Abstract
Cyantraniliprole and chlorantraniliprole are anthranilic diamide insecticides acting on ryanodine receptors. In this study, two camel-derived nanobodies (Nbs, named C1 and C2) recognizing cyantraniliprole as well as chlorantraniliprole were generated. C1-based enzyme-linked immunosorbent assays (ELISAs) for the detection of the two insecticides were developed. The half-maximum signal inhibition concentrations (IC50) of cyantraniliprole and chlorantraniliprole by ELISA were 1.2 and 1.5 ng mL−1, respectively. This assay was employed to detect these two insecticides in soil and vegetables. The average recoveries of cyantraniliprole from both bok choy (Brassica chinensis L.) and soil samples were 90–129%, while those of chlorantraniliprole were in a range of 89–120%. The insecticide residues in soil and bok choy, which were collected from plots sprayed with cyantraniliprole and chlorantraniliprole, were simultaneously detected by the resulting ELISA and a high performance liquid chromatography (HPLC) method, showing a satisfactory correlation. Higher concentrations of chlorantraniliprole than cyantraniliprole were detected in soil and vegetables, which indicates the longer persistence of chlorantraniliprole in the environment.
Keywords: Cyantraniliprole, Chlorantraniliprole, Nanobody, Immunoassay, Bok choy, Soil
Introduction
Cyantraniliprole is an anthranilic diamide insecticide sharing a similar mode of action with chlorantraniliprole, which modulates the ryanodine receptor (RyR). Upon exposure of cyantraniliprole to insects via ingestion and contact, cyantraniliprole binds to the RyR, which is critical for muscle contraction, causing uncontrolled release of calcium ions from muscle cells and affecting calcium homeostasis. It keeps insect muscles contracting for extended periods. Finally, insects become lethargic, paralyzed, and eventually die [1–3]. Cyantraniliprole and chlorantraniliprole are only different in the 4-substituent of the anthranilic core, i.e., cyano and chloro, respectively (Fig. 1). This difference appears to give cyantraniliprole broader target spectrum than chlorantraniliprole. Thus, cyantraniliprole is more commonly used to kill crop pests than chlorantraniliprole.
Fig. 1.

Structures of cyantraniliprole, chlorantraniliprole and the hapten H1.
Cyantraniliprole and chlorantraniliprole are toxic to aquatic invertebrates and highly toxic to bees exposed to direct treatment or residues on blooming crops. Nonetheless, both insecticides have low toxicity to human beings unless people are overexposed to them. In China, the maximum residue limits (MRLs) of cyantraniliprole and chlorantraniliprole in different agro-products are documented. The MRLs of cyantraniliprole in rice, tomato, and cucumber are 0.2 mg kg−1, while those of chlorantraniliprole in cereal and potato are 0.02 mg kg−1 [4]. The analytical methods for the detection of cyantraniliprole or chlorantraniliprole in different samples were generally based on high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS), with high accuracy and sensitivity [5–9]. The HPLC-MS methods are, however, expensive, time-consuming and complicated in sample pretreatment. Therefore, it is attractive to develop a rapid method to detect the insecticide residues in various matrices. Compared with instrumental methods aforementioned, immunoassays, enzyme-linked immunosorbent assays (ELISAs) in particular, are more simple, rapid, and cost-effective and can be used both in the field and for high-throughput detection.
Over the past decades, traditional antibodies such as polyclonal and monoclonal antibodies (pAbs and mAbs) have been extensively employed to develop immunoassays for small-molecules. Recently, nanobody (Nb)-based immunoassays proved to be a great impetus in the detection of environmental compounds (e.g., insecticides) in complicated matrices [10–13]. Because of the nano-scale size (approximately 15 kD), camelid single-domain antibody (VHH) is referred to as Nb, which possesses some advantages over traditional antibodies in terms of size, water solubility, thermal stability, cost of production and ease of genetic modification [14]. The extensive availability of molecular biotechnology allows for the gene engineering of Nbs to facilitate speed of detection, improve analytical sensitivity, and either increase the specificity or even broaden the application range of Nbs. Possibly the greatest advantage of Nbs is that they are essentially immortal analytical tools. For example, Nbs can be stored and recovered in many ways including as stable proteins, plasmids, bacterial stabs or they can even be archived as the primary sequence which can be easily resynthesized.
A mAb-based ELISA has been developed for the selective detection of cyantraniliprole in pakchoi (bok choy) [15]. In contrast to the mAb specific to cyantraniliprole, Nbs recognizing both cyantraniliprole and chlorantraniliprole were raised herein to develop an ELISA for the detection of both insecticides in soil and bok choy, which has a short growth cycle, high yield, and rich vitamins, minerals and cellulose, and plays an important role in Chinese vegetable consumption pattern. Cyantraniliprole and chlorantraniliprole having low toxicity to human beings are permitted to be sprayed on bok choy near the time of harvest. Owing to the short growth cycle, pesticide residues in bok choy could be higher than those in others. In China, MRLs of cyantraniliprole and chlorantraniliprole in Brassica vegetables are 2 mg kg−1 [4]. The Nb-based ELISA could provide a rapid alternative to instrumental methods for the rapid detection of these insecticides in bok choy and soil.
