Purpose:
To assess safety and efficacy outcomes of sutured custom silicone artificial iris and intraocular lens implantation combined with penetrating keratoplasty (triple procedure).
Methods:
Prospective consecutive surgical case series of patients who underwent the triple procedure between 2010 and 2019 at Stein Eye Institute, UCLA, followed up for 1 year minimum. Safety outcomes were changes from preoperative to last follow-up in corrected distance visual acuity (CDVA), endothelial cell count, intraocular pressure (IOP), and postoperative complications. Efficacy outcomes included changes in subjective glare (none to severe), cosmetic appearance (worse to very much improved), and visual function as assessed by the Visual Function Questionnaire-25 at 1-year follow-up.
Results:
Among 82 eyes implanted with an artificial iris, 14 eyes (17.1%) underwent the triple procedure. The median follow-up was 18.1 months (range 12.0–54.9 months). The median CDVA improved from 2.0 log of minimum angle of resolution (logMAR) (range 0.9–2.3 logMAR) to 0.7 logMAR (range 0.2–2.6 logMAR) (P = 0.02). Average endothelial cell count decreased 57.6% (P < 0.01). Six eyes (42.9%) experienced IOP elevations, 13 eyes (92.3%) developed iritis, and 11 eyes (78.6%) underwent secondary surgery. Graft rejection or secondary graft failure occurred in 7 eyes each (50.0%). Cosmesis improved in 12 eyes (85.7%; P < 0.01). The Visual Function Questionnaire-25 score improved from 72 to 77 (P < 0.01). Glare symptoms did not change significantly.
Conclusions:
The triple procedure was effective at improving CDVA, cosmesis, and quality of life; however, it was associated with frequent postoperative complications, of which iritis, IOP elevation, and secondary graft failure were the most common.
Key Words: artificial iris, cornea, penetrating keratoplasty, intraocular lens implantation, triple procedure
Patients with acquired aniridia and aphakia usually experience visual impairment, light and glare sensitivity, and issues related to the cosmetic appearance of their eyes.1,2 Acquired iris defects might result from blunt, penetrating, or surgical trauma; intraocular inflammation; or a variety of rare stationary or progressive disorders.3 Penetrating injuries and surgical trauma are frequently associated with a myriad of ocular comorbidities, such as intraocular pressure (IOP) elevation, intraocular inflammation, aphakia, corneal scarring, corneal endothelial failure, posterior segment damage, and glaucoma. In addition, the emotional consequences of having a deformed body part cannot be ignored.4
Several iris reconstruction lenses, also known as aniridia implants, have been designed to correct traumatic aniridia and aphakia.5,6 Given the general severity of the condition of most eyes that are both aniridic and aphakic, these devices have been reported to be relatively safe and very effective at improving visual acuity and reducing light and glare sensitivity.6,7 However, their limitations include the necessity of a large incision and the lack of color customization, which can lead to poor cosmetic outcomes.5,6
The CustomFlex ArtificialIris (HumanOptics AG, Erlangen, Germany) is a biocompatible silicone prosthesis that was approved by the US and Drug Administration (FDA) in May 2018. It has a diameter of 12.8 mm and a thickness that varies from 0.25 mm in the periphery to 0.4 mm at the pupil margin. It comes in fiber-free and fiber-containing models. The fiber-containing model can be sutured to a lens implant, residual iris tissue, or the sclera. This custom iris is designed to correct iris defects in aphakic or pseudophakic eyes or phakic eyes that will undergo cataract extraction. It is not intended to change eye color as a stand-alone procedure. The front face of the device is hand painted to match a high-quality clinical photograph of the fellow eye. In the setting of bilateral aniridia, a sample photograph of any eye can be used. The back face of the iris is black and opaque. Outcomes of custom artificial iris use for traumatic and congenital iris defects are promising, taking into account the extensive comorbidities presented by such eyes.8,9
To correct concurrent aphakia at the time of custom artificial iris implantation, several techniques have been reported. First, the artificial iris and an intraocular lens (IOL) can be sutured together, or alternatively, the haptics of the IOL can be fed through slits created in the midperiphery of the artificial iris.3,10,11 The IOL in turn can be sutured to the sclera.3 It is also possible to fixate the haptics of an IOL in long scleral tunnels, but the long-term stability of this approach is unknown.12,13 Finally, the iris with its attached IOL can be sutured to residual iris tissue or the sclera.14,15 To correct concomitant corneal scarring or endothelial failure, the feasibility of simultaneous penetrating keratoplasty (PK) has been reported, including short-term follow-up.3,11 This study was designed to evaluate safety and efficacy of a triple procedure, including sutured custom foldable silicone artificial iris and IOL implantation combined with PK, in a larger number of eyes.
