Abstract
Purpose:
A pre-specified statistical model based on four kallikrein markers in blood, commercially available as the 4Kscore, has been shown to accurately detect high grade (>Grade Group 2) prostate cancer in men with moderately elevated PSA. We aimed to assess whether the model predicted prostate cancer metastasis or death in men not subject to PSA screening.
Materials and Methods:
The cohort includes 43,692 unscreened prostate cancer-free men from a Swedish population-based cohort with low rates of PSA screening (Västerbotten Intervention Project). Using cryopreserved blood collected at ages 50 and 60 from men in this cohort, we analyzed the association between PSA and other kallikrein marker levels in blood and risk of prostate cancer metastasis or death.
Results:
There were 308 with metastases, and 172 prostate cancer deaths. Baseline PSA was strongly associated with 20-year risk of prostate cancer death (c-index at age 50: 0.859, 95% CI 0.799, 0.916; age 60: 0.840, 95% CI 0.799, 0.878). Men aged 60 with PSA below median (<1.2 ng/ml) had 0.4% risk of prostate cancer death at 20 years. Among men with moderately elevated PSA (≥2.0 ng/ml), the 4Kscore markedly improved discrimination (c-index 0.767 vs. 0.828 and 0.774 vs. 0.862 in men 50 and 60). Long-term risk of prostate cancer death or metastasis in men with low 4Kscores was very low.
Conclusion:
Screening should focus on men in top PSA-quartile at age 60. Men with elevated PSA but a low 4Kscore can safely be monitored with repeated blood markers in place of immediate biopsy.
Keywords: Prostate cancer, early detection, PSA, reflex test
Introduction
An effective prostate cancer screening program should detect and treat aggressive cancer at an early curable stage to reduce prostate cancer mortality, while avoiding detection of insignificant prostate cancer that is unlikely to progress to cause symptoms, morbidity, or mortality.1 Prostate-specific antigen (PSA) is strongly predictive of risk of distant prostate cancer metastasis or death occurring 20 to 30 years later2. However, although highly sensitive - men aged 60 with a PSA below median have negligible lifetime risk of prostate cancer morbidity or mortality3 - PSA has inadequate specificity, as only a small proportion of all men with modestly elevated PSA develop aggressive prostate cancer4. This results in a significant risk of overdiagnosis5.
A panel of four kallikrein markers measured in blood, commercially available as the 4Kscore, has been shown to improve the specificity to detect aggressive disease compared to PSA alone. Based on the panel of four kallikrein markers, Bryant et al. developed a statistical model for Grade Group 2 (Gleason Score 7) or higher prostate cancer at biopsy 6 that has independently been validated in several retrospective and prospective studies, with a meta-analysis by Zappala et al. reporting on 12 of these studies including 11,134 patients.7 These studies were recently extended to examine whether the panel predicted the long-term risk of metastasis in men not subject to screening, using cryopreserved blood obtained at age 50 and 60 from prostate cancer free men in a large Swedish cohort, the Västerbotten Intervention Project (VIP). In our previous study, men with an elevated PSA, but a low risk from the 4Kscore had an extremely low risk of metastasis within 10 years, suggesting that monitoring rather than immediate biopsy of such men would be safe.8 Updated study outcomes data are now available, which has allowed us to replicate our original analysis of distant metastasis with additional follow-up and events, and to add an analysis of the definitive endpoint of prostate cancer death.
Here we aim to examine the association between PSA, the four kallikrein markers measured at age 50 or 60 in prostate cancer-free men not subject to PSA-screening and the subsequent development of aggressive prostate cancer defined as distant prostate cancer metastasis or prostate cancer death. As levels of microseminoprotein-β (MSP) in blood9 and an inherited genetic variation in the MSMB-gene promoter are associated with risk of prostate cancer diagnosis,9 we also aimed to evaluate whether the addition of MSP could further improve our ability to detect lethal prostate cancers.
