Abstract
Background:
U.S. breast cancer incidence has been changing, as have distributions of risk factors, including body mass index (BMI), age-at-menarche, age-at-first-live-birth and number of live births.
Methods:
Using data for U.S. women from large nationally representative surveys, we estimated risk factor distributions from 1980-2008. To estimate ecologic associations with breast cancer incidence, we fitted Poisson models to age- and calendar-year-specific incidence data from the National Cancer Institute’s Surveillance, Epidemiology and End Results registries from 1980-2011. We then assessed the proportion of incidence attributable to specific risk factors by comparing incidence from models that only included age and calendar period as predictors with models that additionally included age- and cohort-specific categorized mean risk factors. Analyses were stratified by age and race.
Results:
Ecologic associations usually agreed with previous findings from analytic epidemiology. From 1980-2011, compared to the risk factor reference level, increased BMI was associated with 7.6% decreased incidence in women aged 40-44 and 2.6% increased incidence for women aged 55-59. Fewer births were associated with 22.2% and 3.99% increased incidence in women aged 40-44 and 55-59 years, respectively. Changes in age at menarche and age-at-first-live-birth in parous women did not significantly impact population incidence from 1980-2011.
Conclusions:
Changes in BMI and number of births since 1980 significantly impacted U.S. breast cancer incidence.
Impact:
Understanding and quantifying long-term impact of risk factor trends on incidence is important to understand the future breast cancer burden and inform prevention efforts.
Introduction
U.S. breast cancer incidence increased until about 2000, and then decreased slightly. Some of these changes might be from changes in the distribution of known breast cancer risk factors. For example, a drop of 6.7% in 2003 versus 2002 (1), has been attributed to less use of hormone replacement therapy, following the Women’s Health Initiative report in July 2002 (2). Changes in the distributions of other breast cancer risk factors, including reproductive factors such as age-at-first-live-birth or the number of live births, and personal and behavioral characteristics including alcohol consumption and body mass index (BMI) may have influenced these trends also. Understanding the long-term impact of risk factor trends on incidence is important to understand the future breast cancer burden and may inform prevention efforts.
Few epidemiologic studies accrue and follow women long enough to capture secular or birth cohort changes in risk factor distributions. Freedman et al. (3) published trends by birth cohort for smoking and various reproductive variables among women in a U.S. cohort of radiation technologists. Nichols et al. (4) analyzed secular trends in ages at menarche and menopause, and reproductive life span using pooled data from female controls in three population-based case-control studies in Wisconsin, Massachusetts, and New Hampshire. These publications did not evaluate the association of changes in risk factor distributions with breast cancer incidence trends.
We assembled data from U.S. national surveys and analyzed trends in breast cancer risk factor distributions over nearly 30 years for U.S. women overall and in strata (<50 versus ≥50 years old; black versus white women). We used Poisson regression, with allowance for over-dispersion, to estimate ecologic associations of BMI, age-at-menarche, age-at-first-live-birth, and number of births with age- and period-specific breast cancer incidence in U.S. women. Using these associations, we estimated how much breast cancer incidence would have differed from the observed incidence from 1980 to 2011 had risk factors been fixed at reference levels.
We present methods for ecologic analyses, including an attributable risk-like calculation, that are illustrated and motivated by the breast cancer application but have broad potential use.
Methods
Risk Factor Data
We focused on four well-established breast cancer risk factors, BMI, age-at-menarche, number of live births, and age-at-first-live-birth, see e.g. (5).
We obtained risk factor information for women aged ≥20 years at examination and born between 1917 and 1988 from nationally representative surveys described below and in Supplemental Table 1.
National Health and Nutrition Surveys (NHANESs)
NHANES samples are designed to be nationally representative of the civilian, non-institutionalized U.S. population. Participants are selected using a complex, stratified, multistage probability cluster sampling design. Following a home interview, subjects were examined in mobile examination centers (MECs). All information on our variables was obtained at the MECs, and we used MEC sampling weights for all NHANES surveys. To increase the sample size in overlapping age-birth cohort groups for women in the eligible age and birth-year range, we combined data from 7,040 women from the NHANES I, 1971-1975 (6) and 4,147 women from NHANES II, 1976-1980 (7) and revised the weights (8, page 282). From NHANES III (9), 1988-1994, we used data for 7,769 women. We combined 5 two-year NHANES surveys to obtain the continuous NHANES 1999-2008, resulting in 12,309 additional women in the age and birth-year range for our analysis. We adjusted the survey weights for the 10-year period (8, page 282) and harmonized variables as appropriate. Age-at-first-live-birth was not included in NHANES I and II but was obtained from NHANES III and continuous NHANES.
National Health Interview Surveys (NHISs)
The NHIS is a cross-sectional, national survey that measures the health of the U.S. civilian non-institutionalized population. We used data and corresponding weights from 10,691 women in the eligible age and birth cohorts from the NHIS 1987 (10). From NHIS 2000 (11) we used data from 32,463 women, and from NHIS 2005 (11) we used data from 17,283 women. From all three NHIS surveys we obtained information on BMI, age-at-menarche, number of births, and age-at-first-live-birth.
