First, we would like to the thank authors of the new commentary,1 which gave us the chance to reply to critics of our first publication2 entitled “Anosmia in COVID-19 Associated with Injury to the Olfactory Bulbs Evident on MRI” written before the existence of anatomopathologic studies.
Our research group had the opportunity to study and publish 3 other recent articles3-5 about the same subject. In these articles,3-5 we explained why we have interpreted2 the alterations on brain imaging of patients with coronavirus disease 2019 (COVID-19) detected on thin slices of the coronal fat-suppressed T1WI, pre- and/or postcontrast, as olfactory bulb injuries (blood-brain barrier and/or methemoglobin) and not artifacts. However, we will present these explanations again and also comment about some aspects of your Letter,1 as follows:
We understand the reason for your concern about susceptibility artifacts and confess that initially, we also had the same doubts as you expressed in your Letter when for the first time, we were faced with the images of these olfactory bulb injuries in patients with COVID-19.
To make sure that the findings we were identifying in the olfactory bulbs were real and represented an abnormality, we also reviewed the pre- and/or postcontrast fat-suppressed T1WI and STIR MR imaging from our data base of the orbits of healthy subjects obtained before the COVID-19 pandemic, and we explained these findings2,3 in our first publication.2 We used this precaution because we did not find any article in the literature describing the normal aspect of the olfactory bulbs in pre- and postcontrast fat-suppressed T1WI or STIR.
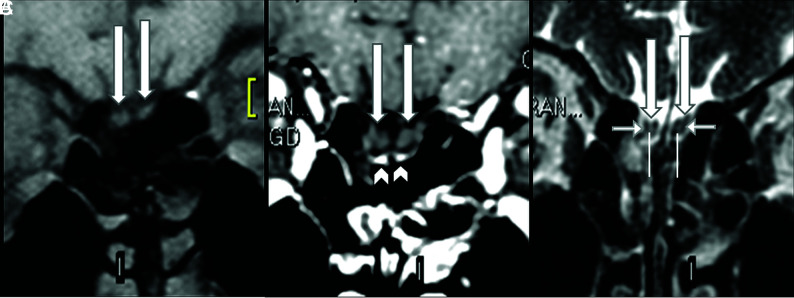
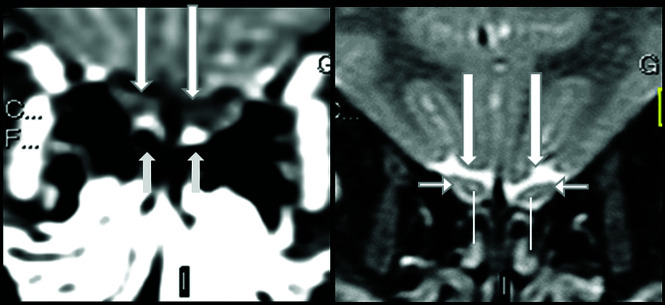
Our research group performed another retrospective study not yet published (performed on 1.5T MR imaging) that show that the normal olfactory bulbs have signal intensity similar to that of the cortical gray matter in the sequence using thin-sliced coronal fat-suppressed T1WI (Fig 1A, arrows) with no contrast enhancement (Fig 1B, and Fig 2A, long arrows). On coronal thin-sliced FSE T2WI, in 90% of olfactory bulbs, the central area has hyperintensity similar to that in the cortical gray matter (Fig 1C and Fig 2B, superior extremity of lines) and the periphery has hypointensity of the white matter (Fig 1C and Fig 2B, horizontal short arrows). This aspect resembles the cell layers observed on histology of normal olfactory bulbs.Susceptibility artifacts due to the interface between the bone and air were found in 81% of images in our study. All of these susceptibility artifacts were bilateral and symmetric, mainly at the topography of the cribriform plate outside and below the olfactory bulbs (Fig 1B, arrowhead and Fig 2A, short arrow) or the parasagittal region adjacent to the crista galli and more frequently on postcontrast sequences. Forty-five percent of patients also presented with a kind of susceptibility artifact at the sphenoidal level that projected inside the base of the frontal lobe, being easily recognized as an artifact as you also showed in your axial T1WI1 performed at 3T. These susceptibility artifacts should not hinder the analyses of olfactory bulbs because they can be recognized.
So, as you seemingly know and in fact show in your figures1 on axial T1WI, artifacts can be easily recognized by radiologists and should not be confounded with enhancement or methemoglobin. The susceptibility artifacts usually have ill-defined margins that eventually vanish and are often bilateral as you have shown in your axial Fig 1.Anyone reviewing our figures (in the first publication,2 in our first reply,3 in our Letter,4 and also in our original study recently published5) will realize that they show true lesions and not artifacts.
In your Letter,1 you wrote about susceptibility artifacts being worse on 3T brain MRI, and they are reduced and disappear in a minor magnetic field MRI machine such as 1.5T.All of our patients’ brain MRIs were performed on a 1.5T machine,2-5 and this was clearly described in the Materials and Methods2 of our article on which you are commenting. Therefore, the brain MRIs of our cases may have fewer artifacts than yours because we used a 1.5T machine and you used a 3T machine. Perhaps this is the reason you have had difficulty with artifacts.
In the figures of your commentary and Letter,1 we were surprised because you showed figures using axial slices on T1WI, which are not adequate to analyze the olfactory bulbs. It would be better if you had shown this artifact in the coronal plane of your figures.I would kindly ask you to look again at the figures in our publications2-5 because we included and analyzed only cases in which there was a sequence with thin slices on the coronal plane.If the indication for brain MR imaging is to evaluate the olfactory bulbs, it is much better to analyze them in a coronal plane or use 3D acquisitions of the brain with reconstructions in the coronal and sagittal planes.
Our MRI findings, as well as other research findings, documented in vivo2,6-8 the hypothesis of Severe Acute Respiratory Syndrome coronavirus 2 in the olfactory bulbs as a cause of anosmia, even before the first anatomopathologic studies were allowed to be performed and published.9,10 And now, recently, the first histopathologic postmortem studies have confirmed our findings.9,10
FIG 1.
Normal olfactory bulbs and susceptibility artifacts seen on 1.5T MR imaging before the COVID-19 pandemic. The coronal precontrast fat-suppressed T1WI (A) and the postcontrast (B) and coronal FSE T2WI (C) demonstrate normal olfactory bulbs (long arrows). The olfactory bulbs are isointense to the cerebral cortex and normally hypointense on pre- (A) and postgadolinium sequences (B) and do not enhance. Susceptibility artifacts on the cribriform plate (B, arrowheads) are bilateral and symmetric below the olfactory bulbs and do not hinder the analysis. On thin-sliced coronal FSE T2WI, the normal olfactory bulbs show a “sandwich-like pattern,” which consists of a hyperintense central area (C, superior extremity of the vertical lines), similar to the cortical gray matter, and a hypointense periphery (C, short horizontal arrows), similar to the white matter that looks like the laminar layers of olfactory bulbs on histology.
FIG 2.
Normal olfactory bulbs and susceptibility artifacts seen on 1.5T MR imaging before the COVID-19 pandemic. The olfactory bulbus are normal, being hypointense, and do not enhance on thin-sliced coronal fat-suppressed postcontrast T1WI (A, long arrows). Susceptibility artifacts are bilateral and symmetric and can be recognized as hyperintensity located outside and adjacent to the inferior periphery of the normal olfactory bulbs, mainly at cribriform plate (A, short arrows). On coronal T2WI, the normal sandwich-like pattern is observed as the central portion of olfactory bulbs showing hyperintensity (B, superior extremity of the line), similar to that of gray matter, and the peripheral portion showing hypointensity, similar to that of white matter (B, short horizontal arrows).
Lee et al9 demonstrated, in an extremely elegant postmortem histopathologic study using much more sophisticated tools (eg, an 11.7T scanner), what we had suggested in vivo previously on the 1.5T MR imaging.2 This postmortem histopathologic study showed microvascular injury with areas of fibrinogen leakage, thinned basal lamina, and hemorrhagic lesions in the brain and olfactory bulbs,9 which can explain our radiologic findings (olfactory bulb enhancement [breakdown of the blood-brain barrier] and/or probable hemorrhagic lesions [methemoglobin]).2-5
In summary, we explained again, point by point, why our findings are not artifacts.2-5 We show new data with the features of a normal olfactory bulb on coronal T2WI which frequently has a hyperintense central area (similar to the cortex) surrounded by a hypointense layer (similar to white matter) and susceptibility artifacts at the cribriform plate on coronal fat suppressed T1WI. We also commented on the Letter1 from our colleagues in Miami. Therefore, in closing, we would like to state that despite sometimes finding artifacts on MR imaging, an experienced radiologist would recognize the artifacts and have the obligation to communicate if find any other important information about the MR images (such as olfactory bulb injury seen in a retrospective study on MR imaging of patients with COVID-19 not investigated for anosmia but investigated for other neurologic complications in the beginning of this terrible pandemic) in an impartial manner. It is our obligation to present our findings to the scientific community without any preconceptions, recognizing MR imaging artifacts and differentiating them from what seemed true COVID-related lesions in the olfactory bulbs. Increasingly, the scientific community is accumulating proof that there is clinical, radiologic2-8 (MR imaging in vivo2-8 and in postmortem9), and anatomopathologic9,10 evidence for the presence of injury to the olfactory bulbs in patients with COVID-19.
References
- 1.Manov JJ, Saigal G, Alfonso M. Susceptibility artifacts in the anterior cranial fossa mimicking hemorrhage in anosmic patients. AJNR Am J Neuroradiol 2021July [Epub ahead of print] 10.3174/ajnr.A7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aragão MF, Leal MC, Cartaxo Filho OQ, et al. Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. AJNR Am J Neuroradiol 2020;41:1703–16 10.3174/ajnr.A6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aragão MF, Leal MC, Fonseca TM, et al. Reply. AJNR Am J Neuroradiol 2020;2:E2–E3 10.3174/ajnr.A6943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aragao MF, Oliveira AD, Lima AR, et al. Virtual biopsy: a reality thanks to advances in radiology. AJNR Am J Neuroradiol 2021March25. [Epub ahead of print] 10.3174/ajnr.a7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aragao M, Leal MC, Andrade PH, et al. Clinical and radiological profiles of COVID-19 patients with neurological symptomatology: a comparative study. Viruses 2021;13:845. 10.3390/v13050845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurendon T, Radulesco T, Mugnier J, et al. Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology 2020;95:224–25 10.1212/WNL.0000000000009850 [DOI] [PubMed] [Google Scholar]
- 7.Chetrit A, Lechien JR, Ammar A, et al. Magnetic resonance imaging of COVID-19 anosmic patients reveals abnormalities of the olfactory bulb: preliminary prospective study. J Infect 2020;81:816–46 10.1016/j.jinf.2020.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strauss SB, Lantos JE, Heier LA, et al. Olfactory bulb signal abnormality in patients with COVID-19 who present with neurologic symptoms. AJNR Am J Neuroradiol 2020;41:1882–87 10.3174/ajnr.A6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med 2021;384:481–83 10.1056/NEJMc2033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 2020;19:919–29 10.1016/S1474-4422(20)30308-2 [DOI] [PMC free article] [PubMed] [Google Scholar]