The authors documented a relatively high rate of epileptogenic lesions in children with new-onset seizures, with the presence of developmental delay and specific seizure types being associated with a higher likelihood of detecting an epileptogenic lesion on neuroimaging.
Abstract
BACKGROUND AND PURPOSE:
There is a paucity of data regarding the incidence of structural brain lesions in children with new-onset unprovoked seizures. Our aim was to determine the frequencies and types of epileptogenic lesions detected on a dedicated epilepsy protocol MR imaging according to age group, the presence of developmental delay, and the number and types of seizures.
MATERIALS AND METHODS:
Consecutive children between 6 months and 18 years of age with new-onset unprovoked seizures were included. The frequencies and types of epileptogenic lesions were determined and then stratified according to sex, age groups, the presence of developmental delay, and the number and types of seizures at presentation. Multivariate analysis was used to identify variables significantly associated with the presence of epileptogenic lesions.
RESULTS:
One thousand children were included. An epileptogenic lesion was identified in 26%, with malformations of cortical development being the most common lesion (32%), followed by hypoxic-ischemic injury (20%) and vascular etiologies (16%). Univariate analysis showed a significant increase in the frequency of epileptogenic lesions with decreasing age, the presence of developmental delay, and the number and types of seizures at presentation. The presence of developmental delay and seizure type at presentation remained significant in a multivariate analysis.
CONCLUSIONS:
We documented a relatively high rate of epileptogenic lesions in children with new-onset seizures, with the presence of developmental delay and specific seizure types being associated with a higher likelihood of detecting an epileptogenic lesion on neuroimaging. This study fulfills the requirements of the study design recommended by the Practice Committee of the American Academy of Neurology, and we hope that our results will assist the relevant societies and committees in formulating neuroimaging guidelines for children with new-onset seizures.
A brain MR imaging is useful in the work-up of patients with new-onset seizures because it can help define the electroclinical syndrome, identify surgically remediable lesions, and assist in predicting medical refractoriness.1,2 In addition, according to the new proposed definition of epilepsy, a brain MR imaging may establish the diagnosis of epilepsy in patients presenting with a single unprovoked seizure.3
There is a paucity of data regarding the frequency of structural brain lesions in children presenting with new-onset unprovoked seizures. Etiologically related neuroimaging abnormalities were identified in 13%–18% of such children, but those studies have several methodologic drawbacks, including the acquisition of head CTs, non-epilepsy protocol brain MRIs, and selection biases.4-6
The practice parameter issued in 2010 and reaffirmed in 2017 by the American Academy of Neurology7 determined that there was insufficient evidence to support a recommendation for routine neuroimaging of children with a first afebrile seizure. To overcome the shortcomings of prior studies and to generate definitive evidence regarding the value of neuroimaging studies in the pediatric population with new-onset seizures, a call was made for prospective data to be collected in sufficiently large samples, allowing adequate statistical power to provide precise estimates with narrow confidence intervals.7 In addition, the American Academy of Neurology practice parameter stressed the importance of stratifying patients by age groups and including consecutive children for the results to be accurate and generalizable.7
The primary aim of this study was to follow the recommendations of the American Academy of Neurology by prospectively assessing the frequencies and types of epileptogenic lesions in a large cohort of consecutive children with new-onset seizures evaluated with a dedicated epilepsy protocol brain MR imaging. The secondary aims were to determine the yields and types of lesions according to sex, the presence and severity of development delay (DD), as well as the number and types of seizure at presentation.
MATERIALS AND METHODS
Study Design and Patient Characteristics
Consecutive children between 6 months and 18 years of age diagnosed with ≥1 unprovoked seizure between November 2010 and April 2017 were included in this study. Those children participated in an ongoing centralized prospective study evaluating patients with new-onset seizures. The details of this study were previously reported.8
Brain MRI and Classification of Neuroimaging Findings
Brain MRIs were obtained from a 1.5 or 3T scanner (Ingenia; Phillips Healthcare) using an imaging-acquisition protocol that included 3D T1 (1 mm slice thickness) and 3D fast fluid-attenuated inversion recovery (FLAIR; 0.9 or 1 mm slice thickness) of the whole brain with multiplanar reconstruction, axial and coronal inversion recovery (2 mm slice thickness), axial T2 TSE and T2 FFE (4 mm slide thickness) and axial diffusion weighted images (4-5 mm slice thickness). The 3D images were obtained with no interslice gap. This protocol satisfies all the recommendations of the recently published Harmonized Neuroimaging of Epilepsy Structural Sequences (HARNESS-MRI)9 except for the lack of acquisition of high in-plane resolution 2D coronal T2-weighted sequences using submillimetric voxel resolution.