Materials and Methods
Study Population
The Västerbotten Intervention Project is an ongoing population-based cohort study initiated in 1985 in which all residents of Västerbotten County, Sweden, are invited to receive a health examination at ages 40, 50 and 60 with blood drawn for cryopreservation.10 For many years, the rate of PSA screening in Sweden was very low, entailing that for much of follow-up, the VIP study is close to a “natural history” study of prostate cancer. We nonetheless planned sensitivity analyses to control for opportunistic screening. Measurements of marker levels (total PSA, free PSA, intact PSA, hK2 and MSP) were performed in a nested case-control set from the VIP cohort. Details on case ascertainment through linkages to national registries of proven accuracy12, case-control matching (ratio 1:3), and laboratory methods are available in the supplemental materials
Statistical Methods
To estimate absolute risk, we used a previously validated2 imputation approach (see supplemental materials for further details). The four kallikrein markers were combined as previously described into a statistical risk prediction model that gives the risk of Grade Group ≥2 prostate cancer (Gleason score ≥7) at prostate biopsy.13 This model is the same as the commercially available 4Kscore™ and locked-down before it was applied to the VIP cohort, making the current study an external validation of a prespecified model. Because the 4Kscore is designed as a reflex test to aid the biopsy decision in men with elevated PSA, our main analyses as to the value of the 4Kscore were in men with higher PSAs. Results for the prespecified model created in the same study containing age, total PSA and free PSA were also presented as a comparison. Prostate cancer death was the outcome in the primary analysis, and a secondary analysis was performed using documented evidence of a distant prostate cancer metastasis as the outcome. Cox proportional hazards models were used to assess the association between PSA, the 4Kscore and time to distant metastasis or prostate cancer death. Participants with date of death or emigration prior to the end of follow up (May 2016) were censored at that date. Absolute risks of prostate cancer metastasis or death at 10, 15 and 20 years were calculated using Kaplan-Meier methods. Discrimination was assessed using the concordance index.
We also investigated adding the biomarker MSP to the 4Kscore. We created Cox regression models including the 4Kscore and MSP for the outcomes of any grade cancer, high grade cancer, distant metastasis and prostate cancer death in each age cohort.
All analyses were conducted in Stata 15 (StataCorp, College Station, TX).
Results
In this cohort of 43,692 men, 2,420 men were diagnosed with prostate cancer, with 131 having metastatic disease at diagnosis, and another 177 developing distant metastases before the date of death. A total of 172 men died from prostate cancer. A total of 27,731 men were followed for 15 or more years without a prostate cancer diagnosis. Cancer and treatment characteristics for 1,924 men with marker measurements available who were diagnosed with prostate cancer are available in eTable 1. Information on the number of events by age cohort is available in eTable 2. Due to the low number of events among men sampled at age 40, further results are presented for the age 50 and age 60 cohorts only.
PSA was strongly associated with risk of prostate cancer death, with a c-index of 0.859 (95% CI 0.799, 0.916) at age 50 and a c-index of 0.840 (95% CI 0.799, 0.878) at age 60 (eFigures 2a and 2b, respectively). Nearly three-quarters of prostate cancer deaths occurred in men with PSA in the top quartile, and only a small number of prostate cancer deaths were in men with PSA below the median (Table 1). Risk of prostate cancer death at 15 or 20 years was at least 10-fold higher among men with PSAs in the top quartile as compared to men with PSAs below the median, with the absolute risk of prostate cancer death within 20 years for men with PSA below the median at age 60 being less than 0.4% and far below population average of 1.8%.
Table 1.
Absolute risk of prostate cancer death by 15 and 20 years of follow-up, and cumulative proportion of prostate cancer deaths, with 95% confidence interval, by PSA levels in cryopreserved blood collected at age 50 or 60 in the Västerbotten Intervention project.
| PSA, ng/ml | Risk of prostate cancer death | Cumulative proportion of prostate cancer deaths, with 95% CI |
||
|---|---|---|---|---|
| 15 years | 20 years | |||
| Age 50 | ||||
| Top Decile | >2.0 | 0.82 (0.41, 1.49) | 1.34 (0.72, 2.28) | 46% (29%, 63%) |
| Top Quartile | >1.3 | 0.45 (0.26, 0.75) | 0.95 (0.59, 1.45) | 75% (61%, 89%) |
| Second Quartile | 0.8–1.3 | 0 (NA) | 0.17 (0.04, 0.54) | 91% (81%, 100%) |
| Third Quartile | 0.6–0.8 | 0.04 (0.00, 0.27) | 0.08 (0.01, 0.34) | 97% (92%, 100%) |
| Fourth Quartile | <0.6 | 0 (NA) | 0 (NA) | 100% |
| Below Median | <0.8 | 0.02 (0.00, 0.14) | 0.04 (0.01, 0.17) | |
| PSA less than 1 | <1.0 | 0.02 (0.00, 0.11) | 0.03 (0.01, 0.14) | |
| Overall | 0.13 (0.07, 0.21) | 0.32 (0.21, 0.46) | ||
| Age 60 | ||||
| Top Decile | >4.0 | 4.63 (3.48, 6.02) | 8.09 (6.08, 10.45) | 53% (45%, 62%) |
| Top Quartile | >2.3 | 2.61 (2.04, 3.29) | 4.75 (3.74, 5.92) | 72% (65%, 80%) |
| Second Quartile | 1.2–2.3 | 0.57 (0.31, 0.99) | 1.32 (0.77, 2.12) | 90% (85%, 95%) |
| Third Quartile | 0.7–1.2 | 0.30 (0.12, 0.66) | 0.57 (0.26, 1.14) | 97% (95%, 100%) |
| Fourth Quartile | <0.7 | 0.09 (0.01, 0.40) | 0.25 (0.07, 0.71) | 100% |
| Below Median | <1.2 | 0.20 (0.09, 0.40) | 0.42 (0.22, 0.74) | |
| PSA less than 1 | <1.0 | 0.18 (0.07, 0.41) | 0.37 (0.18, 0.70) | |
| Overall | 0.92 (0.74, 1.13) | 1.78 (1.46, 2.16) | ||
Among men in the top decile or quartile of PSA, risk of distant metastasis and prostate cancer death also rose with an increasing 4Kscore. Table 2 and eTables 4 and 7 show that the 4Kscore improved discrimination, but only for men with PSAs above median at age 50 or 60. Increases in discrimination were similar for ages 50 and 60 and for different PSA cut-points. The small decrease in discrimination when adding the 4Kscore for men age 50 with the cut-off of PSA ≥3.0 ng/ml is likely a chance finding due to the small number of events using prostate cancer death as the outcome, as this result is not reflected elsewhere in the literature and is not consistent with the results for the other cut-offs, or when distant metastasis is used as the outcome (eTable 7). Like the 4Kscore, the model including age, free and total PSA improved discrimination only in men with total PSA above the median at both age 50 and 60. Discrimination of the 4Kscore was superior to that of the free and total PSA model in these subgroups. For example, the discrimination of total PSA in men with a PSA ≥ 2 at age 60 was 0.774, which increased to 0.817 with the addition of free PSA, but to 0.862 for the 4Kscore.
Table 2.
