Abstract
As advances in diagnostics and therapeutic strategies in oncology have increased the number of cancer survivors, the investigation of the mechanisms associated with long-term cognitive complications of cancer treatment has become an important topic of interest. The neurotoxic effects of chemotherapeutic agents have been described in pre-clinical and clinical research. In vitro and rodent studies have identified some underlying mechanisms contributing to chemotherapy-induced neurotoxicity and cognitive impairment for various chemotherapy drugs and other cancer treatments. However, investigation of the direct biological effects of cancer and other potential contributing factors in the pathogenesis of cancer-related cognitive impairment (CRCI) has only recently come into focus. This review will highlight evidence from pre-clinical tumor-bearing rodent models suggesting that cancer influences the cognitive and behavioral changes reported in human cancer populations through direct or indirect pathways that alter the normal neuroinflammatory responses, induce structural brain deficits, and decrease neurogenesis. We reflect on human clinical cancer research indicating that cognitive and behavioral changes precede cancer treatment in some malignancies. We also highlight implications for future areas of CRCI research based on novel findings on the interplay between cancer, chemotherapy, inflammation, tau pathology, and dysregulation of the microbiota-gut-brain axis.
Keywords: cancer-related cognitive impairment, cancer, chemotherapy, microbiota-gut-brain axis, neuroinflammation
1. Introduction
Cancer-related cognitive impairment (CRCI, also referred to as 'chemo-fog' or 'chemo-brain'), is defined as the neurocognitive deficits experienced by cancer survivors, that may be provoked by cancer and cancer treatments. CRCI is associated with impairments in learning and memory, attention, executive function, and processing speed. Executive function is the control system that manages other cognitive processes; it is regulated by the prefrontal cortex and allows one to mentally organize and regulate information to modulate a response based on environmental cues. Impairments in executive function may result in difficulties in planning, organizing, verbal fluency, processing, storing, and or retrieving information1. These impairments diminish cancer survivors' quality of life (QoL)2.
The earliest clinical reports concerning the emotional and cognitive status of cancer patients date back to the 1970s3-7. Initially, reports attributed the behavioral changes experienced by cancer patients to emotional distress and anxiety associated with the cancer diagnosis8. Reports on psychiatric referrals of hospitalized cancer patients found that patients' cognitive difficulties were often misdiagnosed as depression and were underreported by clinicians and patients3, 9. Weiss et al. provided one of the first detailed reports on the clinical manifestations, incidence, and neurological complications associated with commonly used antineoplastic drugs for the treatment of non-central nervous system (CNS) malignancies, suggesting that the incidence of neurological complications in cancer patients would continue to rise as advances in cancer treatment increased patient survival10, 11. Silberfarb et al. found that cancer patients receiving chemotherapy demonstrated lower cognitive functioning compared to non-chemotherapy cancer patients, independently of depression or anxiety12. However, it was not until the 2000s that neuroimaging and cognitive studies of breast cancer patients convincingly demonstrated that the cognitive detriments experienced by cancer patients had a biological basis13, 14. Research on the neural, molecular, and cellular mechanisms associated with CRCI and cancer contributions to cognitive impairments has also come into focus much more recently15-19.
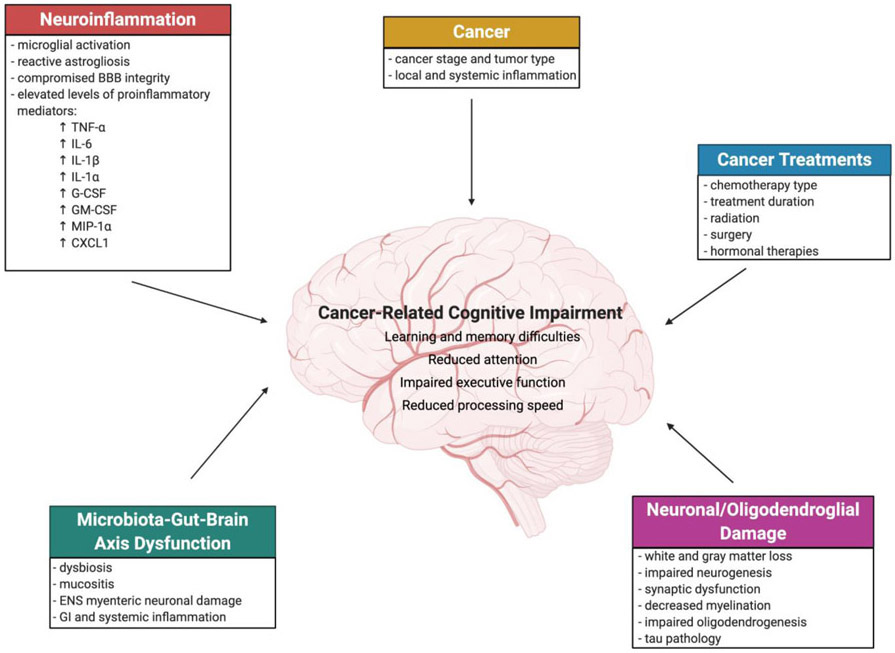
This review highlights CRCI in non-CNS cancers in clinical and pre-clinical studies, focusing on the biological contribution of cancer to impaired cognitive function and behavioral changes. We will also describe potential mechanisms and evidence from multidisciplinary studies supporting the need for insight from interdisciplinary fields to further CRCI research (Fig. 1).
Figure 1. Mechanisms of cancer-related cognitive impairment (CRCI).
A simplified schematic providing an overview of mechanisms of CRCI discussed in this review. Cancer-induced cognitive changes may be associated with local and systemic inflammation, and elevated production of proinflammatory mediators, which can induce neuroinflammation. Cancer treatments, in particular chemotherapy which is discussed here, is associated with neuronal/oligodendroglial damage, neuroinflammation, and microbiota-gut-brain axis dysfunction, which can directly or indirectly induce cognitive decline. Cancer and cancer treatments may exert additive detrimental effects on cognition.
2. Clinical neuroimaging and cognitive studies
The majority of clinical studies on CRCI have focused on breast cancer survivors, as this population regularly reports experiencing cognitive impairments during and after treatment. Acute declines in cognitive function during chemotherapy have been reported in 17% - 75% of chemotherapy-treated breast cancer patients, with 35% of survivors experiencing cognitive impairments years after chemotherapy completion20. In one report, a subset of patients (29%) experienced delayed cognitive decline, which was not present at the time of neurocognitive testing during chemotherapy treatment21. In this same study, 21% of patients demonstrated impairments at baseline cognitive testing, before systemic chemotherapy administration or other cancer treatments, compared to age-matched controls. These findings highlight the need to conduct pre-treatment baseline evaluations in clinical studies of CRCI and suggest that additional factors may influence cognitive decline, such as cancer and diminished cognitive reserve, which may predispose a subset of cancer patients to CRCI and dementia with increasing age21, 22. Cognitive reserve is defined as the overall cognitive capacity and is influenced by genetic and epigenetic factors, education, training, and environmental factors23.
