Abstract
Background/Aims:
The development of decompensation in cirrhosis demarcates a marked change in the natural history of chronic liver disease. HMG-CoA reductase inhibitors (statins) exert pleiotropic effects that reduce inflammation and fibrosis as well as improve vascular reactivity. Retrospective studies uniformly have associated statin utilization with improved outcomes for patients with cirrhosis. Prospective human studies have shown that statins reduce portal hypertension and reduce death in patients with decompensated cirrhosis after variceal hemorrhage when added to standard therapy with an acceptable safety profile. This proposal aims to extend these findings to demonstrate that simvastatin reduces incident hepatic decompensation events among cirrhotic patients at high risk for hepatic decompensation.
Methods:
We will perform the SACRED Trial (NCT03654053), a phase III, prospective, multi-center, double-blind, randomized clinical trial at 11 VA Medical Centers. Patients with compensated cirrhosis with clinically significant portal hypertension will be stratified based upon the concomitant use of nonselective beta-blockers and randomized to simvastatin 40 mg/day versus placebo for up to 24 months. Patients will be observed for the development of hepatic decompensation (variceal hemorrhage, ascites, encephalopathy), hepatocellular carcinoma, liver-related death, death from any cause, and/or complications of statin therapy. Ancillary studies will evaluate patient-reported outcomes and pharmacogenetic corollaries of safety and/or efficacy.
Conclusion:
Statins have a long track-record of safety and tolerability. This class of medications is generic and inexpensive, and thus, if the hypothesis is proven, there will be few barriers to widespread acceptance of the role of statins to prevent decompensation in patients with compensated cirrhosis.
ClinicalTrials.gov Identifier: NCT03654053
Keywords: Human, Cirrhosis, Clinical trial, HMG-CoA reductase inhibitor, Prospective, Veterans
1. Introduction
Cirrhosis remains a major cause of healthcare morbidity and cost in the United States. Nationally, the economic burden of cirrhosis and cirrhosis-related complications is poorly quantified but the best estimate is that cirrhosis results in 150,000 hospitalizations and $4B in annual costs [1]. The dominant etiologies of underlying liver disease in the U.S. Veteran cirrhosis population, including hepatitis C virus (HCV), alcohol, and nonalcoholic fatty liver disease (NAFLD) [2,3], are closely associated with metabolic syndrome, diabetes mellitus, and dyslipidemia [4,5]. Therapy with HMG-CoA reductase inhibitors, statins, are frequently indicated in metabolic syndrome as primary or secondary prevention of cardiovascular disease. However, due to longstanding and incorrect understanding of the safety profile of statins in patients with chronic liver disease, statins remain underutilized in patients with cirrhosis [6].
Statins, independent of cholesterol-lowering effects, have pleiotropic effects that could retard liver disease progression. Statins have been shown to decrease platelet aggregation and activation, increase endothelial nitric oxide synthase (eNOS) activity on endothelial cells, decrease the migration and proliferation of vascular smooth muscle cells, decrease macrophage proliferation and MMP expression, and downregulate vascular inflammation [7]. Statins also might directly induce vascular smooth muscle cell apoptosis [7]. Statins appear to have strong effects on regulating the fibrogenic effect of inflammation in multiple organs including the liver [8,9]. Statins have been shown in animal models to inhibit pro-fibrogenic hepatic stellate cell (HSC) activation and/or induce HSC apoptosis [10–14]. Some of the effect of statins on intrahepatic endothelial function also may be related to their attenuation of Toll-like receptor 4 (TLR4)-mediated impairment of sinusoidal vasodilation [15]. Statins have also been observed to exhibit antineoplastic properties against liver cancer in vitro [16].
Multiple retrospective studies have demonstrated that statins are not only safe for use in cirrhosis, but that hepatitis C (HCV)-infected, hepatitis B (HBV)-infected patients, alcoholic and nonalcoholic steatohepatitis (NASH) patients with cirrhosis exposed to statins have lower risks of liver cancer (adjusted OR 0.74 95%CI 0.64–0.87) [17,18], infections [19], hepatic decompensation (HR 0.55 95%CI 0.39–0.77) and death (HR 0.56 95%CI 0.46–0.69) [17–24].
Proof of concept studies confirm a beneficial effect of statins on vascular reactivity in patients with cirrhosis. Simvastatin enhances nitric oxide production and upon short term exposure increases hepatic blood flow and decreases sinusoidal resistance [25]. In a placebo-controlled proof-of-concept study, longer exposure (1 month, simvastatin 20 mg/day for 15 days then 40 mg for 15 days) decreased hepatic venous portal pressure significantly (compared to placebo) and improved hepatocyte metabolic function [26]. In a multicenter, blinded randomized controlled trial (RCT) of 158 patients with cirrhosis presenting with a first variceal hemorrhage, stratified by Child-Turcotte-Pugh (CTP) Class, receiving standard prophylaxis to prevent re-bleeding (β-blocker and band ligation), the addition of simvastatin (20 mg/day for the first 15 days, 40 mg/d thereafter) given for up to 24 months reduced mortality (22% in placebo vs 6% in patients in the simvastatin group, HR 0.39 95%CI 0.15–0.99; p = 0.030). The survival benefit occurred despite an absence of difference in re-bleeding rates (p = 0.583), but there was reduced death related to variceal hemorrhage events and subsequent infections [21]. A meta-analysis of 3 RCTs testing the effect of statins on rebleeding did suggest that statins might also have an effect on rebleeding with a pooled HR 0.73 (95%CI 0.59–0.91) [27].