Risk factor coding
We defined 71 partially overlapping 5-year birth-cohorts, from 1917 to 1988. The last two birth cohorts only include data up to 1988. We created 13 non-overlapping five-year age categories (20-24, 25-29, … ,80-84). We estimated proportions, means and standard errors (SEs) for each risk factor for each age-cohort group (PROC SURVEYMEANS, PROC SURVEYFREQ, SAS 9.3), using appropriate sampling weights. Missing data and observations with non-positive weights were excluded from analysis. We repeated the computations restricted to white and black women.
We categorized mean exposure values using quintiles from the overall population. For plots that used coarser age categorizations (<40 years, 40-<50 years, 50-<60 years and 60 years or older), mean values of risk factors for 5-year age groups were combined using weights proportional to total population size (from the National Cancer Institute’s (NCI) Surveillance, Epidemiology and End Results (SEER) mortality database) in 5-year age groups and calendar years.
Breast Cancer Incidence
We used breast cancer incidence data for the same thirteen 5-year age groups and six 5-year calendar periods (1982-1986, 1987-1991, …, 2007-2011) spanning 18 partially overlapping 10-year birth-cohorts from the NCI’s SEER 9 and 13 Registries databases (SEER 9-13).
We modeled breast cancer incidence separately for women aged <50 years (“pre-menopausal”) and ≥50 years (“post-menopausal”) and for white and black women to investigate different effects of risk factors and period by age group (“menopausal status”) and race.
We analyzed SEER incidence counts, population sizes at risk, and categorized mean risk factor levels in ecologic units defined by age and period groups.
Statistical analysis
We assumed that the numbers of breast cancer cases Ya,p, for specific 5-year age groups a = 0,1,2,…A, and periods p = 0,1,2,…P, are independent Poisson counts. This working model leads to estimates of the expected counts. However, we relax the independence assumption by allowing for over-dispersion when computing standard errors. For each age and period group, c is the corresponding birth-cohort value, with level Xa,c, for risk factor X. Let Z1a and Z2p be indicator variables that are 1 for age group a and period p, respectively, and 0 otherwise, for a = 1,2,…, A, and p = 1,2,…, P. Reference levels were a = 0 and p = 0. First, we fit a Poisson model to the data that included age and period and the logarithm of the person-years, ηa,p, in cell (a,p), as an offset (PROC GENMOD, SAS 9.3), such that the expected count is , where
| (1) |
For the second model, we added the risk factor Xa,c,
| (2) |
We allowed for over-dispersion in estimating standard errors (option SCALE=PEARSON in PROC GENMOD), as described in detail in the Supplemental Material.
The risk factor categories and corresponding parameter estimates from fitting each risk factor X marginally are shown in Supplemental Tables 2 and 3.
We compared the unadjusted estimated rates from model (1) with the rates λa,p,X0 obtained from model (2) with Xa,c set to the reference level X0. To assess the impact of adjustment for a risk factor over time, we averaged values of and of λa,p,X0 for periods p between 1980 and 1994 and between 1995 and 2011. The division at 1995 was chosen because it is the middle period. We converted these average rates into the percent relative difference (analogous to attributable risk),
| (3) |
The variance computation for Δ(a, p-interval) is described in the Supplemental Material.
We plotted and log10(λa,p,X0) against calendar time to display more refined information on the effect of adjustment for risk factors over time. In particular, we chose two age groups, 40-44 years and 60-64 years to represent pre- and post-menopausal women in these plots. However, all pre-menopausal age groups have the same log-incidence plots, apart from vertical displacements, as do all post-menopausal age groups. Thus, it suffices to present only two age groups. Percent relative differences, Δ, do depend on age group (Table 1). “Statistical significance” refers to two-sided 0.05 level tests.
Table 1:
Values of percent relative difference in incidence, . The three intervals are 1980-1994, 1995-2011, and 1980-2011. Results in the table are based on separate models for women aged <50 and ≥50 years.
