This meta-analysis demonstrated the safety and efficacy of the Woven EndoBridge device in the management of ruptured aneurysms, but further studies are needed.
Abstract
BACKGROUND:
The Woven EndoBridge device has been increasingly used to treat wide-neck aneurysms, particularly ruptured ones.
PURPOSE:
Our aim was to investigate the safety and efficacy of the Woven EndoBridge device in the treatment of ruptured intracranial aneurysms.
DATA SOURCES:
All studies evaluating the outcomes of Woven EndoBridge device use in the treatment of ruptured intracranial aneurysms from inception through 2020 were searched on Ovid Evidence-Based Medicine Reviews, EMBASE, MEDLINE, Scopus, and the Web of Science Core Collection.
STUDY SELECTION:
Eighteen studies encompassing 487 patients with 496 ruptured aneurysms treated with the Woven EndoBridge device were included.
DATA ANALYSIS:
We studied rates of rerupture and retreatment, angiographic outcomes at the last follow-up point, complications, and mortality rates. Data were collected on anticoagulation and antiplatelet use. Meta-analysis was performed using the random effects model.
DATA SYNTHESIS:
The rate of late rebleeding was 1.1% (95% CI, 0.1%–2.1%). The treatment-related perioperative complication rate and the overall clinical complication rate were 13.2% (95% CI, 9.2%–17.2%) and 3.2% (95% CI, 1.6%–4.7%), respectively. Thirteen hemorrhagic (2%; 95% CI, 0.8%–3.3%) and 41 thromboembolic (6.8%; 95% CI, 4.6%–9%) complications occurred. Favorable clinical outcomes were achieved in 85% of patients. Procedure-related mortality and overall mortality rates were 2.1% (95% CI, 0.8%–3.3%) and 11.5% (95% CI, 7%–16%), respectively. At last follow-up, an adequate occlusion rate was 87.3% (95% CI, 82.1%–92.4%) and the retreatment rate was 5.1% (95% CI, 3%–7.3%).
LIMITATIONS:
Our meta-analysis is limited by selection bias and high heterogeneity.
CONCLUSIONS:
This meta-analysis demonstrated the safety and efficacy of the Woven EndoBridge device in the management of ruptured aneurysms, but further studies are needed.
In recent years, the Woven EndoBridge device (WEB; MicroVention) has been increasingly used for the endovascular treatment of wide-neck aneurysms, particularly ruptured ones. One of the advantages of the WEB device in the treatment of ruptured intracranial aneurysms compared with other nontraditional techniques (ie, stent-assisted coiling or flow diversion) is that dual antiplatelet therapy is not necessary.1 A few series have detailed the use of the WEB device in patients with ruptured aneurysms. However, these studies are relatively small and represent early experience. To assess the technical success rate, effectiveness, safety, and early follow-up of patients with aneurysmal SAH treated with the WEB device in the acute phase, we performed a systematic review and meta-analysis of published series.
MATERIALS AND METHODS
Search Strategy
The literature was searched by a medical librarian for “Woven EndoBridge (WEB)” or “flow diverter” combined with “aneurysm” and its variants in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines2 (the PRISMA checklist is provided in the Online Supplemental Data). Search strategies were created using a combination of keywords and standardized index terms. Key words included the following: “Intrasaccular flow diverter or WEB Device,” “Aneurysm, Ruptured/therapy Embolization,” “Therapeutic/instrumentation,” “Endovascular Procedures/instrumentation,” “Intracranial Aneurysm/therapy,” and “Treatment Failure Treatment Outcome.” Searches were run in September 2020 in Ovid Evidence-Based Medicine Reviews, Ovid EMBASE (1974+), Ovid MEDLINE (1946+ including Epub ahead of print, in-process, and other nonindexed citations), Scopus (1970+), and the Web of Science Core Collection (1975+). Results were limited to the English language from 2012+, with most editorials and reviews removed (full search strategy is available in the Online Supplemental Data).
Eligibility Criteria
Inclusion criteria were the following: 1) studies reporting a consecutive series of patients with ruptured intracranial aneurysms treated consecutively with the WEB device with clear reporting of the primary outcomes, and 2) series of at least 5 patients reporting clearly the complications related to the WEB device. Review articles, guidelines, technical notes, comments, and editorials were excluded.