Discrimination of PSA for predicting prostate cancer death compared with a pre-specified model containing age, total PSA and free PSA, and with the 4Kscore.
| Age at blood draw | Subgroup by PSA | PSA centile | Total N | Prostate cancer deaths | C-index based on total PSA | C-index based on total and free PSA | C-index based on 4Kscore | Difference between 4Kscore and total PSA | Difference between 4Kscore and total and free PSA |
|---|---|---|---|---|---|---|---|---|---|
| 50 | All men | 0 | 20524 | 36 | 0.859 | 0.722 | 0.819 | −0.040 | 0.097 |
| PSA<1.0 | <62 | 12598 | 5 | 0.635 | 0.598 | 0.581 | −0.054 | -0.017 | |
| PSA≥1.0 | ≥62 | 7926 | 31 | 0.761 | 0.756 | 0.780 | 0.019 | 0.024 | |
| PSA≥1.5 | ≥82 | 3662 | 23 | 0.735 | 0.779 | 0.788 | 0.053 | 0.009 | |
| PSA≥2.0 | ≥90 | 1980 | 16 | 0.767 | 0.833 | 0.828 | 0.061 | -0.004 | |
| PSA≥3.0 | ≥96 | 714 | 10 | 0.881 | 0.875 | 0.877 | −0.004 | 0.002 | |
| PSA≥4.0 | ≥98 | 390 | 8 | 0.841 | 0.870 | 0.879 | 0.038 | 0.009 | |
| 60 | All men | 0 | 18638 | 144 | 0.840 | 0.758 | 0.859 | 0.019 | 0.102 |
| PSA<1.0 | <40 | 7367 | 9 | 0.630 | 0.603 | 0.530 | −0.100 | -0.074 | |
| PSA≥1.0 | ≥40 | 11271 | 135 | 0.809 | 0.793 | 0.859 | 0.049 | 0.066 | |
| PSA≥1.5 | ≥60 | 7493 | 123 | 0.785 | 0.807 | 0.860 | 0.076 | 0.053 | |
| PSA≥2.0 | ≥71 | 5376 | 111 | 0.774 | 0.817 | 0.862 | 0.087 | 0.045 | |
| PSA≥3.0 | ≥84 | 3057 | 86 | 0.811 | 0.833 | 0.881 | 0.070 | 0.048 | |
| PSA≥4.0 | ≥90 | 1862 | 77 | 0.759 | 0.799 | 0.869 | 0.110 | 0.070 |
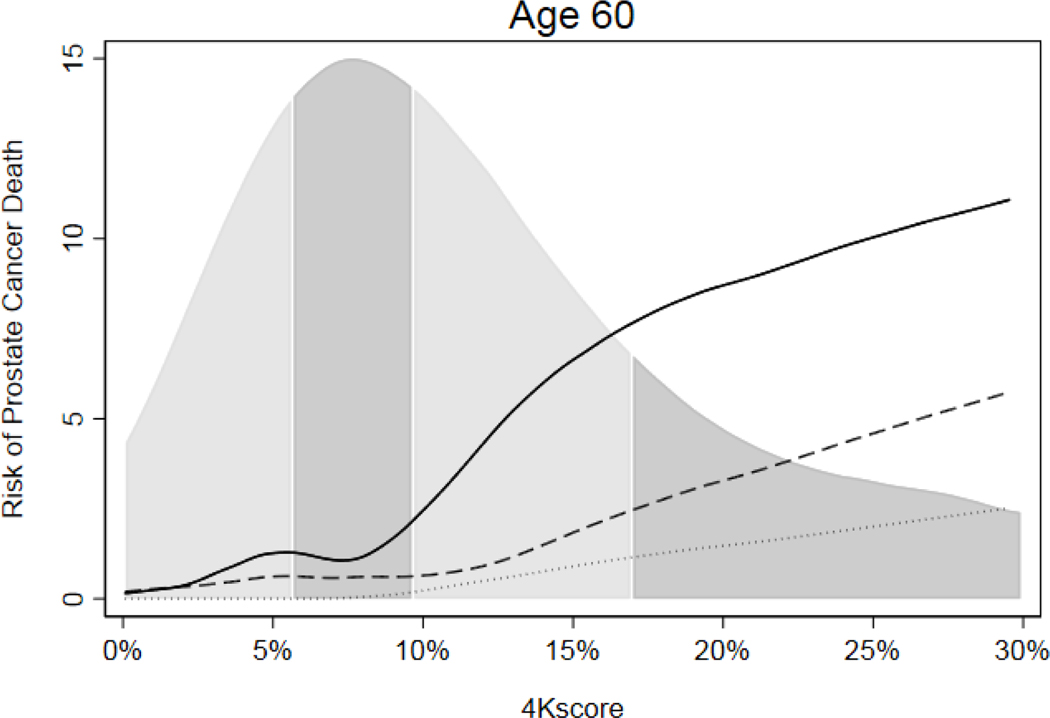
An analysis of the potential impact of using 4Kscore risk to determine biopsy is shown in Table 3,with additional results in eTable 5. The average population risk of prostate cancer death within 20 years was 0.3% and 1.8% for men aged 50 and 60 respectively, with an average 20-year risk of metastasis of 0.8% and 2.9% at age 50 and 60, respectively. Overall, there was low long-term risk of metastasis or death from prostate cancer in men with elevated PSA but low 4Kscore risk, with an average potential biopsy reduction of about 40–60% (Table 3 and eTable 8). The risk of prostate cancer death in men with elevated PSA but low 4Kscore was similar to risk in men without elevated PSA. For instance, a man with a PSA ≥3.0 ng/ml at age 60 and a 4Kscore <7.5% had a zero risk of prostate cancer death within 10 years and 0.55% at 15 years, both well below the population average (0.9% at 15 years) and similar to the risk for a man with PSA 1.5 ng/ml at age 60. A PSA ≥3.0 ng/ml with 4Kscore of 20% at age 60 conferred a similar risk of prostate cancer death as a PSA of 10 ng/ml or higher (Figure 1, eTable 3).
Table 3.