| Values of Δ with standard errors in parentheses | ||||
|---|---|---|---|---|
| Risk factor | Age | 1980-1994 | 1995-2011 | 1980-2011 |
| BMI | 35-39 | −5.09 (1.41) | −8.27 (2.80) | −6.78 (2.12) |
| 40-44 | −5.90 (1.89) | −9.10 (3.32) | −7.60 (2.63) | |
| 45-49 | −6.13 (2.08) | −9.34 (3.54) | −7.84 (2.84) | |
| 50-54 | 1.99 (1.80) | 3.46 (2.50) | 2.81 (2.16) | |
| 55-59 | 1.78 (1.97) | 3.26 (2.64) | 2.60 (2.31) | |
| 60-64 | 1.90 (1.97) | 3.37 (2.63) | 2.72 (2.30) | |
| 65-69 | 1.09 (1.87) | 2.58 (2.50) | 1.91 (2.18) | |
| Age at menarche | 35-39 | −5.87 (4.61) | −6.14 (4.62) | −6.02 (4.61) |
| 40-44 | −5.42 (4.38) | −5.68 (4.40) | −5.56 (4.39) | |
| 45-49 | −6.05 (4.65) | −6.33 (4.65) | −6.20 (4.66) | |
| 50-54 | 0.17 (0.75) | 1.06 (1.30) | 0.67 (1.05) | |
| 55-59 | −0.10 (0.68) | 0.79 (1.22) | 0.39 (0.98) | |
| 60-64 | −0.61 (0.56) | 0.28 (1.05) | −0.12 (0.83) | |
| 65-69 | −0.95 (0.36) | −0.05 (0.82) | −0.45 (0.59) | |
| Number of births | 35-39 | 22.06 (3.84) | 24.09 (4.05) | 23.14 (3.95) |
| 40-44 | 21.08 (3.79) | 23.14 (3.97) | 22.17 (3.88) | |
| 45-49 | 20.73 (3.80) | 22.80 (3.96) | 21.83 (3.87) | |
| 50-54 | 3.36 (1.03) | 3.51 (1.39) | 3.44 (1.22) | |
| 55-59 | 3.91 (0.71) | 4.06 (0.98) | 3.99 (0.85) | |
| 60-64 | 4.17 (0.68) | 4.31 (0.82) | 4.25 (0.73) | |
| 65-69 | 2.61 (0.60) | 2.75 (0.56) | 2.69 (0.54) | |
| Age at first live birth among parous women* | 35-39 | 2.29 (1.37) | 1.79 (2.40) | 2.00 (1.91) |
| * starts in 1981 | 40-44 | 1.77 (1.26) | 1.27 (2.14) | 1.48 (1.69) |
| 45-49 | 1.26 (1.01) | 0.76 (1.90) | 0.98 (1.44) | |
| 50-54 | 0.46 (0.72) | 0.31 (0.66) | 0.37 (0.68) | |
| 55-59 | 0.29 (0.54) | 0.14 (0.52) | 0.20 (0.52) | |
| 60-64 | 0.31 (0.47) | 0.15 (0.43) | 0.22 (0.44) | |
| 65-69 | 0.37 (0.39) | 0.22 (0.27) | 0.28 (0.31) | |
Results
Breast cancer risk factor patterns over time
Sample sizes for risk factor surveys overall and by race are in Supplemental Table 1.
All U.S. women
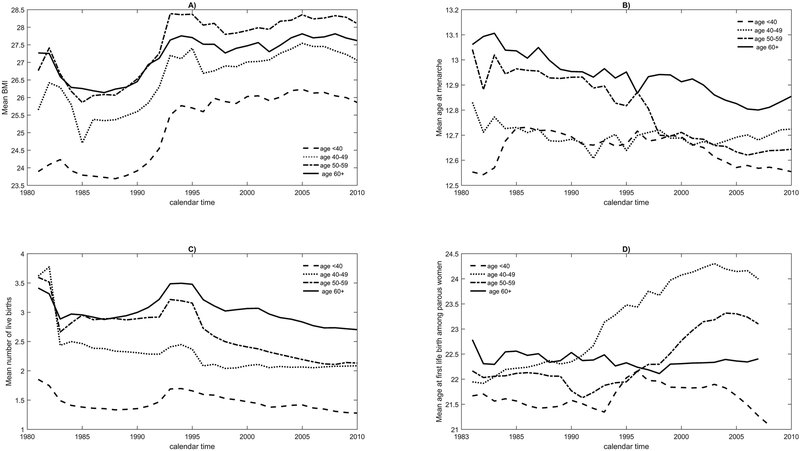
Figure 1 (panels A-D) plots the mean levels of four risk factors over time, weighted to the U.S. female population, separately for age groups <40, 40-<50, 50-<60 and ≥60 years.
Figure 1.
Distribution of mean BMI, mean age at menarche, mean number of births, mean age at first live among parous women from 1980 to 2008 for various age groups, reweighted to the population of all U.S. women.
Figure 1 shows the distribution of breast cancer risk factors in all U.S. women over time in 4 age groups. Panels A) through D) correspond to the distribution of BMI, age at menarche, number of live births and age at first live birth, respectively.
Mean BMI increased for all age groups, and by 1995 mean BMI was and remained elevated compared to the 1980s (Figure 1, panel A). For example, for women aged <40 years, the mean BMI was 23.9 kg/m2 in 1980, 25.7 kg/m2 in 1995, and 25.9 kg/m2 in 2009. Women under age 50 years had lower mean BMIs. Women aged 50-59 had comparable mean BMIs to women aged 60+ years before 1993, but higher BMIs thereafter.
For each calendar time point, mean age-at-menarche was lowest in women under 50 years old and highest in women aged ≥60 years (Figure 1, panel B). For all age groups, however, mean age-at-menarche declined. The strongest drop was seen for women ages 50-60, whose mean age-at-menarche was 13.0 years in 1980, and 12.6 years in 2009.
The mean number of live births also declined overall for all women between 1980 and 2009 (Figure 1 panel C). However, an increase between 1990 and 1997, that is most pronounced among the oldest women, reflects the baby boom after World War II.
Mean age-at-first-live-birth among parous women did not change appreciably over time for women <40 or ≥60 years old (Figure 1, panel D). It increased from 21.9 in 1983 to 24.0 in 2009 for women ages 40-50 and from 22.2 to 23.1 for women ages 50-60, with the steepest increase after 1990.
Risk factor patterns for white and black women
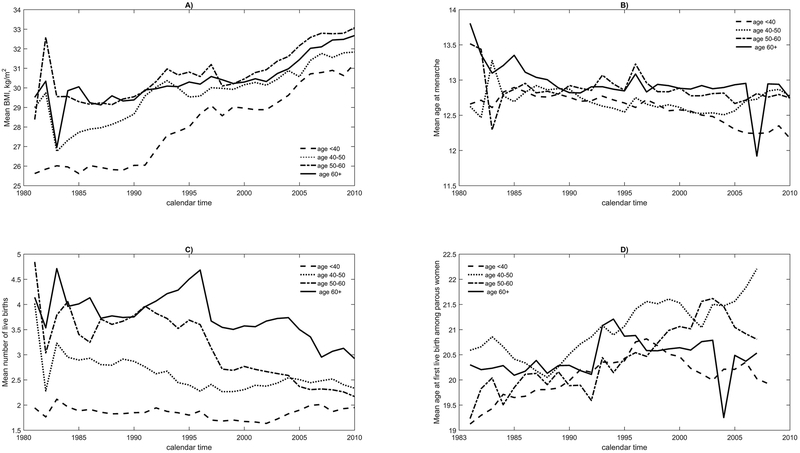
The risk factor patterns for white women (Supplemental Figure S1) largely agree with the overall risk factor patterns (Figure 1). Black women had higher mean BMI levels (Figure 2, panel A) than white women for all age groups for each calendar year. For example, mean BMI in 1980 was 23.6, 25.3, 26.6 and 26.9kg/m2 for white women ages <40, 40-50, 50-60 and 60+ respectively, and the corresponding values were 25.6, 29.0, 28.4 and 29.5kg/m2 for black women. Among black women, mean BMI increased starting in 1980 for all age groups; the increase was much stronger than for white women (compare Figure 2, panel A with Supplemental Figure S1, panel A). The strongest increase was seen for black women aged <40 years, for whom the mean BMI was 25.6 in 1980, 28.7 in 1995, and 31.2 kg/m2 in 2009, while the corresponding values were 23.6, 25.03 and 25.7 kg/m2 for white women aged <40 years. Among black women in age groups <40, 50-59 and ≥60 years, mean age-at-menarche decreased from 12.7 in 1980 to 12.2 in 2009, from 13.5 to 12.7, and from 13.8 to 12.7 respectively (Figure 2, panel B). For black women aged 40-<50 years, mean age-at-menarche barely changed from 12.6 years in 1980 to 12.8 years in 2009. The changes for white women were somewhat smaller than for black women (Supplemental Figure S1, panel B). For white women, the mean number of births decreased for all age groups, with the strongest decline among women aged 40-50 years (Supplemental Figure S1, panel C). Black women had higher mean number of births for all age groups and calendar years compared to white women. From 1980 to 2009, the mean number of births in black women aged <40 years did not change, but it decreased from 4.0 to 2.3 for black women aged 40-50, from 4.8 to 2.2 for black women aged 50-59 and from 4.1 to 2.9 for black women aged ≥60 years (Figure 2, panel C). These decreases are comparable to those among white women. White women of all ages had later mean age-at-first-live-birth than black women (Supplemental Figure S1 and Figure 2, panels D). The largest change was seen for women aged 40-49 years, for whom mean age-at-first-live-birth rose from 22.4 in 1983 to 23.2 in 2009. As for white women, a change in mean age-at-first-live-birth was only seen for black women aged 40-49 years, for whom mean age-at-first-live-birth was 20.6 in 1983 and 22.2 in 2009 (Supplemental Figure S1 and Figure 2, panels D).
Figure 2.
Distribution of mean BMI, mean at menarche, mean number of births, mean age at first live birth among parous women from 1980 to 2008 for various age groups for black U.S. women.
Figure 2 shows the distribution of breast cancer risk factors in black U.S. women over time in 4 age groups. Panels A) through D) correspond to the distribution of BMI, age at menarche, number of live births and age at first live birth, respectively.
Ecologic effects of risk factors on breast cancer incidence
Breast cancer incidence in all U.S. women
We compared incidence rates computed from the unadjusted model (1) to those from the adjusted model (2) with the risk factor set to its lowest (reference) risk level: <25.3kg/m2 for BMI, >13.1 years for age-at-menarche, >2.9 for number of births. The exception was age-at-first-live-birth, for which the referent category was second-to-lowest, (21.6,22.1] (Supplemental Table 2). We graphed results for a woman aged 40-44 years and for a woman aged 60-64 years by adding specific age effects to the intercept and period coefficients. Thus, the results can be interpreted as unadjusted log-incidence rates and log-incidence rates adjusted to the reference level for an ecologic risk factor, for women in those two age groups. However, the same log-incidence patterns would be observed for other pre- and post-age 50 age groups, respectively, apart from vertical displacements.
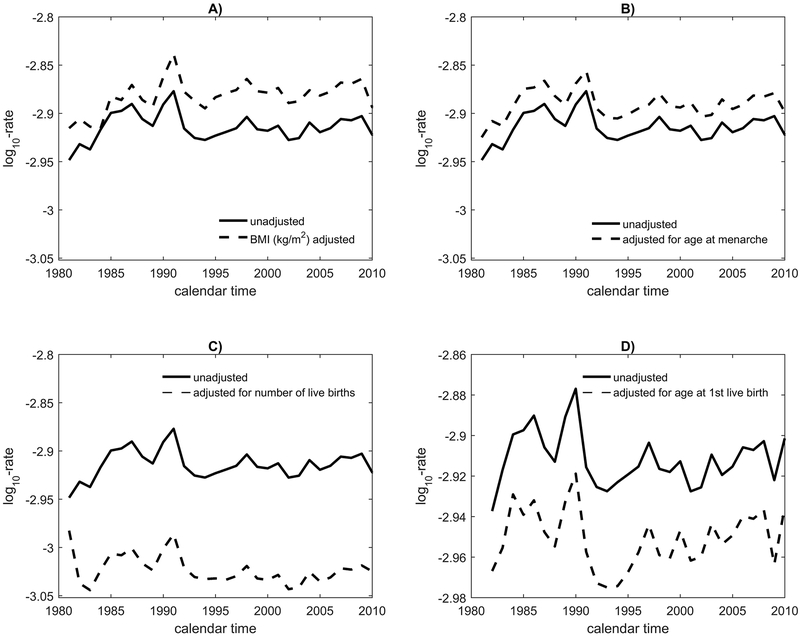
First, we considered all U.S. women ages <50 years (“premenopausal”). For a woman in the 40-44 years age group, breast cancer increased slightly from 1980 to 1985, then dropped slightly and remained constant until 2011. Reference-level incidence from the BMI-adjusted model is higher than unadjusted incidence after 1995, as in Figure 3, panel A, that plots log10-incidence against calendar period. This reflects the facts that higher BMI is associated with lower breast cancer risk for younger women and that population BMI was higher after 1995 for all ages. Before 1995, when BMI levels were lower, adjustment to reference-level BMI increased incidence statistically significantly by 5.9%, and after 1995, by 9.1% (Table 1), consistent with a protective effect of increased BMI in pre-menopausal women. Additional detail on 5-year-age-specific percent relative differences in incidence (with standard errors) is in Table 1. For age-at-menarche (Figure 3, panel B) the adjusted and unadjusted curves differed less. From 1980 to 1994, adjustment to reference-level age-at-menarche increased incidence non-statistically significantly by 5.4%, and from 1995 to 2011 by 5.7%. This non-statistically significant result is not consistent with the facts that age-at-menarche decreased over time and that early age-at-menarche increases breast cancer risk. There is a strong impact of the number of births on incidence (Figure 3, panel C). Adjustment to reference-level for numbers of births statistically significantly reduced incidence by 21.1% before 1995 and by 23.1% between 1995 and 2011 (Figure 3, panel C and Table 1), reflecting the protective effect of more births (and less nulliparity) on breast cancer risk. However, adjustment to reference-level age-at-first-live-birth among parous women had a much smaller non-statistically significant impact; it decreased incidence by 1.8% from 1980 to 1994 and by 1.3% between 1995 and 2011 (Figure 3, panel D and Table 1).
Figure 3.
Plot against calendar time of unadjusted breast cancer log10-incidence and adjusted log10-incidence for women at the reference level of risk factors for a 40-44 year-old woman in the U.S. The reference categories are <25.3 for BMI, (13.1, 13.6] for age at menarche, (2.9,3.61] for number of births and [17.7,21.6] for age at first live birth.
Figure 3 contains observed breast cancer incidence on the log-10 scale in 40-44 year old women and breast cancer incidence that would have been observed when risk factors are held fixed at a low-risk reference category. Panels A) through D) correspond to adjustment for BMI, age at menarche, number of live births and age at first live birth, respectively.
