Abstract
Background:
Epicardial adipose tissue (EAT) volume (cm3) and attenuation (HU) may predict major adverse cardiovascular events (MACE). We aimed to evaluate the prognostic value of fully automated deep-learning-based EAT volume and attenuation measurements quantified from non-contrast cardiac computed tomography (CT).
Methods:
Our study included 2068 asymptomatic subjects (56 ± 9 years, 59% male) from the EISNER trial with long-term follow-up after coronary artery calcium (CAC) measurement. EAT volume and mean attenuation were quantified using automated deep learning software from non-contrast cardiac CT. MACE was defined as myocardial infarction, late (>180 days) revascularization, and cardiac death. EAT measures were compared to CAC score and atherosclerotic cardiovascular disease (ASCVD) risk score for MACE prediction.
Results:
At 14 ± 3 years, 223 subjects suffered MACE. Increased EAT volume and decreased EAT attenuation were both independently associated with MACE. ASCVD risk score, CAC, and EAT volume were associated with increased risk of MACE (Hazard Ratio HR[95%CI]: 1.03[1.01–1.04]; 1.25[1.19–1.30]; and 1.35[1.07–1.68], p<0.01 for all) and EAT attenuation was inversely associated with MACE (HR: 0.83[0.72–0.96], p=0.01), with corresponding Harrell’s C-statistic of 0.76. MACE risk progressively increased with EAT volume≥113 cm3 and CAC≥100 AU and was highest in subjects with both (p<0.02 for all). In 1317 subjects, EAT volume was correlated with inflammatory biomarkers CRP, myeloperoxidase, and adiponectin reduction; EAT attenuation was inversely related to these biomarkers.
Conclusions:
Fully automated EAT volume and attenuation quantification by deep learning from non-contrast cardiac CT can provide prognostic value for the asymptomatic patient, without additional imaging or physician interaction.
Keywords: Computerized Tomography (CT), Prognosis, Cardiovascular Disease, Risk Factors, Computed tomography, artificial intelligence, deep learning, epicardial adipose tissue, coronary artery calcium
Introduction
Coronary artery calcium (CAC) quantification from non-contrast computed tomography (CT) has been shown consistently to add to traditional risk factors for the prediction of future cardiac events in the asymptomatic patient1–4. The prospective, randomized EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) trial5 has reported that CAC measurement improves cardiovascular risk factors modification in asymptomatic patients, without increasing downstream medical testing or costs5,6. In addition to measuring CAC, non-contrast cardiac CT can be used for measuring epicardial adipose tissue (EAT), the visceral adipose tissue surrounding the heart and the coronary arteries7. EAT is a metabolically active fat depot and has been shown to relate to early atherosclerosis8–11 and to predict future adverse cardiovascular events12. However, routine measurement of EAT is time-consuming, requiring 10–15 minutes per scan and is not standard in the clinical reporting of non-contrast cardiac CT.
Deep learning (DL), a subset of machine learning, has been shown to be very effective for automated object detection from a wide range of data as well as image segmentation13,14. Its application to cardiac imaging has been shown to improve diagnostic accuracy compared to standard reporting metrics13,15. We aimed to evaluate the prognostic value of both EAT volume and attenuation quantified by fully automated DL software on non-contrast cardiac CT for future adverse cardiac events.
Methods
The data supporting the findings of this study are available from the corresponding author for checking the reproducibility of the study results.
Study Population
Our study population included 2068 asymptomatic subjects without known coronary artery disease (CAD) enrolled in the EISNER trial (at Cedars-Sinai Medical Center, Los Angeles, California) with available CT image data who completed long-term (over 14 year) prognostic follow-up. These subjects were 78% of the total EISNER trial CT scan group and the EISNER 4 sub-trial. Inclusion criteria for the EISNER trial subjects, as described previously16, were age 45–80 years and intermediate pre-test probability of CAD based on age (>55 years in men, >65 years in women) or the presence of at least one CAD risk factor in younger subjects (age 45–54 years in men or 55–64 years in women). Subjects were excluded if they had prior myocardial infarction (MI), coronary revascularization, cardiomyopathy, peripheral vascular disease, angina, or stroke. Additional exclusion criteria were current pregnancy, prior CAC scanning or invasive coronary angiography, or a medical comorbidity likely to impact outcomes at follow-up such as cancer.
All study subjects underwent a baseline non-contrast CT scan for CAC scoring, as well as clinical and laboratory evaluations. Atherosclerotic cardiovascular disease (ASCVD) risk was calculated using the ACC/AHA guidelines for primary prevention of CV disease from clinical and laboratory data collected at the time of the CT study16. All subjects were prospectively followed for major adverse cardiovascular events (MACE) (including cardiac death, MI, and late revascularization defined as occurring more than 180 days after the CT). Subjects with non-cardiac death were excluded from analysis. Follow-up information was obtained by clinical visits, detailed questionnaires sent by mail, or telephone contact. Reported event information were also verified by the National Death Index query and by comprehensive review of electronic medical, hospital, and death records by 2 independent, experienced cardiologists blinded to clinical factors and CT data. This study was approved by the Institutional Review Board and the subjects gave informed written consent for the use of their data.