Simvastatin when given to patients with early decompensated CTP B patients appears to have an acceptable adverse event profile [21,28]. In the variceal rebleeding study [21], overall rates of adverse events were similar in the simvastatin and placebo arms (Table 1). Two cases of rhabdomyolysis were observed, both in patients with CTP C cirrhosis with total bilirubin levels greater than 5 mg/dl at randomization (Error! Reference source not found.). The SLCO1B1 gene (OATP1B1) is an influx transporter that impacts intracellular accumulation of simvastatin. A nonsynonymous rs4149056 allele in exon 6 has been associated with increased risk for statin-related myopathy and a doubling of the likelihood of drug discontinuation for either myalgia or myonecrosis [29,30]. The association of the rs4149056 allele on myopathy appears highly dependent on age, sex (higher risk in males), body-mass index, and drug dose [31,32]. There are also significant population-level differences in allele frequency based on race [33]. Given a possible increase in the area under the curve (AUC) of statins related to cirrhosis, the identification of a testable genetic predisposition to adverse events could have a significant impact on patient selection.
Table 1.
Treatment-Associated Adverse Events Related to Simvastatin in Decompensated Cirrhosis [21].
| Treatment-Associated Adverse Events | Placebo (N = 79) | Simvastatin (N = 70) |
|---|---|---|
| Overall | 14 (17.7%) | 16 (22.8%) |
| Abdominal Pain | 3 (3.8%) | 0 (0.0%) |
| Ascites | 2 (2.5%) | 2 (2.8%) |
| Asthenia | 3 (3.8%) | 2 (2.8%) |
| Gastrointestinal Hemorrhage | 2 (2.5%) | 1 (1.4%) |
| Gynecomastia | 0 (0.0%) | 2 (2.8%) |
| Hepatic encephalopathy | 1 (1.2%) | 3 (4.2%) |
| Iron-deficiency anemia | 0 (0.0%) | 2 (2.8%) |
| Rhabdomyolysis | 0 (0.0%) | 2 (2.8%)* |
| Toxicity to various agents | 0 (0.0%) | 2 (2.8%) |
Both patients had total bilirubin >5 mg/dl and were Child-Turcotte-Pugh Class C
Decompensation among compensated CTP A cirrhosis patients, defined as the development of variceal hemorrhage, ascites, or hepatic encephalopathy, marks a change in natural history from a relatively benign to a very poor prognosis. Thus, prevention of incident decompensation is the key endpoint of any liver-directed therapy in compensated cirrhosis [34]. To date, no published prospective clinical trials have evaluated the effect of statin therapy on prevention of incident decompensation in patients at high risk of decompensation. The Primary Aims of the SACRED study are to test the hypotheses that statin therapy used in patients with compensated cirrhosis and clinically significant portal hypertension (CSPH) will reduce the incidence of hepatic decompensation, hepatocellular carcinoma and all-cause mortality. Secondary Aims include exploration of the interaction of SLCO1B1 genetic polymorphisms [35] (rs4149056) on safety and clinical efficacy of statin therapy in patients with compensated cirrhosis, and to assess the impact of statin exposure on health-related quality of life in patients with compensated cirrhosis.
2. Materials and methods
2.1. Study design
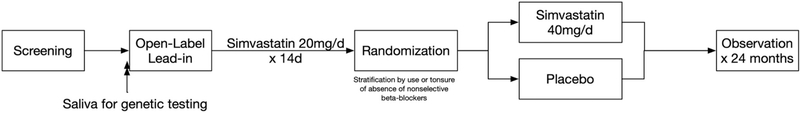
To test the hypothesis that simvastatin therapy administered for up to 2 years in patients with cirrhosis at high risk for hepatic decompensation will safely reduce death, hepatic decompensation, and hepatocellular carcinoma (HCC) and improve patient-reported quality of life, we will perform a phase III, prospective, multi-center, double-blind, randomized clinical trial at 10 VA Medical Centers. (Fig. 1). 500 patients with compensated cirrhosis and CSPH will be stratified based upon the concomitant use of nonselective beta-blockers (NSBB)and randomized to simvastatin 40 mg/day versus placebo for up to 24 months. Patients will be observed for the development of hepatic decompensation (variceal hemorrhage, ascites, encephalopathy), HCC, liver-related death, death from any cause, and/or complications of statin therapy.
Fig. 1.
Study Schema.
2.2. Ethics
The protocol and informed consent documents have been approved by the United States Veterans Affairs Healthcare System Central IRB (Protocol #20–12). This study will be conducted in full accordance with all applicable Research Policies and Procedures for each participating site and all applicable Federal and state laws and regulations including 45 CFR 46, 21 CFR Parts 50, 54, 56, 312, 314, VA Directives and Instructions, and Good Clinical Practice: Consolidated Guidelines approved by the International Conference on Harmonization (ICH).
2.3. Intervention
After screening, all eligible patients who have provided informed consent will be enrolled in the study and will begin a 2-week open-label lead-in phase (Table 2). All patients will be prescribed simvastatin 20 mg orally once daily at bedtime for 2 weeks to assess tolerance of the study drug. Patients who tolerate simvastatin 20 mg daily in the 2-week open-label lead-in will then be randomized to receive either simvastatin 40 mg or a matching placebo to be taken once daily at bedtime for up to 24 months. Study subjects will have research visits scheduled every 12 weeks for research laboratory testing, completion of quality-of-life surveys, assessment of clinical endpoint events, and evaluation of adverse events. The duration was selected based on the prior human clinical trial [21] and statistical considerations. Individuals that discontinue medication due to adverse effects will continue study follow-up to month 24 as scheduled.
Table 2.