Clinical studies have revealed that declines in neurocognitive function correlate with structural brain changes, including lower gray and white matter density in frontal and temporal brain regions and hippocampal atrophy. Magnetic resonance diffusion tensor imaging (DTI), a technique used to map white matter connectivity via the diffusion of water molecules, has been used to examine white matter integrity in chemotherapy-treated patients. A longitudinal study of young premenopausal (43 years at baseline) breast cancer patients found microstructural white matter changes, which significantly correlated with decreased performance on attention and verbal memory tests 3-4 months after chemotherapy completion compared to the baseline assessment24. A follow-up study in the same cohort of patients 3-4 years after chemotherapy completion revealed a recovery to baseline performance in neurocognitive tasks and structural white matter alterations25. Other longitudinal studies in breast cancer survivors have reported a time-dependent recovery in cognitive performance 1-4 years after chemotherapy completion26, 27. In contrast, a separate study examining the long-term effects of adjuvant chemotherapy on neurocognitive function in breast cancer survivors (n=196) 20 years after treatment found that they performed worse on tasks assessing verbal memory, processing speed, and psychomotor speed, compared to a large population-based group without a history of cancer (n=1,509)28. Together these findings point to the discrepancies between different methodologies used in CRCI studies and suggest that a subset of cancer survivors are vulnerable to long-term neurological sequelae that may detrimentally affect their quality of life years after completing chemotherapy treatment.
Several studies have also examined hippocampal volume alterations and their association with cognitive function. Apple et al. performed a cross-sectional study that examined hippocampal volume in 16 breast cancer survivors 18 months after adjuvant chemotherapy (doxorubicin, paclitaxel) and estrogen-blockade therapy (tamoxifen) compared to 18 healthy controls29. Cancer survivors had significantly smaller hippocampal volumes than controls, increased self-reported cognitive difficulties, and episodic memory impairments. Given the study's cross-sectional design, it is unclear whether the breast cancer survivors experienced cognitive decline or hippocampal atrophy following chemotherapy completion relative to before treatment. It is also unclear whether chemotherapy, tamoxifen, or a combination of both, contributed to changes in hippocampal volume as tamoxifen has been shown to impair working memory and induce structural changes in the prefrontal cortex and hippocampus independently of chemotherapy30, 31. Bergouignan et al. also observed hippocampal atrophy (8% volume reduction) and impairments in episodic memory in breast cancer survivors, 18-36 months after treatment completion (surgery, chemotherapy, radiation) relative to controls32. Functional neuroimaging studies of breast cancer survivors revealed altered hippocampal connectivity 5-6 months after chemotherapy treatment using resting-state functional magnetic resonance imaging (Rs-fMRI), which correlated with executive function, planning, and working memory impairments33, 34.
The clinical evidence shows that there is a neurobiological basis underlying the cognitive impairments associated with CRCI. In particular, these neurocognitive impairments are consistent with dysfunction in the hippocampus and frontal cortex brain regions35. Multiple factors may influence the development of CRCI, including age, class of chemotherapeutic agents, and other therapeutic strategies (radiation, hormone-blockade therapies, surgery). However, there are discrepancies in the literature regarding clinical neuropsychological assessments, which should be addressed using validated neuroscientific approaches to investigate CRCI23. Subjective reports of cognitive impairments do not necessarily correlate with objective neuropsychological testing results, Horowitz et al. attribute this to the diffuse brain damage associated with CRCI, which may be under-detected, given that these tests were initially designed to detect focal lesions23. The effects of cognitive reserve, cancer, epigenetic, and genetic factors on cognition may be masked in cross-sectional comparisons (e.g., to cancer patients not treated with chemotherapy or healthy controls) compared to longitudinal assessments where comparisons are made to a patient's pre-treatment baseline. The lack of a standardized battery of cognitive tests to examine CRCI also contributes to the broad estimate (17% - 75%) of CRCI prevalence in the literature.
3. Tumor-bearing rodent models in pre-clinical studies
Multiple interweaved factors have been proposed to underlie cancer patients' cognitive and behavioral changes, including age, tumor biology, genetic risk factors, cancer treatments, and emotional distress associated with the cancer diagnosis. Studies using tumor-bearing rodent models who are not treated with chemotherapy, yet experience attention, learning, and memory-related behavioral changes suggest that biological factors associated with cancer contribute to cognitive changes in CRCI, independently of chemotherapy and the emotional distress associated with a life-altering diagnosis36-38.
Tumor-bearing rodent models are needed to examine cancer's potential contributions to cognitive function and its interplay with cancer treatments39, 40. Although there have been advances in developing tumor-bearing rodent models of CRCI in the past decade37, 41-43, examination of delayed behavioral changes in cancer-bearing rodents treated with clinically used chemotherapy regimens is limited36, 44. Winocur et al. compared the neurotoxicity of methotrexate (MTX) + 5-fluorouracil (5-FU) and subsequent development of cognitive impairments in the FVB/N-Tg (MMTV-neu) 202 Mul/J female transgenic mouse model of breast cancer to non-cancerous wild-type controls36. Tumor-bearing mice and wild-type controls treated with MTX + 5-FU had impairments in hippocampal- and frontal-cortex dependent cognitive tasks. Notably, tumor-bearing mice not treated with chemotherapy also had impaired performance in these tasks. Non-treated tumor-bearing mice exhibited increased production of circulating pro-inflammatory cytokines, i.e., interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and monocyte chemoattractant protein-1 (MCP-1)45. To examine if the factors secreted by tumor cells induce cognitive impairment, Walker et al. injected 4T1.2. tumor cell-conditioned medium into BALB/c mice daily for nine days46. 4T1.2. is an epithelial breast cancer cell line syngeneic to BALB/c mice. Mice injected intraperitoneally with tumor cell-conditioned media did not have significantly different body mass or locomotion changes; however, on day nine of tumor conditioned media injection, they showed deficits in hippocampal- and frontal cortex-dependent learning, compared to mice injected with the non-tumor conditioned medium. On day nine of conditioned-media injection, mice that received tumor-cell conditioned media showed deficits in hippocampal- and frontal cortex-dependent learning memory46. Additional investigation is necessary to determine the duration of these impairments after discontinuing systemic exposure to tumor-conditioned medium in this model. Comparison of plasma cytokine levels in mice with tumors and mice that were injected with tumor cell-conditioned medium revealed a similar inflammatory profile, with elevated levels of interleukin-1 alpha (IL-1α), IL-6, granulocyte colony-stimulating factor (G-CSF), and the chemoattractant macrophage inflammatory protein-1 alpha (MIP-1α). In a separate study, Yang et al. showed that female BALB/c mice bearing colon carcinoma cell peripheral tumors (colon carcinoma cell line, CT26) demonstrated a 1.68-fold increase in plasma corticosterone levels and significantly higher adrenal gland weights compared to the non-tumor controls, 14 days post-tumor inoculation37. These changes were accompanied by elevated mRNA expression of IL-6 and TNF-α pro-inflammatory cytokines in the hippocampus and high IL-6 levels in plasma.
Although further studies are needed to examine the combined effect of cancer and cancer treatment on cognitive function long-term, there is strong evidence implicating inflammatory mechanisms in CRCI, which will be discussed in the following section.
4. Neuroinflammation
Inflammation is one of the hallmarks of cancer, and the connection between inflammation and changes in learning and memory have been described in aging and neurological disorders independently of cancer and its treatment47-51. Increased circulating levels of pro-inflammatory cytokines have been found to correlate with cognition changes in breast52, 53, and testicular cancers54. Chemotherapeutic agents, many of which cannot cross the BBB or do so at low concentrations, can indirectly induce neuroinflammation via an increase in pro-inflammatory cytokines in the periphery. Although the BBB remains poorly understudied in the context of cancer and CRCI, it is plausible that systemic inflammation induced by specific chemotherapy agents and cancer may disrupt or alter the integrity of the BBB55. Tumor-induced production of pro-inflammatory cytokines as well as those elicited by chemotherapy may contribute to behavioral changes that affect cognitive function in cancer patients40, 56, 57, since many of the upregulated cytokines during cancer progression can cross the BBB, including TNF-α58, IL-659, and interleukin-1 beta (IL-1β)60.