Cardiac CT
Non-contrast CT scans were acquired using either an Electron Beam CT (EBCT) scanner (GE Healthcare, Milwaukee, WI, USA) or a 4-slice CT scanner (Somatom Volumezoom, Siemens Medical Solutions, Erlangen, Germany) with prospective electrocardiogram (ECG) triggering and a tube voltage of 120 kVp. Raw data were reconstructed at a slice thickness of either 2.5 or 3.0 mm. Each scan was analyzed using commercially available semi-automatic calcium scoring software (ScImage, Inc., Los Altos, CA, USA) by expert cardiologists to measure the total Agatston CAC score17.
Deep Learning Based EAT Quantification
EAT is defined as all adipose tissue enclosed by the visceral pericardium. EAT volume and attenuation were quantified using a fully automated DL algorithm incorporated into QFAT research software (version 2.0, Cedars-Sinai Medical Center, Los Angeles, CA) (Figure 1 and Figure 2). This fully automated method was first trained on 850 cardiac CT scans from multiple scanners, protocols, and sites and was shown to perform as accurately as an independent expert reader18. Using this method, the pericardium was automatically segmented from the non-contrast CT datasets. The limits of the heart were automatically defined as the pulmonary artery bifurcation (superior limit) to the posterior descending artery (inferior limit). EAT volume (reported in cm3) and mean attenuation (reported in Hounsfield units [HU]) were automatically calculated from three-dimensional fat voxels between the HU limits of ([−190, −30] HU) enclosed by the visceral pericardium.
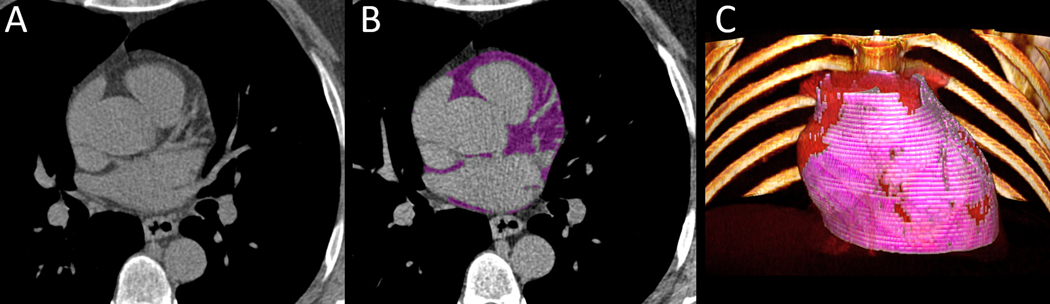
Figure 1:
Case example of a 53-year-old asymptomatic male baseline cardiac CT without coronary artery calcium (CAC Score=0 AU) with late revascularization after 2 years (A). Automated segmentation by deep learning on baseline cardiac CT (EAT=148 cm3, purple) (B). 3D volume rendering of EAT from QFAT software shown in pink (C).
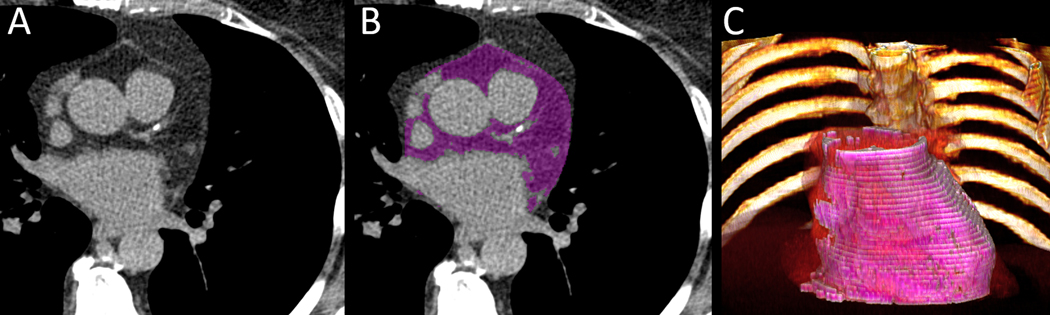
Figure 2:
Case example of a 63-year-old asymptomatic female baseline cardiac CT with low coronary artery calcium (CAC Score=43.9 AU), who had cardiac death after 3 years (A). Automated segmentation by deep learning on baseline cardiac CT (EAT=186 cm3, purple) (B). 3D volume rendering of EAT from QFAT software shown in pink (C).
Serum Biomarkers
In a subset of 1317 subjects, serum biomarkers were measured from blood samples extracted at the time of the baseline CAC scan. These samples were immediately centrifuged and stored at −80 degrees Celsius until assayed. Adipocytokines and inflammatory serum biomarkers including C-reactive protein (CRP), interleukin 6 (IL-6), endothelial plasminogen activator inhibitor 1 (PAI-1), adiponectin, myoglobin, D-dimer, myeloperoxidase (MPO), endothelial cell-selective adhesion molecule (ESAM), and matrix metallopeptidase 9 (MMP-9) were examined in relation to EAT volume and attenuation.