The MRIs were interpreted by a neuroradiologist blinded to the clinical data with vast experience in the neuroimaging of patients with epilepsy.
MR imaging findings were classified as epileptogenic on the basis of previously published criteria.10-12 Epileptogenic lesions were classified into the following categories: malformations of cortical development (MCD), mesial temporal sclerosis (MTS), hypoxic-ischemic injury (moderate or severe periventricular leukomalacia [PVL] or hypoxic brain injury), vascular lesions, tumoral, neurocutaneous syndromes (NCS), metabolic disorder, and others (eg, postinfectious or posttraumatic encephalomalacia with gliosis, leukodystrophy, and large arachnoid cysts exerting mass effect).
The cases of PVL were graded according to the following scoring system:13 severe PVL, diffuse white matter signal abnormality with cystic changes; moderate PVL, diffuse white matter abnormality without cystic changes; and mild PVL, isolated white matter abnormality. We considered only moderate or severe PVL as epileptogenic.14
MR imaging abnormalities consisting of isolated subcortical lesions or abnormal signal, nonspecific white matter hyperintensities, mild PVL, hydrocephalus, and brain atrophy were considered incidental findings.
Assessment of Intellectual or Global Developmental Delay
All children were evaluated for the presence and severity of DD. Children younger than 6 years of age were assessed with the Denver Development Screening Test.15 Older children were evaluated according to the Diagnostic and Statistical Manual of Mental Disorders criteria that stratify intellectual disability into mild, moderate, severe, and profound on the basis of deficits in intellectual functioning as well as difficulties in conceptual, social, and practical areas of living.16 For our analysis, we included only 3 groups of DD (mild, moderate, or severe) by combining children with severe and profound delays into a single category.
Classification of Seizure Types
The seizures were classified according to the recent International League Against Epilepsy operational classification.17,18 Children were stratified into the following groups based on a detailed description of the seizure semiologies experienced at the time of initial evaluation:
Group 1: Children with epileptic spasms in clusters or frequent tonic or atonic seizures
Group 2: Children with focal-onset seizures (focal aware or focal impaired awareness) with or without focal-to-bilateral tonic-clonic seizures
Group 3: Children with unknown-onset tonic-clonic seizures
Group 4: Children with frequent absence seizures and/or myoclonic seizures with or without generalized-onset tonic-clonic seizures
Group 5: Children with unclassified seizure types. This category included children who experienced what used to be labeled “dialeptic seizures” semiologically characterized by a loss of awareness and motionlessness that did not allow a definite distinction based on the semiologic description alone between absence seizures and focal impaired-awareness seizures.
Ethics Approval
This study was approved by the American University of Beirut Medical Center institutional review board, and all parents signed an informed consent form. Additionally, children between 7 and 17 years of age signed an assent form.
Statistical Analyses
We calculated the percentage of children with an epileptogenic lesion and compared the frequencies and types of lesions according to age groups (0–2 years, 2–5 years, 5–10 years, 10–15 years, 15–18 years), DD, and types and number of seizures at baseline.
For continuous variables, descriptive statistics, including mean, median, range, percentage, and 95% confidence interval were calculated. Statistical analyses were performed using the χ2 test or Fisher exact test for categoric variables. Significant P values were set at <.05. Variables that showed a significant association with the presence of an epileptogenic lesion in univariate analyses were entered into a multivariate model.
In addition, a recursive partition analysis was performed to identify variables associated with higher or lower probabilities of detecting epileptogenic lesions. For this analysis, we used the χ2 Automatic Interaction Detector with cross-validation. At each step, the χ2 Automatic Interaction Detector algorithm chooses the independent variable that has the strongest interaction with the dependent variable using P values with a Bonferroni correction as splitting criteria. The final result is a decision tree with various nodes that can be used to predict the probability of detecting an epileptogenic lesion in each subgroup.
RESULTS
Patient Demographics
Of the 1160 consecutive children enrolled in this study, 160 were excluded for the following reasons: 94 because a brain MR imaging was not yet performed for financial or other reasons, 63 who underwent a non-epilepsy protocol brain MR imaging, and 3 children in whom a brain MR imaging was contraindicated. Therefore, a total of 1000 children (boys = 58.1%, girls = 41.9%) with a mean age of 7.8 years (range, 6 months to 17.9 years) were included. The number of seizures at initial evaluation, seizure types, and psychomotor development are shown in Table 1.