Materials and methods
Reagents
Formulation of cyantraniliprole (suspension concentrate (SC), 10%) and chlorantraniliprole (SC, 200 g L−1) were purchased from Dupont (China). N-hydroxysuccinimide (NHS), dicyclohexylexylcarbodiimide (DCC), complete and incomplete Freund’s adjuvant, keyhole limpet hemocyanin (KLH), bovine serum albumin (BSA), 3,3′,5,5′-tetramethylbenzidine (TMB), polyethylene glycol 8000 (PEG 8000), isopropyl-β-D-thiogalactopyranoside (IPTG), imidazole and rabbit anti-M13 fd phage mAb were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). The goat anti-rabbit-horseradish peroxidase (HRP) was bought from ZSGB-BIO Co. (Beijing, China). The phagemid vector pComb3X was a generous gift from Dr. Barbas (The Scripps Research Institute, La Jolla, CA). Electrocompetent cells of E.coli ER2738 were acquired from Lucigen Corporation (Middleton, WI). All restriction enzymes, T4 DNA ligase and M13KO7 helper phage were bought from New England Biolabs, Inc. (Ipswich, MA). DNA polymerase was purchased from Tsingke Biological Technology Lt. (Beijing, China). The goat anti-HA tag IgG-HRP conjugate was purchased from F. Hoffmann-La Roche Ltd. (Basel, Switzerland). LeukoLOCK™ Total RNA Isolation System, HisPur Ni-NTA resin and Nunc MaxiSorp flat-bottom 96 well microtiter plates were purchased from Thermo Fisher Scientific Inc. (Rockford, IL). PrimeScript™ II 1st Strand cDNA Synthesis Kit was from Takara Biological Inc. (Beijing, China).
Conjugation of hapten to carrier proteins and animal immunization
The hapten H1 (Fig. 1) of cyantraniliprole was synthesized according to the route illustrated in a previous study [15]. The conjugate of H1 and KLH was used as the immunogen (H1-KLH), while H1 coupling to BSA was used as the coating antigen (H1-BSA). A 3-year-old male Bactrian camel was immunized subcutaneously with H1-KLH four times biweekly. Freund’s complete adjuvant was used in the first injection and Freund’s incomplete adjuvant was used for the rest of injections.
Construction of a phage-displayed Nb library
The blood lymphocytes were collected 7 days after the last immunization. The total mRNA was extracted following the protocol of LeukoLOCK™ Total RNA Isolation System and then reversely transcribed into cDNA. Nb genes were amplified by polymerase chain reaction (PCR) using primers listed in Table 1. The Nb fragments were ligated into the plasmid pComb3Xss via restriction enzyme Sfi I and T4 DNA Ligase. Then the ligated phage was electroporated into competent cells of E.coli ER2738. M13KO7 helper phages were added for infection and the phages were precipitated with PEG-NaCl (20% PEG 8000, 2.5 mol L−1 NaCl), followed by resuspension with phosphate buffered saline (PBS, 0.01 mol L−1 phosphate, 0.137 mol L−1 NaCl, and 0.003 mol L−1 KCl; pH 7.4). The size of the Nb library was evaluated by calculating plaques formed on plates and the diversity was identified by sequencing 30 clones randomly selected. Finally, the phage-displayed Nb library was stored in −80 °C until use.
Table 1.
Primers used for library construction.
| Primers | Sequence | |
|---|---|---|
| First round | GSP-RT | 5’-CGCCATCAATRTACCAGTTGA-3’ |
| LP-leader | 5’-GTGGTCCTGGCTGCTCTW-3’ | |
| Second round | F | 5’-CATGCCATGACTGTGGCCCAGGCGGCCCAGKTGCAGCTCGTGGAGTC-3’ |
| R3 | 5’-CATGCCATGACTCGCGGCCGGCCTGGCCTGGTTGTGGTTGTGGTTGTGG-3’ | |
| R4 | 5’-CATGCCATGACTCGCGGCCGGCCTGGCCGCTGGGGTCTTCGCTGTGGTGCG-3’ | |
| R5 | 5’-CATGCCATGACTCGCGGCCGGCCTGGCCCTTGCATACTTCATTCGTTCCTG-3’ | |
Selection of anti-cyantraniliprole Nbs
The detailed procedure for biopanning positive clones was available in our previous study [11]. Briefly, one well of a microtiter plate was coated with 100 μL of H1-BSA (50 μg mL−1) in carbonate buffer (CB, 0.05 mol L−1 Na2CO3, and 0.05 mol L−1 NaHCO3; pH 9.6) at 4 °C overnight. The next day, the coated well was blocked with 200 μL of gelatin (0.01 g mL−1) in CB for 1 h at ambient temperature. Two additional wells were blocked with BSA (0.03 g mL−1) in order to eliminate the clones specific to the carrier protein. A 100-μL aliquot of phage-displayed Nb library was added into the well coated with H1-BSA and incubated for 2 h with gentle shaking at ambient temperature. After washing 10 times with PBST (0.05% Tween-20 in PBS), this well was eluted with 100 μL of cyantraniliprole in PBS (1000 ng mL−1) for 1 h at ambient temperature. The eluent was evenly transferred to the two BSA-blocked wells and then collected (approximately 95 μL) after 1 h incubation. A part of the eluent (10 μL) was used to determine the phage titer and the remainder was amplified with M13KO7 helper phage for the next round of panning. The panning procedure was repeated four times, and the amount of cyantraniliprole and coating antigen was decreased gradually in each round of panning. The concentrations of cyantraniliprole were 500, 250 and 125 ng mL−1 for the 2nd, 3rd and 4th panning, respectively, while the concentrations of H1-BSA were 25, 12.5 and 6.25 μg mL−1, respectively. After each round of panning, 24 clones (96 clones in total) were selected randomly and tested for their binding capacity to cyantraniliprole by a competitive phage ELISA. Positive Nbs demonstrating high binding capacity to cyantraniliprole were expressed and purified.
Nb-based competitive ELISAs for insecticides
A microtiter plate was coated with H1-BSA (100 μL/well) at 4 °C overnight and washed with PBST three times. The plate was blocked with BSA (0.01 g mL−1) at ambient temperature for 1 h. A serial dilution of cyantraniliprole or chlorantraniliprole (50 μL/well) was added to the plate and Nbs in PBS (50 μL/well) were subsequently added. The plate was incubated at ambient temperature for 1 h and then washed with PBST. Goat anti-HA tag IgG-HRP (100 μL/well) was added into wells and incubated at ambient temperature for 1 h. After washing, a solution of TMB (100 μL/well) was added into the plate and the reaction was stopped by adding 50 μL of 2 M H2SO4. The absorbance was read at 450 nm on a microtiter plate reader. The half-maximum signal inhibition concentrations (IC50), limit of detection (LOD, IC10) and linear range (IC20–IC80) were obtained from a four-parameter logistic equation from SigmaPlot 10.0.