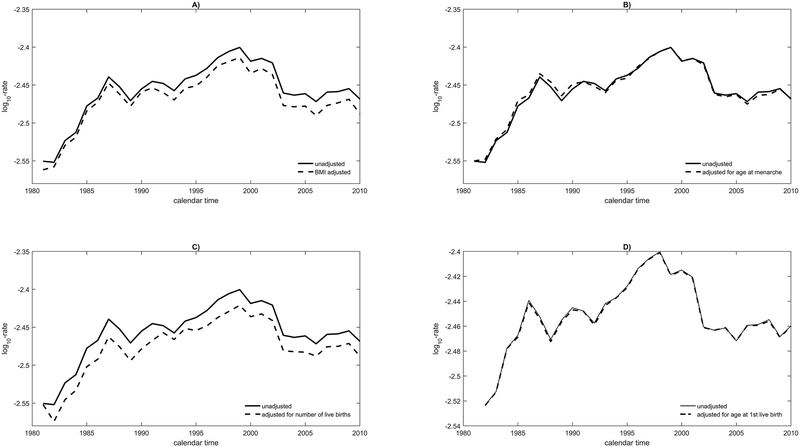
Next, we considered all U.S. women aged ≥50 years (“postmenopausal”). Panels A-D of Figure 4 show the log10-incidence trends for women aged 60-64 years. Incidence increased strongly until 2002, then fell slightly and remained nearly constant, but still higher than in 1980. Adjustment to reference-level BMI decreased incidence non-statistically significantly by 3.4% after 1995 and by 1.9% before then (Figure 4, panel A and Table 1), consistent with BMI’s positive association with breast cancer risk in post-menopausal women. Adjustment to reference-level age-at-menarche had virtually no impact on incidence; it increased incidence by 0.6% between 1980 and 1995 and decreased it by 0.3% after 1995 (Figure 4, panel B and Table 1). Adjustment to reference-level numbers of births decreased incidence statistically significantly by 4.2% between 1980 and 1994 and by 4.3% between 1995 and 2011 (Figure 4, panel C and Table 1). Changes in age-at-first-live-birth had virtually no impact on breast cancer incidence (Figure 4, panel D and Table 1).
Figure 4.
Plot against calendar time of unadjusted breast cancer log10-incidence and adjusted log10-incidence for women at the reference level of risk factors for a 60-64 year-old woman in the U.S. The reference categories are <25.3 for BMI, (13.1, 13.6] for age at menarche, (2.9,3.61] for number of births and (21.6,22.1] for age at first live birth.
Figure 4 contains observed breast cancer incidence on the log-10 scale in 60-64 year old women and breast cancer incidence that would have been observed when risk factors are held fixed at a low-risk reference category. Panels A) through D) correspond to adjustment for BMI, age at menarche, number of live births and age at first live birth, respectively.
Breast cancer incidence in white and black women
For women aged <50 years, unadjusted breast cancer incidence was similar in white and black women (Supplemental Figure S2). However, adjustment to reference-level for BMI and number of births had a stronger impact on incidence in black than in white women (Supplemental Figure S2, panels A and C). For a black woman aged 40-44 years, adjustment to reference-level BMI increased incidence by 14.7% before 1995 and by 21.0% thereafter (Supplemental Table 4). For a white woman aged 40-44 years, adjustment increased incidence only by 4% both before and after 1995 (Supplemental Table 5). From 1980 to 1994, adjustment to reference-level number of live births (Supplemental Figure S2, panel C) decreased risk by 21.6% for white women and by 37.1% for black women aged 40-44 years, and from 1995 to 2011 the decrease was 24.2% for white women and 38.2% for black women (Supplemental Tables 4 and 5).
Among women aged 60-64 years, black women had noticeably lower unadjusted breast cancer incidence than white women (Supplemental Figure S3). Adjustment to reference-level BMI decreased incidence among black women by a non-statistically significant 1% before 1995 and 2.4% after 1995. For white women, the decrease was 2% (non-statistically significant) before 1995, but 5.1% (statistically significant) after 1995 (Supplemental Figure S3, panel A; Supplemental Tables 4 and 5). Adjustment for age-at-menarche did not impact rates appreciably in either racial group. Adjusting to the reference-level number of live births reduced breast cancer incidence among 60-64 year-old black women by 2.1% (statistically significant) before 1995 and by 0.4% (not statistically significant) after 1995. Among white women statistically significant decreases were found: 3.3% before 1995 and 2.9% after 1995. For both time periods, adjusting to reference-level age-at-first-live-birth resulted in <1% decrease in incidence for white women, but in statistically significant 2% decreases for black women aged 60-64 years (Supplemental Figure S3, panels C and D; Supplemental Tables 4 and 5).
Discussion
We presented methods for ecologic analyses (also see Supplemental Material) and describe how secular changes in the distributions of well-established breast cancer risk factors are associated with trends in breast cancer incidence in U.S. women. We used these associations to estimate what the incidence rates might have been if BMI, age-at-menarche, number of births, and age-at-first-live-birth had been fixed at low-risk reference-levels from 1980 to 2011. Although there have been several studies of trends in risk factor prevalence, we are unaware of previous efforts to estimate their ecologic associations with breast cancer incidence trends. We concluded that trends in numbers of live births and BMI were strongly associated with breast cancer incidence in all U.S. women. These associations were especially strong in premenopausal women in whom decreases in numbers of births may have increased breast cancer incidence by 20%, and increases in BMI may have may have decreased incidence by about 7% from 1980 to 2011, with stronger effects after 1995, when BMI levels were particularly high. In women aged ≥50 years, adjustment to reference level BMI reduced breast cancer incidence by approximately 3%, consistent with the known positive association of BMI with breast cancer incidence in postmenopausal women. Adjustment for the risk factors we studied produced smaller percentage changes in incidence in postmenopausal women, but because rates are higher in postmenopausal women, the public health impact could nonetheless be appreciable. The ecologic associations were stronger in black than in white women, possibly reflecting stronger secular trends in their risk factor distributions.