Absolute risk (%) of prostate cancer death by PSA and 4Kscore. Risk of prostate cancer death by 4Kscore in men with PSA ≥3.0 ng/ml at age 50 were not presented due to a limited number of events. The second column (N) represents the number of men in each group, indicating potential number of biopsy reductions. For example, there are 3057 men with PSA ≥3.0 ng/ml at age 60 and recommending biopsy only for those with a 4Kscore of ≥7.5% would result in 38% of these men avoiding biopsy.
| PSA Group | N | 10 Years | 15 Years | 20 Years | Prostate cancer deaths per 10,000 men at 10 years | Prostate cancer deaths per 10,000 men at 20 years |
|---|---|---|---|---|---|---|
| Age 50 | ||||||
| PSA≥0 | 20524 | 0.04 (0.01 – 0.08) | 0.13 (0.07 – 0.21) | 0.32 (0.21 – 0.46) | 4 | 32 |
| PSA≥1.5 | 3662 | 0.13 (0.04 – 0.35) | 0.56 (0.30 – 0.97) | 1.07 (0.64 – 1.70) | 2 | 19 |
| 4Kscore ≥7.5% | 348 (10%) | 1.33 (0.38 – 3.52) | 2.76 (1.09 – 5.78) | 3.93 (1.73 – 7.56) | 2 | 7 |
| <7.5% | 3314 (90%) | 0 (NA) | 0.32 (0.12 – 0.72) | 0.76 (0.37 – 1.41) | 0 | 12 |
| PSA≥2.0 | 1980 | 0.24 (0.08 – 0.65) | 0.86 (0.43 – 1.56) | 1.40 (0.76 – 2.39) | 2 | 13 |
| 4Kscore ≥7.5% | 345 (17%) | 1.35 (0.38 – 3.56) | 2.79 (1.10 – 5.85) | 3.98 (1.75 – 7.66) | 2 | 7 |
| <7.5% | 1635 (83%) | 0 (NA) | 0.43 (0.12 – 1.17) | 0.83 (0.29 – 1.95) | 0 | 6 |
| PSA≥3.0 | 714 | 0.66 (0.20 – 1.76) | 1.13 (0.42 – 2.53) | 2.24 (0.94 – 4.56) | 2 | 8 |
| Age 60 | ||||||
| PSA≥0 | 18638 | 0.33 (0.24 – 0.44) | 0.92 (0.74 – 1.13) | 1.78 (1.46 – 2.16) | 33 | 178 |
| PSA≥2.0 | 5376 | 0.93 (0.67 – 1.27) | 2.38 (1.86 – 2.98) | 4.51 (3.59 – 5.58) | 27 | 130 |
| 4Kscore ≥7.5% | 2422 (45%) | 2.01 (1.44 – 2.74) | 4.40 (3.40 – 5.59) | 7.81 (6.08 – 9.82) | 26 | 102 |
| <7.5% | 2955 (55%) | 0.02 (0.00 – 0.22) | 0.62 (0.28 – 1.23) | 1.65 (0.87 – 2.87) | 1 | 28 |
| PSA≥3.0 | 3057 | 1.47 (1.04 – 2.03) | 3.17 (2.41 – 4.08) | 5.83 (4.46 – 7.43) | 24 | 96 |
| 4Kscore ≥7.5% | 1906 (62%) | 2.33 (1.64 – 3.22) | 4.67 (3.52 – 6.04) | 8.56 (6.52 – 10.94) | 24 | 88 |
| <7.5% | 1152 (38%) | 0 (NA) | 0.55 (0.13 – 1.73) | 1.02 (0.25 – 3.03) | 0 | 8 |
Figure 1.
Risk of prostate cancer death by 4Kscore within 10 years (dotted line), 15 years (dashed line) and 20 years (solid line) among men with PSA ≥3.0 ng/ml at age 60, with distribution of 4Kscore by quartile.