Optimization of Nb-based ELISAs
The effects of variables including organic solvent, pH and ionic strength on Nb-based ELISAs for cyantraniliprole were evaluated. Methanol was added into PBS to form the final percentage of 0–20% (v/v). Values of pH in PBS were adjusted in a range of 4.5–10.5. In order to evaluate the effect of ionic strength, the percentage of NaCl in PBS was shifted from 0.4% to 12.8%.
Cross-reactivity
The specificity of Nbs was evaluated by determining their cross-reactivity (CR) with a group of compounds including cyantraniliprole, chlorantraniliprole and structural analogues. The CR was calculated as follows: CR (%)=[IC50 (cyantraniliprole)/IC50 (tested compound)]×100.
Field experiment of insecticide application
The field experiment to evaluate the persistence of these insecticides in the environment was carried out in Jintang Town located in Zhejiang Province, China. Cyantraniliprole (SC, 10%) was sprayed onto bok choy growing in plots at three levels: 30 (recommended by the manufacturer), 60 and 120 g a.i. ha−1. Each level of application in triplicate plots was sprayed twice with 7-day interval between the spraying and the control group was sprayed with water. Likewise, chlorantraniliprole (SC, 200 g L−1) was applied to other plots at the same levels as those of cyantraniliprole. Bok choy and soil (depths 0–10 cm) samples were evenly collected from each plot on the 7th day after the 2nd spraying. All vegetable and soil samples were packed in plastic bags and sealed. They were stored at −20 °C until analysis.
Sample preparation and analysis
The sample preparation was available from a previou study [8] with slight modification. Briefly, bok choy samples were chopped and 10 g samples were weighed into a 50-mL centrifuge tube and homogenized. A volume of methanol (10 mL) was added, followed by the addition of 6 g anhydrous magnesium sulfate and 2 g sodium chloride. The mixture was vortexed for 2 min and then ultrasonicated for 20 min. After centrifugation at 6000 rpm for 15 min, the solvent layer was collected and filtered through a NYLON 66 0.22-μm organic phase microporous filter membrane. The solvent was evaporated under a gentle stream of nitrogen and the residue was redissolved in PBS prior to ELISAs. Air-dried soil samples were ground and sieved through a 20-mesh screen. Soil samples (10 g) were weighted and added into a centrifuge tube. The insecticides in soil were extracted in the same way as that used for bok choy and residues were also dissolved in PBS for ELISAs.
Sample treatment for an HPLC method was similar to that for ELISA except that the extract was further cleaned up. The supernatant was transferred to an EP tube containing 50 mg PSA and 150 mg anhydrous magnesium sulfate and was vortexed for 1 minute. Then the tube was centrifuged at 5000 rpm for 10 minutes. The supernatant was filtered with a 0.22 μm organic filter membrane for HPLC.
The HPLC analysis was performed on an Agilent 1260 system equipped with an Eclipse XDB-C18 column (5 μm, 4.6 mm × 150 mm), quaternary pump, autosampler (ALS) and diode array detector. The performance conditions were described by Farag [16] with slight modification. Briefly, the flow rate of the mobile phase (methanol/water=55/45, v/v) was 0.8 mL/min and the injection volume was 10 μL. The column temperature was 35 °C and the detection wavelength was 220 nm.
Results and discussion
Animal immunization
It is well known that heavy-chain only antibodies (HCAbs) are much lower in concentration than conventional antibodies in the sera of camels. This shortcoming could lead to weak immune responses of HCAbs to antigens and haptens in particular. Thus, generating Nbs for small molecules through direct in vivo production is difficult even though multiple such approaches have succeeded. The hapten of cyantraniliprole has been efficiently used to raise mAbs specific to this insecticide and thereby was employed herein to generate Nbs with potential novel properties. The camel antisera exhibited increasing titers with the boosting immunization, having a titer of 1×107 after the 4th injection. The antisera also showed a high binding affinity to cyantraniliprole, with an inhibition ≥ 75% in the presence of 100 ng mL−1 cyantraniliprole. The features of antisera provided a bright prospect for the construction of a high quality Nb library.
Construction of Nb library
In the construction of the library, complete amplification of Nb genes from lymphocytes is a critical step to increase the diversity of the library. Three reverse primers frequently employed in previous studies were assumed to only amplify Nb fragments from three allotypes (IgG2b, IgG3b and IgG3a) [12]. In this study, two novel reverse primers R3 and R4 along with a previously employed reverse primer R5 [12] (Table 1), potentially able to amplify Nb gene fragments of IgG2, IgG3b and IgG3a, were employed to improve the diversity of Nb library and thereby enhance the success rate of Nb generation to a certain extent.
The size of the Nb library was estimated to be 1.8×107 colony forming units (cfu). The insertion ratio of Nb gene was 100%, according to the colony PCR of 30 individual clones randomly selected. The sequences of 30 individual clones selected in this study are unique (data not shown), indicating the high diversity of the constructed library.
Selection of anti-cyantraniliprole Nbs
In an attempt to obtain Nbs with high binding affinity to cyantraniliprole, the concentration of coating antigen H1-BSA and cyantraniliprole were reduced in the process of panning. The recovery of phages increased gradually through the first to the fourth round of panning (Table S1), indicative of the enrichment of specific clones. Totally, 16 clones showed a strong binding capacity (OD>1.0) to the coating antigen and a strong inhibition (>70%) in the presence of 1000 ng mL−1 cyantraniliprole, so they were identified as positive clones. All of the positive clones were sequenced and only two clones, named C1 and C2, had unique sequences in hinge region (Fig. S1). According to the sequences of hinge region, Nbs C1 and C2 belong to subtype IgG3a and IgG3b, respectively.
Effects of physicochemical properties on Nb-based ELISAs
In this study, the effects of pH, methanol, and NaCl on Nb-based ELISAs for cyantraniliprole were evaluated. For Nb C1-based ELISA, when the buffer pH values were < 6.5, the A0 values and sensitivities declined sharply (Fig. 2a). There was no significant effect on the IC50 with pH values in the range of 7.5–9.5. As the concentration of methanol ranged from 0% to 20%, the IC50 values increased from 1.2 to 50.9 ng mL−1 (Fig. 2b), suggesting the Nb is sensitive to this solvent. Fortunately, because both cyantraniliprole and chlorantraniliprole are relatively water-soluble compounds, high levels of water miscible solvents are not necessary in the assay buffer to retain the insecticides in solution. The moderate resistance to methanol may make this Nb useful in obtaining reservable binding or using post solid phase clean up. Nb C1 demonstrated high tolerability to ionic strength, because reasonable curves and Ao/IC50 ratios were obtained even under the high level of NaCl (1.6–6.4%) (Fig. 2c).
Fig. 2.
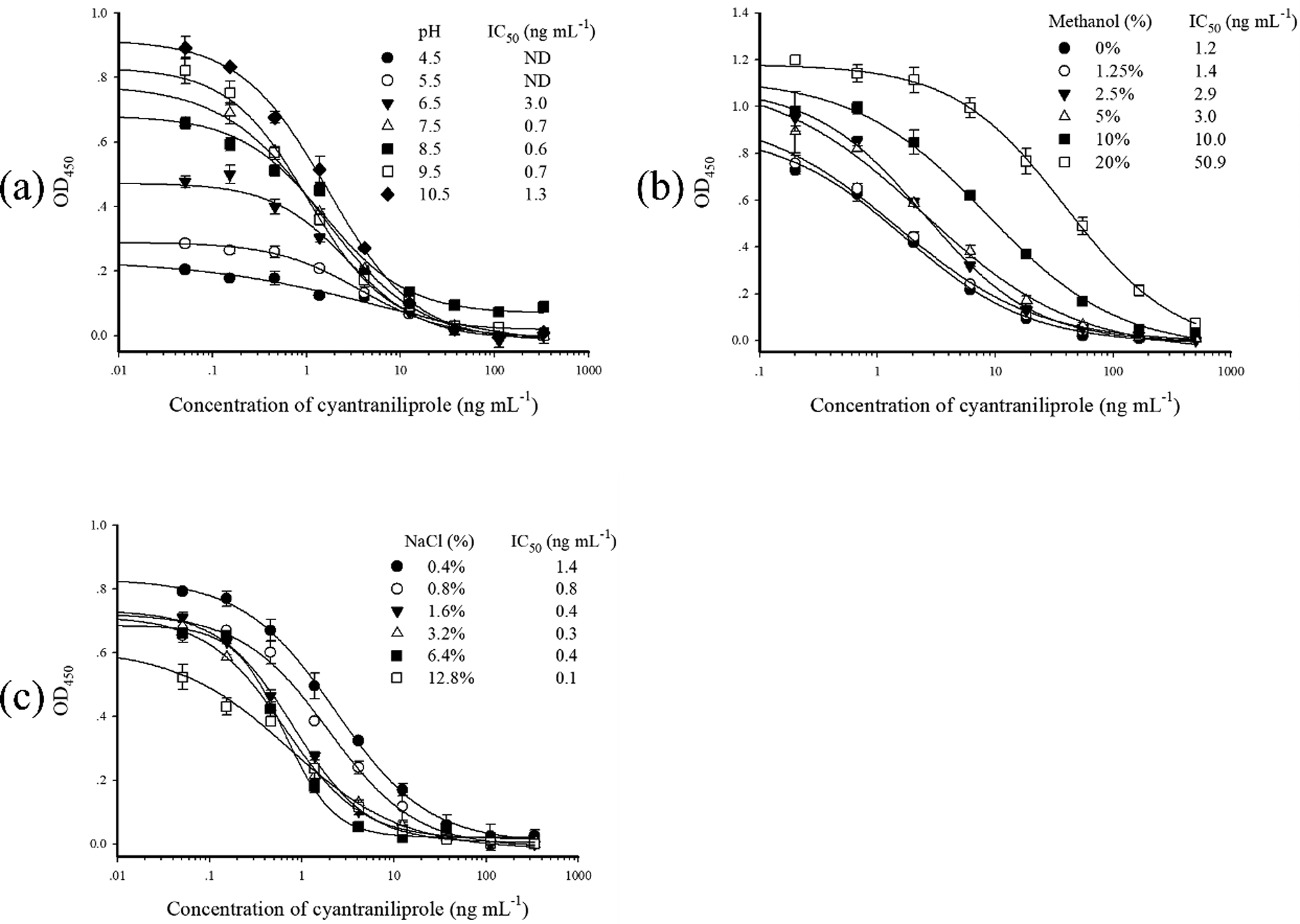
Effect of pH (a), methanol (b) and NaCl (c) on Nb C1-based ELISA for cyantraniliprole. The data are average of three replicates. ND: not detectable.
The Nb C2-based ELISA exhibited similar changes to those of C1-based ELISAs in response to the variables of pH and methanol in assay buffer (Fig. S2a and S2b). However, C2 showed less tolerance to high ionic strength than C1 (Fig. S2c). The binding affinity of C2 to cyantraniliprole was dramatically reduced when the concentration of NaCl was > 0.8%. Probably, high concentration of salt ions binds to the charged groups of epitopes or paratopes, impeding the combination of antigen and antibody, and consequently leading to a decrease in sensitivity [17]. The isoelectric point (pI) of two Nbs were computed by using the web program (https://web.expasy.org/compute_pi), and the pI of C1 and C2 was 8.81 and 7.96, respectively. Although the calculated pI might be different with the experimental pI, it also provided a certain reference value. Thus, there might be different amounts of charges between two Nbs in the assay buffer, which may lead to the different salt tolerance.