MATERIAL AND METHODS
This prospective, interventional, nonrandomized case series was approved in 2 parts by the institutional review board of the University of California, Los Angeles (#13-001270; #19-001389). Patients were recruited from the practice of the senior author (K.M.M.), who performed the artificial iris and IOL implantations. Two senior corneal surgeons (A.J.A. and S.X.D.) performed the PKs. All procedures were performed at the Stein Eye Institute between March 30, 2010, and June 30, 2019. The minimum follow-up interval was 1 year. Compassionate use device exemptions were obtained from the US FDA for patients implanted between March 30, 2010, and March 1, 2015. Patients implanted between March 1, 2015, and April 31, 2019 participated in the formal FDA clinical trial. Patients implanted after April 31, 2019, received FDA-approved devices. Data collection was compliant with the Health Insurance Portability and Accountability Act, and all research adhered to the tenets of the Declaration of Helsinki. Written permission for participation in the studies, the surgical procedure itself, and the use of full-face photographs for educational presentations and publications was obtained from all subjects.
To be included in the study, patients had to be 18 years or older at the time of enrollment and have1 a large acquired iris defect, significant glare, photophobia, contrast loss, blurred vision, and dissatisfaction with nonsurgical options2; significant corneal edema and/or scarring requiring a PK over a lamellar keratoplasty, and3 aphakia, crystalline lens or IOL dislocation, or poor capsular bag support making it impossible to passively implant an IOL in the capsular bag or ciliary sulcus. Exclusion criteria included eyes with small iris defects or a lens or corneal status that did not meet the inclusion criteria.
Subjective Measurements
Light sensitivity, glare, and cosmesis symptoms were scored using a questionnaire, preoperatively and at each follow-up appointment to 1 year. Subjective daytime and nighttime glare and light sensitivity were assessed by having patients rate their symptoms on a scale of 0 (none) to 5 (severe). Cosmesis was graded 3 months after implantation on a scale of worse to very much improved. Quality of life related to visual activities was scored preoperatively and at each postoperative follow-up appointment to 1 year using the Visual Function Questionnaire-25 (VFQ-25).16
Artificial Iris Sizing
For the patients in this study, each artificial iris was implanted at full diameter.
IOL Power Calculation
Biometry measurements were obtained using a Lenstar LS900 (Haag-Streit, Bern, Switzerland) or IOL Master 500 (Carl Zeiss Meditec AG, Jena, Germany). Lens power calculation was performed using the Sanders-Retzlaff-Kraff/theoretical (SRK/T) or Barrett formulas. Scheimpflug tomography (Pentacam; Oculus, Wetzlar, Germany) images were also obtained preoperatively. However, because the cornea was replaced in each case, we used an average keratometry value of 44.0 diopters (D) to calculate IOL power for the anticipated posttransplant state.
Surgical Technique
All surgeries were performed under general anesthesia. The devices were fiber containing and, as mentioned previously, were implanted at full size without an iridectomy. Pockets of Hoffman et al17 were placed in the thickest parts of the sclera because many eyes had scleral thinning. The corneal surgeon placed a Flieringa ring in all cases except subject 3 and removed the corneal button. An IOL was sutured to the back face of the artificial iris at the 2 optic–haptic junctions using 10-0 Prolene. For the first 5 cases, after any necessary anterior vitrectomy (AV), glaucoma tube shunt modification, or native iris trimming, the IOL was sutured in the open sky configuration to the sclera using 9-0 Prolene at 2 closely spaced points inside the pockets of Hoffman et al. For the remaining cases, CV-8 Gore-Tex suture was attached to the periphery of the artificial iris in horizontal mattress fashion 90 degrees away from the apices of the haptics and then secured to the sclera within the pockets of Hoffman et al at more widely separated points. Care was taken to assure that the knots were not exposed. The donor cornea was sized 0.5 mm larger than the trephination and secured to the host cornea using sixteen 10-0 nylon sutures (Ethicon; Johnson & Johnson, NJ). Final centration of the artificial iris implant was evaluated by the senior surgeon (K.M.M.) and adjusted as needed.