Study Selection Process
Titles and abstracts were screened for inclusion by 1 author using EndNote (Clarivate Analytics). Full-text articles were retrieved for the included abstracts and screened by the same author and were reviewed and confirmed by the senior author. In studies that included both ruptured and unruptured aneurysms, we abstracted only data from patients with ruptured aneurysms.
Data Extraction and Outcome Measures
We extracted baseline patient characteristics from each study, including aneurysm location, aneurysm size, number of aneurysms, sex, mean age, and Hunt and Hess score at admission.
To evaluate WEB device efficacy, we identified our primary and secondary outcomes. The primary one was rebleeding (rerupture) of the aneurysm after the deployment of the WEB device. This primary outcome was chosen because the primary goal of treating a ruptured aneurysm is to prevent rerupture. Secondary outcomes included occlusion status of the aneurysm at the last follow-up point available in each study, retreatment rates, safety outcomes including WEB device–related hemorrhagic and thromboembolic complications, favorable clinical outcome represented by mRS scores between 0 and 2 at last follow-up, and procedure-related and overall mortality rates. If the patients had been followed up for >1 year, 1-year occlusion status was selected as the end point. Short-term follow-up was defined as <1-year follow-up, while ≥1-year follow-up was considered midterm follow-up. Low-quality studies included studies with a high and moderate risk of bias, and studies with a low risk of bias were identified as high-quality studies. Adequate occlusion was assessed according the occlusion scale mentioned in each study, which generally included complete aneurysm occlusion or small neck remnant. Data were also collected concerning anticoagulation and antiplatelet use.
Study Risk of Bias Assessment
We used the Newcastle-Ottawa Quality Assessment Scale for case-control studies tool to assess the risk of bias in our included studies. Although this tool was designed for the comparative studies, we modified it, focusing on 5 questions: 1) Did the study include all patients or consecutive patients versus a selected sample; 2) was the study retrospective or prospective; 3) was angiographic and clinical follow-up satisfactory, thus allowing ascertainment of all outcomes; 4) were outcomes clearly reported; and, 5) were the operators treating the patients, the same ones who assessed angiographic and clinical outcomes?
Statistical Analysis
The cumulative incidence (event rate per patient at the end of the study) for each study was estimated, along with 95% confidence intervals. Because we anticipated marked heterogeneity in the populations and interventions across various included studies, a random effects model was used to pool incidence rates across studies. The I2 statistic was used to express the proportion of inconsistency not attributable to chance. Analyses including subgroup meta-analysis were conducted using OpenMeta[Analyst] open-source statistical software (http://www.cebm.brown.edu/openmeta/).
RESULTS
Study Selection and Characteristics
After removing duplicates, we found 1701 articles. After we excluded nonrelevant articles by the screening of the title and abstract, 69 articles were included for full-text screening. Eighteen studies (19 articles)3-21 were included in our qualitative and quantitative analyses. The results of 1 included study were published in 2 separate articles that had to be included to cover the total characteristics and outcomes of the sample.8,9 Overall, 487 patients with 496 aneurysms were considered. The mean age was 57 years; 387 (83.6%) and 76 (16.4%) of these aneurysms were located in the anterior (commonly in anterior communicating artery, MCA, and posterior communicating artery) and posterior (commonly in the basilar artery and PICA) circulations, respectively. The mean width of the ruptured aneurysms, reported in 10 studies, was 5.6 mm. The mean height of the ruptured aneurysms (consistently reported in 5 studies) was 6.0 mm. Most aneurysms were wide-neck. Two hundred seventy-five (74.7%) patients were admitted with Hunt and Hess scores of 1–3, and 93 patients (25.3%) experienced severe SAH (Hunt and Hess score, 4–5). Forty-one additional interventions, consisting mainly of coiling or stent placement, were used for incompletely occluded ruptured aneurysms. A summary of the data of the included studies and reported baseline characteristics of the ruptured aneurysms are provided in the Online Supplemental Data. The flow diagram for study selection is provided in the Online Supplemental Data.