Nb-based ELISAs for cyantraniliprole
The typical calibration curves of Nb-based (C1 and C2) competitive ELISAs for cyantraniliprole were both generated in PBS (pH 7.5) containing 0.8% NaCl and none solvents (Fig. 3). The C1-based ELISA had a linear range of 0.2–5.7 ng mL−1 (IC20-IC80), an IC50 of 1.2 ng mL−1 and a limit of detection (LOD) of 0.1 ng mL−1 (IC10). The sensitivity of C2-based ELISA is approximately 2-fold lower than that of C1-based ELISA, with an IC50 of 2.3 vs 1.2 ng mL−1.
Fig. 3.
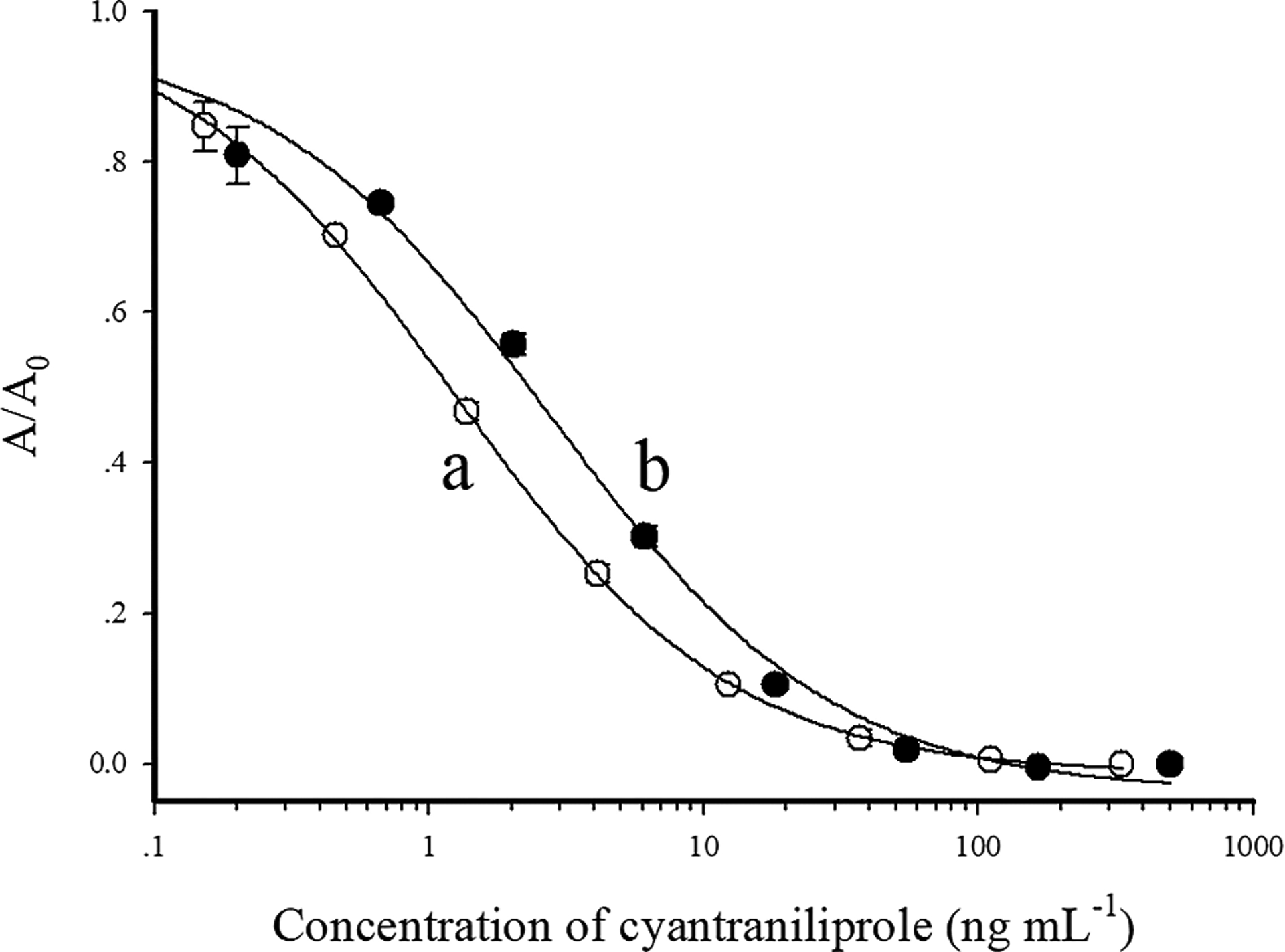
Calibration curves of Nb C1-based ELISA (a) and Nb C2-based ELISA (b) for cyantraniliprole. The data are average of three replicates.
The amino acid sequences of two Nbs obtained in this study were distinguished to each other in their second position (L vs V) and hinge regions (Fig. S1). Nonetheless, the difference resulted in distinct binding affinities of the Nbs to cyantraniliprole and different amounts of the Nbs expressed in Top10F′ cells of E. coli, 2.85 mg C1 vs 18 mg C2 in 500 mL of Top10F′ culture media. Since the properties of leucine and valine are similar, the different features of two Nbs are mainly originated from the discrepancy of hinge regions. One possible reason is that the C1 hinge region contains more rare codons than C2. High frequency of rare codons in the expression host could lead to translational errors that reduce the quantity or quality of the protein expression [18–21]. Owing to the small size of Nbs, the hinge region at the end of Nbs may play an important role in the stability and activity of Nbs. The polarity and pondus hydrogenii of different amino acids in hinge regions could lead to discrepancy in intermolecular forces and chemical bonds. In addition, the cysteine in the hinge region of C1 may disturb the formation of an original disulfide bond in the Nb, resulting in the variation of conformation and consequently, stability and avidity of this Nb. Further studies are needed to verify how the hinge region affects the structure and function of Nbs.