Study Interventions.
| Study Phase |
Screening |
Lead-in1 |
Intervention |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit Number | 0 | 1 | 2 | 3 | 4 | 54 | 6 | 74 | 8 | 94 | 10 |
| Study Days | −44 to −14 | −14 ± 2 | 0 | 90 ± 7 | 180 ± 7 | 270 ± 7 | 360 ± 14 | 450 ± 14 | 540 ± 14 | 630 ± 14 | 720 ± 14 |
| Review of prior Esophago-gastroduodenoscopy (EGD) | X2 | X | |||||||||
| Informed Consent | X | ||||||||||
| Review Inclusion/Exclusion Criteria | X | ||||||||||
| Demographics/Medical History | X | X | X | X | X | X | X | X | X | X | |
| Physical Examination | X | X3 | X3 | X | X | X | X | X | X | X | X |
| Vital Signs: BP, HR, RR | X | X3 | X | X | X | X | X | X | X | X | X |
| Height and Weight | X | X3 | X | X | |||||||
| Pregnancy Test (female only) | X | X3 | X | X | X | X | X | X | X | X | |
| Prior/Concomitant Medications | X | X | X | X | X | X | X | X | X | X | X |
| Clinical Laboratory Evaluation | X | X | X | X | X | X | X | X | X | X | X |
| Health-related QOL (PROMIS-29) | X | X | X | X | X | ||||||
| Ultrasound and AFP (standard clinical care) | X | X5 | X | X | X | X | |||||
| Randomization | X | ||||||||||
| Saliva collection for genetic testing | X | ||||||||||
| MoCA Test | X | ||||||||||
| Dispense Investigational Product | X1 | X | X | X | X | X | X | X | X | ||
| IP Compliance | X | X | X | X | X | X | X | X | X | ||
| Adverse Event / Unanticipated Problems Assessment | X | X | X | X | X | X | X | X | X | ||
| Alcohol use screening (AUDIT-C) and counselling | X | X | X | X | X | X | X | X | X | X | |
Open-label simvastatin 20 mg orally once daily.
EGD performance will be left to the discretion of local providers per local practices. EGD within 2 years of enrolment will be acceptable for identifying varices which is one of several possible inclusion criteria. EGD may be repeated at any time by the treating clinician for clinical indications.
Can defer if screening visit within 30 days.
Visit may be done virtually based upon local site capacity and patient preference; laboratory evaluation will need to be completed and pharmacy dispensing/drug accountability performed.
can defer if US and AFP have been done within the last 6 months.
2.4. Endpoint rationale
Prevention of decompensation is the key endpoint of any liver-directed therapy [36], as the median survival in the compensated state exceeds 10 years but median survival in the decompensated state approximates 1.5 years [34,36]. The primary study endpoint for this trial is the development of the composite endpoint of incident hepatic decompensation defined as occurrence of any one of the following events: variceal hemorrhage, ascites, hepatic encephalopathy or HCC. Endpoints will be adjudicated by two independent site investigators pursuant to detailed criteria (see Supplemental Methods). Secondary study endpoints inclu clinically relevant events such as liver-related death, receipt of liver transplantation, survival free from major adverse cardiac events (MACE: acute myocardial infarction, unstable angina, acute ischemic stroke, or coronary revascularization), change in patient health-related quality from baseline to month 12 [as assessed by the PROMIS-29 questionnaire (PROMIS)]. Multiple safety endpoints will also be addressed including progression to CTP Score > 9 points (CTP C status), development of muscle-related complications (myalgia, myopathy, myonecrosis, myonecrosis with acute kidney injury and myoglobinuria [clinical rhabdomyolysis]), and hepatotoxicity defined as Grade ≥ 3 liver toxicity per Common Terminology Criteria for Adverse Events (CTCAE) v5.0 (≥ 5 times upper limit of normal as defined by local laboratory). Details of the monitoring plan for muscle-related complications are included in supplemental material.
2.5. Rational for patient selection
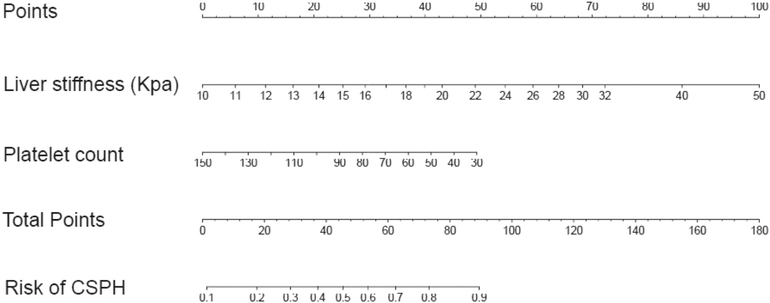
Veterans with compensated cirrhosis (i.e., no history of variceal hemorrhage, no ascites, and no hepatic encephalopathy) due to chronic viral hepatitis, alcohol and/or non-alcoholic fatty liver with a high risk of decompensation will be recruited. Patients with compensated cirrhosis more likely to decompensate are those with clinically significant portal hypertension (CSPH), defined as a hepatic venous pressure gradient (HVPG) ≥10 mmHg. Patients without CSPH have a 90% event-free survival over 4 years [37]. Patients with CSPH but without esophageal varices develop decompensation at a rate of 7–10% per year [38]. Patients with CSPH and varices that have not bled are at the highest risk of decompensation with a 19% risk of decompensation and 5% risk of death over 2 years [39]. The presence of CSPH can be determined noninvasively by: a) the presence of gastroesophageal varices on endoscopy [34]; b) by the presence of portosystemic collaterals on imaging [40]; or c) by a combination of liver stiffness measurement (Vibration Controlled Transient Elastography [VCTE] or other similar technologies) and platelet count [41,42]. We will utilize validated clinical predictors such as the presence of varices, liver stiffness, and hypersplenism to identify individuals with compensated cirrhosis at high risk for hepatic decompensation. General inclusion criteria include: cirrhosis due to chronic viral hepatitis, alcohol and/or non-alcoholic fatty liver; compensated cirrhosis (i.e., no history of variceal hemorrhage, absence of overt ascites, absence of history of overt non-precipitated encephalopathy), age ≥ 18 and ≤ 80; and competence to provide informed consent. To enrich for patients with increased risk of developing cirrhosis decompensation during follow-up, patients will be required to have any one of the following features: esophageal varices on endoscopy within the prior 3 years; visible portosystemic collaterals on imaging as determined by a radiologist at the local site; VCTE (Fibroscan) ≥ 25 kPa; platelet count ≤110 K/mm3; or ≥ 44 total points (∼50% risk of clinically significant portal hypertension) using the ANTICIPATE nomogram (Fig. 2) [41].