Statistical Analysis
Continuous variables are reported as mean ± standard deviation (SD) or median (interquartile range), as appropriate. Categorical variables are reported as frequencies (in percent) and compared with the Chi-Square test. Wilcoxon rank sum test or two-sample t-tests were used to compare groups, as appropriate. Distributions of CAC score and EAT volume were not normally distributed and thus described as mean ± standard deviation (SD) after normalization with logarithmic adjustment. Base-2 logarithmic transformation was used for CAC score and EAT volume, which represents doubling of the covariate 19. EAT attenuation was normally distributed and was examined per-5 HU increase. We used the time to first event in our analysis. After examining variable correlation, association of EAT features with MACE events was assessed using stepwise-adjusted Cox regression with adjustments for 1) ASCVD risk score 2) CAC, and 3) EAT. Since EAT measures are from a specific fat depot, we also examined association of EAT features with obesity measures such as waist circumference (cm), and body mass index (BMI, kg/m2). The proportional hazards assumption was assessed with Schoenfeld residuals and Harrell’s C-statistic was reported. In a sub-analysis, we examined the relationship of EAT with MACE by gender. We also examined quartiles of EAT and the standard CAC categories (0, 1–99, 100–400, >400) in the EISNER population. We investigated the prognostic value of standard threshold of CAC (CAC≥100 AU) as well as the EAT threshold of ≥113 cm3 determined by maximum Youden’s index (defined as J=sensitivity + specificity −1). Spearman rank correlations were performed to examine the relationship between EAT measurements and serum biomarkers. When appropriate, differences in means were examined with one-way Analysis of variance (ANOVA) analysis, with Bonferroni corrections between pairs. The Kaplan Meier survival curves for all subjects stratified by EAT volume ≥113 cm3 and CAC ≥100 AU were calculated; the log-rank test was used to test differences in survival between groups. All analyses were performed using Stata/IC version 15.1 (StataCorp LP, College Station, Texas). A p-value of 0.05 was considered statistically significant.
RESULTS
A total of 2,068 subjects (age 56.1 ± 9.1 years; 59% male) were included. EAT metrics were successfully processed by the DL software in all subjects. MACE were observed in 223 (11%) subjects at a mean follow-up of 13.9 ± 3 years. In those who suffered MACE, 42 subjects (19%) experienced MI, 145 (65%) subjects underwent late revascularization, and 36 subjects (16%) had cardiac death. Subjects who experienced MACE were older, had a higher BMI, higher prevalence of hypertension and diabetes, and higher ASCVD risk score. All other baseline characteristics are shown in Table 1.
Table 1:
Baseline characteristics of the study population. Results are shown as mean ± standard deviation, n (%) or median (interquartile range).
| Total Subjects N=2068 | MACE + N=223 | MACE – N=1845 | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 56.1 ± 9.1 | 60.0 ± 9.9 | 55.6 ± 8.8 | <0.001* |
| Male | 1226 (59%) | 155 (70%) | 1071 (58%) | 0.001* |
| BMI (kg/m2) | 26.6 ± 4.9 | 27.7 ± 5.2 | 26.5 ± 4.8 | <0.001* |
| Waist circumference (cm) | 36.1 ± 5.4 | 37.2 ± 5.7 | 35.9 ± 5.3 | 0.03* |
| Cardiovascular risk factors | ||||
| ASCVD risk score (%) | 7.5 ± 7.6 | 12.8 ± 11.2 | 6.8 ± 6.8 | <0.001* |
| Diabetes mellitus | 119 (6%) | 20 (9%) | 99 (5%) | 0.03* |
| Hypertension | 834 (40%) | 133 (60%) | 701 (38%) | <0.001* |
| Hyperlipidemia | 1439 (70%) | 168 (75%) | 1271 (69%) | 0.05 |
| Smoking | 91 (4%) | 10 (4%) | 81 (4%) | 0.55 |
| Family history of CAD | 623 (30%) | 77 (35%) | 546 (30%) | 0.13 |
| Medications | ||||
| Statin | 447 (22%) | 58 (26%) | 389 (21%) | 0.10 |
| Aspirin | 127 (6%) | 26 (12%) | 101 (5%) | 0.01* |
| ACE/ARB | 163 (8%) | 32 (14%) | 131 (7%) | <0.001* |
| HTN medication | 345 (17%) | 64 (29%) | 281 (15%) | <0.001†=* |
| Baseline measurements | ||||
| Total cholesterol (mg/dL) | 210.9 ± 40.1 | 212.7 ± 43.6 | 210.7 ± 39.7 | 0.71 |
| LDL-cholesterol (mg/dL) | 131.3 ± 37.2 | 135.5 ± 45.8 | 130.7 ± 36.0 | 0.32 |
| CAC score (AU) * | 0 (0.0–56.4) | 116.3 (6.3–527.0) | 0 (0–35.5) | <0.001* |
| EAT volume (cm3) | 78.5 (55.9–106.0) | 90.6 (67.4–128.7) | 77.0 (54.7–103.3) | <0.001* |
| EAT attenuation (HU) | −73.8 ± 4.8 | −75.4 ± 5.13 | −73.6 ± 4.7 | <0.001* |
Abbreviations: ACE: angiotensin-converting enzyme, ARB: angiotensin II receptor blocker, ASCVD: atherosclerotic cardiovascular disease, BMI: body mass index, CAC: coronary artery calcium, CAD: coronary artery diseaseEAT: epicardial adipose tissue, HTN: hypertension, LDL: low-density lipoprotein, MACE: major adverse cardiovascular events
P-values <0.05 are statistically significant.
Cardiac CT Measurements
CAC was present in 984 (48%) subjects at baseline. In subjects with CAC, the median CAC score was 63.8 [Interquartile range (IQR) 18.2 to 208.9] AU. The baseline CAC score was significantly higher in subjects who developed MACE compared to those who did not (116.3 vs. 0 AU, p<0.001). CAC categories were higher in subjects who suffered MACE (p<0.001). In long-term follow-up, 45 subjects with no coronary calcium experienced MACE compared to 60 subjects with CAC 1–99 AU, 54 subjects with CAC 100–400 AU, and 64 subjects with CAC>400 AU.