As the resident macrophages in the CNS, microglia play an essential role in neuroimmune surveillance and CNS homeostasis maintenance. Microglia are involved in various processes including synaptic pruning, programmed cell death, neurogenesis, myelin turnover, and phagocytic debris removal, functions which are critical for the maturation and plasticity of neural circuits61. Release of pro-inflammatory cytokines and immune mediators under systemic inflammation can activate microglia and increase the local production of pro-inflammatory cytokines in the brain62. Under transient systemic inflammation, microglia migrate to the cerebral vasculature to maintain BBB integrity; however, prolonged inflammation phenotypically alters microglia, resulting in the phagocytosis of astrocytic end-feet and compromises BBB integrity, resulting in widespread neuroinflammation and a leaky BBB63. Activated microglia and astrocytes are observed in many mouse models of Alzheimer's disease (AD)64, traumatic brain injury (TBI)65, ischemia, and stroke66.
Microglia depletion can mitigate neuroinflammation in rodent models of CRCI. Gibson et al. showed that microglia depletion using the colony-stimulating factor 1 receptor (CSF1R) inhibitor, PLX5622, reduced astrocyte reactivity, normalized myelination, and prevented MTX-induced cognitive deficits in a juvenile mouse model67. PLX5622 has also been shown to ameliorate cognitive deficits in rodent models of AD68, cranial radiation69, and TBI70. Microglial activation and increased pro-inflammatory cytokine/chemokine gene expression in the brain has also been observed following paclitaxel chemotherapy71. Loman et al. examined the changes in peripheral immune system activation, cognition, and microglia immune reactivity in mice treated with paclitaxel71. Paclitaxel-treated mice had elevated levels of IL-1β, TNF-α, IL-6, and the chemoattractant Cxcl1 in the plasma, which was associated with increased expression of IL-1β, TNF-α, IL-6, Cxcl1 mRNA transcription in the brain, and correlated with fatigue and cognitive impairments, but no evidence of microglial activation. Interestingly in rodent models of paclitaxel-induced peripheral neuropathy, astrocytic activation but not microglial activation has been observed in the spinal dorsal horn72, suggesting that although paclitaxel may induce neuroinflammation, microglial activation may not be involved or may only be transiently activated shortly following paclitaxel exposure. Microglial inflammation plays a central role in the pathobiology of neurological sequelae of many commonly used cancer therapies, including radiation69, MTX73, and cyclophosphamide74, doxorubicin75, but prolonged microglial activation and neuroinflammation have not been observed following exposure to cisplatin76 and 5-FU77 in rodent models.
CNS inflammation is associated with breakdown of the BBB and recruitment of peripheral immune cells in multiple neurodegenerative disorders78, 79. Astrocytes are integral to the formation and maintenance of the BBB, and bidirectional crosstalk between astrocytes and microglia modulate CNS inflammation through the secretion of cytokines and inflammatory mediators and recruitment of peripheral immune cells into the CNS78, 80 . Astrocytes promote the activation of microglia through the secretion of IL-6, granulocyte-macrophage colony–stimulating factor (GM-CSF), TNF-α, and other signaling factors 78, 81, 82. The secretion of IL-1α , TNF-α, and complement component 1q (C1q) has been shown to induce a neurotoxic phenotype in reactive astrocytes in models of lipopolysaccharide (LPS)-induced neuroinflammation83. Based on the role of astrocytes in mediating neuroinflammation and maintaining BBB integrity, astrocyte involvement in CRCI is plausible yet pre-clinical evidence in chemotherapy-treated rodents and non-CNS tumor-bearing rodents is limited. Demers et al. found that systemic inflammation including elevated levels of plasma IL-6 and blood neutrophils in a subcutaneous model of Lewis lung carcinoma in C57BL/6 mice was associated with an increase in astrocyte activation and fibrin accumulation in the blood vessels of the brains of tumor-bearing mice compared to controls, however there was no significant change in BBB integrity at 17 days post-transplantation84. Gibson et al. demonstrated that microglia activation induced by MTX exposure in C57BL/6 mice, increased astrocyte reactivity which was associated with neuronal and oligodendrocyte cell death, and impaired oligodendroglial differentiation85.
Among other functions, astrocytes provide vital support for neurons in health and during development, they secrete neurotrophic factors, i.e., brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), to regulate synaptogenesis, neuronal differentiation, and neuronal survival86. In a recent study, English et al. examined the neuroprotective effects of astrocytes in mitigating cisplatin-induced neurotoxicity. Exposure of primary cortical neurons to cisplatin depolarized neuronal mitochondrial membrane potential, altered neuronal calcium dynamics, and reduced neuronal survival. The co-culture of cisplatin-treated neurons with astrocytes restored neuronal calcium dynamics, mitochondrial membrane potential, and increased neuronal survival via astrocytic mitochondrial transfer via the mitochondrial Rho GTPase-1 protein (Miro-1)-dependent pathway87. Notably this and other studies have shown that neurons are more sensitive to cisplatin than astrocytes, which suggests that astrocytic mitochondrial transfer might mitigate neuronal mitochondrial damage and neurotoxicity in the brain after acute cisplatin exposure15, 87. However, extensive neuronal damage under chronic cisplatin exposure and potential reactive astrogliosis may contribute to the development of CRCI, although further investigation in rodent models is clearly warranted.
Additional studies in tumor-bearing rodents are needed to elucidate the individual and combined effects of cancer and chemotherapy on neuroinflammatory processes and subsequent effects on learning and memory.
5. Neuronal/Oligodendroglial Damage
Neurogenesis is central to the integrity and plasticity of the hippocampus; reduced neurogenesis in aging and neurodegenerative disorders has been linked to cognitive impairments and mood disorders (anxiety, depression)88, 89. Impaired hippocampal neurogenesis following chemotherapy treatment may contribute to cognitive changes associated with CRCI90. Neural stem/progenitor cells (NSC) are crucial to intact memory, and hippocampal function throughout life; toxicity to NSC and decreased neurogenesis may contribute to the neurocognitive impairments experienced by cancer survivors16. In humans, postmortem analysis of hippocampal tissue from medulloblastoma or leukemia patients within 2-23 years following chemotherapy completion revealed a profound reduction in neurogenesis. The number of immature neurons (DCX+) was decreased 10 to 100-fold in patients receiving chemotherapy plus radiation relative to age- and sex-matched control subjects91.
Rodent studies have elucidated the effects of chemotherapy on hippocampal NSC and neurons. Our studies and others have shown both in vitro and in vivo that neural progenitor cells and post-mitotic neural cells, including neurons and oligodendrocytes, are preferentially susceptible to damage by diverse types of chemotherapeutic agents, as compared to human cancer cell lines15, 92-94. At clinically relevant concentrations that kill 40-80% of cancer cells, the DNA targeting agent, cisplatin, has been shown to reduce the viability of human and rat primary CNS progenitor cells by 70-100%15. Sub-lethal doses of cisplatin and other DNA-targeting agents reduce the self-renewal capacity of neural progenitor cells, decrease the number of SOX2+ progenitor cells in the dentate gyrus, and induce dendritic branching and spine density loss in hippocampal neurons15, 92, 94.