Table 1:
Demographic variables of the 1000 children included in the study
| Demographics | |
|---|---|
| Age | |
| Mean | 7.8 yr |
| Range | 6 mo to 17.9 yr |
| Sex | |
| Male/female | 581/419 |
| No. of seizures (%) | |
| 1 seizure | 315 (31.5%) |
| Multiple seizures | 685 (68.5%) |
| Seizure type (No.) (%) | |
| Focal-onset seizures | 484 (48.4%) |
| Unknown-onset tonic-clonic seizures | 260 (26.0%) |
| Absence and/or myoclonus with or without generalized tonic-clonic seizures | 130 (13.0%) |
| Spasms, tonic or atonic seizures | 93 (9.3%) |
| Unclassified | 33 (3.3%) |
| Psychomotor development (No.) (%) | |
| Normal | 777 (77.7%) |
| Delay | 223 (22.3%) |
| Mild DD | 70 (7.0%) |
| Moderate DD | 63 (6.3%) |
| Severe/profound DD | 90 (9.0%) |
MR Imaging Findings
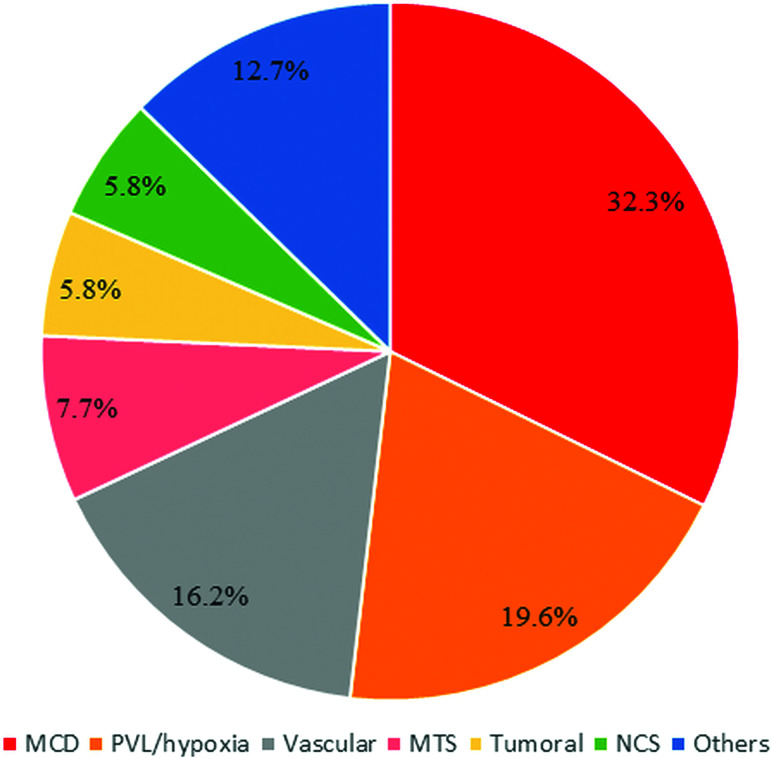
Epileptogenic lesions were detected on the brain MR imaging of 260 children (26%; 95% CI, 23.4%–28.8%). The frequencies of epileptogenic lesions stratified by etiologic categories are shown in Fig 1. The most common type of epileptogenic lesion was MCD detected in 84 children. The most frequent abnormalities in this group consisted of focal cortical dysplasia (47.6%), followed by polymicrogyria (22.6%), heterotopia (9.5%), multiple congenital malformations (8.3%), lissencephaly (7.1%), holoprosencephaly (2.4%), and septo-optic dysplasia (2.4%). Evidence of a hypoxic-ischemic injury was detected in 19.6% of children with etiologically relevant lesions on neuroimaging. Epileptogenic lesions associated with a vascular etiology were detected in 16.2% of children. Most (81%) had evidence of a prior ischemic infarction involving the cortex, 14% had a cavernoma, and 5% had an arteriovenous malformation. The next most common type of epileptogenic lesion was MTS, accounting for 7.7% of cases, followed by tumoral etiologies and NCS, each diagnosed in 5.8% of children. The tumors consisted of 8 neuroglial tumors, 4 infiltrative astrocytomas, 2 hypothalamic hamartomas, and 1 epidermoid cyst. Twelve of the 15 children with NCS were diagnosed with tuberous sclerosis, with the remaining 3 diagnosed with neurofibromatosis. The rest of the identified epileptogenic lesions consisted of posttraumatic or postinfectious encephalomalacia with cortical gliosis (n = 14), metabolic disorder (n = 9), leukodystrophy (n = 6), and large arachnoid cysts exerting mass effect (n = 4).