EAT volume and attenuation are derived from the same voxels in the CT data and were strongly inversely correlated (Spearman’s r = −0.76, p<0.0001). EAT volume also correlated moderately with BMI (Spearman’s r = 0.64, p<0.001). The baseline median EAT volume was 78.5 (IQR 55.9 to 106.0) cm3. EAT volume was significantly higher in subjects with MACE (90.6 [IQR 67.4 to 128.7] cm3) than subjects without (77.0 [IQR 54.7 to 103.3] cm3, p<0.001). In our population, median EAT volume progressively increased with CAC score: 78.5 cm3 for CAC 0 AU, 90.6 cm3 for CAC 1–99 AU, 95.7 cm3 for CAC 100–400 AU, and 107.0 cm3 for CAC>400 AU; p<0.001.
Mean EAT attenuation was −73.8 ± 4.8 HU and was significantly lower in subjects with MACE than those without (−75.4 vs. −73.6 HU, p<0.001). Mean EAT attenuation was lower with increased CAC: −73.4 HU for CAC 0 AU, −74.1 HU for CAC 1–99 AU, −74.9 HU for CAC 100–400 AU, and −75.3 HU for CAC>400 AU; with significant differences in means between CAC 0 AU, CAC 100–400 AU and CAC>400 AU (p<0.02).
CAC and EAT as MACE Predictors
Both EAT volume (cm3) and CAC score (AU) were independent predictors of future MACE. In multivariate analysis adjusted for ASCVD risk score, CAC score, and EAT volume were associated with increased risk of MACE (Table 2, Model 1, p<0.03 for all); the corresponding Harrell’s C-statistic was 0.76. After stratifying EAT volume and CAC score by quartiles, the highest EAT volume quartile was a significant predictor of MACE (HR: 2.03 [95% CI: 1.32–3.12], p=0.001). The associations for EAT volume, EAT attenuation and CAC score persisted when 1-SD increase in CAC and EAT measures were considered. EAT attenuation (HU) was also predictive of MACE. In multivariable analysis, EAT attenuation was inversely associated with MACE (Table 2, Model 2), independent of risk factors. The Harrell’s C-statistic was 0.76. From Youden’s index analysis, the prognostic threshold for EAT volume was ≥ 113 cm3 and for EAT attenuation was ≤ −77.0 HU.
Table 2:
Multivariable Cox analysis of EAT volume and attenuation for MACE.
| Model 1 - with EAT volume | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| ASCVD risk score | 1.03 (1.01–1.04) | <0.001† |
| CAC score (AU) * | 1.25 (1.19–1.30) | <0.001† |
| EAT volume (cm3) * | 1.35 (1.07–1.68) | 0.009‡ |
| Model 2 - with EAT attenuation | Hazard Ratio (95% CI) | P-value |
| ASCVD risk score | 1.02 (1.01–1.04) | <0.001† |
| CAC score (AU) | 1.25 (1.20–1.30) | <0.001† |
| EAT attenuation (HU) * | 0.83 (0.72–0.96) | 0.01† |
| Model 3 - with EAT volume | Hazard Ratio (95% CI) | P-value |
| Waist circumference (cm) | 1.03 (0.97–1.08) | 0.34 |
| BMI (kg/m2) | 0.97 (0.92–1.02) | 0.24 |
| EAT volume (cm3) | 1.77 (1.29–2.42) | <0.001† |
| Model 4 - with EAT attenuation | Hazard Ratio (95% CI) | P-value |
| Waist circumference (cm) | 1.04 (0.99–1.1) | 0.11 |
| BMI (kg/m2) | 0.97 (0.92–1.02) | 0.32 |
| EAT attenuation (HU) | 0.76 (0.64–0.91) | 0.003† |
CAC score (AU) and EAT volume (cm3) two-fold increase/doubling. EAT attenuation per-5 HU increase.
P-values <0.05 are statistically significant.
Abbreviations: ASCVD: atherosclerotic cardiovascular disease, BMI: body mass index, CAC: coronary artery calcium, CI: confidence interval, EAT: epicardial adipose tissue
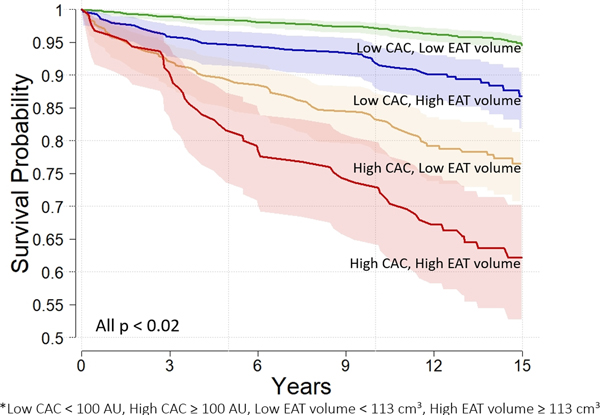
The Kaplan Meier survival curves for subjects stratified by high EAT volume (≥113 cm3) and CAC≥100 AU are shown in Figure 3 (p<0.02 for all). Risk of future MACE increased with higher EAT volume and CAC and was highest in subjects with both high EAT and high CAC (Figure 3). Subjects at risk for MACE over the 15 years are shown in Supplement Table 5.