Chronic treatment with clinically equivalent doses of cyclophosphamide or doxorubicin impaired performance on hippocampus-dependent tasks in treated rats compared to untreated controls. Assessment of hippocampal neurogenesis at three weeks post-treatment revealed a 47% - 53% decrease in DCX+ neurons and an 81%-88% drop in BrdU-NeuN+ cells compared to the saline-treated controls95. The DCX+ neurons displayed abnormal dendritic morphology and ectopic migration. These results suggest that a decline in hippocampal neurogenesis is associated with disrupted hippocampal-based cognitive function in CRCI and that neuroprotective strategies to preserve hippocampal neurogenesis may be useful in ameliorating CRCI95. In a follow-up study, human NSC transplantation in the rat model of chronic cyclophosphamide-treatment reduced cognitive impairments74. The grafted NSC survived (8%) and differentiated along neuronal and astroglial lineages, and importantly enhanced neuronal dendritic arborization and spine density. As only 8% of transplanted NSC survived, a plausible mechanism for reversing the deleterious effects of CRCI with stem cells may be through trophic support via secretion of neurotrophic factors (e.g., BDNF, NGF) that preserve neuronal integrity and synaptic plasticity.
Clinical neuroimaging studies have revealed a loss of white matter structural integrity in breast cancer survivors96, 97. Chemotherapy-induced white matter damage, including loss of oligodendrocytes, and demyelination has been widely described for MTX and 5-FU77, 85. Gibson et al. showed that frontal lobe postmortem tissue samples of individuals treated with chemotherapy contain fewer oligodendrocytes and oligodendrocyte precursor cells (OPC) than samples from control subjects. Also, MTX treatment in mice decreased myelin sheath thickness in the white matter and a long-term decrease in proliferating and mature OPC compared to control mice, suggesting that OPC survival and maturation are impaired85. Notably, stimulating BDNF-TrkB signaling, using the small molecule LM22A-4, rescued MTX-induced myelin loss and cognitive impairment98.
Using a cellular approach, Chiu et al. demonstrated that nasal mesenchymal stem cells (MSC), administered after cisplatin discontinuation, migrated into the brain, restored white matter integrity, and reversed cisplatin-induced cognitive impairments, including deficits in working memory, spatial recognition, and executive functioning99. Cisplatin altered the brain expression profiles of genes involved in oxidative phosphorylation and mitochondrial function. Also, MSC administration restored mitochondrial respiratory function and morphology following cisplatin treatment in brain synaptosomes. Further studies revealed that MSC nasal administration prevented the cisplatin-induced loss of hippocampal neural progenitor cells in mice. Mitochondrial transfer to NSC restores cisplatin-induced mitochondrial integrity changes by normalizing membrane potential and respiratory function and promoting NSC survival100. Although further investigation on how MSC-derived mitochondria communicate with the damaged acceptor NSC cellular machinery to improve NSC survival and rescue mitochondrial function is needed, these studies, along with others, crucially demonstrate the role of impaired neurogenesis and neuronal damage in CRCI.
6. Tau pathology
The microtubule-associated protein tau, which is abundantly expressed in neurons of the CNS, plays an essential role in the assembly and stability of axonal microtubules and regulates neurite outgrowth101. Tau hyperphosphorylation and aggregation in neurofilament tangles reduce microtubule stability and decrease axonal transport102. Tau dysregulation is involved in the pathogenesis of various neurodegenerative diseases, notably in AD, TBI, and Parkinson's disease (PD) 103. Elevated cerebrospinal fluid (CSF) levels of tau have been used as a biomarker of AD, and correlate with impaired cognitive function in neurocognitive testing104.
Although tau has been typically studied in neurodegenerative diseases, it is not exclusive to neurodegeneration, rather elevated tau levels are recognized as a biomarker of brain injury. Recent studies in acute lymphoblastic leukemia (ALL) and breast cancer survivors have examined the association between tau and cognition105-107. Protas et al. assessed tau levels in CSF samples from children with ALL treated with intrathecal MTX and assessed cognitive function 3.7 years after diagnosis. Elevated tau levels in the CSF after consolidation chemotherapy were associated with decreased verbal memory 3.7 years after diagnosis106. A follow-up study found that the high CSF tau levels measured at treatment completion correlated with poor performance in neurocognitive tasks six years after treatment completion, suggesting delayed chronic cognitive complications in ALL survivors105. Henneghan et al. explored the association between serum levels of tau, amyloid-β, and pro-inflammatory cytokines in chemotherapy-treated breast cancer survivors107. Findings suggest an interplay between tau and inflammatory responses to influence cognitive functioning, although further studies are needed to determine whether serum tau levels correlate with cognitive impairments in breast cancer survivors.
Tau pathology has also been linked to cisplatin-induced cognitive impairments in pre-clinical studies. Cisplatin treatment in C57BL/6 mice induced a significant increase in α-tubulin deacetylation and accumulation of hyperphosphorylated tau in the brain, which indicates disruption of microtubule stability108. Cisplatin administration in adult (7-8 month-old) C57BL/6 mice resulted in delayed cognitive impairments and an increased tau expression in the hippocampus 4 months after cisplatin discontinuation. The increase in hippocampal tau clusters was associated with a decreased synaptic integrity in cisplatin-treated mice compared to age-matched 11-12 month-old control mice109. Mouse models of MTX-induced CRCI have also provided evidence of tau dysregulation. MTX treatment in 2 week-old C57BL/6 pups resulted in transient increases in CSF tau and reduced hippocampal cell proliferation, which resulted in hippocampus-dependent cognitive impairments in adulthood, 3-4 months after MTX completion110. These findings suggest that tau dysregulation is indicative of neuronal damage and synaptic dysfunction associated with chemotherapy treatment. Additional studies are needed to determine whether the acute changes in tau levels observed in cancer patients and pre-clinical models result in long-lasting aberrant tau (accumulation or hyperphosphorylation) in the brain and whether serum tau levels can serve as a potential biomarker of CRCI.
7. Microbiota-gut-brain axis dysregulation
The microbiota-gut-brain axis refers to the bidirectional communication system between gut microbes, the enteric nervous system (ENS), and the CNS via neural, endocrine, immune, and humoral pathways111, 112. Chemotherapy has been shown to adversely affect the gut microbiota composition in pediatric and adult cancer populations. Gastrointestinal (GI) issues are frequent and dose-limiting toxicities of chemotherapy treatment that may reduce cancer treatment adherence and affect survival. Chemotherapy-induced GI toxicities are due to disruption of the gut microbiota, termed dysbiosis, inflammation (intestinal and systemic), and mucositis, which is the ulceration of the oral/GI tract mucosal cell lining. Mucositis affects over 80% of cancer patients treated with high-dose chemotherapy113, 114. Huang et al. examined changes in the intestinal microbiota composition of 36 pediatric ALL patients treated with high-dose MTX, compared to 36 healthy age-matched controls115. Intestinal microbes decreased in the stool samples of the ALL group post-chemotherapy completion by 29.6% compared to the controls. MTX-induced changes in the gut microbiota composition were evident one-week post-treatment completion and at least 9 months after cessation of chemotherapy in a separate study of ALL survivors116. Additional studies suggest that chemotherapy treatment results in reduced gut microbiota diversity, leading to mucositis, inflammation, and long-term microbiota dysbiosis117, 118.