We used nationally representative survey data to estimate average risk factor levels over time. Others have studied these risk factors, sometimes using the same data sources. For example, Komlos et al. (12) estimated BMI trends by birth cohort (1882–1986), ethnicity and gender using NHANES data. McDowell et al. (13) used NHANES data from 1999 to 2004 to show declines in mean age-at-menarche in women born before 1920 compared to women born in 1980-84, with larger declines seen among black women. Krieger et al. (14) used NHANES and National Health Examination Surveys (NHES) to show that BMI increased and age-at-menarche fell from 1959 to 2008, with patterns varying by race/ethnicity and socioeconomic status. Using census data, Martin et al. (15) demonstrated declines in the birth rate among U.S. women from 1980 to 2011, with stronger decreases for black women than for white women, similar to the pattern we observed. Compared to these papers, our analysis covers a longer time window, and by combining different surveys provides more precise standard errors for risk factor prevalence.
We found ecologic associations with risk factors that agreed qualitatively, with a few exceptions, with findings from analytic epidemiologic studies, although some of the ecologic associations were not statistically significant and the sign of the association varied in some categories (Supplemental Tables 2 and 3). BMI was inversely associated with breast cancer risk in women aged <50 years, and positively associated in women aged ≥50, in accord with analytic studies (e.g. 16-20). Early age-at-first-live-birth was also protective in our ecologic study, in line with analytic studies (e.g. 21). We did not find significant associations with age-at-menarche, however, unlike analytic studies (e.g. 22). However, we want to stress that while the general agreement of our results with that from analytic studies is reassuring, we do not estimate individual level risk factor associations for breast cancer in our ecologic investigation. To do that, much stronger assumptions on a model and additional individual level risk factor information on subsets of individuals in the ecologic unit would be needed for some analyses, e.g. those described in (23).
In other settings, ecologic associations are often confounded by factors that differ among the various populations studied (e.g. 24). We are studying a single large region, the U.S.. Our ecologic units are defined by age and period and may be less subject to unmeasured confounding than studies based on regional units. Nonetheless, an unmeasured risk factor that varied with period and was associated with e.g. BMI could confound the ecologic association of BMI with breast cancer incidence. For example, changes in screening practice or unmeasured changes in the racial or ethnic composition of the population could confound our results. Several facts argue against confounding by screening, however. First, increased BMI is protective in premenopausal but adverse in postmenopausal women, and the effects of BMI are stronger after 1995, whereas screening increased in the 1980’s (19,20). Moreover, protective ecologic associations with number of births were stronger in women under age 50 years, who were less likely to be screened, and were nearly constant across calendar time (Figures 3 and 4). Although secular change in the racial/ethnic composition of the population is a potential confounder, the key features we found were confirmed in separate analyses of white and black women.
Few papers have attempted formal analyses to estimate associations of time trends in disease incidence with ecologic exposure information. One key paper by Holford (25) showed that most of the period and cohort contributions to lung cancer incidence trends in Connecticut could be explained by a multiplicative ecologic model that included the proportion of current and ex-smokers and the mean duration of smoking. Here again, a single region was studied, and the ecologic units were defined by period and cohort (or equivalently by age and period). These considerations do not eliminate the possibility that other unmeasured confounders may account for the plausible ecologic associations we found, but they suggest robustness to confounding in studies with ecologic units defined by time, compared to regional ecologic units.
Strengths of our study include use of large population-based surveys for risk factors and large numbers of breast cancer cases in registry-based incidence data that allow us to stratify the analysis by age and race. As we adjust for one-year period effects, our model accommodates abrupt changes in breast cancer incidence, like the drop in 2002, following the publication of results on hormone replacement therapy from the Women’s Health Initiative (2). This study also has limitations. As previously discussed, our ecologic associations may be influenced by unmeasured confounders that are associated with various age-, period-, and cohort units. We modeled each risk factor marginally and did not assess joint effects. We did not comprehensivly evaluate some other important risk factors, such as hormone replacement therapy use or physical activity. We did also not utilize information on mammographic screening, which was widely implemented in the 1980s and contributed variably to increased breast cancer incidence for different age groups (26). We also limited race-specific analyses to white and black women, because there were insufficient data for other groups. Finally, comparison to women at the reference level of risk factors is a hypothetical counterfactual construct, analogous to that used for attributable risk calculations.
In summary, we provided data on trends in breast cancer risk factors, found ecologic associations with breast cancer in line with results from analytic studies, and used data on risk factor prevalence and ecologic associations to estimate the impact that these risk factors might have had on population incidence rates.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the National Cancer Institute. We thank Ms. Mary Alice Anderson for help with identifying risk factor variables from the surveys and Dr. William Anderson for providing the SEER incidence data.
Footnotes
Disclosure of Potential Conflicts of Interest
Yenny Webb-Vargas reports compensated relationships with Genentech, Inc. and the National Cancer Institute, NIH
The other authors declare no potential conflicts of interest.