Primary Outcomes
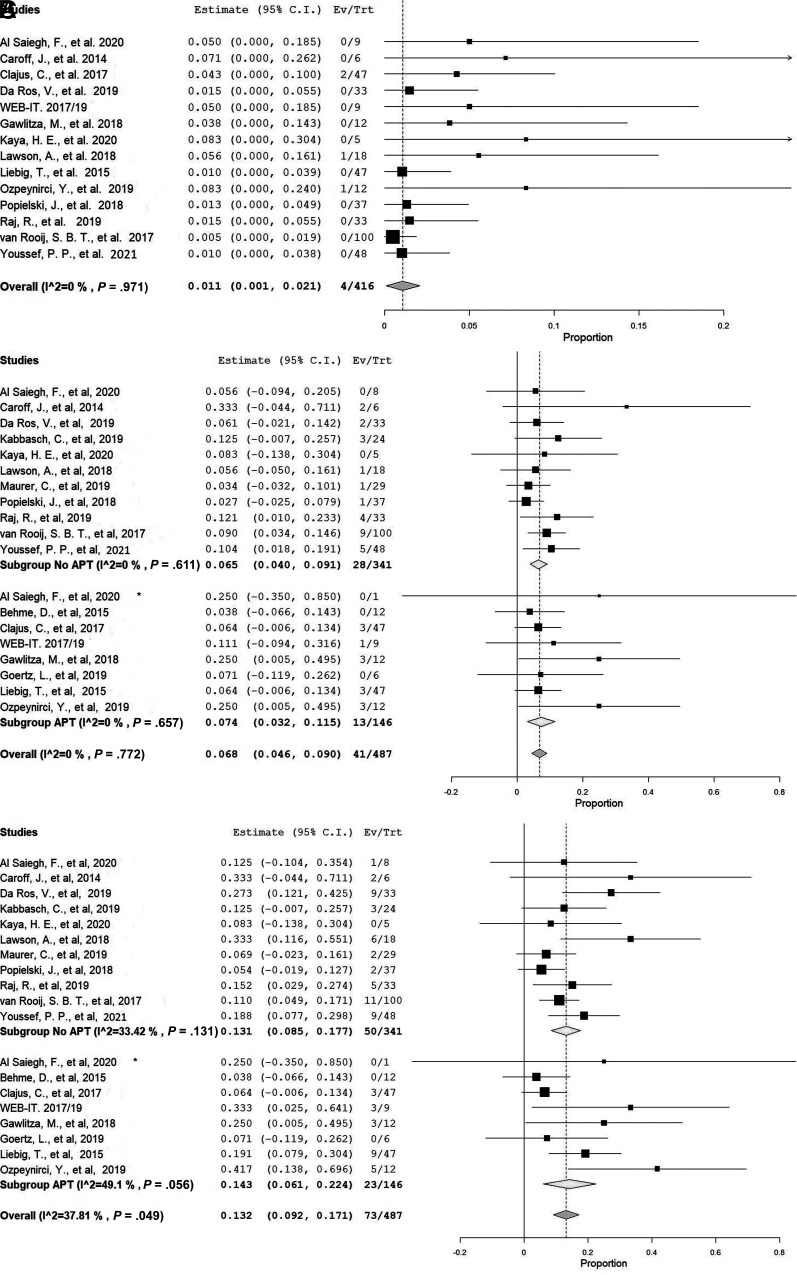
The primary outcome (aneurysm rerupture after treatment with the WEB device) occurred in 1.1% of cases (95% CI, 0.1%–2.1%) (4/423 aneurysms): 0.8% (95% CI, 0.3%–2%) and 1.7% (95% CI, 0.1%–3.5%) in the studies with short- and midterm follow-ups, respectively. The rate of rerupture per month during follow-up was 0.2% (95% CI, 0.0%–0.3%).