Figure 3:
The relationship between EAT volume (cm3) and CAC (AU) and MACE are shown. Subjects were stratified by CAC and EAT as follows: Low CAC < 100 AU, High CAC ≥ 100 AU, Low EAT < 113 cm3, High EAT ≥ 113 cm3. Risk of future MACE increased with increase in EAT volume and CAC and was highest in subjects with both high EAT and high CAC.
EAT as a Predictor of MACE in Low-Risk Subjects
In subjects without CAC on baseline CT (n=1084), 45 developed MACE over long-term follow-up. The median EAT volume was significantly higher in the MACE group with no CAC than those without MACE (87.2 vs 70.6 cm3, p<0.01). Mean EAT attenuation at baseline was significantly lower in subjects without CAC who developed MACE than those who did not (−75.02 vs −73.3 HU, p=0.014). After adjusting for ASCVD risk score and gender, EAT volume in these low-risk subjects was significantly associated with risk of MACE (HR: 1.81 [95% CI: 1.13–2.92], p=0.01); however, EAT attenuation did not reach significance (HR: 0.76 [95% CI: 0.55–1.03], p = 0.08).
EAT as a Predictor of hard events (MI and Cardiac Death)
78 subjects (4%) experienced MI or cardiac death. In multivariable analysis, EAT volume was significantly associated with future MI and cardiac death [HR 1.49 (95% CI: 1.00–2.21)] as shown in Table 3 (Model 1). When adjusted for obesity parameters (BMI [kg/m2] and waist circumference [cm]), EAT volume remained an independent predictor of MI and cardiac death (Table 3, Model 3). EAT attenuation was also significantly associated with MI and cardiac death after adjustment of obesity measures (Table 3, Model 4).
Table 3:
Multivariable Cox analysis of EAT volume and attenuation for hard events (Myocardial infarction and cardiac death).
| Model 1 - with EAT volume | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| ASCVD risk score | 1.04 (1.02–1.07) | <0.001† |
| CAC score (AU) * | 1.21 (1.12–1.30) | <0.001† |
| EAT volume (cm3) * | 1.49 (1.00–2.21) | 0.046† |
| Model 2 - with EAT attenuation | Hazard Ratio (95% CI) | P-value |
| ASCVD risk score | 1.05 (1.03–1.07) | <0.001† |
| CAC score (AU) | 1.22 (1.13–1.30) | <0.001† |
| EAT attenuation (HU) * | 0.79 (0.63–1.01) | 0.057 |
| Model 3 - with EAT volume | Hazard Ratio (95% CI) | P-value |
| Waist circumference (cm) | 0.96 (0.86–1.04) | 0.314 |
| BMI (kg/m2) | 1.01 (0.93–1.10) | 0.851 |
| EAT volume (cm3) | 2.97 (1.70–5.21) | <0.001† |
| Model 4 - with EAT attenuation | Hazard Ratio (95% CI) | P-value |
| Waist circumference (cm) | 0.99 (0.91–1.08) | 0.792† |
| BMI (kg/m2) | 1.02 (0.94–1.10) | 0.675† |
| EAT attenuation (HU) | 0.65 (0.49–0.87) | 0.004† |
CAC score (AU) and EAT volume (cm3) two-fold increase/doubling. EAT attenuation per-5 HU increase.
P-values <0.05 are statistically significant.
Abbreviations: ASCVD: atherosclerotic cardiovascular disease, BMI: body mass index, CAC: coronary artery calcium, CI: confidence interval, EAT: epicardial adipose tissue
EAT and Obesity
Standard measures of obesity (BMI [kg/m2] and waist circumference [cm]) were not independently predictive of MACE by Cox regression. After adjusting for obesity measures, EAT volume (cm3) remained a significant independent risk factor for future MACE (Table 2, Model 3. Additionally, a lower EAT attenuation (HU) at baseline was associated with a higher risk of MACE (Table 2, Model 4).
EAT and Gender
MACE occurred in 155 (70%) males and 68 (30%) females. After adjusting for CAC, increased EAT volumes (cm3) were associated with higher risk of MACE separately, in both males (HR: 1.18 [95% CI: 1.10–1.26], p<0.001) and females (HR: 1.17 [95% CI: 1.03–1.32], p=0.02). There were no significant differences in EAT attenuation and MACE between males and females.
EAT and Levels of serum biomarkers
In bivariate correlation analysis, EAT volume was weakly but significantly correlated with serum levels of CRP, PAI-1, adiponectin, myoglobin, D-dimer, and MPO (Table 4, p<0.01 for all). EAT attenuation was inversely correlated with the same biomarkers as EAT volume, plus IL-6, ESAM, and MMP-9 (p<0.05 for all), as summarized in Table 4.
Table 4:
Relationship of EAT volume and attenuation with levels of serum biomarkers.
| Serum Biomarkers | Correlation (EAT volume*) | P-value | Correlation (EAT attenuation) | P-value |
|---|---|---|---|---|
| CRP | 0.18 | <0.001* | −0.19 | <0.001* |
| IL-6 | −0.05 | 0.08 | 0.06 | 0.02* |
| PAI-1 | 0.38 | <0.001* | −0.33 | <0.001* |
| Adiponectin | −0.19 | <0.001* | 0.23 | <0.001* |
| Myoglobin | 0.21 | <0.001* | −0.08 | 0.004* |
| D-dimer | 0.08 | 0.003* | −0.08 | 0.006* |
| MPO | 0.10 | <0.001* | −0.09 | 0.001* |
| ESAM | 0.05 | 0.05 | −0.07 | 0.02* |
| MMP-9 | 0.05 | 0.08 | −0.06 | 0.04* |
Abbreivations: CRP: C-reactive protein, EAT: epicardial adipose tissue, ESAM: endothelial cell-selective adhesion molecule, IL6: interleukin 6, MMP-9: matrix metallopeptidase 9, MPO: myeloperoxidase, PAI-1: endothelial plasminogen activator inhibitor 1
P-values <0.05 are statistically significant.