Fig. 2.
ANTICIPATE Nomogram for stratification of patient risk for Clinically Significant Portal Hypertension (CSPH).
Patients will be excluded if there is a contraindication to utilization of statin (pregnancy, drug-drug-interactions), prior decompensation without hepatic re-compensation, CTP C status (safety), poor prognosis (life-limiting comorbidities), uncured hepatitis C infection, or an independent indication for statin therapy (i.e., unethical to assign to placebo arm). Specific exclusions to prevent inclusion of patients who have already met the primary endpoint include prior/ongoing hepatic decompensation with ascites or hepatic encephalopathy within the previous 1 year; prior variceal hemorrhage confirmed endoscopically within the previous 2 years; HCC; CTP Stage > B7 cirrhosis; or prior receipt of organ transplant. Exclusions for safety include prior exposure to any statin within 1 year; allergy or sensitivity to any statin preparation; need for concomitant administration of potent inhibitors of CYP3A4 enzymes; or pregnancy or anticipated pregnancy within 2 years. Exclusions related to independent indication for a statin include any independent indication for statin therapy [43], such as any form of clinical atherosclerotic cardiovascular disease (ASCVD); LDL-C levels ≥190 mg/dl; diabetes mellitus, age 40 to 75 years, with LDL-C levels of 70–189 mg/dl; patients without diabetes, age 40 to 75 years, with an estimated 10-year ASCVD risk ≥12%.
2.6. Rationale for choice and dose of simvastatin
The only published randomized controlled trials utilizing a statin preparation in cirrhosis have utilized simvastatin with a 20 mg lead-in followed by 40 mg daily dose [21,26]. These studies demonstrated both efficacy and safety. For this reason, simvastatin 40 mg per day was selected for the present study [21]. No cases of myonecrosis with acute kidney injury and myoglobinuria (clinical rhabdomyolysis) were observed in patients with CTP A cirrhosis at this dose. In the LiverHope study [28], 19% of CTP B patients developed significant muscle and liver enzyme elevations on simvastatin 40 mg and for this reason no CTP B patients will be recruited for the present study.
2.7. Allocation to interventional group
Randomization 1:1 to either simvastatin or placebo will be performed by using a computer-generated algorithm block-of-4 randomization schema stratified by the presence or absence of NSBB use at baseline. Randomization will be performed by the VA Cooperative Studies Program (CSP) central pharmacy using an Interactive Web Response System (IWRS) software program with direction from the study biostatistician. The investigational drug pharmacy at each local site will receive bar-coded drug kits that will be assigned at each dispensing by the CSP central pharmacy according to randomization assignment.
2.8. Sample size estimation
It is expected that approximately 500 subjects will be enrolled in order to produce 467 evaluable subjects. To estimate event rates, we retrospectively identified 15,395 statin-naïve Veterans with compensated CTP A cirrhosis without an independent indication for statin use likely to have portal hypertension (platelet counts ≤150 K/mm3) from our existing cohort [18]. Exclusion criteria included prior diabetes, CAD, prior cardiovascular revascularization, or stroke. In this population, the rate of a composite endpoint of death, decompensation, or HCC was 0.15 at 1 year (15 per 100 patient-years) and 0.27 at 2 years of follow-up. Overall death rates were 0.06 and 0.14, HCC 0.045 and 0.075, decompensation 0.06 and 0.12 at 1 and 2 years, respectively. Based on the event rates estimated by our preliminary data and similar prior studies, a sample size of 467 subjects would provide power to detect a significant difference in the primary composite outcome if the event rate is 10% per year in the placebo arm and the hazard ratio of the effect approximates 0.56. (Table 3) If the HR were similar to the findings of the prior prospective trial (0.39), the sample size needed would be 296 subjects.
Table 3.
Sample Size Estimates.
| Event Rate - 2 years | Power | HR | Sample Size |
|---|---|---|---|
| 0.12 | 0.80 | 0.48 | 486 |
| 0.20 | 0.80 | 0.48 | 292 |
| 0.28 | 0.80 | 0.48 | 209 |
| 0.12 | 0.80 | 0.56 | 779 |
| 0.20 | 0.80 | 0.56 | 467 |
| 0.28 | 0.80 | 0.56 | 334 |
| 0.12 | 0.80 | 0.60 | 1003 |
| 0.20 | 0.80 | 0.60 | 602 |
| 0.28 | 0.80 | 0.60 | 430 |
2.9. Statistical plan and data analysis
For each primary analysis, the core analysis will consist of two parts. First, Cox regression will be used, with the factor of interest being treatment/placebo. The Cox models will be stratified by NSBB use and adjusted for any covariate for which lack of balance was identified in preliminary analysis. The second part of the main analysis will consist of comparing area under the treatment and placebo survival curves. If no covariate adjustment is implied by the preliminary analysis, then weighted Nelson-Aalen survival curves will be compared (with the weight based on the marginal NSBB distribution). If covariate adjustment is deemed necessary by the preliminary analysis, then the methods of Chen et al. [44] will be applied. Each of the secondary endpoints will be viewed as time-to-event, due to the potential for each to be censored, and with follow-up expected to vary across subjects. We will analyze time to each of the secondary endpoints using Cox regression, stratified by NSBB, and adjusted for any covariates identified during preliminary analysis as being unbalanced. The secondary endpoints will be considered a family of group comparisons for which the familywise error rate, termed the false discovery rate (FDR), will be controlled at 5%. For these secondary endpoints with the FDR set at 5%, only endpoints with p-values less than 0.0083 or 0.0085 would be declared significant by Bonferroni or Sidak corrections, respectively.
A priori subgroup analysis is planned for: baseline NSBB use; baseline varices or absence of varices; age ≥ 65 vs. <65 years; baseline cholesterol levels (quartiles of total and LDL-cholesterol levels). The Mantel-Haenszel procedure will be applied to test for the linearity of effects across subgroups, while the chi-square test will be applied to test for heterogeneity of effects among the subgroups. Analysis of the PROMIS-29 domains and overall scores will be conducted using time-weighted averages (Twa), and summarized by descriptivestatistics (mean, standard deviation by exposure, stratified by baseline presence of varices). Statistically significant differences between treatment groups and between outcomes will be evaluated by ANOVA with effects for treatment. We will test for differences with respect to each adjustment covariate by SLCO1B1 gene variant status (presence vs. absence). The Wilcoxon rank sum test will be used for continuous covariates, while the Chi-square test will be used for categorical predictors. Next, we will use logistic regression to evaluate the association between the SLCO1B1 status and each outcome of myalgia, myopathy and myonecrosis. For each outcome, we will assess the improvement in risk discrimination by comparing the area under the ROC curve (via the C index) for models with and without SLCO1B1 status.
2.10. Special safety considerations
The study protocol will allow continued administration of simvastatin after a first hepatic decompensation event if that event is a) variceal bleeding, b) minimal hepatic encephalopathy, or c) HCC. However, simvastatin will be discontinued once a subject experiences ascites, hospitalization for hepatic encephalopathy, or a second hepatic decompensation event. The rationale for continuing the intervention until Child-Turcotte-Pugh C status (the second primary endpoint) is that there is evidence that continuing a statin after initial variceal hemorrhage improves outcome, and there is strong rationale to expect ongoing benefits of statin after development of HCC and hepatic encephalopathy.
2.10.1. Myalgia or myopathy
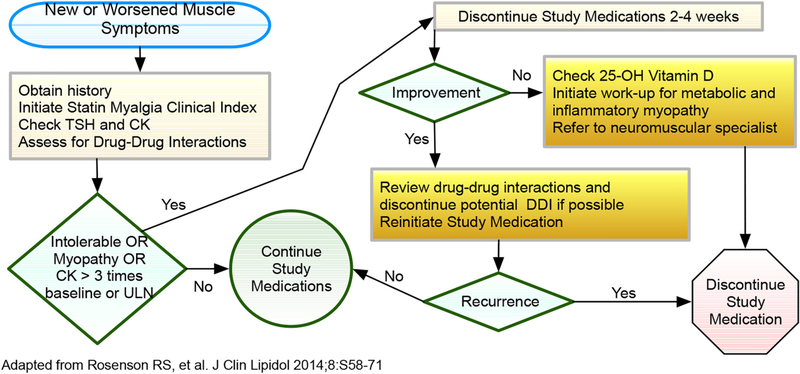
Patients who develop muscle-related symptoms during the lead-in phase will not be randomized. For those who develop muscle pain or weakness after randomization, a standardized approach (Fig. 3) will be adopted for evaluation. We will utilize the Statin Myalgia Clinical Index [46] (SMCI, Table 4) to assess the likelihood that myalgia symptoms are statin related. In the absence of known triggers (e.g., strenuous activity), if a patient develops symptoms (myalgia or myopathy) or a serum level of CK >5-fold their baseline which is confirmed on repeat testing, the study medication will be permanently discontinued. Myonecrosis with myoglobinuria or acute renal failure (increase in serum creatinine ≥0.5 mg/dl [clinical rhabdomyolysis]) will also result in permanent discontinuation of study medication.
Fig. 3.
Schema for Assessment and Management of Muscle Symptoms.
Table 4.
Statin myalgia clinical index score (SMCI) [46]
| Regional distribution/pattern | POINTS |
|---|---|
| Clinical symptoms (new or increased unexplained muscle symptoms) | |
| Symmetric hip flexors/thigh aches | 3 |
| Symmetric calf aches | 2 |
| Symmetric upper proximal aches | 2 |
| Non-specific asymmetric, intermittent | 1 |
| Temporal pattern | |
| Symptoms onset <4 weeks | 3 |
| Symptoms onset 4–12 weeks | 2 |
| Symptoms onset >12 weeks | 1 |
| Dechallenge | |
| Improves upon withdrawal (<2 wks) | 2 |
| Improves upon withdrawal (2–4 wks) | 1 |
| Does not improve upon withdrawal | 0 |
| Rechallenge | |
| Symptoms reoccur upon rechallenge < 4 wks | 3 |
| Symptoms reoccur upon rechallenge 4–12 wks | 1 |
| Statin myalgia clinical index score | |
| Probable | 9–11 |
| Possible | 7–8 |
| Unlikely | <7 |
2.10.2. Statin-related hepatotoxicity/Drug-induced liver injury (DILI)
In the event of routine or symptom-related identification of abnormal liver enzymes, the algorithm delineated in Chalasani et al. [47] will be utilized to assess and closely monitor these findings.
2.10.3. Other adverse events of special interest
Post-marketing studies of various statins identified pancreatitis as a potential adverse event highly likely or probably related to statins in certain individual patients. Post-marketing studies of various statins also identified interstitial lung disease as a potential adverse event highly likely or probably related to statins. Autoimmune hepatitis has been observed with simvastatin use. Any of these events will prompt immediate study drug discontinuation and specific management.