Cross-reactivity
It is not surprising that both C1 and C2 have negligible cross reactivity with most of the tested compounds (<0.1%) except for cyantraniliprole and chlorantraniliprole (Table S2), because the structures of these two insecticides are only different in the 4-substituent of the anthranilic core (cyan vs chloro) but are quite different from the other compounds tested. The IC50 values of chlorantraniliprole by C1-based and C2-based ELISA were 1.5 ng mL−1 and 2.7 ng mL−1, respectively. Compared with Nbs C1 and C2, a mAb generated from the same hapten H1 was highly selective to cyantraniliprole, with a little cross reactivity to chlorantraniliprole [15]. These results suggested that by using the same hapten to immunize animals, it is possible to obtain antibodies with different properties due to the difference of animal species and generation techniques. The high cross reactivity of Nbs with both cyantraniliprole and chlorantraniliprole allows for screening two insecticides in matrices simultaneously, whereas, the specific mAb is more suitable for the selective detection of cyantraniliprole.
Nb-based ELISAs for chlorantraniliprole
The typical calibration curves of Nb-based (C1 and C2) competitive ELISAs for chlorantraniliprole were also generated under the same conditions as those for cyantraniliprole (Fig. S3). The C1-based ELISA for chlorantraniliprole had a linear range of 0.4–6.1 ng mL−1, an IC50 of 1.5 ng mL−1 and a LOD of 0.2 ng mL−1, while the IC50 of C2-based ELISA for chlorantraniliprole is 2.7 ng mL−1.
Accuracy and precision of Nb-based ELISAs
Because the C1-based ELISA is more sensitive than the C2-based ELISA for the detection of both cyantraniliprole and chlorantraniliprole, the former assay was applied to the sample detection in this study. Matrix effects which are inevitable in sample analysis may reduce the sensitivity and reliability of immunoassays. Dilution of sample extracts with assay buffer is a common approach to eliminate the matrix effect on immunoassays. Regarding to vegetable and soil extracts, a respective 20-fold and 5-fold dilution with PBS was required to generate a standard curve overlapping that generated in assay buffer, indicative of the minimum matrix effect. The current assays are sensitive enough that such dilutions can be tolerated and still have adequate detection levels. Improved assays could allow further dilution in the future.
The accuracy and precision of assays for insecticides spiked in bok choy and soil samples were evaluated. Cyantraniliprole or chlorantraniliprole was spiked into blank vegetable samples to reach final concentrations of 10, 40, and 160 ng g−1, and into blank soil samples at three levels of 2.5, 10, and 40 ng g−1 (Table 2). The average recoveries of cyantraniliprole from bok choy and soil were 90–129% and the coefficient variations (CVs) ranged from 2.1% to 7.2%. The recoveries of chlorantraniliprole in those samples were in a range of 89–120%, with CVs of 1.6–7.4% (Table 2).
Table 2.
Recoveries of cyantraniliprole and chlorantraniliprole from vegetable and soil samples by the C1-based ELISA (n=3).
| Samples | Spiked level (ng g−1) | Average recovery(CV), % | ||
|---|---|---|---|---|
| cyantraniliprole | chlorantraniliprole | cyantraniliprole | chlorantraniliprole | |
| bok choy | 0 | 0 | <LOD | <LOD |
| 10 | 10 | 129(3.2) | 120(3.8) | |
| 40 | 40 | 99(2.1) | 97(1.6) | |
| 160 | 160 | 109(3.3) | 104(4.1) | |
| soil | 0 | 0 | <LOD | <LOD |
| 2.5 | 2.5 | 127(7.2) | 120(7.0) | |
| 10 | 10 | 101(5.6) | 100(2.5) | |
| 40 | 40 | 90(2.7) | 89(7.4) | |
Sample analysis
The Nb-based ELISA was subsequently used for the detection of cyantraniliprole and chlorantraniliprole residues in bok choy and soil samples collected from experimental plots (Table S3). We found the levels of insecticide residues in bok choy and soil increased as the spray dose was raised, but all of them were below the MRLs (2 mg kg−1). The findings indicated that it is quite safe to harvest bok choy 7 days after the application of both insecticides at the recommended level, 30 g a.i. ha−1. It was obvious that cyantraniliprole and chlorantraniliprole were degraded rapidly in the environment, as shown in bok choy and soil, and the degradation rate of cyantraniliprole was apparently faster than that of chlorantraniliprole, resulting in lower concentrations of cyantraniliprole than those of chlorantraniliprole in all the samples (Table S3). These results are entirely consistent with other studies [5, 6, 22, 23]. Data from Pesticide Properties DataBase (PPDB) also show the degradation rate of chlorantraniliprole is faster than that of cyantraniliprole [24]. Being of environmentally friendly insecticide, both cyantraniliprole and chlorantraniliprole are widely employed to control insects in agricultural production, but cyantraniliprole is more extensively used than chlorantraniliprole.
Meanwhile, all of the samples were analyzed by an HPLC method (Table S3). As shown in Fig. 4, the results by HPLC correlated well with those of Nb-based ELISA for cyantraniliprole (R2 = 0.864) and chlorantraniliprole (R2 = 0.997). Thus, the resulting C1-based ELISA proved to be a valid method to detect two insecticides in both bok choy and soil samples.
Fig. 4.