Discussion
In this study, EAT volume and attenuation quantified by automated DL algorithms using standard non-contrast cardiac CT images provided additional prognostic value for prediction of future adverse cardiac events, in addition to ASCVD risk and CAC scores. EAT volume≥113 cm3 was associated with a greater risk of adverse outcomes, with the highest MACE risk in subjects with EAT volume≥113 cm3 and CAC≥100 AU. The EAT volume and attenuation quantifications were automatically measured using DL software applied to standard non-contrast coronary calcium scoring images, without the need for additional image acquisition. By automating EAT quantification methods, this valuable clinical information can be acquired without additional training or measurements for the physician or technologist. This measurement saves physician time significantly as DL-based quantification requires <30 seconds for complete EAT and thoracic adipose tissue analysis compared to 10–15 minutes per case using semi-automated software.
Importantly, in this study we evaluated high-risk thresholds for EAT volume (≥113 cm3) and EAT attenuation (≤ −77.0 HU) for prediction of MACE. This is in line with abnormal EAT volume thresholds reported previously (100 cm3 from the Framingham study20 and 125 cm3 from a previous report of the EISNER study19 with EAT measurement over a longer heart extent up to the diaphragm). In our study, the algorithm was trained on expert tracings from the pulmonary artery bifurcation to the posterior descending artery, which spans the heart and the major coronary arteries. Our findings show that these easily identifiable superior and inferior limits are prognostically relevant and could become the standard in clinical practice.
Quantification of EAT from non-contrast cardiac CT has been shown to provide independent clinical data beyond CAC scoring; yet, it is not routinely reported in most laboratories7,10,12. The Heinz Nixdorf Recall Study assessed the role of EAT quantification for predicting fatal and nonfatal coronary events in 4093 patients over an 8-year follow-up12. In this study, increased EAT volume was associated with an increased risk of adverse cardiac events, independent of CAC score and underlying CV risk (HR: 1.35 [95% CI: 1.07–1.68]). Rajani et al. also reported that increased EAT volume was associated with high-risk plaque characteristics, including non-calcified plaque and severe stenosis7. In that study, which included 402 patients who underwent coronary CTA, EAT volume was an independent predictor of high-risk plaque characteristics, regardless of cardiovascular risk factors (OR: 1.7 [95% CI: 0.9–3.4], p=0.04). Our population showed that increased EAT volume and lower EAT attenuation was also associated with increased MACE, independent of CAC and ASCVD risk. However, our study is unique in that it is the first report of EAT quantification performed using a fully automated DL approach, omitting the need for additional measurements by the physician. DL algorithms allow for direct segmentation and analysis of images for clinical reporting and potentially, for outcome prediction. In cardiac CT, DL has been shown to be effective for automated coronary calcium scoring for both low-dose CT and coronary CT angiography21,22 as well as direct measurement of luminal stenosis from coronary CT angiography23. Our study extends these prior studies by reporting the prognostic value of an imaging biomarker (EAT) measured using a fully automated DL algorithm in a prospective trial. Such fully automated reporting of EAT metrics from standard non-contrast cardiac images has the potential to become the new standard of EAT analysis in daily clinical practice as this technique can be used without additional work to the physician or technologist.
Quantification of CAC on non-contrast cardiac CT improves patient risk classification compared to stratification by CV risk factors alone16. In patients with elevated ASCVD risk scores, the absence of coronary artery calcium can re-classify these patients as low-risk for future adverse cardiac events and warrant cessation of statins or aspirin therapy16,24. Conversely, increased CAC scores in otherwise low-risk individuals not only warrants medication and lifestyle modifications, but also improves adherence to risk-reducing therapy5. In a previous publication using the EISNER trial, it was shown that subjects who underwent CAC scanning had greater reductions in systolic BP, LDL cholesterol, and reduced waist circumference compared to those without CAC scans5. Additionally, there was a direct proportional relationship between the magnitude of baseline CAC score and the degree of reduction in blood pressure, cholesterol and waist size. In our study population, there was collinearity between EAT and CAC and therefore there was non-significant increase in Harrell’s C-statistic when EAT was added to a model with both ASCVD and CAC. However, both CAC and EAT were independently predictive of MACE. Notably in our study, EAT predicted MACE in patients with no coronary calcium and also correlated with inflammatory circulating biomarkers, suggesting a different mechanism than CAC for long-term MACE. These findings suggest independent predictive value of this metric in identifying subjects at increased risk for future events and in whom risk reduction therapy such as lifestyle modification, statin therapy, and anti-inflammatory therapy should be initiated.