2.11. Interim analysis/study stopping rules
During the study, one interim analysis will be performed after 200 patients have completed 1 year of the study protocol. Data will be analyzed with the double scope of verifying the correctness of the assumptions made for sample size estimation regarding the primary endpoint event rate and to perform a formal efficacy analysis. The data monitoring committee (DMC) will decide whether to end or modify the study if the comparisons in the study have provided “proof beyond reasonable doubt” that for all patients the treatment is clearly indicated or clearly contraindicated in terms of a net difference in the incidence of major end-points [48]. The DMC may also recommend discontinuation of the trial in the event that excess adverse events of special interest (AESI) are associated with the simvastatin arm. For this study, DILI and myonecrosis associated with acute kidney injury and myoglobinuria (clinical rhabdomyolysis) will be evaluated as AESI. A Bayesian approach will be adopted to guide continued patient accrual in response to any safety signal [49]. A maximum tolerated AESI rate of 4% will be adopted (RU = 0.04). If P (π > RU | data) > τ1 using τ1 = 0.9, the DMC will recommend stopping the study for safety considerations.
2.12. Expected findings
We expect to find that simvastatin 40 mg once daily significantly reduces the incidence of hepatic decompensation events and HCC in patients with compensated cirrhosis and CSPH. This would correspond to 27% of the placebo arm meeting the primary endpoint compared to 15% in the simvastatin arm over two years. Concomitantly, we expect low rates of treatment-related adverse events, < 3% serious in nature.
2.13. Potential pitfalls and alternative approaches
2.13.1. Enrollment
Eleven clinical sites have been selected based on large hepatology practices with sufficient cirrhosis populations such that enrollment of 50 patients over 2 years is feasible. However, due to inclusion and exclusion criteria many patients with cirrhosis may not be candidates. The sample size calculation utilized was based on a relatively conservative HR of 0.56. If the effect size is similar to the prior prospective study (HR 0.39), the study may be overpowered and at interim analysis the study size can be reduced. Would enrollment rates fell behind expected targets, alternative approaches may include addition of more trial sites and/or liberalization of the inclusion/exclusion criteria.
2.14. Antiviral therapy for hepatitis C
It is unethical not to treat patients with hepatitis C cirrhosis with direct acting antivirals to cure the infection. High treatment rates of patients with hepatitis C could reduce event rates in patients with SVR12 (cure). For this reason, identification of CSPH status will only occur after hepatitis C direct-acting antiviral treatment. Data suggest that treated patients with CSPH do not have markedly reduced decompensation rates [50]. Published data suggest that many (if not the majority of) formerly HCV-infected veterans continue to consume alcohol and/or exhibit evidence of co-existing NAFLD, and thus remain at high risk for hepatic decompensation [51].
2.15. Small effect size
If the effect size is less significant than the projected HR 0.56, the study might be underpowered to find a statistically significant difference in outcomes (Type II error). Such false negative findings would provide misleading clinical information, suggesting that statins provide limited if any benefit in patients with compensated cirrhosis and should not be routinely prescribed.
2.16. Higher than expected AEs are observed
Simvastatin was selected for this study due to the existing clinical trial in a similar population showing efficacy and safety. A different statin formulation might be considered for future research were the safety of simvastatin not supported by the present study.
2.17. Generalizability
The veteran population with cirrhosis differs from the US cirrhosis population. Veterans with cirrhosis are older, nearly exclusively male, and have higher rates of alcohol abuse and diabetes mellitus. The nature of presumed biological effect of statins on endothelial function are unlikely to be significantly impacted by these factors; thus, we do not anticipate that the specific population under study will impact generalizability of findings.
3. Conclusion
Cirrhosis-related decompensation events are burdensome to patients and tremendously costly to the US healthcare system. Strong preclinical data demonstrate that HMG-CoA reductase inhibitors exert pleiotropic effects that reduce inflammation and fibrosis as well as improve vascular reactivity. Retrospective studies uniformly have associated statin utilization with improved outcomes. Prospective human studies have shown that statins reduce portal hypertension and reduce death in patients with decompensated cirrhosis after variceal hemorrhage when added to standard therapy. This proposal aims to extend these findings to demonstrate that simvastatin reduces incident hepatic decompensation events among patients with cirrhosis at high risk for hepatic decompensation. This prospective randomized double-blind multicenter study will provide the highest quality evidence to inform the medical community as to the utility and safety of statin therapy in patients with cirrhosis. Ancillary studies will evaluate patient-reported outcomes and pharmacogenetic corollaries of safety and/or efficacy. Statins have a long track-record of safety and tolerability. This class of medications is generic and inexpensive, and thus, if the hypothesis is proven, there will be few barriers to widespread acceptance. The investigative team has proposed an ambitious but feasible study that will have extremely high impact for patients with cirrhosis and the clinicians who care for them.
Supplementary Material
Acknowledgments
Grant support
United States Department of Veterans Affairs CSR&D Merit Review I01-CX002010. The sponsor had no role in the design of the clinical trial and will play no role in data collection, analysis, interpretation, writing or publication.
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2021.106367.