FIG 1.
Frequencies of epileptogenic lesions stratified by etiologic categories.
Frequencies of Epileptogenic Lesions According to Sex
The frequencies of epileptogenic lesions were not statistically significant between boys (26%) and girls (26%).
Frequencies of Epileptogenic Lesions According to the Number of Seizures at Presentation
At the initial evaluation, 315 children (31.5%) presented with a single seizure. Those who presented with ≥2 seizures were significantly more likely to have an epileptogenic lesion (193/685, 28.2%; 95% CI, 24.9%–31.7%) compared with those with a single seizure (67/315, 21.3%; 95% CI, 17.1%–26.1%; P = .021). There was, however, no significant difference in the types of identified epileptogenic lesions between those 2 groups.
Frequencies of Epileptogenic Lesions According to Age Groups
There was a gradual and significant reduction in the frequencies of epileptogenic lesions with ascending age groups (Table 2) (P < .001). In addition, the predominant subtype of epileptogenic lesions varied according to age groups. For instance, hypoxic-ischemic lesion, which was the most common substrate in children younger than 2 years of age and accounting for 31.7% of epileptogenic lesions in that age group, gradually declined to account for 15.0% of lesions in the 5–10 year age group and 0% in the 15–18 year age group (Table 2). On the other hand, there was a gradual increase in the frequencies of MTS and tumoral etiologies with ascending age groups, peaking in the 15–18 year age group (Table 2). Other types of epileptogenic lesions did not show an apparent age-related pattern. However, children with MCD presented with seizures before 15 years of age.
Table 2:
Frequencies of epileptogenic lesion subtypes stratified by age groupsa
| 0–2 Years (n = 169) | 2–5 Years (n = 189) | 5–10 Years (n = 281) | 10–15 Years (n = 257) | 15–18 Years (n = 104) | |
|---|---|---|---|---|---|
| Epileptogenic lesions | 82 (48.5%) | 57 (30.2%) | 60 (21.4%) | 47 (18.3%) | 14 (13.5%) |
| MCD | 22 (26.8%) | 20 (35.1%) | 24 (40.0%) | 18 (38.3%) | 0 (0.0%) |
| Hypoxic-ischemic | 26 (31.7%) | 14 (24.6%) | 9 (15.0%) | 2 (4.3%) | 0 (0.0%) |
| Vascular | 14 (17.1%) | 10 (17.5%) | 6 (10.0%) | 9 (19.1%) | 3 (21.4%) |
| MTS | 2 (2.4%) | 1 (1.8%) | 5 (8.3%) | 9 (19.1%) | 3 (21.4%) |
| NCS | 8 (9.8%) | 1 (1.8%) | 4 (6.7%) | 1 (2.1%) | 1 (7.1%) |
| Tumoral | 0 (0.0%) | 3 (5.3%) | 4 (6.7%) | 4 (8.5%) | 4 (28.6%) |
| Other | 10 (12.2%) | 8 (14.0%) | 8 (13.3%) | 4 (8.5%) | 3 (21.4%) |
95% CI for the percentages of epileptogenic lesions in the 0–2 year, 2–5 year, 5–10 year, 10–15 year, and 15–18 year age groups were 41.1%–56.0%, 24.1%–37%, 17.0%–26.5%, 14.0%–23.5%, and 8.2%–21.3%, respectively.
Frequencies of Epileptogenic Lesions According to the Presence of DD
Two hundred twenty-three children had evidence of DD at the initial evaluation, with a significant difference in frequency across the age groups (P < .001). DD was highest in the 0–2 year group (60.9%), followed by the 2–5 year group (29.1%), the 5–10 year group (12.8%), the 10–15 year group (8.9%), and finally the 15–18 year group (5.8%).
The frequency of epileptogenic lesions was significantly higher in children with DD (127/223, 57.0%; 95% CI, 50.4%–63.3%) compared with those with normal development (133/777, 17.1%; 95% CI, 14.6%–19.9%; P < .001).There was also a significant increase in the frequencies of epileptogenic lesions as the severity of DD worsened (P < .001).
Frequencies of Epileptogenic Lesions According to the Type of Seizures Groups
There was a significant difference in the frequencies of identified epileptogenic lesions according to the seizure types at presentation (P < .001, Table 3). The lowest yield of detecting a lesion was in the group of patients with absences and/or myoclonus with or without generalized tonic-clonic seizures (6.2%), and the highest yield was in children who presented with epileptic spasms in clusters or frequent tonic or atonic seizures (65.6%). The frequency of epileptogenic lesions in children with focal-onset seizures was 30.6% (Table 3).