Data Collection
Data collected included demographic information, the preoperative state of the eye including the lens and cornea, and previous surgical details. Postoperative follow-up examinations were conducted at 1 day, 2 weeks, 1 month, 3, 6 and 12 months, and every 6 months thereafter. All patients came to all scheduled appointments during the first postoperative year and completed all questionnaires. Additional appointments were scheduled as needed. Baseline was defined as the date of surgery and final follow-up was defined as the last examination on record at the time of data collection for this study.
Safety measures included loss of corrected distance visual acuity (CDVA), changes in endothelial cell count (ECC), IOP, intraoperative and postoperative complications, and secondary surgical interventions. Safety measures were compared between baseline and the last follow-up visit. IOP elevation was defined as an IOP ≥24 mm Hg or as an increase of 8 mm Hg from the baseline IOP. A rejection episode was defined as the presence of any cells in the anterior chamber (AC), not present at baseline, associated with keratic precipitates on the donor corneal endothelium, and/or an increased corneal thickness, evaluated clinically and with a handheld pachymeter (Pachmate 2; DGH Technology, Exton, PA).
Efficacy outcomes included subjective evaluation of daytime and nighttime light and glare sensitivity (1 = none to 5 = severe), subjective evaluation of cosmesis (1 = worse to 5 = very much improved), and the VFQ-25. Efficacy outcomes were compared between baseline and 1 year after the triple procedure. Artificial iris centration was assessed by the surgeon who performed the implantation at the 1-year follow-up examination. It was measured at a slitlamp biomicroscope about the horizontal and vertical corneal diameters.
Statistical Analyses
All statistical analyses were performed using Excel 2019 for Mac (Microsoft Corp, Redmond, WA) with XLSTAT software version 2019.5592 (Addinsoft, Paris, France). For statistical analysis, CDVAs were converted to the base 10 log of minimum angle of resolution (logMAR). Nonparametric Wilcoxon signed-rank test analysis was performed for samples larger than 5. A P value of 0.05 or less was considered statistically significant.
RESULTS
Among 83 artificial irises implanted in 82 eyes of 78 patients by the senior surgeon (K.M.M.), 14 eyes (17.1%) of 14 patients (17.9%) underwent the triple procedure. All patients were pseudophakic after surgery, and every patient returned for every scheduled examination during the first postoperative year.
Demographic and Surgical Details
Demographic and ocular comorbidity information are summarized in Table 1. Ten of the 14 patients (71.4%) were men. The mean age at surgery was 53.4 ± 26.1 years. The most common etiologies of the iris defects were blunt and surgical trauma. The median number of preoperative comorbidities was 5 (range 3–8). All 14 eyes (100%) had preoperative glaucoma, including 7 eyes (50.0%) with tube shunts. Five eyes (35.7%) were status postretinal detachment (RD) repair. Nine eyes (64.3%) were aphakic, 3 (21.4%) were pseudophakic with dislocated posterior chamber IOLs, and 2 (14.3%) had visually significant cataracts.
TABLE 1.