The intestinal microbiota's ability to influence CNS function, cognition, and behavior has sparked interest recently. Alterations in gut microbiota have been implicated in the pathogenesis of various neurological disorders involving changes in cognition and emotional behavior which include, AD119, autism spectrum disorders (ASDs)120, Major Depressive Disorder (MDD)121, 122, PD123, and schizophrenia124. These disorders are also associated with neuroinflammation. There are multiple pathways proposed by which intestinal mucositis and decreased GI microbiota diversity provoked by chemotherapy treatment may contribute to CRCI, including endocrine and neural signaling pathways via the enteric nervous system125 and systemic inflammation118, 126. Pre-clinical studies of rodent models treated with platinum-based chemotherapy (cisplatin, carboplatin, oxaliplatin) have described oxidative stress and mitochondrial dysfunction as the underlying mechanisms of cognitive impairments94, 127, 128, peripheral neuropathy129-131, and neural cell damage elicited by these agents15, 92. However, the influence of chemotherapy on the ENS function and the extent to which GI dysfunction influences cognition and behavior needs further exploration. The ENS is a large component of the autonomic nervous system and is responsible for the GI tract's physiological functions, including motility, secretion of gastrointestinal enzymes, and blood flow132. Located within the GI tract wall, the ENS contains 200-600 million neurons, which are distributed into two types of ganglia, myenteric and submucosal plexuses133. Using a mouse model, Wafai et al. demonstrated that chronic oxaliplatin administration in BALB/c mice increased the expression of neuron nitric oxide synthase (nNOS), decreased myenteric neurons of the distal colon, and reduced colonic motility134. Chronic cisplatin administration reduced GI and colonic motor function, reduced the number of colonic myenteric neurons, and increased nNOS+ myenteric neurons135. Similar findings have been reported for 5-FU, which is the first-line chemotherapy for colorectal cancer. McQuade et al. found that increased GI transit and acute intestinal inflammation (increased levels of fecal lipocalin-2 and CD45+ leukocytes in the colon) preceded myenteric neuronal loss and reduced colonic motility in BALB/c mice treated with a clinically relevant regimen of 5-FU136.
Using a clinically-relevant regimen of paclitaxel chemotherapy in adult female BALB/c mice, Loman et al. found that paclitaxel transiently increased expression of pro-inflammatory cytokine (IL-1β, IL-6, and TNF-α) and chemokine (CXCL1) mRNA levels in the brain (hypothalamus, hippocampus), circulating levels of LPS binding protein (LBP), IL-1β, TNF-α, and CXCL171, and impaired context discrimination. Paclitaxel-treated mice had altered microbiota composition and increased colon crypt depth. Reductions in fecal and colonic microbiome diversity and loss of colonic tissue integrity have been shown in other studies to contribute to anxiety and depression137, 138. In a recent study, Zhang et al. found that 5-FU treatment in rats significantly increased immobility time in the tail suspension test and decreased sucrose consumption in the sucrose preference test, two common rodent behavioral tasks used to assess rodent depression-like behavior139. In addition to these behavioral changes and reduced gut microbiome diversity, 5-FU increased gamma-aminobutyric acid (GABA) levels, the most abundant inhibitory neurotransmitter in the CNS. Fecal microbiota transplants from healthy controls into 5-FU treated rats reversed depressive-like behaviors.
The microbiota-gut-brain axis plays a significant role in the development of various neurological disorders. Current studies have demonstrated that chemotherapy may induce ENS toxicities and gut microbiota changes, may influence neurocognitive function. Further studies are warranted to elucidate the effects of cancer and cancer treatment (chemotherapy, radiation, immunotherapy) on gut microbial composition in cancer patients and pre-clinical rodent models. Understanding the mechanisms by which chemotherapy alters ENS and GI microbiome diversity is also of interest for developing new treatment strategies that can diminish CRCI and GI toxicities.
8. Summary and conclusions
As the number of cancer survivors continues to rise, an improved understanding of the biological mechanisms associated with CRCI becomes more crucial for developing potential therapeutic strategies to minimize the cognitive and neurotoxic adverse sequelae of cancer and cancer treatments in survivors. In this review, we have highlighted clinical and pre-clinical evidence of non-CNS tumors affecting cognition and the potential mechanisms by which tumors may, directly and indirectly, influence cognitive function. We have also highlighted recent findings implicating cancer-induced inflammation, tau pathology, neuronal/oligodendroglial damage, and microbiota-gut-brain axis dysfunction in the pathogenesis of CRCI.
Several challenges remain in the clinical and pre-clinical CRCI research settings. Currently, standardized neurocognitive assessments of CRCI in patients are lacking, and subjective complaints of CRCI do not correlate with the results of neurocognitive evaluations, which is reflected in the reported range (17% – 75%) of cancer survivors who experience CRCI23. Although clinical neuropsychological tests have found significant effects of cancer treatment on cognition, these measures do not account for the subtle cognitive difficulties associated with CRCI as they were developed to diagnose severe brain pathologies, such as AD, PD, and TBI. Recent neuroimaging longitudinal studies in breast cancer survivors have revealed that structural changes in white matter integrity correlate with altered cognitive function24, 25. Such studies have demonstrated the essential yet challenging need of examining baseline cognitive performance and structural MRI analysis in cancer patients before initiating treatment, in addition to assessment at multiple time-points during and after treatment completion. In pre-clinical studies, the use of tumor-bearing rodent models treated with clinically used cancer regimens is needed to characterize further the mechanisms associated with CRCI and test therapeutic interventions. Interdisciplinary approaches spanning diverse methodologies, including microbiology, gut biology, basic neuroscience, aging biology, and tumor biology, will be essential to leverage the field of CRCI and help develop therapeutic interventions to improve the quality of life of cancer survivors.
Acknowledgments
Financial support: This work was supported by the National Institute for Neurological Diseases and Stroke Award (NINDS/NIH) [NS072234], the National Center for Advancing Translational Sciences, NIH [UL1 TR001414], and the UCI Cancer Center Award [P30CA062203] from the National Cancer Institute to DB; and NIH Grants U18 EB029354 and HL147562 to KG. The NIH MBRS-IMSD training grant [GM055246] and the NINDS/NIH pre-doctoral fellowship [NS082174] provided support for N. Lomeli. The NIH MBRS-IMSD training grant [GM055246] provided support for J. Lepe. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of Potential Conflicts of Interest: KG reports grants from Grifols, Cyclerion, and 1910 Genetics, and honorarium from Novartis, Tautona Group, and CSL Behring, outside the scope of the submitted work. NL, JL, DB do not have any potential conflicts of interest to disclose.
9. References
- 1.Diamond A Executive functions. Annual review of psychology. 2013;64: 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy SJ, Krull KR, Wefel JS, Janelsins M. Cognitive Changes in Cancer Survivors. American Society of Clinical Oncology Educational Book. 2018: 795–806. [DOI] [PubMed] [Google Scholar]
- 3.Levine PM, Silberfarb PM, Lipowski ZJ. Mental disorders in cancer patients. A study of 100 psychiatric referrals. Cancer. 1978;42: 1385–1391. [DOI] [PubMed] [Google Scholar]
- 4.Achté KA, Vauhkonen ML. Cancer and the psyche. Ann Med Intern Fenn. 1967;56: Suppl 49:45–33. [PubMed] [Google Scholar]
- 5.Holland J Psychological Aspects of Oncology. Medical Clinics of North America. 1977;61: 737–748. [DOI] [PubMed] [Google Scholar]
- 6.Peck A Emotional reactions to having cancer. CA: a cancer journal for clinicians. 1972;225: 284–291. [DOI] [PubMed] [Google Scholar]
- 7.Weisman AD. Early diagnosis of vulnerability in cancer patients. Am J Med Sci. 1976;271: 187–196. [DOI] [PubMed] [Google Scholar]
- 8.Craig TJ, Abeloff MD. Psychiatric symptomatology among hospitalized cancer patients. The American Journal of Psychiatry. 1974;131: 1323–1327. [PubMed] [Google Scholar]
- 9.Schottenfeld D, Robbins GF. Quality of survival among patients who have had radical mastectomy. Cancer. 1970;26: 650–655. [DOI] [PubMed] [Google Scholar]
- 10.Weiss HD, Walker MD, Wiernik PH. Neurotoxicity of commonly used antineoplastic agents (first of two parts). N Engl J Med. 1974;291: 75–81. [DOI] [PubMed] [Google Scholar]
- 11.Weiss HD, Walker MD, Wiernik PH. Neurotoxicity of commonly used antineoplastic agents (second of two parts). N Engl J Med. 1974;291: 127–133. [DOI] [PubMed] [Google Scholar]
- 12.Silberfarb PM, Philibert D, Levine PM. Psychosocial aspects of neoplastic disease: II. Affective and cognitive effects of chemotherapy in cancer patients. Am J Psychiatry. 1980;137: 597–601. [DOI] [PubMed] [Google Scholar]
- 13.Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20: 485–493. [DOI] [PubMed] [Google Scholar]
- 14.Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry. 2003;8: 201–216. [PubMed] [Google Scholar]
- 15.Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13: 1285–1295. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich J, Prust M, Kaiser J. Chemotherapy, cognitive impairment and hippocampal toxicity. Neuroscience. 2015;309: 224–232. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser J, Bledowski C, Dietrich J. Neural correlates of chemotherapy-related cognitive impairment. Cortex. 2014;54: 33–50. [DOI] [PubMed] [Google Scholar]
- 19.Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res. 2012;227: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. International Review of Psychiatry. 2014;26: 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116: 3348–3356. [DOI] [PubMed] [Google Scholar]
- 22.Kesler SR, Rao V, Ray WJ, Rao A. Probability of Alzheimer's disease in breast cancer survivors based on gray-matter structural network efficiency. Alzheimers Dement (Amst). 2017;9: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horowitz TS, Suls J, Treviño M. A Call for a Neuroscience Approach to Cancer-Related Cognitive Impairment. Trends in Neurosciences. 2018;41: 493–496. [DOI] [PubMed] [Google Scholar]
- 24.Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30: 274–281. [DOI] [PubMed] [Google Scholar]
- 25.Billiet T, Emsell L, Vandenbulcke M, et al. Recovery from chemotherapy-induced white matter changes in young breast cancer survivors? Brain Imaging and Behavior. 2018;12: 64–77. [DOI] [PubMed] [Google Scholar]
- 26.Schagen SB, Muller MJ, Boogerd W, et al. Late effects of adjuvant chemotherapy on cognitive function: a follow-up study in breast cancer patients. Ann Oncol. 2002;13: 1387–1397. [DOI] [PubMed] [Google Scholar]
- 27.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28: 4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koppelmans V, Breteler MMB, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological Performance in Survivors of Breast Cancer More Than 20 Years After Adjuvant Chemotherapy. Journal of Clinical Oncology. 2012;30: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 29.Apple AC, Ryals AJ, Alpert KI, et al. Subtle hippocampal deformities in breast cancer survivors with reduced episodic memory and self-reported cognitive concerns. Neuroimage Clin. 2017;14: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, He X, Tao L, et al. The Working Memory and Dorsolateral Prefrontal-Hippocampal Functional Connectivity Changes in Long-Term Survival Breast Cancer Patients Treated with Tamoxifen. Int J Neuropsychopharmacol. 2017;20: 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature reviews. Cancer 2007;7: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergouignan L, Lefranc JP, Chupin M, Morel N, Spano JP, Fossati P. Breast cancer affects both the hippocampus volume and the episodic autobiographical memory retrieval. PLoS One. 2011;6: e25349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng H, Li W, Gong L, et al. Altered resting-state hippocampal functional networks associated with chemotherapy-induced prospective memory impairment in breast cancer survivors. Scientific Reports. 2017;7: 45135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng Y, Tuluhong D, Shi Z, et al. Postchemotherapy hippocampal functional connectivity patterns in patients with breast cancer: a longitudinal resting state functional MR imaging study. Brain Imaging and Behavior. 2019. [DOI] [PubMed] [Google Scholar]
- 35.Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;39: 297–304. [DOI] [PubMed] [Google Scholar]
- 36.Winocur G, Berman H, Nguyen M, et al. Neurobiological Mechanisms of Chemotherapy-induced Cognitive Impairment in a Transgenic Model of Breast Cancer. Neuroscience. 2018;369: 51–65. [DOI] [PubMed] [Google Scholar]
- 37.Yang M, Kim J, Kim JS, et al. Hippocampal dysfunctions in tumor-bearing mice. Brain Behav Immun. 2014;36: 147–155. [DOI] [PubMed] [Google Scholar]
- 38.Casaril AM, Domingues M, Bampi SR, et al. The antioxidant and immunomodulatory compound 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole attenuates depression-like behavior and cognitive impairment developed in a mouse model of breast tumor. Brain, Behavior, and Immunity. 2020;84: 229–241. [DOI] [PubMed] [Google Scholar]
- 39.Winocur G, Johnston I, Castel H. Chemotherapy and cognition: International cognition and cancer task force recommendations for harmonising preclinical research. Cancer Treatment Reviews. 2018;69: 72–83. [DOI] [PubMed] [Google Scholar]
- 40.Schrepf A, Lutgendorf SK, Pyter LM. Pre-treatment effects of peripheral tumors on brain and behavior: Neuroinflammatory mechanisms in humans and rodents. Brain, Behavior, and Immunity. 2015;49: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pyter LM, Cochrane SF, Ouwenga RL, Patel PN, Pineros V, Prendergast BJ. Mammary tumors induce select cognitive impairments. Brain, behavior, and immunity. 2010;24: 903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seigers R, Pourtau L, Schagen SB, et al. Inhibition of hippocampal cell proliferation by methotrexate in rats is not potentiated by the presence of a tumor. Brain Res Bull. 2010;81: 472–476. [DOI] [PubMed] [Google Scholar]
- 43.Yang M, Kim JS, Kim J, et al. Acute treatment with methotrexate induces hippocampal dysfunction in a mouse model of breast cancer. Brain Res Bull. 2012;89: 50–56. [DOI] [PubMed] [Google Scholar]
- 44.Pyter LM, Suarez-Kelly LP, Carson WE 3rd, Kaur J, Bellisario J, Bever SR Novel rodent model of breast cancer survival with persistent anxiety-like behavior and inflammation. Behav Brain Res. 2017;330: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cytokines in breast cancer development and progression. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2015;35: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker AK, Chang A, Ziegler AI, Dhillon HM, Vardy JL, Sloan EK. Low dose aspirin blocks breast cancer-induced cognitive impairment in mice. PLoS One. 2018;13: e0208593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Czerniawski J, Guzowski JF. Acute neuroinflammation impairs context discrimination memory and disrupts pattern separation processes in hippocampus. J Neurosci. 2014;34: 12470–12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czerniawski J, Miyashita T, Lewandowski G, Guzowski JF. Systemic lipopolysaccharide administration impairs retrieval of context-object discrimination, but not spatial, memory: Evidence for selective disruption of specific hippocampus-dependent memory functions during acute neuroinflammation. Brain Behav Immun. 2015;44: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clausen F, Hånell A, Björk M, et al. Neutralization of interleukin-1β modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. European Journal of Neuroscience. 2009;30: 385–396. [DOI] [PubMed] [Google Scholar]
- 50.Sartori AC, Vance DE, Slater LZ, Crowe M. The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. J Neurosci Nurs. 2012;44: 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sudduth TL, Schmitt FA, Nelson PT, Wilcock DM. Neuroinflammatory phenotype in early Alzheimer's disease. Neurobiology of Aging. 2013;34: 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel SK, Wong AL, Wong FL, et al. Inflammatory Biomarkers, Comorbidity, and Neurocognition in Women With Newly Diagnosed Breast Cancer. J Natl Cancer Inst. 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams AM, Shah R, Shayne M, et al. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. Journal of Neuroimmunology. 2018;314: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amidi A, Agerbaek M, Wu LM, et al. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. 2016. [DOI] [PubMed] [Google Scholar]
- 55.Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain, Behavior, and Immunity. 2017;60: 1–12. [DOI] [PubMed] [Google Scholar]
- 56.Pomykala KL, Ganz PA, Bower JE, et al. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain imaging and behavior. 2013;7: 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nature Reviews Cancer. 2008;8: 887–899. [DOI] [PubMed] [Google Scholar]
- 58.Kim KS, Wass CA, Cross AS, Opal SM. Modulation of blood-brain barrier permeability by tumor necrosis factor and antibody to tumor necrosis factor in the rat. Lymphokine and cytokine research. 1992;11: 293–298. [PubMed] [Google Scholar]
- 59.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2: 241–248. [DOI] [PubMed] [Google Scholar]
- 60.Argaw AT, Zhang Y, Snyder BJ, et al. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177: 5574–5584. [DOI] [PubMed] [Google Scholar]
- 61.Wake H, Moorhouse AJ, Miyamoto A, Nabekura J. Microglia: actively surveying and shaping neuronal circuit structure and function. Trends in Neurosciences. 2013;36: 209–217. [DOI] [PubMed] [Google Scholar]
- 62.Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haruwaka K, Ikegami A, Tachibana Y, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nature Communications. 2019;10: 5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crapser JD, Spangenberg EE, Barahona RA, Arreola MA, Hohsfield LA, Green KN. Microglia facilitate loss of perineuronal nets in the Alzheimer's disease brain. EBioMedicine. 2020;58: 102919–102919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gold EM, Vasilevko V, Hasselmann J, et al. Repeated Mild Closed Head Injuries Induce Long-Term White Matter Pathology and Neuronal Loss That Are Correlated With Behavioral Deficits. ASN neuro. 2018;10: 1759091418781921–1759091418781921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. Journal of Neuroinflammation. 2019;16: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gibson E, Monje M. Effect of cancer therapy on neural stem cells: implications for cognitive function. Curr Opin Oncol. 2012;24: 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dagher NN, Najafi AR, Kayala KMN, et al. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. Journal of Neuroinflammation. 2015;12: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Acharya MM, Green KN, Allen BD, et al. Elimination of microglia improves cognitive function following cranial irradiation. Scientific Reports. 2016;6: 31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henry RJ, Ritzel RM, Barrett JP, et al. Microglial Depletion with CSF1R Inhibitor During Chronic Phase of Experimental Traumatic Brain Injury Reduces Neurodegeneration and Neurological Deficits. The Journal of Neuroscience. 2020;40: 2960–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loman BR, Jordan KR, Haynes B, Bailey MT, Pyter LM. Chemotherapy-induced neuroinflammation is associated with disrupted colonic and bacterial homeostasis in female mice. Sci Rep. 2019;9: 16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H, Yoon S-Y, Zhang H, Dougherty PM. Evidence That Spinal Astrocytes but Not Microglia Contribute to the Pathogenesis of Paclitaxel-Induced Painful Neuropathy. The Journal of Pain. 2012;13: 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seigers R, Timmermans J, van der Horn HJ, et al. Methotrexate reduces hippocampal blood vessel density and activates microglia in rats but does not elevate central cytokine release. Behav Brain Res. 2010;207: 265–272. [DOI] [PubMed] [Google Scholar]
- 74.Acharya MM, Martirosian V, Chmielewski NN, et al. Stem Cell Transplantation Reverses Chemotherapy-Induced Cognitive Dysfunction. Cancer Res. 2015;75: 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allen BD, Apodaca LA, Syage AR, et al. Attenuation of neuroinflammation reverses Adriamycin-induced cognitive impairments. Acta Neuropathologica Communications. 2019;7: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou W, Kavelaars A, Heijnen CJ. Metformin Prevents Cisplatin-Induced Cognitive Impairment and Brain Damage in Mice. PLOS One. 2016;11: e0151890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han R, Yang YM, Dietrich J, Luebke A, Mayer-Pröschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. Journal of Biology. 2008;7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Linnerbauer M, Wheeler MA, Quintana FJ. Astrocyte Crosstalk in CNS Inflammation. Neuron. 2020;108: 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rothhammer V, Quintana FJ. Control of autoimmune CNS inflammation by astrocytes. Seminars in Immunopathology. 2015;37: 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541: 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wheeler MA, Jaronen M, Covacu R, et al. Environmental Control of Astrocyte Pathogenic Activities in CNS Inflammation. Cell. 2019;176: 581–596.e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palpagama TH, Waldvogel HJ, Faull RLM, Kwakowsky A. The Role of Microglia and Astrocytes in Huntington's Disease. Frontiers in molecular neuroscience. 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity. 2017;46: 957–967. [DOI] [PubMed] [Google Scholar]
- 84.Demers M, Suidan GL, Andrews N, Martinod K, Cabral JE, Wagner DD. Solid peripheral tumor leads to systemic inflammation, astrocyte activation and signs of behavioral despair in mice. PLoS One. 2018;13: e0207241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gibson EM, Nagaraja S, Ocampo A, et al. Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell. 2019;176: 43–55 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Allen NJ, Bennett ML, Foo LC, et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486: 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.English K, Shepherd A, Uzor NE, Trinh R, Kavelaars A, Heijnen CJ. Astrocytes rescue neuronal health after cisplatin treatment through mitochondrial transfer. Acta Neuropathol Commun. 2020;8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94: 10409–10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nature reviews. Neuroscience 2017;18: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stone JB, DeAngelis LM. Cancer-treatment-induced neurotoxicity—focus on newer treatments. Nature Reviews Clinical Oncology. 2015;13: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62: 515–520. [DOI] [PubMed] [Google Scholar]
- 92.Andres AL, Gong X, Di K, Bota DA. Low-doses of cisplatin injure hippocampal synapses: a mechanism for 'chemo' brain? Exp Neurol. 2014;255: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gong X, Schwartz PH, Linskey ME, Bota DA. Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy. Neurology. 2011;76: 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lomeli N, Di K, Czerniawski J, Guzowski JF, Bota DA. Cisplatin-induced mitochondrial dysfunction is associated with impaired cognitive function in rats. Free Radic Biol Med. 2017;102: 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Christie LA, Acharya MM, Parihar VK, Nguyen A, Martirosian V, Limoli CL. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18: 1954–1965. [DOI] [PubMed] [Google Scholar]
- 96.Matsos A, Loomes M, Zhou I, et al. Chemotherapy-induced cognitive impairments: White matter pathologies. Cancer Treat Rev. 2017;61: 6–14. [DOI] [PubMed] [Google Scholar]
- 97.Feng Y, Zhang XD, Zheng G, Zhang LJ. Chemotherapy-induced brain changes in breast cancer survivors: evaluation with multimodality magnetic resonance imaging. Brain imaging and behavior. 2019;13: 1799–1814. [DOI] [PubMed] [Google Scholar]
- 98.Geraghty AC, Gibson EM, Ghanem RA, et al. Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron. 2019;103: 250–265.e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chiu GS, Boukelmoune N, Chiang ACA, et al. Nasal administration of mesenchymal stem cells restores cisplatin-induced cognitive impairment and brain damage in mice. Oncotarget. 2018;9: 35581–35597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boukelmoune N, Chiu GS, Kavelaars A, Heijnen CJ. Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathol Commun. 2018;6: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodríguez-Martín T, Cuchillo-Ibáñez I, Noble W, Nyenya F, Anderton BH, Hanger DP. Tau phosphorylation affects its axonal transport and degradation. Neurobiol Aging. 2013;34: 2146–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol. 2017;133: 665–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Edwards G 3rd, Zhao J, Dash PK, Soto C, Moreno-Gonzalez I. Traumatic Brain Injury Induces Tau Aggregation and Spreading. J Neurotrauma. 2020;37: 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andreasen N, Minthon L, Davidsson P, et al. Evaluation of CSF-tau and CSF-Aβ42 as Diagnostic Markers for Alzheimer Disease in Clinical Practice. Archives of Neurology. 2001;58: 373–379. [DOI] [PubMed] [Google Scholar]
- 105.Krawczuk-Rybak M, Grabowska A, Protas PT, Muszynska-Roslan K, Holownia A, Braszko J. Intellectual functioning of childhood leukemia survivors--relation to Tau protein--a marker of white matter injury. Adv Med Sci. 2012;57: 266–272. [DOI] [PubMed] [Google Scholar]
- 106.Protas PT, Muszynska-Roslan K, Holownia A, et al. Negative correlation between cerebrospinal fluid tau protein and cognitive functioning in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;53: 105–108. [DOI] [PubMed] [Google Scholar]
- 107.Henneghan A, Haley AP, Kesler S. Exploring Relationships Among Peripheral Amyloid Beta, Tau, Cytokines, Cognitive Function, and Psychosomatic Symptoms in Breast Cancer Survivors. Biol Res Nurs. 2020;22: 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma J, Huo X, Jarpe MB, Kavelaars A, Heijnen CJ. Pharmacological inhibition of HDAC6 reverses cognitive impairment and tau pathology as a result of cisplatin treatment. Acta Neuropathol Commun. 2018;6: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chiang ACA, Huo X, Kavelaars A, Heijnen CJ. Chemotherapy accelerates age-related development of tauopathy and results in loss of synaptic integrity and cognitive impairment. Brain Behav Immun. 2019;79: 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elens I, Dekeyster E, Moons L, D'Hooge R. Methotrexate Affects Cerebrospinal Fluid Folate and Tau Levels and Induces Late Cognitive Deficits in Mice. Neuroscience. 2019;404: 62–70. [DOI] [PubMed] [Google Scholar]
- 111.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nature reviews. Neuroscience 2011;12: 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Annals of gastroenterology. 2015;28: 203–209. [PMC free article] [PubMed] [Google Scholar]
- 113.Lalla RV, Bowen J, Barasch A, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120: 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100: 2026–2046. [DOI] [PubMed] [Google Scholar]
- 115.Huang Y, Yang W, Liu H, et al. Effect of high-dose methotrexate chemotherapy on intestinal Bifidobacteria, Lactobacillus and Escherichia coli in children with acute lymphoblastic leukemia. Experimental Biology and Medicine. 2012;237: 305–311. [DOI] [PubMed] [Google Scholar]
- 116.Chua LL, Rajasuriar R, Lim YAL, Woo YL, Loke Pn, Ariffin H. Temporal changes in gut microbiota profile in children with acute lymphoblastic leukemia prior to commencement-, during-, and post-cessation of chemotherapy. BMC cancer. 2020;20: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chua LL, Rajasuriar R, Azanan MS, et al. Reduced microbial diversity in adult survivors of childhood acute lymphoblastic leukemia and microbial associations with increased immune activation. Microbiome. 2017;5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jordan KR, Loman BR, Bailey MT, Pyter LM. Gut microbiota-immune-brain interactions in chemotherapy-associated behavioral comorbidities. Cancer. 2018;124: 3990–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhuang ZQ, Shen LL, Li WW, et al. Gut Microbiota is Altered in Patients with Alzheimer's Disease. J Alzheimers Dis. 2018;63: 1337–1346. [DOI] [PubMed] [Google Scholar]
- 120.Fowlie G, Cohen N, Ming X. The Perturbance of Microbiome and Gut-Brain Axis in Autism Spectrum Disorders. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sanada K, Nakajima S, Kurokawa S, et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. Journal of Affective Disorders. 2020;266: 1–13. [DOI] [PubMed] [Google Scholar]
- 122.Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity. 2015;48: 186–194. [DOI] [PubMed] [Google Scholar]
- 123.Sampson TR, Debelius JW, Thron T, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell. 2016;167: 1469–1480.e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125: 926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deleemans JM, Chleilat F, Reimer RA, et al. The chemo-gut study: investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; study protocol. BMC cancer. 2019;19: 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chiu GS, Maj MA, Rizvi S, et al. Pifithrin-mu Prevents Cisplatin-Induced Chemobrain by Preserving Neuronal Mitochondrial Function. Cancer Res. 2017;77: 742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fardell JE, Vardy J, Monds LA, Johnston IN. The long-term impact of oxaliplatin chemotherapy on rodent cognition and peripheral neuropathy. Behav Brain Res. 2015;291: 80–88. [DOI] [PubMed] [Google Scholar]
- 129.Podratz JL, Knight AM, Ta LE, et al. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011;41: 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology. 2006;27: 992–1002. [DOI] [PubMed] [Google Scholar]
- 131.Maj MA, Ma J, Krukowski KN, Kavelaars A, Heijnen CJ. Inhibition of Mitochondrial p53 Accumulation by PFT-μ Prevents Cisplatin-Induced Peripheral Neuropathy. Frontiers in molecular neuroscience. 2017;10: 108–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nature Reviews Gastroenterology & Hepatology. 2016;13: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817: 39–71. [DOI] [PubMed] [Google Scholar]
- 134.Wafai L, Taher M, Jovanovska V, Bornstein JC, Dass CR, Nurgali K. Effects of oxaliplatin on mouse myenteric neurons and colonic motility. Frontiers in neuroscience. 2013;7: 30–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vera G, Castillo M, Cabezos PA, et al. Enteric neuropathy evoked by repeated cisplatin in the rat. Neurogastroenterol Motil. 2011;23: 370–378, e162-373. [DOI] [PubMed] [Google Scholar]
- 136.McQuade RM, Stojanovska V, Donald E, Abalo R, Bornstein JC, Nurgali K. Gastrointestinal dysfunction and enteric neurotoxicity following treatment with anticancer chemotherapeutic agent 5-fluorouracil. Neurogastroenterol Motil. 2016;28: 1861–1875. [DOI] [PubMed] [Google Scholar]
- 137.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36: 305–312. [DOI] [PubMed] [Google Scholar]
- 138.Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota's effect on mental health: The gut-brain axis. Clinics and practice. 2017;7: 987–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang F, Chen H, Zhang R, et al. 5-Fluorouracil induced dysregulation of the microbiome-gut-brain axis manifesting as depressive like behaviors in rats. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2020;1866: 165884. [DOI] [PubMed] [Google Scholar]
- 140.Figure 1 was created using BioRender.com.