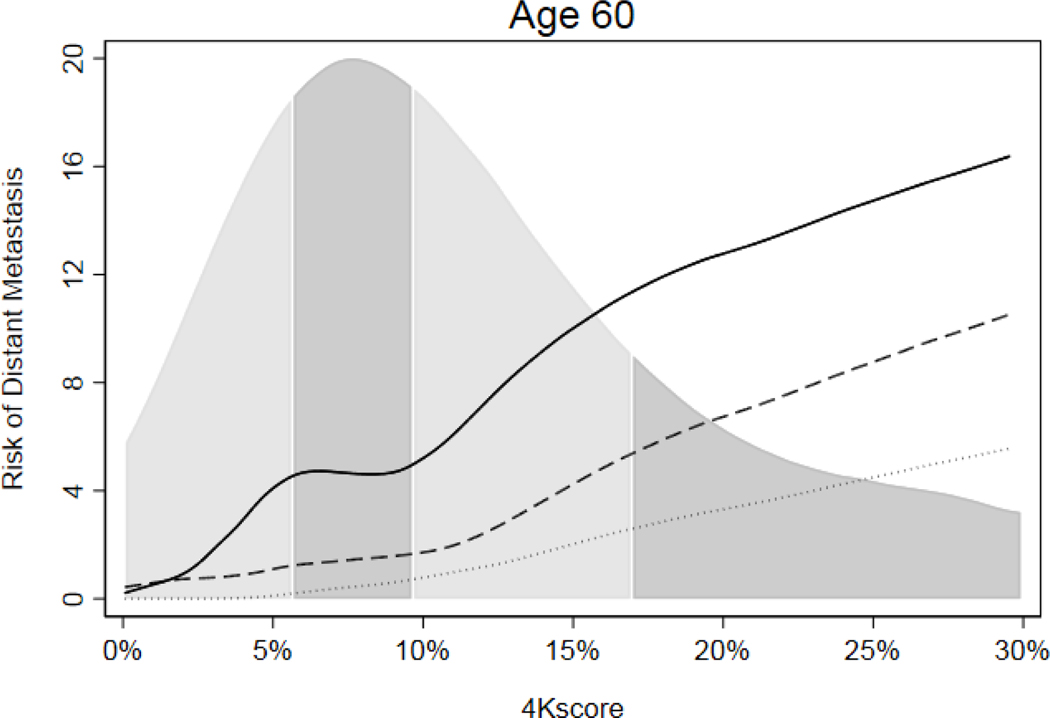
Results of all analyses were consistent when using distant metastasis as an outcome (eTables 6–9, Figure 2 and eFigures 1 and 2) and when taking into account opportunistic screening (eTables 10–13 and eFigures 3 and 4). For the distant metastasis endpoint, the 4Kscore increase in discrimination was more similar between older and younger men, suggesting that the differences seen in the main analyses for death may be related to chance fluctuations associated with low event numbers. We then investigated whether adding levels of MSP in blood could improve the discrimination of the 4Kscore panel. We found no evidence that MSP was significantly associated with time to any grade prostate cancer diagnosis, Grade Group 2 or higher cancer diagnosis, distant metastasis or death from prostate cancer when added to 4Kscore for men at age 50 or age 60, either among all men (eTable 14) or when excluding men diagnosed with prostate cancer in 2006 or later (eTable 15).
Figure 2.
Risk of distant metastasis by 4Kscore within 10 years (dotted line), 15 years (dashed line) and 20 years (solid line) among men at age 60 with PSA ≥3.0 ng/ml, with distribution of 4Kscore by quartile.
Discussion
We have shown a strong association between PSA measurements in midlife and the long-term risk of lethal prostate cancer using a large population-based cohort with low rates of opportunistic PSA testing. Among men with elevated PSA, the 4Kscore added considerable prognostic value with an assessment of clinical utility suggesting that use of the 4Kscore could reduce the number of prostate biopsies, and hence also reduce overdiagnosis, with a very low risk of missing a lethal prostate cancer at a curable stage. This study adds to a growing body of evidence supporting risk stratification screening practices to reduce both unnecessary PSA testing and prostate biopsies while still identifying lethal prostate cancers at a very early and curable stage.
Among men with a PSA measurement at age 50 or 60, most deaths in this cohort occurred in those with PSA in the top quartile. As expected, these results are consistent with the results of our prior study in the same cohort which used the outcome of distant metastasis and found that nearly 70% of metastatic cases occurred in men with PSA in the top quartile.8 Similarly, in the Malmö Preventive Project (MPP), another Swedish population-based study, 51% of metastatic cancers occurred in men with PSA in the top quartile at age 45–49 (PSA ≥1.06 ng/ml).2 Comparable results have been found in a US cohort, where 65% of lethal prostate cancers occurred in men who had PSA in the top quartile (≥1.40 ng/ml) between age 50 and 54.14
For men in their 60s, we also found that men with PSAs below the median (1.2 ng/ml) have <1% risk of distant metastasis8 or prostate cancer death at 20 years. An analysis of 60-year-old men in the MPP cohort also supports this conclusion, reporting a 1.6% risk of distant metastasis and a 0.9% risk of prostate cancer death at 25 years for men with a PSA at the median (1.06 ng/ml).3 Men in their 60s are at high risk for overdiagnosis and overtreatment, with Vickers et al. estimating that 43% of excess prostate cancer diagnoses between 1987 and 2000 were among men in their 60s.15
We were unable to identify a subgroup of men at age 40 at high risk for lethal prostate cancer. While PSA has been shown to predict cancer diagnosis,16 distant metastasis and prostate cancer death up to 25 years after blood draw, most men are diagnosed in their mid-sixties and few men die of prostate cancer by age 65.17 Even among men with the highest PSA measurements at age 40, 20 year risk of metastasis was less than 1%.8
Our results also provide additional evidence of the clinical benefit associated with the 4Kscore as a reflex test. Men with elevated PSA but a low risk from the 4Kscore have a very low risk of death at 10 years (generally zero) or 15 years (<007E;0.5%), similar to the risk for men without elevated PSA. Given the long lead time of prostate cancer, this suggests that such men can safely avoid biopsy and instead by monitored with repeated blood markers. Our findings on the 4Kscore are reasonably similar to the estimates from the Malmö Diet and Cancer cohort, where samples were taken less recently and included a larger age range.18
We found no evidence that adding MSP to the 4Kscore adds to the prediction of any cancer-related outcome. However, there are conflicting findings in the literature19,20,21,22, and further investigation into the utility of MSP for improving prediction of prostate cancer is warranted, particularly regarding the cause of the heterogeneous findings.