Demographic Information and Ocular Comorbidities
| Demographic Information | Ocular Comorbidities | |||||||||
| ID | Age* (Sex), Ethnicity | Eye | Etiology of Iris Defect | Type/Extent of Iris Defect | Cornea Status/Lens Status | Posterior Segment Status | Dystrophy | Blepharoptosis | Glaucoma (n Medications) | Previous Glaucoma Surgery |
| 1 | 61 (M), Caucasian | OS | Blunt trauma, ruptured globe | Complete aniridia/100% | RK, scar/aphakia | — | No | Yes, 2 mm | Yes (4) | No |
| 2 | 67 (M), Caucasian | OD | Blunt trauma, no rupture | Partial aniridia/75%–100% | MCE, scar/aphakia | RD repair (PPV, SO injection, and SO removal) | No | No | Yes (3) | No |
| 3 | 54 (M), Caucasian | OS | Blunt trauma,penetrating trauma | Partial aniridia/50%–74% | MCE, scar/aphakia | RD repair (scleral buckle) | Exotropia | No | Yes (2) | No |
| 4 | 71 (F), Caucasian | OD | Blunt trauma, ruptured globe | Partial aniridia/75%–100% | MCE, scar/aphakia | RD repair (PPV, SO injection) | Exotropia | Yes, 3 mm | Yes (1) | No |
| 5 | 28 (F), Caucasian | OD | Blunt trauma, penetrating trauma | Complete aniridia/100% | MCE/Morcher 67B, fibrotic membrane | — | Exotropia | Yes, 3 mm | Yes (2) | Yes, 1 tube shunt |
| 6 | 60 (F), Hispanic | OD | Surgical trauma, cosmetic artificial iris | Mydriasis/100% | Failed PK/posterior chamber IOL, fibrotic membrane | — | Exotropia | Yes, 5 mm | Yes (5) | Yes, 1 tube shunt, tube shunt revision |
| 7 | 18 (M), Caucasian | OD | Surgical trauma, repeat PK | Mydriasis/100% | Failed PK/cataract, zonular instability | — | No | No | Yes (0) | Yes, 2 tube shunts |
| 8 | 63 (M), Asian | OD | Acute angle closure | Mydriasis/100% | MCE, scar/cataract, posterior subluxation | Diabetic retinopathy, PRP | Exotropia | No | Yes (0) | Yes, 2 tube shunts |
| 9 | 48 (M), Caucasian | OS | Surgical trauma, repeat PK | Mydriasis/100% | Failed PK/posterior chamber IOL, anterior subluxation | Chronic uveitis | No | Yes, 2 mm | Yes (1) | Yes, 1 tube shunt |
| 10 | 83 (M), Caucasian | OS | Blunt trauma, ruptured globe | Complete aniridia/100% | Failed PK/aphakia | — | No | No | Yes (3) | No |
| 11 | 55 (M), Caucasian | OS | Surgical trauma, repeat PK | Mydriasis/100% | Failed PK/aphakia | — | No | No | Yes (1) | Yes, 1 tube shunt, tube shunt removal, cyclophotocoagulation |
| 12 | 47 (F), Caucasian | OS | Blunt trauma, penetrating trauma | Iridodialysis/50%–74% | Scar/aphakia | RD repair (scleral buckle), macular scarring | No | No | Yes (3) | Yes, 1 tube shunt |
| 13 | 37 (M), Caucasian | OS | Blunt trauma, ruptured globe | Partial aniridia/75%–100% | Failed PK/aphakia | — | No | No | Yes (0) | Yes, 1 tube shunt |
| 14 | 55 (M) Caucasian | OS | Blunt trauma, penetrating trauma | Partial aniridia/75%–100% | Scar/aphakia | RD repair (PPV, SO injection, and SO removal) | No | No | Yes (2) | No |
At the time of surgery.
ID, patient identity; Kpro, keratoprosthesis (Landers 7.2 mm); MCE, microcystic epithelial corneal edema; PPV, pars plana vitrectomy; PK, penetrating keratoplasty; PRP, panretinal photocoagulation; RD, retinal detachment; RK, radial keratotomy; SO, silicone oil.
Surgical information are tabulated in Table 2. No intraoperative complications occurred. The average host trephination diameter was 8.5 mm (range 7.75–9.0 mm). A concomitant AV was performed in 10 eyes (71.4%), and residual native iris tissue was trimmed in 4 eyes (28.6%). Three dislocated IOLs were explanted, and 2 cataracts were extracted in open-sky fashion. The average IOL power was 14.7 D (range 7–24 D). Correct artificial iris centration was achieved at the end of the surgery in 13 cases (92.3%). Subject 12 had a tube shunt in the AC that prevented good centration.
TABLE 2.
Surgical Information
| ID | Host/Donor Trephination (mm) | IOL Model | IOL Power (D) | Site of IOL Fixation | Site of Artificial Iris Fixation | Other Concurrent Procedures |
| 1 | 8.0/8.5 | CZ70BD | 13.5 | Sclera | IOL | AV |
| 2 | 8.0/8.5 | CZ70BD | 15.5 | Sclera | IOL | AV |
| 3 | 8.0/8.5 | CZ70BD | 17 | Sclera | IOL | AV |
| 4 | 8.0/8.5 | CZ70BD | 14.5 | Sclera | IOL | None |
| 5 | 7.25/7.75 | CZ70BD | 24 | