting event than in the first. However, because of the small sample size, no significance could be attributed to the change in the concentration of endocrine correlates between the two nesting events. CI was significantly lower in July than in August (ANOVA, F1,14 = 7.656, P > 0.05) (Fig. 6). Neither SCL, CCL nor body weight varied significantly from July to August 2018.
Figure 4. Testosterone concentration (n = 66) was significantly lower in July than in August. Boxplot shown with standard deviation, median and data points. Data points displayed with jitter to avoid overlap. Whiskers showing highest and lowest observations.

Figure 5. Concentration of VTG (n = 52) was significantly higher during the third week of sampling. Boxplot shown with standard deviation, median and data points. Data points displayed with jitter to avoid overlap. Whiskers showing highest and lowest observations.

Figure 6. CI of Green sea turtles nesting at Tortuguero was significantly lower in July than in August. Boxplot shown with standard deviation, median and distribution of data points. Data points displayed with jitter to avoid overlap. Whiskers showing highest and lowest observations.

Finally, there was positive relationship between weight and SCL (linear regression, r2 = 0.613, F1,226 = 358.103, P < 0.005) and CCL (linear regression, r2 = 0.599, F1,13 = 19.430, P < 0.005) (Figs 7 and 8). However, CI was not significantly correlated to any of the other body measurements nor to the concentrations of T and VTG.
Figure 7. Green sea turtle weight (n = 14) was positively correlated to SCL (r2 = 0.613). Scatter plots shown with the regression line and 95% confidence interval.

Figure 8. Green sea turtle weight (n = 15) was positively correlated to CCL (r2 = 0.599). Scatter plots shown with the regression line and 95% confidence interval.

Discussion
We sampled blood from female green sea turtles during four weeks at the start of the nesting season at Tortuguero, which lasts up to 4 months. Concentration of Testosterone increased significantly from July to August, and VTG concentration, which was elevated during the whole sampling period, significantly increased during the third week of sampling. Interestingly, we sampled blood twice from the same female with an 11-day interval and observed that both T and VTG concentrations almost doubled between the first and second encounter. VTG concentration in Tortuguero green sea turtles shown in our study were up to 40 000 times greater than basal VTG concentrations in juvenile Green (Herbst et al. 2003) and adult female loggerhead sea turtles that were not preparing to breed (Smelker et al. 2014). Moreover, VTG concentration in nesting Tortuguero green sea turtles were akin to that of juvenile Green (Herbst et al. 2003) and Kemp’s Ridley (Lepidochelys kempii; Heck et al. 1997) sea turtles injected with E2 and of nesting Loggerheads (Smelker et al. 2014; Myre et al. 2016). As reptilian VTG estimated half-life is between 15 and 25 days (Bast and Gibson 1985), the results of our study showed that one of the components of vitellogenesis, the production of VTG (Ho 1987), was still taking place early into the nesting season of Tortuguero green sea turtles. Whether VTG produced early in the nesting season of Tortuguero green sea turtles was still being incorporated in ovarian follicles requires further research.
Elevated concentrations of gonadal steroids in loggerhead sea turtles early in the nesting season (Wibbels et al. 1990), the follicular hierarchy observed in necropsied nesting green sea turtles (Limpus et al. 2003) and pre-ovulatory surges in estradiol concentration in captive loggerhead sea turtles (Kakizoe et al. 2010) led authors to suggest that there may be continued follicular development early into the nesting season. In contrast, leatherback sea turtles appeared to arrive at the nesting beach with fully developed ovaries, as no hierarchy in follicular size was detected via ultrasonography (Rostal et al. 1996). Likewise, ultrasonography of captive Kemp’s Ridleys concluded that the entire complement of ovarian follicles was fully developed by the time of mating (Rostal et al. 1990, 1997; Rostal 2005). However, leatherback sea turtles travel long distances from their foraging areas to their nesting grounds (Shillinger et al. 2008; Benson et al. 2011), presumably having ample time to complete ovarian development prior to arrival at nesting beaches. Kemp’s Ridley sea turtles have lower clutch frequency and size than Tortuguero green turtles (Van Buskirk and Crowder 1994; Rostal et al. 1997; Bjorndal 1999; Rostal 2005; Shaver et al. 2016) and may afford the space in the coelomic cavity to hold the full complement of mature follicles for the nesting season all at once. In summary, due to distinct constraints for reproduction of different sea turtle species and populations, timing of completion of yolk deposition into ovarian follicles may vary within the sea turtle superfamily Chelonioidea.
Concentration of VTG in our study was negatively correlated with female green sea turtle body weight, which may be due to sampling larger turtles earlier into their nesting effort, when their VTG concentrations were higher. Relationships between body size and reproductive phenology are found throughout Reptilia. For example, smaller female viviparous lizards reproduce later than their larger counterparts, presumably due to having to divide energy between growth and reproduction (Bauwens and Verheyen 1985). Additionally, larger female diamond-backed terrapins (Malaclemys terrapin) nest earlier than their smaller counterparts, which may allow them to have access to the fittest males in the population (Wolfe et al., 2021, in review). Measuring a different metric of the sea turtle reproductive output, Hatase and Tsukamoto (2008) found that the size of female loggerhead sea turtles influences reproductive frequency, with larger individuals having shorter remigration intervals than smaller ones. Larger female green sea turtles grow slower than their smaller conspecifics (Bjorndal et al. 2000) and may be able to reach the energetic threshold necessary for reproduction sooner and nest earlier in the season than smaller individuals. Additionally, if larger female green sea turtles swim faster, they may arrive earlier at Tortuguero than smaller females.
Our data are consistent with previous studies that have examined the morphometry of green sea turtles. Mean SCL of female Tortuguero green sea turtles was similar to values reported by Bjorndal et al. (2000) for adult green sea turtles throughout the Caribbean Sea. The mean CCL of our study was close to that reported for adult green sea turtles nesting in the Southern Great Barrier Reef (Limpus and Chaloupka 1997) and on Cyprus (Broderick et al. 2003). Green sea turtle CI in our study was similar to other populations (Bjorndal et al. 2000; Thomson et al. 2009; Labrada-Martagón et al. 2010). Moreover, in agreement with Bjorndal et al. (2000), CI was not significantly correlated with SCL, CCL or weight in our study. Mean body weight was unsurprisingly strongly correlated to green sea turtle SCL and CCL. From the standpoint of morphometry, our results suggest that our findings with reproductive biomarkers of gonadal function may be applicable to other green sea turtle populations.
Mean concentration of VTG was higher and mean concentration of T was lower than previously reported for loggerhead sea turtles (Wibbels et al. 1990; Smelker et al. 2014; Myre et al. 2016). Owens (1980) and Wibbels et al. (1990) reported that green sea turtles have an overall lower concentration of gonadal steroids than other sea turtle species, and dietary intake has previously been cited to affect concentrations of gonadal steroids and overall reproductive output in female reptiles (Limpus and Nicholls 1988; Chaloupka et al. 2008; Lovern and Adams 2008). Finally, both Wibbels et al. (1990) and Smelker et al. (2014) used a radioimmunoassay to measure T in their samples, whereas we used ELISAs, which complicate direct comparisons. Radioimmunoassay and ELISA have been reported to yield slightly different, but correlated results when used to measure concentration of gonadal and adrenal steroids (Lewis et al. 1986; Khatun et al. 2009).
Understanding the reproductive physiology of endangered species is crucial for conservation, and ours was the first study investigating reproductive biomarkers of endangered green sea turtles at their most important breeding ground in the Atlantic Basin. The information provided here can be used to guide further studies regarding the fluctuation of reproductive biomarkers during the nesting cycle and the timing of completion of yolk deposition into the ovarian follicles of free-ranging sea turtles.