Secondary Outcomes
A total of 285 patients from 16 studies had angiographic occlusion outcomes reported. Adequate occlusion was achieved after short-term follow-up in 193 of 216 patients (91.7%; 95% CI, 87.4%–95.9%) and after midterm follow-up in 77 of 94 patients (77%; 95% CI, 68.6%–85.4%). In total, 245 patients showed adequate occlusion (87.3%; 95% CI, 82.1%–92.4%) at last follow-up. Adequate occlusion was reported in 78.1% (95% CI, 69.4%–86.8%) among studies with high quality (low risk of bias) and 93.4% (95% CI, 90%–96.9%) among studies with low quality (high or moderate risk of bias). The mean length of follow-up ranged from 3 to 15 months, with a median of 7 months. During follow-up, 27 aneurysms required retreatment (5.1%; 95% CI, 3%–7.3%), 5.8% (95% CI, 2.8%–8.7%) at short-term and 6% (95% CI, 1.2%–10.9%) at midterm follow-up. The treatment-related perioperative complication rate was 13.2% (95% CI, 9.2%–17.1%). Only a minority of these complications (24.4%; 95% CI, 14.5%–34.3%) resulted in prolonged clinical deterioration or permanent neurologic deficits; thus, the overall clinical complication rate related to WEB device deployment was 3.2% (95% CI, 1.6%–4.7%), 13 hemorrhagic (2%; 95% CI, 0.8%–3.3%) and 41 thromboembolic (6.8%; 95% CI, 4.6%–9%) complications. At short- and midterm follow-up, 210 of 250 patients (85%; 95% CI, 78%–92.1%) and 55 of 64 patients (86.7%; 95% CI, 78.5%–95%) showed favorable clinical outcomes (mRS 0–2), respectively. Twelve patients died in the perioperative period as a result of procedure-related complications (2.1%; 95% CI, 0.8%–3.3%). The all-cause mortality rate was 11.5% (95% CI, 7%–16%).
Antiplatelet Therapy
Routine antiplatelet therapy (APT) was not used in 10 included studies, while in 8 studies, APT was routinely given before and after the procedure either as monotherapy or dual therapy for variable time intervals. Many studies reported the use of dual antiplatelet therapy for 1–2 months in cases in which a thromboembolic complication occurred or an additional device was deployed. In those who received any APT, the rate of thromboembolic and treatment-related overall complications was 7.4% (95% CI, 3.2%-11.5%) and 14.3% (95% CI, 6.1%-22.4%), respectively. In studies in which no routine APT was used, the rates of thromboembolic and treatment-related overall complications were 6.5% (95% CI, 4%-9.1%) and 13.1% (95% CI, 8.5%-17.7%), respectively. The procedure-related primary and secondary outcomes and details of the APT use are explained in the Online Supplemental Data. The forest plots of the meta-analysis of the outcomes are shown in the Figure and the Online Supplemental Data.
FIGURE.
A, Forest plot with a random effects model shows the late rebleeding rate. B, Forest plot with a random effects model shows the rate of treatment-related perioperative complications grouped by APT use. C, Forest plot with a random effects model shows the rate of thromboembolic complications grouped by APT use. Ev/Trt indicates Event (Outcome, complication)/Treated patient.
Risk of Bias
Of 18 studies, the risk of bias was low in 5, moderate in 10, and high in 3 studies. The smallest study had 5 patients with 5 ruptured aneurysms, and the largest study included 100 patients with 106 aneurysms (100 ruptured aneurysms).
DISCUSSION
This systematic review and meta-analysis of 18 cohort studies demonstrates that primary treatment of ruptured intracranial aneurysms with the WEB device is both safe and effective, with rerupture rates of approximately 1% and a perioperative complication rate of 13%, with most of these not resulting in additional clinical deficits. At long-term follow-up, >85% of the aneurysms showed adequate obliteration, suggesting that in general, the obliteration rate of ruptured aneurysms treated with the WEB device is high. These findings are important because they suggest that the WEB device could potentially be used routinely in the treatment of ruptured wide-neck bifurcation aneurysms.