References
- [1].Kaplan DE, Chapko MK, Mehta R, et al. Healthcare costs related to treatment of hepatocellular carcinoma among veterans with cirrhosis in the United States, Clin. Gastroenterol. Hepatol. (July262017), 10.1016/j.cgh.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kaplan DE, Dai F, Aytaman A, et al. Development and performance of an algorithm to estimate the child-turcotte-pugh score from a national electronic healthcare database, Clin. Gastroenterol. Hepatol. (July152015), 10.1016/j.cgh.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN, Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013, Gastroenterology. 149 (6) (November 2015), 10.1053/j.gastro.2015.07.056, 1471–1482 e5. [DOI] [PubMed] [Google Scholar]
- [4].El-Serag HB, Hampel H, Javadi F, The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence, Clin. Gastroenterol. Hepatol. 4 (3) (March 2006) 369–380. [DOI] [PubMed] [Google Scholar]
- [5].Jepsen P, Lash TL, Vilstrup H, The clinical course of alcoholic cirrhosis: development of comorbid diseases. A Danish nationwide cohort study, Liver Int. (April282016), 10.1111/liv.13151. [DOI] [PubMed] [Google Scholar]
- [6].Blais P, Lin M, Kramer JR, El-Serag HB, Kanwal F, Statins are underutilized in patients with nonalcoholic fatty liver disease and dyslipidemia, Dig. Dis. Sci. 61 (6) (June 2016) 1714–1720, 10.1007/s10620-015-4000-6. [DOI] [PubMed] [Google Scholar]
- [7].Sadowitz B, Maier KG, Gahtan V, Basic science review: statin therapy—part I: the pleiotropic effects of statins in cardiovascular disease, Vasc. Endovasc. Surg. 44 (4) (May 2010) 241–251, 10.1177/1538574410362922. [DOI] [PubMed] [Google Scholar]
- [8].Vieira JM Jr., Mantovani E, Rodrigues LT, et al. Simvastatin attenuates renal inflammation, tubular transdifferentiation and interstitial fibrosis in rats with unilateral ureteral obstruction, Nephrol. Dial. Transplant. 20 (8) (August 2005) 1582–1591, 10.1093/ndt/gfh859. [DOI] [PubMed] [Google Scholar]
- [9].Yamamoto C, Fukuda N, Jumabay M, et al. Protective effects of statin on cardiac fibrosis and apoptosis in adrenomedullin-knockout mice treated with angiotensin II and high salt loading, Hypertens. Res. 34 (3) (March 2011) 348–353, 10.1038/hr.2010.243. [DOI] [PubMed] [Google Scholar]
- [10].Tokunaga T, Ikegami T, Yoshizumi T, et al. Beneficial effects of fluvastatin on liver microcirculation and regeneration after massive hepatectomy in rats, Dig. Dis. Sci. 53 (11) (November 2008) 2989–2994, 10.1007/s10620-008-0241-y. [DOI] [PubMed] [Google Scholar]
- [11].El-Ashmawy NE, El-Bahrawy HA, Shamloula MM, Ibrahim AO, Antifibrotic effect of AT-1 blocker and statin in rats with hepatic fibrosis, Clin. Exp. Pharmacol. Physiol. 42 (9) (September 2015) 979–987, 10.1111/1440-1681.12446. [DOI] [PubMed] [Google Scholar]
- [12].Abraldes JG, Rodriguez-Vilarrupla A, Graupera M, et al. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats, J. Hepatol. 46 (6) (2007) 1040–1046, 10.1016/j.jhep.2007.01.020. [DOI] [PubMed] [Google Scholar]
- [13].Moreno M, Ramalho LN, Sancho-Bru P, et al. Atorvastatin attenuates angiotensin II-induced inflammatory actions in the liver, Am. J. Physiol. Gastrointest. Liver Physiol. 296 (2) (February 2009) G147–G156, 10.1152/ajpgi.00462.2007. [DOI] [PubMed] [Google Scholar]
- [14].Busnelli M, Manzini S, Froio A, et al. Diet induced mild hypercholesterolemia in pigs: local and systemic inflammation, effects on vascular injury - rescue by high-dose statin treatment, PLoS One 8 (11) (2013), e80588, 10.1371/journal.pone.0080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].La Mura V, Pasarin M, Meireles CZ, et al. Effects of simvastatin administration on rodents with lipopolysaccharide-induced liver microvascular dysfunction, Hepatology. 57 (3) (March 2013) 1172–1181, 10.1002/hep.26127. [DOI] [PubMed] [Google Scholar]
- [16].Zhang W, Wu J, Zhou L, Xie HY, Zheng SS, Fluvastatin, a lipophilic statin, induces apoptosis in human hepatocellular carcinoma cells through mitochondria-operated pathway, Indian J. Exp. Biol. 48 (12) (December 2010) 1167–1174. [PubMed] [Google Scholar]
- [17].El-Serag HB, Johnson ML, Hachem C, Morgana RO, Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes, Gastroenterology 136 (5) (May 2009) 1601–1608, 10.1053/j.gastro.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kaplan DE, Serper MA, Mehta R, et al. Effects of hypercholesterolemia and statin exposure on survival in a large national cohort of patients with cirrhosis, Gastroenterology 156 (6) (May 2019) 1693–1706, 10.1053/j.gastro.2019.01.026, e12. [DOI] [PubMed] [Google Scholar]
- [19].Motzkus-Feagans C, Pakyz AL, Ratliff SM, Bajaj JS, Lapane KL, Statin use and infections in veterans with cirrhosis, Aliment. Pharmacol. Ther. 38 (6) (September 2013) 611–618, 10.1111/apt.12430. [DOI] [PubMed] [Google Scholar]
- [20].Mohanty A, Tate JP, Garcia-Tsao G, Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C-related compensated cirrhosis, Gastroenterology. 150 (2) (February 2016) 430–440, 10.1053/j.gastro.2015.10.007, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abraldes JG, Villanueva C, Aracil C, et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce Rebleeding but increases survival in patients with cirrhosis, Gastroenterology 150 (5) (May 2016) 1160–1170, 10.1053/j.gastro.2016.01.004, e3. [DOI] [PubMed] [Google Scholar]
- [22].Kumar S, Grace ND, Qamar AA, Statin use in patients with cirrhosis: a retrospective cohort study, Dig. Dis. Sci. 59 (8) (August 2014) 1958–1965, 10.1007/s10620-014-3179-2. [DOI] [PubMed] [Google Scholar]
- [23].Chang FM, Wang YP, Lang HC, et al. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: a population-based study, Hepatology. 66 (3) (September 2017) 896–907, 10.1002/hep.29172. [DOI] [PubMed] [Google Scholar]
- [24].Stokkeland K, Lageborn CT, Ekbom A, et al. Statins and angiotensin-converting enzyme inhibitors are associated with reduced mortality and morbidity in chronic liver disease, Basic Clin. Pharmacol. Toxicol. 122 (1) (January 2018) 104–110, 10.1111/bcpt.12844. [DOI] [PubMed] [Google Scholar]