Funding
This work was supported by the National Geographic Society [EC-51596-18], Sea Turtle Conservancy, Sigma Xi Grant in Aid of Research, American Museum of Natural History, IDEAWILD, CORBANA and Varcli Pinares S.A. Kenneth (Major) Dyson Professorship to R.A.V.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors thank the community and national park rangers of Tortuguero, Costa Rica, for their support. The author thanks all STC research assistants and staff and all the personnel from ASVO Tortuguero and ASOPROTOMA.
REFERENCES
- Allen CD, Robbins MN, Eguchi T, Owens DW, Meylan AB, Meylan PA, Kellar NM, Schwenter JA, Nollens HH, LeRoux RA et al. (2015) First assessment of the sex ratio for an East Pacific green sea turtle foraging aggregation: validation and application of a testosterone ELISA. PLoS One 10: p.e0138861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast RE, Gibson R (1985) Characterization of reptilian vitellogenin: subunit composition and molecular weights of vitellogenin from the colubrid snake Thamnophis sirtalis. Comp Biochem Physiol 80B: 409–418. [Google Scholar]
- Bauwens D, Verheyen RF (1985) The timing of reproduction in the lizard Lacerta vivipara: differences between individual females. J Herpetol 19: 353–364. 10.2307/1564263. [DOI] [Google Scholar]
- Benson SR, Eguchi T, Foley DG, Forney KA, Bailey H, Hitipeuw C, Samber BP, Tapilatu RF, Rei V, Ramohia P et al. (2011) Large-scale movements and high-use areas of western Pacific leatherback turtles, Dermochelys coriacea. Ecosphere 2: 1–27. 10.1890/ES11-00053.1. [DOI] [Google Scholar]
- Bergink EW, Wallace RA (1974) Precursor-product relationship between amphibian vitellogenin and the yolk proteins, lipovitellin and phosvitin. J Biol Chem 249: 2897–2903. [PubMed] [Google Scholar]
- Bjorndal KA, Wetherall JA, Bolten AB, Mortimer JM (1999) Twenty-six years of green turtle nesting at Tortuguero, Costa Rica: an encouraging trend. Conserv Biol. 126–134. [Google Scholar]
- Bjorndal KA, Bolten AB, Chaloupka MY (2000) Green sea turtle somatic growth model: evidence for density dependence. Ecol Appl 10: 269–282. 10.1890/1051-0761(2000)010[0269:GTSGME]2.0.CO;2 [DOI] [Google Scholar]
- Bjorndal KA, Carr A (1989) Variation in clutch size and eggs size in the green turtle nesting population at Tortuguero, Costa Rica. Herpertologica 45: 181–189. 10.1093/nar/15.12.4737. [DOI] [Google Scholar]
- Broderick AC, Glen F, Godley BJ, Hays GC (2003) Variation in reproductive output of marine turtles. J Exp Mar Biol Ecol 288: 95–109. 10.1016/S0022-0981. [DOI] [Google Scholar]
- Bruno RS, Restrepo JA, Valverde RA (2020, 2020) Effects of El Niño Southern Oscillation and local ocean temperature on the reproductive output of green turtles (Chelonia mydas) nesting at Tortuguero, Costa Rica. Mar Biol 167: 128. 10.1007/s00227-020-03749-z. [DOI] [Google Scholar]
- Callard IP, Klotz KL (1973) Sensitivity of parameters of estrogen action in the iguanid lizard Dipsosaurus dorsalis. Gen Comp Endocrinol 21: 314–321. 10.1016/0016-6480(73)90063-4. [DOI] [PubMed] [Google Scholar]
- Carr AF (1954) The passing of the fleet. AIBS Bulletin 4: 17–19. 10.2307/1292357. [DOI] [Google Scholar]
- Chaloupka MY, Kamekazi N, Limpus CJ (2008) Is climate change affecting the population dynamic of the endangered Pacific loggerhead sea turtle? J Exp Mar Biol Ecol 356: 136–143. 10.1016/j.jembe.2007.12.009. [DOI] [Google Scholar]
- Garcia-Varela R, Quilez GZ, Harrison E (2014) Report on the 2014 Green sea turtle Program at Tortuguero, Costa Rica. Sea Turtle Conservancy.
- Hamann, M, Limpus CJ, Owens DW (2003) Reproductive cycles of males and females. In PL Lutz, JA Musick, J Wyneken, eds, The Biology of Sea Turtles, Vol 2. CRC Press, Boca Raton, pp. 135–162. [Google Scholar]
- Hatase H, Tsukamoto K (2008) Smaller longer, larger shorter: energy budget calculations explain intrapopulation variation in remigration intervals for loggerhead sea turtles (Caretta caretta). Can J Zool 86: 595–600. 10.1139/Z08-035. [DOI] [Google Scholar]
- Heck J, MacKenzie DS, Rostal D, Medler K, Owens DW (1997) Estrogen induction of plasma vitellogenin in the Kemp’s Ridley sea turtle (Lepidochelys Kempi). Gen Comp Endocrinol 107: 280–288. 10.1006/gcen.1997.6930. [DOI] [PubMed] [Google Scholar]
- Herbst LH, Siconoff-Baez L, Torelli JH, Klein PA, Kerben MJ, Schumacher IM (2003) Induction of vitellogenesis by estradiol 17B and development of enzyme-linked immunosorbent assays to quantify plasma vitellogenin levels in green turtles (Chelonia mydas). Comp Biochem Physiol B 135: 551–563. [DOI] [PubMed] [Google Scholar]
- Ho SM (1987) Endocrinology of vitellogenesis. In D Norris, R Jones, eds, Hormones in Fishes, Amphibians, and Reptiles. Plenum Press, New York and London, pp. 145–169. [Google Scholar]
- Ho SM, Kleis S, McPherson R, Heisermann GJ, Callard IP (1982) Regulation of vitellogenesis in reptiles. Herpetologica 38: 40–50. [Google Scholar]
- Ho SM, L’Italien J, Callard IP (1980) Studies on reptilian yolk: Chrysemys vitellogenin and phosvitin. Comp Biochem Physiol 65: 130–144. 10.1016/0305-0491(80)90122-4. [DOI] [Google Scholar]
- Ho SM, Danko D, Callard IP (1981) Effect of exogenous estradiol-17β on plasma vitellogenin levels in male and female Chrysemys and its modulation by testosterone and progesterone. Gen Comp Endocrinol 43: 413–421. 10.1016/0016-6480(81)90224-0. [DOI] [PubMed] [Google Scholar]
- Kakizoe Y, Fujiwara M, Akune Y, Kanou Y, Saito T, Uchida I (2010) Cyclical changes of plasma sex steroids in captive breeding loggerhead turtles (Caretta caretta). J Zoo Wildl Med 41: 643–648. 10.1638/2009-0254.1. [DOI] [PubMed] [Google Scholar]
- Khatun S, Nara S, Tripathi V, Rangari K, Chaube SK, Kariya KP, Kumar S, Shrivastav TG (2009) Development of ELISA for measurement of progesterone employing 17-a-OH-P-HRP as enzyme label. J Immunoassay Immunochem 30: 186–196. 10.1080/15321810902782889. [DOI] [PubMed] [Google Scholar]
- Labrada-Maratagón V, Méndez-Rodríguez LC, Gardner SC, Cruz-Escalona VH, Zenteno-Savín T (2010) Health indices of the green sea turtle (Chelonia mydas) along the Pacific coast of Baja California Sur, Mexico. II. Body condition index. Chelonian Conserv Biol 9: 173–183. 10.2744/CCB-0807.1. [DOI] [Google Scholar]
- Lewis JG, Manley L, Townsend CJ, Elder PA (1986) An enzyme-linked immunosorbent assay (ELISA) for urinary free cortisol. Clinica Chimica Acta 159: 205–209. 10.1016/0009-8981(86)90053-7. [DOI] [PubMed] [Google Scholar]
- Licht P, Rainey W, Cliffton K (1980) Serum gonadotropin and steroids associated with breeding activities in the green sea turtle, Chelonia mydas. Gen Comp Endocrinol 40: 116–122. 10.1016/0016-6480(80)90102-1. [DOI] [PubMed] [Google Scholar]