While a limitation is the use of an ethnically homogeneous cohort in our analysis, we believe our results may be broadly applicable. It has been shown that both the relationship between PSA and aggressive prostate cancer, and the predictive ability of the 4Kscore, are similar in black and white men.23,24 It is also known that prostate cancer death rates are higher in Sweden that in the United States. Given this knowledge, the risk estimates presented here are likely overestimates of the risk of prostate cancer death from the US population and hence the reported benefits of stratified screening are more conservative.
Conclusions
This study adds to a large body of evidence supporting risk stratification of prostate cancer screening using PSA and the pre-specified statistical model based on a panel of four kallikrein markers in blood commercially available as the 4Kscore. Screening for men in their 50s and 60s should be focused on those with PSAs in the top quartile. Men with low PSA can be screened less frequently in their 50s, and cease screening altogether in their 60s. Regular screening for men aged less than 45 is not warranted. Men with an elevated PSA but a low 4Kscore (e.g. <7.5%) are at very low long-term risk of prostate cancer metastasis and death and can safely be monitored with repeated blood markers in place of immediate biopsy.
Supplementary Material
Acknowledgments
We thank all participants included in the VIP. We also thank all scientists and organizations behind the cohort and Åsa Ågren and her team at the department of biobank research for coordinating the VIP data. We also thank Mona Hassan Al Battat for performing the blood marker measurements at Dr. Lilja’s laboratory at Lund University, Malmö, Sweden.
We also thank the Northern Sweden part of the National Prostate Cancer Register (NPCR) of Sweden in particular Johan Styrke and Camilla Thellenberg Karlsson for providing clinical data for the prostate cancer cases.
Funding
This work was supported in part by the National Institutes of Health/National Cancer Institute (NIH/NCI) with a Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center [P30 CA008748], a SPORE grant in Prostate Cancer to Dr. H. Scher [P50-CA92629], a R01 grant to Dr. R. Klein [R01 CA175491], the Sidney Kimmel Center for Prostate and Urologic Cancers, David H. Koch through the Prostate Cancer Foundation. This work was also supported in part by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Program in UK, the Västerbotten county council, the Swedish Cancer Society (CAN 2017/559), and the Swedish Research Council (VR-MH project no. 2016-02974), and General Hospital in Malmö Foundation for Combating Cancer.
Abbreviations
- PSA
prostate specific antigen
- VIP
Västerbotten Intervention Project
- ISUP
International Society of Urologic Pathology
- MSP
microseminoprotein-β
Footnotes
Declarations
Ethics approval and consent to participate
All participants gave written informed consent at the time of recruitment, and the project was approved by the research ethics board at Umeå University (research authorization number 2009-1436-31).
Availability of data and materials
A full dataset along with the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper. Express written permission must be sought from the authors for any other data use.
Competing interests
Hans Lilja holds a patent on assays to measure intact PSA and is together with Andrew Vickers named on a patent for a statistical method to detect prostate cancer that has been commercialized as 4Kscore test by OPKO Health. Andrew Vickers and Hans Lilja receive royalties from sales of the test. Hans Lilja has stock and Andrew Vickers has stock options in OPKO Health.