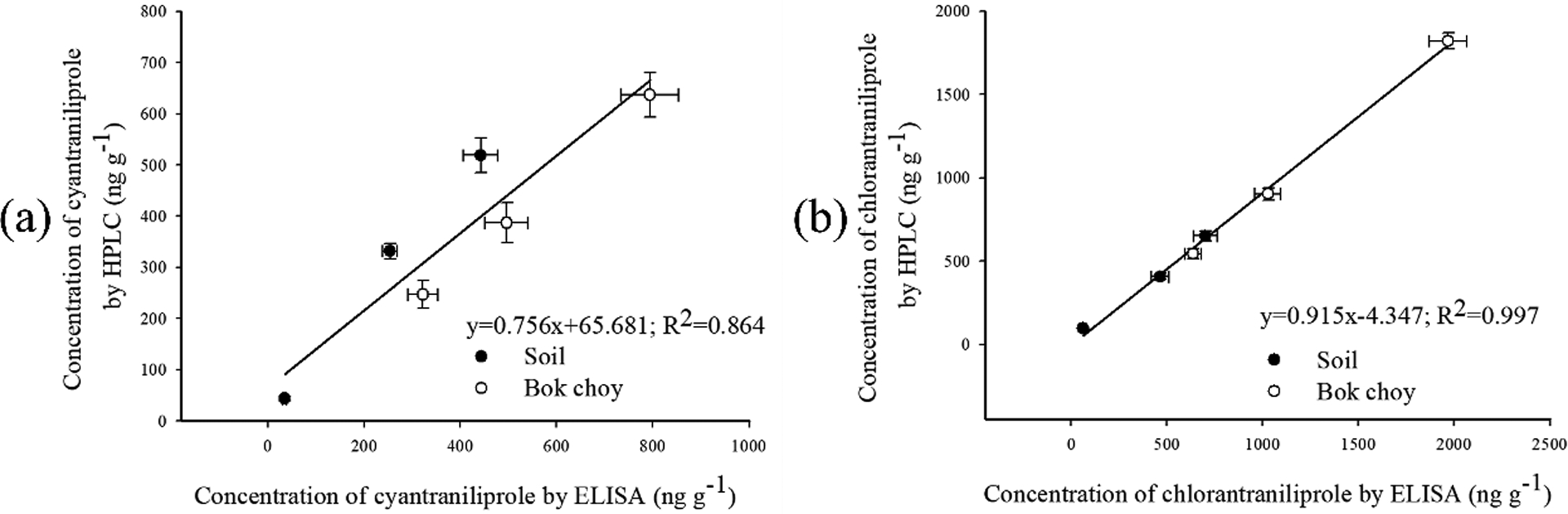
Correlation between ELISA and HPLC for cyantraniliprole and chlorantraniliprole in real samples. (a) cyantraniliprole in soil and bok choy samples; (b) chlorantraniliprole in soil and bok choy samples.
Conclusion
Compared with traditional antibodies, Nb has the advantages of small size, good solubility, good stability, low cost and ease of genetic modification. In addition, the simple and convenient ways of storing and recovering are its greatest advantages as an analytical tool. Two Nbs C1 and C2 selective to insecticides cyantraniliprole and chlorantraniliprole were isolated from a diverse library constructed by using two new reverse primers (R3 and R4) and a previously employed reverse primer (R5). Nb C1-based ELISA, which showed higher sensitivity to cyantraniliprole and chlorantraniliprole than Nb C2-based ELISA, was used to detect the insecticides in vegetable and soil samples, while the mAb-based ELISA method developed previously was only selective to cyantraniliprole.[citation] The accuracy and precision of C1-based ELISA were satisfactory. The resulting assay method agreed well with an HPLC method for the detection of insecticides in real world samples (bok choy and soil) which were collected from a trial of insect control by spraying cyantraniliprole and chlorantraniliprole on bok choy. Although this class of compounds is generally quite toxic to bees, the Brassica plants described here do not require pollination for production and generally, the plants are harvested before blooming so toxicity to bees is seldom an issue. Therefore, this is a good example of a “green” use of a pesticide. This investigation demonstrated that Nb-based ELISA is suitable for monitoring the cyantraniliprole and chlorantraniliprole residues in environmental matrices.
Supplementary Material
Funding information
This work was supported in part by the Key Project of Inter-Governmental International Scientific and Technological Innovation Cooperation (2016YFE0108900 and 2019YFE0115800), the National Key Research and Development Program of China (2016YFD0800606 and 2018YFC1602900), the National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program (P42ES04699), NIEHS RIVER Award (R35ES030443), and the USDA Hatch Project (HAW05044-R).