- Limpus CJ, Chaloupka MY (1997) Nonparametric regression modelling of green sea turtles growth rates (Southern Great Barrier Reef). Mar Ecol Prog Ser 149: 23–34. 10.3354/meps149023. [DOI] [Google Scholar]
- Limpus CJ, Miller JD, Parmenter CJ, Limpus DJ (2003) The green turtle, Chelonia mydas, population of Raine Island and the Northern Great Barrier Reef: 1843–2001. Mem Queensl Mus 49: 349–440. [Google Scholar]
- Limpus CJ, Nicholls N (1988) The Southern Oscillation regulates the annual numbers of green sea turtles (Chelonia Mydas) breeding around northern Australia. Aust Wild Res 15: 157–161. 10.1071/WR9880157. [DOI] [Google Scholar]
- Lovern MB, Adams AL (2008) The effects of diet on plasma and yolk steroids in lizards (Anolis carolinensis). Integr Comp Biol 48: 428–436. 10.1093/icb/icn058. [DOI] [PubMed] [Google Scholar]
- Miller JD (1997) Reproduction in sea turtles. In PL Lutz, JA Musick, eds, The Biology of Sea Turtles, Vol. 1. CRC Press. Boca Raton, Florida, pp. 199–222. [Google Scholar]
- Mosconi G, Carnevali O, Carletta R, Nabissi M, Polzonetti-Magni AM (1998) Gilthead seabream (Sparus Aurata) vitellogenin: purification, partial characterization, and validation of an enzyme-linked immunosorbent assay (ELISA). Gen Comp Endocrinol 110: 252–261. 10.1006/gcen.1998.7075. [DOI] [PubMed] [Google Scholar]
- Myre BL, Guertin J, Selcer K, Valverde RA (2016) Ovarian dynamics in free-ranging loggerhead sea turtles (Caretta Caretta). Copeia 104: 921–929. 10.1643/CP-16-393. [DOI] [Google Scholar]
- Owens DW (1980) The comparative reproductive physiology of sea turtles. Amer Zool 20: 549–563. 10.1093/icb/20.3.549. [DOI] [Google Scholar]
- Owens DW, Ruiz GJ (1980) New methods of obtaining blood and cerebrospinal fluid from marine turtles. Herpetologica 36: 17–12. [Google Scholar]
- Owens DW, Morris YA (1985) The comparative endocrinology of sea turtles. Copeia 1985: 723–735. 10.2307/1444766. [DOI] [Google Scholar]
- Polzonetti-Magni AM, Mosconi G, Soverchia L, Kikuyama S, Carnevali O (2004) Multihormonal control of vitellogeneis in lower vertebrates. Int Rev Cytol 239: 1–46. 10.1016/S0074-7696(04)39001-7. [DOI] [PubMed] [Google Scholar]
- Rostal DC (2005) Seasonal reproductive biology of the Kemp’s Ridley sea turtle (Lepidochelys kempii): comparison of captive and wild populations. Chelonian Conserv Biol 4: 788–800. [Google Scholar]
- Rostal DC, Robeck TR, Owens DW, Kraemer DC (1990) Ultrasound imaging of ovaries and eggs in Kemp’s ridley sea turtles (Lepidochelys kempi). J Zoo Wildl Med 21: 27–35. [Google Scholar]
- Rostal DC, Paladino FV, Patterson RM, Spotila J (1996) Reproductive physiology of nesting Leatherback turtles (Dermochelys coriacea) at Las Baulas National Park, Costa Rica. Chelonian Conserv Biol 2: 230–236. [Google Scholar]
- Rostal DC, Grumbles JS, Byles RA, Marquez MR, Owens DW (1997) Nesting physiology of Kemp’s ridley sea turtles, Lepidochelys kempii, at Rancho Nuevo, Tamaulipas, Mexico, with observations on population estimates. Chelonian Conserv Biol 2: 538–554. [Google Scholar]
- Rostal DC, Owens DW, Grumbles JS, MacKenzie DS, Amoss MS (1998) Seasonal reproductive cycle of the Kemp’s ridley sea turtle (Lepidochelys Kempi). Gen Comp Endocrinol 109: 232–243. 10.1006/gcen.1997.7026. [DOI] [PubMed] [Google Scholar]
- Shaver DJ, Rubio C, Walker JS, George J, Amos AF (2016) Kemp's Ridley sea turtle (Lepidochelys kempii) nesting on the Texas coast: geographic, temporal, and demographic trends through 2014. Kemp's Ridley sea turtle (Lepidochelys kempii) nesting on the Texas coast: geographic, temporal, and demographic trends through 2014. Gulf Mex Sci 33: 1–21. 10.18785/goms.3302.04. [DOI] [Google Scholar]
- Shillinger GL, Palacios DM, Bailey H, Bograd SJ, Swithenbank AM, Gaspar P, Wallace BP, Spotila JR, Paladino FV, Piedra R et al. (2008) Persistent leatherback turtle migrations present opportunities for conservation. PLoS Biol 6: 1408–1416. 10.1371/journal.pbio.0060171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifuentes-Romero I, Vázquez-Boucard C, Sierra-Beltrán AP, Gardner SC (2006) Vitellogenin in black turtle (Chelonia Mydas Agassizii): purification, partial characterization, and validation of an enzyme-linked immunosorbent assay for its detection. Environ Toxicol Chem 25: 477–485. 10.1897/05-063R2.1. [DOI] [PubMed] [Google Scholar]
- Smelker K, Smith L, Arendt M, Schwenter J, Rostal D, Selcer K, Valverde R (2014) Plasma vitellogenin in free-ranging loggerhead sea turtles (Caretta Caretta) of the northwest Atlantic Ocean. J Mar Biol 2014: 1–10. 10.1155/2014/748267. [DOI] [Google Scholar]
- Thomson JA, Burkholder D, Heithaus MR, Dill LM (2009) Validation of a rapid visual-assessment technique for categorizing the body condition of green sea turtles (Chelonia mydas) in the field. Copeia 2: 251–255. 10.1643/CE-07-227. [DOI] [Google Scholar]
- Troëng S, Rankin E (2005) Long-term conservation efforts contribute to positive green sea turtle nesting trend at Tortuguero, Costa Rica. Biol Conserv 121: 111–116. 10.1016/j.biocon.2004.04.014. [DOI] [Google Scholar]
- Urist MR, Schjeide AO (1961) The partition of calcium and protein in the blood of oviparous vertebrates during estrus. J Gen Physiol 44: 743–756. 10.1085/jgp.44.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk J, Crowder LB (1994) Life-history variation in marine turtles. Copeia 1994: 66–81. [Google Scholar]
- Wibbels T, Owens DW, Limpus CJ, Reed PC, Amoss MS (1990) Seasonal changes gonadal steroids associated with migration, mating, and nesting in the loggerhead sea turtle (Caretta caretta). Gen Comp Endocrinol 79: 154–164. 10.1016/0016-6480(90)90099-8. [DOI] [PubMed] [Google Scholar]
- Wolfe S, Donini J, Valverde RA (2021) Plasma vitellogenin and testosterone in diamond-backed terrapins (Malaclemys terrapin) during the nesting season in Coastal New Jersey. Submitted to Copeia.
- Wyneken J (2001) The anatomy of sea turtles. U.S. Department of Commerce NOAA technical memorandum NMFS-SEFSC, 1–172.