One commonly used technique for the treatment of wide-neck bifurcation aneurysms is stent-assisted coiling. Several series have recently been published examining the efficacy of stent-assisted coiling in the treatment of ruptured aneurysms.22 In a recently published meta-analysis by Bsat et al,22 the rates of postinterventional rebleeding (2.5%) and hemorrhagic complications (8.7%) were higher than the rates of these complications in the setting of WEB device deployment according to our meta-analysis. The risk of thromboembolic complications in the meta-analysis of Bsat et al was 9.1%, and this is also higher than that found in our study (6.8%). Another meta-analysis published in 2019 compared the rate of perioperative complications for ruptured intracranial aneurysms treated with stent-assisted coiling with the rate of those treated with simple coiling and reported a 20.2% complication rate for the stent-assisted coiling group versus 13.1% for the coiling-only group.23 Nevertheless, the rate of perioperative complications in our meta-analysis was lower (13.2%) than that in the stent-assisted coiling group and similar to the that in the coiling group. Thus, intrasaccular flow-diversion treatment with the WEB may be safer than stent-assisted coiling and as safe as primary coiling for treating wide-neck bifurcation aneurysms.
Prevention of rebleeding is the primary goal in the treatment of ruptured aneurysms. The rate of rebleeding in our meta-analysis is similar to that reported in the Analysis of Recanalization after Endovascular Treatment of Intracranial Aneurysm (ARETA) study,24,25 a large prospective, multicenter study conducted to assess the recanalization of ruptured intracranial aneurysms after endovascular treatment with coiling and balloon-assisted coiling. The risk of rebleeding in 753 patients with ruptured aneurysms in the ARETA study was 1% (95% CI, 0.3%–1.7%). These patients had 78 thromboembolic (10.4%) and 28 hemorrhagic (3.7%) complications, similar to the results of our study. In the International Subarachnoid Aneurysm Trial (ISAT),26 1073 patients underwent coil embolization for ruptured aneurysms. The rate of rebleeding was 1.9% in the first 30 days after the treatment and 0.8% at follow-up (30 days to 1 year); 92% of aneurysms showed adequate occlusion (66%, complete occlusion; 26%, neck remnant or subtotal occlusion), and the overall mortality rate after 1 year was 8.1%. In comparison with ISAT, in our meta-analysis, the overall rebleeding (1%) and adequate occlusion (87%) rates were similar, an outcome especially remarkable given that the aneurysms treated with the WEB device were mostly wide-neck. The overall mortality rate (11.5%) was slightly higher. Nevertheless, 25% of our study patients were admitted with severe SAH compared with 12% in ISAT, and procedure-related mortality was around 2%. From these results, we can infer that the WEB may be as safe as simple coiling and balloon-assisted coiling to treat acutely ruptured intracranial aneurysms.
One interesting issue that has been brought up recently is whether there are differences in occlusion rates and clinical outcomes between ruptured and unruptured aneurysms treated with the WEB. In a study published by Pierot et al,27 which included cumulative data from 3 clinical trials comprising >90% unruptured aneurysms (WEB Clinical Assessment of Intrasaccular Aneurysm Therapy [WEBCAST],28 WEBCAST-2,29 and the French Observatory30), the rate of adequate occlusion at 1 year was found to be 79.1%, which is similar to the adequate occlusion rate (77%) of ruptured aneurysms at midterm follow-up in our meta-analysis. In the WEBCAST study, 85.4% of the treated unruptured aneurysms had adequate occlusion at short-term follow-up (6 months), which is only slightly less than that in our study of ruptured aneurysms (91.7%). This can be referred to prothrombotic milieu in the setting of SAH that may accelerate aneurysm thrombosis.31 The rate of retreatment in unruptured aneurysms at 1-year follow-up was 6.9%,27 which is also similar to that in our study (6%). Thromboembolic adverse events occurred in 14.4% of the patients in the cumulative data of Pierot et al, which is twice as high as that in our results (6.8%). However, the symptomatic thromboembolic events with clinical sequelae were reported as 3%, which is similar to the rate (3.2%) of overall clinical complications in our meta-analysis. Furthermore, the rate (2%) of hemorrhagic complications in this study is also similar to that in the previous study with unruptured aneurysms (1.8%). These results demonstrate that the WEB device has a high efficacy and feasibility in the management of ruptured wide-neck cerebral aneurysms, similar to what was confirmed in the past for unruptured wide-neck aneurysms.
The main advantage of using the WEB device over stent or flow-diversion techniques is the absence of the requirement for periprocedural dual antiplatelet treatment. This is especially important in the acute phase of aneurysmal SAH when patients often require invasive procedures such as placement of external ventricular drains or lumbar drains and ventriculoperitoneal shunts, procedures that can be complicated by hemorrhage in the setting of dual antiplatelet therapy. While there was no notable difference in thromboembolic (non-APT, 6.5%; APT, 7.4%) and overall complication (non-APT, 13.1%; APT, 14.3%) rates between patients with and without routine nonuse of antiplatelet therapy in our meta-analysis, at our center, we continue to place these patients on antiplatelet therapy with high-dose aspirin and clopidogrel (Plavix) because we believe that this prevents severe acute thrombosis after WEB device placement in ruptured aneurysms.
Limitations
All the included studies in this meta-analysis were uncontrolled, and many of them were retrospective and single-center. Thus, our meta-analysis is limited by selection bias and the heterogeneity that arises from the variability in aneurysm morphologies, patient scenarios, operator experience, and practice protocols (eg, pre- and postoperative antiplatelet therapy application methods). Despite our effort to exclude overlapping patient populations, the possibility of overlap in patients among the studies remains. The mean follow-up periods among the included studies were variable and modest in terms of length. However, we tried to reduce this variety by selecting the last follow-up point or 1-year follow-up results. Most of the studies in the literature lack the stratification of outcomes based on aneurysm rupture status. Among those that investigated outcomes on the basis of the rupture status, many did not report the impact of important variables such as the type and size of the WEB device and baseline patient morbidity in the treatment outcomes. The rate of adequate occlusion in studies with low quality, mostly due to self-assessment of the angiographic and clinical outcomes, was higher (93.4%) than that of high-quality-studies (78.1%). This finding raises the question of bias that results from including such low-quality studies. Furthermore, the relatively small sample of patients with reported angiographic outcomes, absence of a single and common standard method for assessment of aneurysm occlusion, lack of a control group, and the short duration of follow-up may affect the reliability of the results concerning the safety and durability of aneurysm occlusion. Therefore, the overall certainty in the evidence at present is rated as low.32 Finally, this meta-analysis has concentrated on only ruptured aneurysms, which can be a limitation. However, the main advantage of this meta-analysis was the thorough assessment of WEB device efficacy and safety in acute subarachnoid hemorrhage situations.
CONCLUSIONS
Our meta-analysis of 18 studies including around 500 ruptured aneurysms treated with the WEB device demonstrated the safety and efficacy of the WEB device in the management of wide-neck ruptured aneurysms. Further studies and randomized clinical trials with longitudinal follow-up directly comparing the WEB with other established techniques (ie, coiling, clipping, stent-assisted coiling, and so forth) are needed.
ABBREVIATION:
- APT
antiplatelet therapy
Footnotes
Disclosures: Giuseppe Lanzino—UNRELATED: Consultancy: Superior Medical Experts, Nested Knowledge. Waleed Brinjikji—RELATED: Consulting Fee or Honorarium: MicroVention, Comments: consultant and proctor for MicroVention*; UNRELATED: Board Membership: Marblehead Medical LLC, Comments: owner; Consultancy: Cerenovus.* *Money paid to the institution.
References
- 1.Mine B, Pierot L, Lubicz B. Intrasaccular flow-diversion for treatment of intracranial aneurysms: the Woven EndoBridge. Expert Rev Med Devices 2014;11:315–25 10.1586/17434440.2014.907741 [DOI] [PubMed] [Google Scholar]
- 2.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Saiegh F, Hasan D, Mouchtouris N, et al. Treatment of acutely ruptured cerebral aneurysms with the Woven EndoBridge device: experience post-FDA approval. Neurosurgery 2020;87:E16–22 10.1093/neuros/nyaa092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behme D, Berlis A, Weber W. Woven EndoBridge intrasaccular flow disrupter for the treatment of ruptured and unruptured wide-neck cerebral aneurysms: report of 55 cases. AJNR Am J Neuroradiol 2015;36:1501–06 10.3174/ajnr.A4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caroff J, Mihalea C, Dargento F, et al. Woven Endobridge (WEB) device for endovascular treatment of ruptured intracranial wide-neck aneurysms: a single-center experience. Neuroradiology 2014;56:755–61 10.1007/s00234-014-1390-7 [DOI] [PubMed] [Google Scholar]
- 6.Clajus C, Strasilla C, Fiebig T, et al. Initial and mid-term results from 108 consecutive patients with cerebral aneurysms treated with the WEB device. J Neurointerv Surg 2017;9:411–17 10.1136/neurintsurg-2016-012276 [DOI] [PubMed] [Google Scholar]
- 7.Da Ros V, Bozzi A, Comelli C, et al. Ruptured intracranial aneurysms treated with Woven EndoBridge intrasaccular flow disruptor: a multicenter experience. World Neurosurg 2019;122:e498–505. 10.1016/j.wneu.2018.10.088 [DOI] [PubMed] [Google Scholar]
- 8.Fiorella D, Boulos A, Turk AS, et al. The safety and effectiveness of the LVIS stent system for the treatment of wide-necked cerebral aneurysms: final results of the pivotal US LVIS trial. J Neurointerv Surg 2019;11:357–61 10.1136/neurintsurg-2018-014309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arthur AS, Molyneux A, Coon AL, et al. The safety and effectiveness of the Woven EndoBridge (WEB) system for the treatment of wide-necked bifurcation aneurysms: final 12-month results of the pivotal WEB Intrasaccular Therapy (WEB-IT) study. J Neurointerv Surg 2019;11:924–30 10.1136/neurintsurg-2019-014815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gawlitza M, Soize S, Januel A-C, et al. Treatment of recurrent aneurysms using the Woven EndoBridge (WEB): anatomical and clinical results. J Neurointerv Surg 2018;10:629–33 10.1136/neurintsurg-2017-013287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goertz L, Liebig T, Siebert E, et al. Extending the indication of Woven EndoBridge (WEB) embolization to internal carotid artery aneurysms: a multicenter safety and feasibility study. World Neurosurg 2019;126:e965–74. 10.1016/j.wneu.2019.02.198 [DOI] [PubMed] [Google Scholar]
- 12.Kabbasch C, Goertz L, Siebert E, et al. WEB embolization versus stent-assisted coiling: comparison of complication rates and angiographic outcomes. J Neurointerv Surg 2019;11:812–16 10.1136/neurintsurg-2018-014555 [DOI] [PubMed] [Google Scholar]
- 13.Kaya HE, Bakdık S, Keskin F, et al. Endovascular treatment of intracranial aneurysms using the Woven EndoBridge (WEB) device: retrospective analysis of a single center experience. Clin Imaging 2020;59:25–29 10.1016/j.clinimag.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 14.Lawson A, Molyneux A, Sellar R, et al. Safety results from the treatment of 109 cerebral aneurysms using the Woven EndoBridge technique: preliminary results in the United Kingdom. J Neurosurg 2018;128:144–53 10.3171/2016.9.JNS152849 [DOI] [PubMed] [Google Scholar]
- 15.Liebig T, Kabbasch C, Strasilla C, et al. Intrasaccular flow disruption in acutely ruptured aneurysms: a multicenter retrospective review of the use of the WEB. AJNR Am J Neuroradiol 2015;36:1721–27 10.3174/ajnr.A4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurer C, König I, Berlis A, et al. Two-center experience in the endovascular treatment of intracranial aneurysms using the Woven EndoBridge 17 device including midterm follow-up results: a retrospective analysis. AJNR Am J Neuroradiol 2019;40:1517–22 10.3174/ajnr.A6177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozpeynirci Y, Braun M, Pala A, et al. WEB-only treatment of ruptured and unruptured intracranial aneurysms: a retrospective analysis of 47 aneurysms. Acta Neurochir (Wien) 2019;161:1507–13 10.1007/s00701-019-03988-0 [DOI] [PubMed] [Google Scholar]