- [25].Zafra C, Abraldes JG, Turnes J, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis, Gastroenterology. 126 (3) (March 2004) 749–755. [DOI] [PubMed] [Google Scholar]
- [26].Abraldes JG, Albillos A, Banares R, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial, Gastroenterology. 136 (5) (May 2009) 1651–1658, 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- [27].Kim RG, Loomba R, Prokop LJ, Singh S, Statin use and risk of cirrhosis and related complications in patients with chronic liver diseases: a systematic review and meta-analysis, Clin. Gastroenterol. Hepatol. 15 (10) (October 2017) 1521–1530, 10.1016/j.cgh.2017.04.039, e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pose E, Napoleone L, Amin A, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial, Lancet Gastroenterol. Hepatol. 5 (1) (January 2020) 31–41, 10.1016/S2468-1253(19)30320-6. [DOI] [PubMed] [Google Scholar]
- [29].Group SC, Link E, Parish S, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study, N. Engl. J. Med. 359 (8) (August 21 2008) 789–799, 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- [30].Voora D, Shah SH, Spasojevic I, et al. The SLCO1B1*5 genetic variant is associated with statin-induced side effects, J. Am. Coll. Cardiol. 54 (17) (October 20 2009) 1609–1616, 10.1016/j.jacc.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bakar NS, Neely D, Avery P, Brown C, Daly AK, Kamali F, Genetic and clinical factors are associated with statin-related myotoxicity of moderate severity: a case-control study, Clin. Pharmacol. Ther. 104 (1) (July 2018) 178–187, 10.1002/cpt.887. [DOI] [PubMed] [Google Scholar]
- [32].Nguyen KA, Li L, Lu D, et al. A comprehensive review and meta-analysis of risk factors for statin-induced myopathy, Eur. J. Clin. Pharmacol. 74 (9) (September 2018) 1099–1109, 10.1007/s00228-018-2482-9. [DOI] [PubMed] [Google Scholar]
- [33].Santos PC, Soares RA, Nascimento RM, et al. SLCO1B1 rs4149056 polymorphism associated with statin-induced myopathy is differently distributed according to ethnicity in the Brazilian general population: Amerindians as a high risk ethnic group, BMC Med. Genet. 12 (October 12 2011) 136, 10.1186/1471-2350-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J, Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American association for the study of liver diseases, Hepatology 65 (1) (January 2017) 310–335, 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- [35].Molokhia M, Bhatia S, Nitsch D, Genetic determinants of statin-associated myopathy, Perinat. Med. 5 (5) (September 2008) 481–494, 10.2217/17410541.5.5.481. [DOI] [PubMed] [Google Scholar]
- [36].Abraldes JG, Garcia-Tsao G, The design of clinical trials in portal hypertension, Semin. Liver Dis. 37 (1) (February 2017) 73–84, 10.1055/s-0036-1597891. [DOI] [PubMed] [Google Scholar]
- [37].Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis, Gastroenterology. 133 (2) (August 2007) 481–488, 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- [38].Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis, N. Engl. J. Med. 353 (21) (November 24 2005) 2254–2261, 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- [39].D’Amico G, Pasta L, Morabito A, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients, Aliment. Pharmacol. Ther. 39 (10) (May 2014) 1180–1193, 10.1111/apt.12721. [DOI] [PubMed] [Google Scholar]
- [40].Augustin S, Pons M, Maurice JB, et al. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease, Hepatology. 66 (6) (December 2017) 1980–1988, 10.1002/hep.29363. [DOI] [PubMed] [Google Scholar]
- [41].Abraldes JG, Bureau C, Stefanescu H, et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: the “Anticipate” study, Hepatology. 64 (6) (December 2016) 2173–2184, 10.1002/hep.28824. [DOI] [PubMed] [Google Scholar]
- [42].Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT, American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases, Gastroenterology 152 (6) (May 2017) 1544–1577, 10.1053/j.gastro.2017.03.016. [DOI] [PubMed] [Google Scholar]
- [43].Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines, J. Am. Coll. Cardiol. 63 (25 Pt B) (July 1 2014) 2889–2934, 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- [44].Chen PY, Tsiatis AA, Causal inference on the difference of the restricted mean lifetime between two groups, Biometrics. 57 (4) (December 2001) 1030–1038, 10.1111/j.0006-341x.2001.01030.x. [DOI] [PubMed] [Google Scholar]
- [46].Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA, The National Lipid Association’s muscle safety expert P. an assessment by the statin muscle safety task force: 2014 update, J. Clin. Lipidol. 8 (3 Suppl) (May-Jun 2014) S58–S71, 10.1016/j.jacl.2014.03.004. [DOI] [PubMed] [Google Scholar]
- [47].Chalasani N, Regev A, Drug-induced liver injury in patients with preexisting chronic liver disease in drug development: how to identify and manage? Gastroenterology 151 (6) (December 2016) 1046–1051, 10.1053/j.gastro.2016.10.010. [DOI] [PubMed] [Google Scholar]
- [48].Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples, Br. J. Cancer 35 (1) (January 1977) 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zohar S, Teramukai S, Zhou Y, Bayesian design and conduct of phase II single-arm clinical trials with binary outcomes: a tutorial, Contemp. Clin. Trials 29 (4) (July 2008) 608–616, 10.1016/j.cct.2007.11.005. [DOI] [PubMed] [Google Scholar]
- [50].El-Sherif O, Jiang ZG, Tapper EB, et al. Baseline factors associated with improvements in decompensated cirrhosis after direct-acting antiviral therapy for hepatitis C virus infection, Gastroenterology 154 (8) (June 2018) 2111–2121, 10.1053/j.gastro.2018.03.022, e8. [DOI] [PubMed] [Google Scholar]
- [51].Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB, Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents, Gastroenterology 153 (4) (October 2017) 996–1005, 10.1053/j.gastro.2017.06.012, e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.