Footnotes
Complicance with ethical standards
The animal experiments were approved by the China Agricultural University Animal Care and Use Committee. All procedures performed in this research involving Bactrian camels were in accordance with the ethical standards of the China Agricultural University Animal Care and Use Committee. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Cordova D, Benner EA, Sacher MD, Rauh JJ, Sopa JS, Lahm GP, Selby TP, Stevenson TM, Flexner L, Gutteridge S, Rhoades DF, Wu L, Smith RM, Tao Y. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic Biochem Physiol. 2006;84:196–214. 10.1016/j.pestbp.2005.07.005 [DOI] [Google Scholar]
- 2.Lahm GP, Stevenson TM, Selby TP, Freudenberger JH, Cordova D, Flexner L, Bellin CA, Dubas CM, Smith BK, Hughes KA, Hollingshaus JG, Clark CE, Benner EA. Rynaxypyr™: A new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorganic Med Chem Lett. 2007;17:6274–6279. 10.1016/j.bmcl.2007.09.012 [DOI] [PubMed] [Google Scholar]
- 3.Sattelle DB, Cordova D, Cheek TR. Insect ryanodine receptors: molecular targets for novel pest control chemicals. Invertebr Neurosci. 2008;8:107. 10.1007/s10158-008-0076-4 [DOI] [PubMed] [Google Scholar]
- 4.GB 2763–2019, national Food Safety Standard Maximum Residue Limits for Pesticides in Foods. National standards of the People’s Republic of China. [Google Scholar]
- 5.Dong F, Liu X, Xu J, Li J, Li Y, Shan W, Song W, Zheng Y. Determination of cyantraniliprole and its major metabolite residues in vegetable and soil using ultra-performance liquid chromatography/tandem mass spectrometry. Biomed Chromatogr. 2011;26(3):377–383. 10.1002/bmc.1669 [DOI] [PubMed] [Google Scholar]
- 6.Hu X, Zhang C, Zhu Y, Wu M, Cai X, Ping L, Li Z. Determination of Residues of Cyantraniliprole and Its Metabolite J9Z38 in Watermelon and Soil Using Ultra-Performance Liquid Chromatography/Mass Spectrometry. J AOAC Int. 2013;96:1448–1452. 10.5740/jaoacint.12-423 [DOI] [PubMed] [Google Scholar]
- 7.Singh B, Kar A, Mandal K, Kumar R, Sahoo SK. Development and Validation of QuEChERS Method for Estimation of Chlorantraniliprole Residue in Vegetables. J Food Sci. 2012;77:T208–T215. 10.1111/j.1750-3841.2012.02801.x [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Feng N, Tang C, Qin D. Determination of cyantraniliprole and its major metabolite residues in pakchoi and soil using ultra-performance liquid chromatography-tandem mass spectrometry. Bull Environ Contam Toxicol. 2012;89:845–852. 10.1007/s00128-012-0752-2 [DOI] [PubMed] [Google Scholar]
- 9.Tian F, Qiao C, Luo J, Guo L, Pang T, Pang R, Li J, Wang C, Wang R, Xie H. Development and validation of a method for the analysis of five diamide insecticides in edible mushrooms using modified QuEChERS and HPLC-MS/MS. Food Chem. 2020;333:127468. 10.1016/j.foodchem.2020.127468 [DOI] [PubMed] [Google Scholar]
- 10.He T, Wang Y, Li P, Zhang Q, Lei J, Zhang Z, Ding X, Zhou H, Zhang W. Nanobody-based enzyme immunoassay for aflatoxin in agro-products with high tolerance to cosolvent methanol. Anal Chem. 2014;86:8873–8880. 10.1021/ac502390c [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Wang K, Wu S, Wang Z, Ding G, Hao X, Li QX, Li J, Gee SJ, Hammock BD, Xu T. Development of an immunoassay for the detection of carbaryl in cereals based on a camelid variable heavy‐chain antibody domain. J Sci Food Agric. 2019;99:4383–4390. 10.1002/jsfa.9672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K, Liu Z, Ding G, Li J, Vasylieva N, Li QX, Li D, Gee SJ, Hammock BD, Xu T. Development of a one-step immunoassay for triazophos using camel single-domain antibody–alkaline phosphatase fusion protein. Anal Bioanal Chem 2019;411:1287–1295. 10.1007/s00216-018-01563-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang K, Vasylieva N, Wan D, Eads DA, Yang J, Tretten T, Barnych B, Li J, Li QX, Gee SJ, Hammock BD, Xu T. Quantitative Detection of Fipronil and Fipronil-Sulfone in Sera of Black-Tailed Prairie Dogs and Rats after Oral Exposure to Fipronil by Camel Single-Domain Antibody-Based Immunoassays. Anal Chem. 2019;91:1532–1540. 10.1021/acs.analchem.8b04653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muyldermans S. Nanobodies: Natural Single-Domain Antibodies. Annu Rev Biochem. 2013;82:775–797. 10.1146/annurev-biochem-063011-092449 [DOI] [PubMed] [Google Scholar]
- 15.Zhang R, Liu K, Cui Y, Zhang W, He L, Guo S, Chen Y, Li QX, Liu S, Wang B. Development of a monoclonal antibody-based ELISA for the detection of the novel insecticide cyantraniliprole. RSC Adv. 2015;5:35874–35881. 10.1039/c5ra01127b [DOI] [Google Scholar]
- 16.Malhat FM. Determination of Chlorantraniliprole Residues in Grape by High-Performance Liquid Chromatography. Food Anal Methods. 2012;5:1492–1496. 10.1007/s12161-012-9400-z [DOI] [Google Scholar]
- 17.Reverberi R, Reverberi L. Factors affecting the antigen-antibody reaction. Blood Transfus. 2007;5:227–240. 10.2450/2007.0047-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han JH, Choi YS, Kim WJ, Jeon YH, Lee SK, Lee BJ, Ryu KS. Codon optimization enhances protein expression of human peptide deformylase in E. coli. Protein Expr Purif. 2010;70:224–230. 10.1016/j.pep.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 19.Sørensen HP, Mortensen KK. Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol. 2005;115:113–128. 10.1016/j.jbiotec.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 20.Kane JF. Effects of rare codon clusters on high-level expression of heterologous proteins in Escherichia coli. Curr Opin Biotechnol. 1995;6:494–500. 10.1016/0958-1669(95)80082-4 [DOI] [PubMed] [Google Scholar]
- 21.Tegel H, Tourle S, Ottosson J, Persson A. Increased levels of recombinant human proteins with the Escherichia coli strain Rosetta(DE3). Protein Expr Purif. 2010;69:159–167. 10.1016/j.pep.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 22.Malhat F, Abdallah H, Hegazy I. Dissipation of chlorantraniliprole in tomato fruits and soil. Bull Environ Contam Toxicol. 2012;88:349–351. 10.1007/s00128-011-0465-y [DOI] [PubMed] [Google Scholar]
- 23.Zhang JM, Chai WG, Wu YL. Residues of chlorantraniliprole in rice field ecosystem. Chemosphere. 2012;87:132–136. 10.1016/j.chemosphere.2011.11.076 [DOI] [PubMed] [Google Scholar]
- 24.Pesticide Property DataBase. Agriculture & Environment Research Unit (AERU), University of Herfordshire (UK). http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