- 18.Popielski J, Berlis A, Weber W, et al. Two-center experience in the endovascular treatment of ruptured and unruptured intracranial aneurysms using the WEB device: a retrospective analysis. AJNR Am J Neuroradiol 2018;39:111–January 10.3174/ajnr.A5413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raj R, Rautio R, Pekkola J, et al. Treatment of ruptured intracranial aneurysms using the Woven EndoBridge device: a two-center experience. World Neurosurg 2019;123:e709–16. 10.1016/j.wneu.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 20.van Rooij SB, van Rooij WJ, Peluso JP, et al. WEB treatment of ruptured intracranial aneurysms: a single-center cohort of 100 patients. AJNR Am J Neuroradiol 2017;38:2282–87 10.3174/ajnr.A5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youssef PP, DornbosD, III, Peterson J, et al. Woven EndoBridge (WEB) device in the treatment of ruptured aneurysms. J Neuronterv Surg 2021;13:443–46 10.1136/neurintsurg-2020-016405 [DOI] [PubMed] [Google Scholar]
- 22.Bsat S, Bsat A, Tamim H, et al. Safety of stent-assisted coiling for the treatment of wide-necked ruptured aneurysm: a systematic literature review and meta-analysis of prevalence. Interv Neuroradiol 2020;26:547–56 10.1177/1591019920945059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Zuo Q, Tang H, et al. Stent assisted coiling versus non-stent assisted coiling for the management of ruptured intracranial aneurysms: a meta-analysis and systematic review. J Neurointerv Surg 2019;11:489–96 10.1136/neurintsurg-2018-014388 [DOI] [PubMed] [Google Scholar]
- 24.Pierot L, Barbe C, Nguyen HA, et al. Intraoperative complications of endovascular treatment of intracranial aneurysms with coiling or balloon-assisted coiling in a prospective multicenter cohort of 1088 participants: Analysis of Recanalization after Endovascular Treatment of Intracranial Aneurysm (ARETA) study. Radiology 2020;295:381–89 10.1148/radiol.2020191842 [DOI] [PubMed] [Google Scholar]
- 25.Pierot L, Barbe C, Herbreteau D, et al. Rebleeding and bleeding in the year following intracranial aneurysm coiling: analysis of a large prospective multicenter cohort of 1140 patients: Analysis of Recanalization after Endovascular Treatment of Intracranial Aneurysm (ARETA) study. J NeuroIntervent Surg 2020;12:1219–25 10.1136/neurintsurg-2020-015971 [DOI] [PubMed] [Google Scholar]
- 26.Molyneux AJ, Kerr RS, Yu L-M, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809–17 10.1016/S0140-6736(05)67214-5 [DOI] [PubMed] [Google Scholar]
- 27.Pierot L, Moret J, Barreau X, et al. Safety and efficacy of aneurysm treatment with WEB in the cumulative population of three prospective, multicenter series. J Neurointerv Surg 2018;10:553–59 10.1136/neurintsurg-2017-013448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierot L, Costalat V, Moret J, et al. Safety and efficacy of aneurysm treatment with WEB: results of the WEBCAST study. J Neurosurg 2016;124:1250–56 10.3171/2015.2.JNS142634 [DOI] [PubMed] [Google Scholar]
- 29.Pierot L, Gubucz I, Buhk JH, et al. Safety and efficacy of aneurysm treatment with the WEB: results of the WEBCAST 2 study. AJNR Am J Neuroradiol 2017;38:1151–55 10.3174/ajnr.A5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierot L, Moret J, Turjman F, et al. WEB treatment of intracranial aneurysms: clinical and anatomic results in the French Observatory. AJNR Am J Neuroradiol 2016;37:655–59 10.3174/ajnr.A4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamilton MG, Dold ON. Spontaneous disappearance of an intracranial aneurysm after subarachnoid hemorrhage. Can J Neurol Sci 1992;19:389–91 [PubMed] [Google Scholar]
- 32.Murad MH, Montori VM, Ioannidis JPA, et al. How to read a systematic review and meta-analysis and apply the results to patient care: users’ guides to the medical literature. JAMA 2014;312:171–79 10.1001/jama.2014.5559 [DOI] [PubMed] [Google Scholar]