Table 3:
Frequencies of epileptogenic lesions stratified by seizure type at presentation
| Seizure Types | Epileptogenic Lesions |
|---|---|
| Absence and/or myoclonus with or without generalized tonic-clonic seizures (n = 130) | 8 (6.2%) |
| Unknown-onset tonic-clonic seizures (n = 260) | 38 (14.6%) |
| Unclassified (n = 33) | 5 (15.2%) |
| Focal-onset seizures (n = 484) | 148 (30.6%) |
| Spasms, tonic or atonic seizures (n = 93) | 61 (65.6%) |
Multivariate Analysis
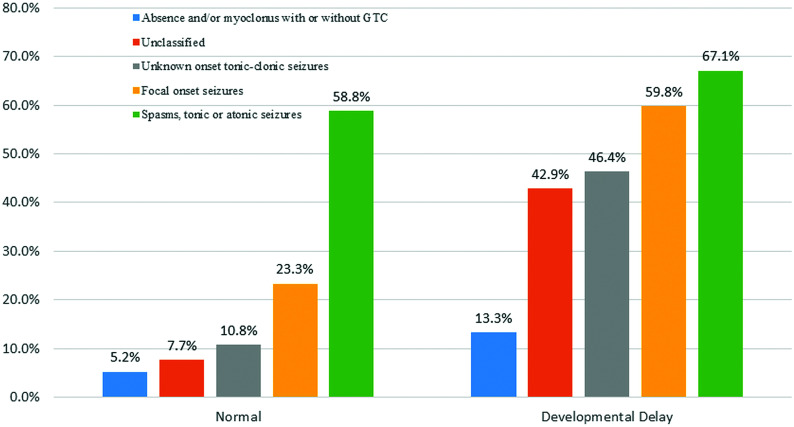
A logistic regression analysis with an epileptogenic lesion as the dependent variable and all significant variables in the univariate analyses (≥1 seizure on presentation, age groups, presence of DD, and seizure types) as independent variables showed that only the presence of DD (odds ratio = 4.3, P < .001) and seizure types on presentation (odds ratio = 2.3, P < .001) remained significant. The frequencies of epileptogenic lesions stratified by seizure types and the presence or absence of DD are shown in Fig 2.
FIG 2.
Percentages of children with epileptogenic lesions stratified according to seizure types and the presence or absence of developmental delay. GTC indicates generalized tonic-clonic seizures.
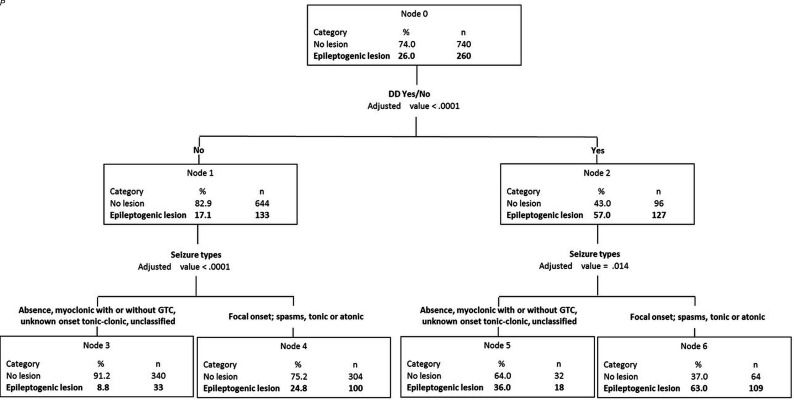
The recursive analysis identified the same 2 variables (the presence or absence of DD and seizure type) that partitioned the patients into a decision tree with 4 groups (Fig 3). The highest yield of detecting epileptogenic lesions (63.0%) was in the group of children with DD who presented with focal-onset seizures or with epileptic spasms or tonic or atonic seizures. The frequency of epileptogenic lesions in children without DD who presented with the same seizure types was 24.8%. The corresponding yields for children with and without DD who presented with other seizure types were 36% and 8.8%, respectively.
FIG 3.
Recursive partition analysis stratified children into 4 groups based only on the presence of DD and seizure types. GTC indicates generalized tonic-clonic seizures.
DISCUSSION
This is the largest study that prospectively evaluated brain MR imaging findings in consecutive children with new-onset unprovoked seizures using a dedicated imaging protocol. We documented that the yield of identifying epileptogenic lesions in that patient population is 26% (95% CI, 23.4%–28.8%).