At present, non-contrast cardiac CT is used to measure CAC, a measure of underlying CAD and total plaque burden. We now know that inflammation plays a key role in atherogenesis, and non-invasive detection of epicardial adipose inflammation could identify patients at risk for developing CAD and predict future adverse cardiovascular events10,25,26. The independent association of EAT with MACE is consistent with previous reports that greater EAT volume and lower attenuation reflect metabolically active adipose tissue and systemic inflammation10. Prior studies have suggested that epicardial and pericoronary adipose tissue may precipitate coronary atherosclerosis through its direct contact with the adventitia of the underlying coronary arteries10,26,27. Current data suggests that pericoronary adipose tissue attenuation is of value in contrast-enhanced coronary CT Angiography, but less in non-contrast CT where the non-calcified plaque, vessel wall and adipose tissue vascularity cannot be assessed. On the other hand, EAT attenuation has been shown to maintain its prognostic value from non-contrast cardiac CT. Goeller et al. recently reported that a lower EAT attenuation and greater EAT volumes measured from non-contrast CT were associated with circulating inflammatory biomarkers and may be associated with early plaque formation10,26. In our population, subjects without CAC who experienced MACE had greater EAT volumes than subjects who did not. Further, increased EAT volume and decreased EAT attenuation were independent predictors of MACE, with the same C-statistic; suggesting that mean EAT attenuation may provide a surrogate global biomarker for underlying vascular inflammation. Importantly, our results also show that automatically measured EAT volume was correlated with inflammatory biomarkers CRP, MPO and reduced expression of adiponectin, and also to D-dimer and myoglobin (related to cardiovascular events). EAT attenuation was inversely related to these biomarkers, consistent with the negative hazard ratio for MACE.
We acknowledge several limitations in our study. Long-term follow-up could be obtained in 2068 (78%) of subjects. This was a single-center community-based study of asymptomatic subjects with no prior history of CAD or significant co-morbidity, with CT scans performed during 1998–2005. Our results are therefore representative of the asymptomatic population undergoing coronary calcium scoring for cardiovascular risk assessment and may need re-evaluation in symptomatic subjects undergoing coronary calcium scoring or coronary CT angiography. As this is a long-term follow-up trial, non-contrast CT images were acquired on older CT scanners than 64+-detector CT scanners18. However, the DL software was able to successfully process all imaging data in this population and has been shown to maintain its accuracy when applied to CT images acquired with 64+-detector CT18. While in line with other studies, the EAT thresholds in this study require external validation in independent studies for generalizability.
Conclusions
EAT volume and attenuation can be measured automatically from non-contrast cardiac CT using deep learning algorithms. Both EAT volume and attenuation measurements predict MACE in asymptomatic subjects, independent of traditional risk factors and CAC score. These findings suggest that automatic reporting of EAT metrics can provide additional prognostic information from standard non-contrast cardiac images; importantly, these imaging metrics can be obtained without physician interaction.
Supplementary Material
CLINICAL PERSPECTIVE.
Epicardial adipose tissue (EAT) has been previously shown to provide additional prognostic value when measured from non-contrast cardiac computed tomography (CT) compared to standard metrics such as the coronary artery calcium (CAC) and atherosclerotic cardiovascular disease (ASCVD) risk score. In this study, we used fully automated deep learning (DL) software to quantify EAT volume and attenuation from non-contrast cardiac CT. These deep learning algorithms were able to automatically generate EAT metrics in under 30 seconds, without any additional imaging post-processing techniques or work for the physician. When the automated EAT metrics were applied to 2068 asymptomatic subjects from the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) trial with long-term follow-up after CAC measurement, increased EAT volume (mm3) and decreased EAT attenuation (HU) were independently associated with major adverse cardiovascular events (MACE) when adjusted for ASCVD risk, CAC score, gender, and markers of obesity. This study shows the prognostic value of fully automated EAT volume and attenuation quantification by deep learning from non-contrast cardiac CT. Such information can provide prognostic value for the asymptomatic patient, without additional imaging or physician interaction.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr. John D. Friedman, Dr. Louise E.J. Thomson, Dr. Sean Hayes, and Ms. Romalisa Miranda-Peats for their contributions to the EISNER trial and Dr. Andrew Lin for his assistance with the manuscript.
Sources of Funding
This work was funded by National Institute of Health/National Heart, Lung, and Blood Institute grant 1R01HL133616 (to Dr. Dey) and in part by Bundesministerium für Bildung und Forschung (01EX1012B, Spitzencluster Medical Valley), and a grant from the Miriam and Sheldon G. Adelson Medical Research Foundation.