References
- 1.Hugosson J, Roobol MJ, Mansson M, et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. European urology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40–55 and long term risk of metastasis: case-control study. BMJ (Clinical research ed). 2013;346:f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vickers AJ, Cronin AM, Bjork T, et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ (Clinical research ed). 2010;341:c4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet (London, England). 2014;384(9959):2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. Journal of the National Cancer Institute. 2003;95(12):868–878. [DOI] [PubMed] [Google Scholar]
- 6.Bryant RJ, Sjoberg DD, Vickers AJ, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. Journal of the National Cancer Institute. 2015;107(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zappala SM, Scardino PT, Okrongly D, Linder V, Dong Y. Clinical performance of the 4Kscore Test to predict high-grade prostate cancer at biopsy: A meta-analysis of us and European clinical validation study results. Reviews in urology. 2017;19(3):149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stattin P, Vickers AJ, Sjoberg DD, et al. Improving the Specificity of Screening for Lethal Prostate Cancer Using Prostate-specific Antigen and a Panel of Kallikrein Markers: A Nested Case-Control Study. European urology. 2015;68(2):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith Byrne K, Appleby PN, Key TJ, et al. The role of plasma microseminoprotein-beta in prostate cancer: an observational nested case-control and Mendelian randomization study in the European prospective investigation into cancer and nutrition. Annals of oncology : official journal of the European Society for Medical Oncology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallmans G, Agren A, Johansson G, et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort - evaluation of risk factors and their interactions. Scandinavian journal of public health Supplement. 2003;61:18–24. [DOI] [PubMed] [Google Scholar]
- 11.Pilebro B, Johansson R, Damber L, Damber JE, Stattin P. Population-based study of prostate-specific antigen testing and prostate cancer detection in clinical practice in northern Sweden. Scandinavian journal of urology and nephrology. 2003;37(3):210–212. [DOI] [PubMed] [Google Scholar]
- 12.Tomic K, Berglund A, Robinson D, et al. Capture rate and representativity of The National Prostate Cancer Register of Sweden. Acta oncologica (Stockholm, Sweden). 2015;54(2):158–163. [DOI] [PubMed] [Google Scholar]
- 13.Parekh DJ, Punnen S, Sjoberg DD, et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. European urology. 2015;68(3):464–470. [DOI] [PubMed] [Google Scholar]
- 14.Preston MA, Batista JL, Wilson KM, et al. Baseline Prostate-Specific Antigen Levels in Midlife Predict Lethal Prostate Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(23):2705–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vickers AJ, Sjoberg DD, Ulmert D, et al. Empirical estimates of prostate cancer overdiagnosis by age and prostate-specific antigen. BMC medicine. 2014;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilja H, Ulmert D, Björk T, et al. Long-term prediction of prostate cancer up to 25 years before diagnosis of prostate cancer using prostate kallikreins measured at age 44 to 50 years. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(4):431–436. [DOI] [PubMed] [Google Scholar]
- 17.Ulmert D, Cronin AM, Björk T, et al. Prostate-specific antigen at or before age 50 as a predictor of advanced prostate cancer diagnosed up to 25 years later: a case-control study. BMC medicine. 2008;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sjoberg DD, Vickers AJ, Assel M, et al. Twenty-year Risk of Prostate Cancer Death by Midlife Prostate-specific Antigen and a Panel of Four Kallikrein Markers in a Large Population-based Cohort of Healthy Men. European urology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haiman CA, Stram DO, Vickers AJ, et al. Levels of beta-microseminoprotein in blood and risk of prostate cancer in multiple populations. Journal of the National Cancer Institute. 2013;105(3):237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun K, Sjoberg DD, Vickers AJ, Lilja H, Bjartell AS. A Four-kallikrein Panel Predicts High-grade Cancer on Biopsy: Independent Validation in a Community Cohort. European urology. 2016;69(3):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim EH, Andriole GL, Crawford ED, et al. Detection of High Grade Prostate Cancer among PLCO Participants Using a Prespecified 4-Kallikrein Marker Panel. The Journal of urology. 2017;197(4):1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assel M, Sjöblom L, Murtola TJ, et al. A Four-kallikrein Panel and beta-Microseminoprotein in Predicting High-grade Prostate Cancer on Biopsy: An Independent Replication from the Finnish Section of the European Randomized Study of Screening for Prostate Cancer. European urology focus. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preston MA, Gerke T, Carlsson SV, et al. Baseline Prostate-specific Antigen Level in Midlife and Aggressive Prostate Cancer in Black Men. European urology. 2019;75(3):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Punnen S, Freedland SJ, Polascik TJ, et al. A Multi-institutional Prospective Trial in the Veterans Affairs Health System confirms the 4Kscore maintains its Predictive value among African American Men. The Journal of urology. 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.