Three previous studies identified etiologically related neuroimaging abnormalities in 13%–18% of children with new-onset seizures.4-6 Those studies had several methodologic shortcomings, including evaluating children with a mixture of head CT and non-epilepsy protocol brain MRIs,4,5 selection bias because neuroimaging studies were not systematically performed but only as clinically indicated,4,5 and exclusion of children with DD.6 For instance, epileptogenic lesions were detected in 18% of 411 children who presented with a first afebrile seizure.4 In that study, neuroimaging was only performed in 53% of children, with most studies consisting of head CTs. In a community-based study of 613 children with newly diagnosed epilepsy, neuroimaging studies (approximately two-thirds had a standard brain MR imaging, and one-third underwent a head CT) were obtained in 80%, with etiologically relevant abnormalities detected in 13%.5 A subsequent prospective study performed brain MR imaging in 281/349 children with a first-recognized seizure.6 Significant abnormalities or those potentially related to seizures were identified in 14% of those children. The relatively low yield of epileptogenic lesions in that study can be explained by the fact that children younger than 6 years of age and those with moderate or severe DD were excluded.6
The most common epileptogenic lesion in our study was MCD, followed by hypoxic-ischemic injuries and vascular lesions. Because of the large number of children enrolled, our study is the first to stratify the pathologic substrate on brain MR imaging according to age groups. We found that in children 2 years of age and younger, the most common underlying etiology was hypoxic-ischemic injury and MCD. Those results are concordant with those of a smaller study conducted in children younger than 2 years of age with newly diagnosed epilepsy in whom the most common pathologic substrate was developmental brain malformations.19 We also found that MCD was one of the most common epileptogenic lesions in the 2–15 year age group, while MTS was most frequent in the 10–18 year age group presenting with new-onset seizures.
This is also the first study that documented a gradual increase in the frequency of epileptogenic lesions with decreasing age as well as with the presence and severity of DD. For young children, our results are overall similar to those recently reported in a study that evaluated the frequency of etiologically relevant neuroimaging abnormalities in children with early-life epilepsy, most of whom were evaluated with an epilepsy protocol brain MR imaging.20 In that study, 40% of children 3 years of age and younger were found to have epileptogenic lesions, with a frequency of 61% in those with DD compared with 24% in children with normal development.20 These results are very similar to ours, because in the group of children younger than 2 years of age, we identified an epileptogenic lesion in 48.5%, with a 65% frequency in children with DD compared with 23% in developmentally healthy children. Two other studies that evaluated the yield of neuroimaging in children younger than 2 years of age with new-onset seizures showed similar results, with etiologically relevant abnormalities detected in 42%21 and in 51%19 of children. In our study, the highest frequency of DD was in children younger than 2 years of age, with 61% of children with new-onset seizures having concomitant DD. This finding is consistent with those in previous studies that showed that a substantial proportion of children presenting with seizures in early life have associated DD.22,23
Our data showed that there was a significant difference in the frequencies of epileptogenic lesions according to the seizure types experienced by the child at the time of evaluation. We stratified the seizure types into various groups based on the fact that certain seizure types are known to occur in generalized genetic epilepsy, and others, in focal epilepsy and epileptic encephalopathies. As would be expected, the frequency of detecting an epileptogenic lesion was lowest in children who presented with absence and/or myoclonic seizures with or without generalized tonic-clonic seizures and highest in those who experienced epileptic spasms in clusters or frequent tonic or atonic seizures.
Using logistic regression, we found that only the presence of DD (odds ratio = 4.3), as well as the seizures types (odds ratio = 2.3), remained significantly associated with the presence of an epileptogenic lesion. Because the highest frequency of children with DD was in those younger than 2 years of age, it is not surprising that the age group did not achieve statistical significance in the multivariate model. The results of the recursive partitioning analysis were concordant with those of the logistic regression and provided a tree with 6 nodes based on the seizure types and the presence or absence of DD. The expected yield of detecting an epileptogenic lesion in each of the various nodes could be used as a decision tree to determine when brain MR imaging should be performed.
We purposefully avoided including the electroencephalography results in our analyses because our aim was to establish the yield of neuroimaging based on the clinical presentation alone. Furthermore, because a presumed electroclinical syndrome could be modified by the presence of an epileptogenic lesion on the MR imaging, including the electroencephalography results as a variable can lead to circular reasoning. For example, a developmentally healthy child presenting with an opercular seizure (focal-aware seizure) and found to have rolandic maturational epileptiform discharges on the electroencephalography would be initially diagnosed as a case of self-limited epilepsy with centrotemporal spikes. The diagnosis on that same child would be changed to structural focal epilepsy if the MR imaging were to reveal a cavernoma in the inferior frontal rolandic cortex.
The strength of our study is that it evaluated a large cohort of consecutive children referred for new-onset unprovoked seizures and who underwent a HARNESS brain MR imaging protocol.9 The acquisition of MRIs was centralized, the neuroimaging studies were obtained shortly after the seizure onset, and the studies were interpreted by an experienced neuroradiologist who was blinded to the clinical data. In addition, we were very conservative in defining epileptogenic lesions and stratified the types and frequencies of lesions according to the seizure types experienced by the child at the time of the initial evaluation. We elected not to include children who underwent a non-epilepsy protocol MR imaging to have a set of uniform data, especially because it was previously shown that up to 65% of studies interpreted as having normal findings would reveal a relevant lesion when a high-quality study was performed.24,25
CONCLUSIONS
Ideally, we believe that brain MR imaging should be performed in every child with new-onset unprovoked seizures, especially when sedation is not required, for several reasons: First, it would be in keeping with the new International League Against Epilepsy classification of the epilepsies, which emphasizes the need to consider the etiology at each step of diagnosis, including a structural etiology, which should preferably be evaluated with brain MR imaging and that will help with the syndromic classification.9,18 In addition, for children who present with a single, unprovoked seizure, the presence of specific structural brain lesions could satisfy the diagnosis of epilepsy 9,17 and will also guide the need for treatment. Furthermore, the pathologic substrate identified on neuroimaging can assist with the prognosis and accelerate referral to a specialized epilepsy center.9 When brain MR imaging is not readily available, as in developing nations where the resources might be scarce or in case of financial constraints, it would be useful to have guidelines to recommend when brain MR imaging should be performed in children with new-onset, unprovoked seizures. The practice parameter issued in 2000 and reaffirmed in 2017 by the American Academy of Neurology, the Child Neurology Society, and the American Epilepsy Society for the evaluation of a first nonfebrile seizure in children asserts that brain MR imaging is the preferred technique and that it should be seriously considered in any child with psychomotor delay, focal-onset seizure, and younger than 1 year of age.7 The League Against Epilepsy Guidelines recommend imaging (MR imaging is preferred over CT when available) for infants and children with recent-onset epilepsy whenever localization-related epilepsy is known or suspected, when the epilepsy classification is in doubt, or when an epilepsy syndrome with a remote symptomatic cause is suspected.26 However, the authors of the practice parameter stressed that there is insufficient evidence available from the published studies for issuing evidence-based guidelines pertaining to neuroimaging in children with new-onset seizures. They also stressed the need for a large prospective study that enrolls consecutive children using a standardized MR imaging acquisition protocol and that stratifies the findings according to age groups. Our study fulfills those requirements, and we hope that our results will assist the relevant societies and committees in formulating neuroimaging guidelines for children with new-onset seizures.
ABBREVIATIONS:
- DD
developmental delay
- MCD
malformations of cortical development
- MTS
mesial temporal sclerosis
- NCS
neurocutaneous syndromes
- PVL
periventricular leukomalacia
Footnotes
R. Hourani and W. Nasreddine contributed equally to this work.
Data are available on reasonable scientific request.