Abbreviations
- ASCVD
Atherosclerotic cardiovascular disease
- BMI
Body mass index
- CAC
Coronary artery calcium
- CAD
Coronary artery disease
- CT
Computed tomography
- CV
Cardiovascular
- DL
Deep learning
- EAT
Epicardial adipose tissue
- MACE
Major adverse cardiovascular events
- MI
Myocardial infarction
Footnotes
Disclosures
Evann Eisenberg– No disclosures
Priscilla A. McElhinney– No disclosures
Frederic Commandeur– No disclosures
Xi Chen– No disclosures
Sebastien Cadet– No disclosures
Markus Goeller – No disclosures
Aryabod Razipour– No disclosures
Heidi Gransar– No disclosures
Stephanie Cantu– No disclosures
Robert J.H. Miller– No disclosures
Piotr J. Slomka– No disclosures
Nathan D. Wong– No disclosures
Alan Rozanski– No disclosures
Stephan Achenbach– No disclosures
Balaji K. Tamarappoo– No disclosures
Daniel S. Berman– No disclosures
Damini Dey–research grant from the NIH/NHLBI 1R01HL133616
References
- 1.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–165. [DOI] [PubMed] [Google Scholar]
- 2.Raggi P, Callister TQ, Cooil B, He ZX, Lippolis NJ, Russo DJ, Zelinger A, Mahmarian JJ. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron-beam computed tomography. Circulation. 2000;101:850–855. [DOI] [PubMed] [Google Scholar]
- 3.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807–814. [DOI] [PubMed] [Google Scholar]
- 4.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 5.Rozanski A, Gransar H, Shaw LJ, Kim J, Miranda-Peats L, Wong ND, Rana JS, Orakzai R, Hayes SW, Friedman JD, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing: the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw LJ, Min JK, Budoff M, Gransar H, Rozanski A, Hayes SW, Friedman JD, Miranda R, Wong ND, Berman DS. Induced Cardiovascular Procedural Costs and Resource Consumption Patterns After Coronary Artery Calcium Screening: Results From the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) Study. J Am Coll Cardiol. 2009;54:1258–1267. [DOI] [PubMed] [Google Scholar]
- 7.Rajani R, Shmilovich H, Nakazato R, Nakanishi R, Otaki Y, Cheng VY, Hayes SW, Thomson LE, Friedman JD, Slomka PJ, et al. Relationship of epicardial fat volume to coronary plaque, severe coronary stenosis, and high-risk coronary plaque features assessed by coronary CT angiography. J Cardiovasc Comput Tomogr. 2013;7:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang I-C, Park HE, Choi S-Y. Epicardial adipose tissue contributes to the development of non-calcified coronary plaque: a 5-year computed tomography follow-up study. J Atheroscler Thromb. 2016:36467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dey D, Wong ND, Tamarappoo B, Nakazato R, Gransar H, Cheng VY, Ramesh A, Kakadiaris I, Germano G, Slomka PJ, et al. Computer-aided non-contrast CT-based quantification of pericardial and thoracic fat and their associations with coronary calcium and Metabolic Syndrome. Atherosclerosis. 2010;209:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goeller M, Achenbach S, Marwan M, Doris MK, Cadet S, Commandeur F, Chen X, Slomka PJ, Gransar H, Cao JJ, et al. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr. 2018;12:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. [DOI] [PubMed] [Google Scholar]
- 12.Mahabadi AA, Berg MH, Lehmann N, Kalsh H, Bauer M, Kara K, Dragano N, Moebus S, Jockel KH, Erbel R, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388–1395. [DOI] [PubMed] [Google Scholar]
- 13.Dey D, Slomka PJ, Leeson P, Comaniciu D, Shrestha S, Sengupta PP, Marwich TH. Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:1317–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Commandeur F, Goeller M, Betancur J, Cadet S, Doris M, Chen X, Berman DS, Slomka PJ, Tamarappoo BK, Dey D. Deep Learning for Quantification of Epicardial and Thoracic Adipose Tissue From Non-Contrast CT. IEEE Trans Med Imaging. 2018;37:1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Betancur J, Commandeur F, Motlagh M, Sharir T, Einstein AJ, Bokhari S, Fish MB, Ruddy TD, Kaufmann P, Sinusas AJ, et al. Deep Learning for Prediction of Obstructive Disease From Fast Myocardial Perfusion SPECT: A Multicenter Study. JACC Cardiovasc Imaging. 2018;11:1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation. 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 18.Commandeur F, Goeller M, Razipour A, Cadet S, Hell MM, Kwiecinski J, Chen X, Chang H, Marwan M, Achenbach S, et al. Fully automated CT quantification of epicardial adipose tissue using deep learning: a multicenter study. Radiol Artif Intelle. 2019;1:e19004 doi. 10.1148/ryai.2019190045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda-Peats R, Ramesh A, Wong ND, Shaw LJ, Slomka PJ, et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS.. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. [DOI] [PubMed] [Google Scholar]
- 21.Lessmann N, van Ginneken B, Zreik M, de Jong PA, de Vos BD, Viergever MA, Isgum I. Automatic Calcium Scoring in Low-Dose Chest CT Using Deep Neural Networks With Dilated Convolutions. IEEE Trans Med Imaging. 2018;37:615–625. [DOI] [PubMed] [Google Scholar]
- 22.Wolterink JM, Leiner T, de Vos BD, van Hamersvelt RW, Viergever MA, Isgum I. Automatic coronary artery calcium scoring in cardiac CT angiography using paired convolutional neural networks. Med Image Anal. 2016;34:123–136. [DOI] [PubMed] [Google Scholar]
- 23.Hong Y, Commandeur F, Cadet S, Goeller M, Doris M, Chen X, Kwiecinski J, Berman D, Slomka P, Chang H, et al. Deep learning-based stenosis quantification from coronary CT angiography. SPIE. 2019;1094921–1094930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahabadi AA, Mohlenkamp S, Lehmann N, Kalsch H, Dykun I, Pundt N, Moebus S, Jockel KH, Erbel R. CAC Score Improves Coronary and CV Risk Assessment Above Statin Indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10:143–153. [DOI] [PubMed] [Google Scholar]
- 25.Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou ET, Herdman L, Margaritis M, Shirodaria C, Kampoli AM, Akoumianakis I, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9:eaal2658. [DOI] [PubMed] [Google Scholar]
- 26.Goeller M, Achenbach S, Cadet S, Kwan AC, Commandeur F, Slomka PJ, Gransar H, Albrecht MH, Tamarappoo BK, Berman DS, et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared With Stable Coronary Artery Disease. JAMA Cardiol. 2018;3:858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010;210:150–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.