REFERENCES
- 1.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007; 19;356:1670–1674. [DOI] [PubMed] [Google Scholar]
- 2.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288:321–333. [DOI] [PubMed] [Google Scholar]
- 3.Freedman DM, Tarone RE, Doody MM, et al. Trends in reproductive, smoking, and other chronic disease risk factors by birth cohort and race in a large occupational study population. Ann Epidemiol 2002; 12: 363–369. [DOI] [PubMed] [Google Scholar]
- 4.Nichols HB, Trentham-Dietz A, Hampton JM, et al. From menarche to menopause: Trends among U.S. women born from 1912 to 1969. Am J Epidemiol. 2006; 164: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 5.Ban KA, Godellas CV. Epidemiology of Breast Cancer. Surg Oncol Clin N Am 2014; 23: 409–422. [DOI] [PubMed] [Google Scholar]
- 6.Engel A, Murphy RS, Maurer K et al. Plan and Operation of the HANES I Augmentation Survey of Adults 25-74 Years. 1978. Vital and health statistics. Ser. 1, Programs and collection procedures, (14). [PubMed] [Google Scholar]
- 7.Cox C, Rothwell S, Madans J, et al. Plan and operation of the NHANES I Epidemiologic Follow-up Study, 1987. Vital Health Stat Ser. 1992; 1 27:1–190 [PubMed] [Google Scholar]
- 8.Korn EL, Graubard BI. Analysis of Health Surveys. Wiley Series in Probability and Statistics. 1999. New York: Wiley [Google Scholar]
- 9.Ezzati TM, Massey JT, Waksberg J, et al. Sample Design: Third National Health and Nutrition Examination Survey. 1992. Vital and health statistics. Ser. 2, Data Evaluation and Methods Research, 113. [PubMed] [Google Scholar]
- 10.Massey J, Moore T, Parsons V et al. Design and Estimation for the National Health Interview Survey, 1985-94. 1989. National Center for Health Statistics. Vital and health statistics 2, 110. [PubMed] [Google Scholar]
- 11.Botman S, Moore T, Moriarty C, et al. Design and Estimation for the National Health Interview Survey, 1995-2004. National Center for Health Statistics. 2000. Vital and health statistics 2, 130. [PubMed] [Google Scholar]
- 12.Komlos J, Brabec M. The trend of BMI values of U.S. adults by deciles, birth cohorts 1882-1986 stratified by gender and ethnicity. Economics and Human Biology, 201; 9: 234–250. [DOI] [PubMed] [Google Scholar]
- 13.McDowell MA, Brody DJ, Hughes JP. J Adolesc Health. Has age at menarche changed? Results from the National Health and Nut J Adolesc Health. 2007; 40:227–231. [DOI] [PubMed] [Google Scholar]
- 14.Krieger N, Kosheleva A, Waterman PD, et al. 50-year trends in U.S. socioeconomic inequalities in health: U.S.-born Black and White Americans, 1959-2008. Int J Epidemiol. 2014. 43(4):1294–313. doi: 10.1093/ije/dyu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin JA, Hamilton BE, Osterman MJK, et al. Births: Final data for 2012. Natl Vital Stat Rep. 62(9), 2013. [PubMed] [Google Scholar]
- 16.Trentham-Dietz A, Newcomb PA, Storer BE, et al. Body size and risk of breast cancer. Am J Epidemiol. 1997; 145:1011–1019. [DOI] [PubMed] [Google Scholar]
- 17.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997; 278: 1407–1411. [PubMed] [Google Scholar]
- 18.Gaudet MM, Carter BD, Patel AV, et al. Waist circumference, body mass index, and postmenopausal breast cancer incidence in the Cancer Prevention Study-II Nutrition Cohort. Cancer Causes & Control 2014; 25: 737–745 [DOI] [PubMed] [Google Scholar]
- 19.Berstad P, Coates RJ, Bernstein L, et al. A Case-Control Study of Body Mass Index and Breast Cancer Risk in White and African-American Women. Cancer Epidemiol Biomarkers Prev 2010; 19: 1532–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer JR, Adams-Campbell LL, Boggs DA, et al. A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomarkers Prev 2007; 16: 1795–1802. [DOI] [PubMed] [Google Scholar]
- 21.MacMahon B, Cole P, Lin TM, et al. Age at first birth and breast cancer risk Bull World Health Organ 1970; 43: 209–221. [PMC free article] [PubMed] [Google Scholar]
- 22.Collaborative Group on Hormonal Factors in Breast Cancer, Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012; 13: 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prentice RL, Sheppard L. Aggregate data studies of disease risk factors. Biometrika. 1995. 82: 113–125. [Google Scholar]
- 24.Subramanian SV, Jones K, Kaddour A, et al. Revisiting Robinson: The perils of individualistic and ecologic fallacy. Int. J. Epidemiol. 2009; 38: 342–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holford TR, Zhang Z, Zheng T, et al. A model for the effect of cigarette smoking on lung cancer incidence in Connecticut. Stat Med. 1996;15:565–80. [DOI] [PubMed] [Google Scholar]
- 26.Breen N, Gentleman JF, Schiller JS. Update on mammography trends, Cancer, 2011; 117: 2209–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.