References
- 1.Semah F, Picot MC, Adam C, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology 1998;51:1256–62 10.1212/WNL.51.5.1256 [DOI] [PubMed] [Google Scholar]
- 2.Stephen LJ, Kwan P, Brodie MJ. Does the cause of localisation‐related epilepsy influence the response to antiepileptic drug treatment? Epilepsia 2002;42:357–62 10.1046/j.1528-1157.2001.29000.x [DOI] [PubMed] [Google Scholar]
- 3.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55:475–82 10.1111/epi.12550 [DOI] [PubMed] [Google Scholar]
- 4.Shinnar S, O’Dell C, Mitnick R, et al. Neuroimaging abnormalities in children with an apparent first unprovoked seizure. Epilepsy Res 2001;43:261–69 10.1016/S0920-1211(00)00206-0 [DOI] [PubMed] [Google Scholar]
- 5.Berg AT, Testa FM, Levy SR, et al. Neuroimaging in children with newly diagnosed epilepsy: a community-based study. Pediatrics 2000;106:527–32 10.1542/peds.106.3.527 [DOI] [PubMed] [Google Scholar]
- 6.Kalnin AJ, Fastenau PS, Degrauw TJ, et al. Magnetic resonance imaging findings in children with a first recognized seizure. Pediatr Neurol 2008;39:404–14 10.1016/j.pediatrneurol.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirtz D, Ashwal S, Berg A, et al. Practice parameter: evaluating a first nonfebrile seizure in children: report of the quality standards subcommittee of the American Academy of Neurology, the Child Neurology Society, and the American Epilepsy Society. Neurology 2000;55:616–23 10.1212/WNL.55.5.616 [DOI] [PubMed] [Google Scholar]
- 8.Arabi M, Dirani M, Hourani R, et al. Frequency and stratification of epileptogenic lesions in elderly with new onset seizures. Front Neurol 2018;9:995. 10.3389/fneur.2018.00995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernasconi A, Cendes F, Theodore WH, et al. Recommendations for the use of structural magnetic resonance imaging in the care of patients with epilepsy: a consensus report from the International League Against Epilepsy Neuroimaging Task Force. Epilepsia 2019;60:1054–68 10.1111/epi.16324 [DOI] [PubMed] [Google Scholar]
- 10.Chuang NA, Otsubo H, Chuang SH. Magnetic resonance imaging in pediatric epilepsy. Top Magn Reson Imaging 2002;13:39–60 10.1097/00002142-200202000-00004 [DOI] [PubMed] [Google Scholar]
- 11.Urbach H. Imaging of the epilepsies. Eur Radiol 2005;15:494–500 10.1007/s00330-004-2629-1 [DOI] [PubMed] [Google Scholar]
- 12.Moosa AN, Wyllie E. Focal epileptogenic lesionsHandb Clin Neurol 2013;111:493–510 10.1016/B978-0-444-52891-9.00053-1 [DOI] [PubMed] [Google Scholar]
- 13.Volpe JJ. Confusions in nomenclature: “periventricular leukomalacia” and “white matter injury”: identical, distinct, or overlapping? Pediatr Neurol 2017;73:3–6 10.1016/j.pediatrneurol.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 14.Humphreys P, Deonandan R, Whiting S, et al. Factors associated with epilepsy in children with periventricular leukomalacia. J Child Neurol 2007;22:598–605 10.1177/0883073807302599 [DOI] [PubMed] [Google Scholar]
- 15.Frankenburg WK, Dodds J, Archer P, et al. Denver II Training Manual. Denver Developmental Materials; 1992 [Google Scholar]
- 16.American Psychiatric Association. DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). American Psychiatric Association; 2013 [Google Scholar]
- 17.Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:522–30 10.1111/epi.13670 [DOI] [PubMed] [Google Scholar]
- 18.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:512–21 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eltze CM, Chong WK, Cox T, et al. A population‐based study of newly diagnosed epilepsy in infants. Epilepsia 2013;54:437–45 10.1111/epi.12046 [DOI] [PubMed] [Google Scholar]
- 20.Coryell J, Gaillard WD, Shellhaas RA, et al. Neuroimaging of early life epilepsy. Pediatrics 2018;142:e20180672. 10.1542/peds.2018-0672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh D, Chang T, Tsuchida T, et al. New-onset afebrile seizures in infants: role of neuroimaging. Neurology 2010;74:150–56 10.1212/WNL.0b013e3181c91847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Battaglia D, Rando T, Deodato F, et al. Epileptic disorders with onset in the first year of life: neurological and cognitive outcome. Eur J Paediatr Neurol 1999;3:95–103 10.1016/S1090-3798(99)90096-X [DOI] [PubMed] [Google Scholar]
- 23.Altunbaşak Ş, Incecik F, Hergüner Ö, et al. Prognosis of patients with seizures occurring in the first 2 years. J Child Neurol 2007;22:307–13 10.1177/0883073807300540 [DOI] [PubMed] [Google Scholar]
- 24.Knake S, Triantafyllou C, Wald L, et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology 2005;65:1026–31 10.1212/01.wnl.0000179355.04481.3c [DOI] [PubMed] [Google Scholar]
- 25.Kokkinos V, Kallifatidis A, Kapsalaki EZ, et al. Thin isotropic FLAIR MR images at 1.5 T increase the yield of focal cortical dysplasia transmantle sign detection in frontal lobe epilepsy. Epilepsy Res 2017;132:1–7 10.1016/j.eplepsyres.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 26.Gaillard WD, Chiron C, Cross JH, et al. ILAE, Committee for Neuroimaging, Subcommittee for Pediatric. Guidelines for imaging infants and children with recent‐onset epilepsy. Epilepsia 2009;50:2147–53 10.1111/j.1528-1167.2009.02075.x [DOI] [PubMed] [Google Scholar]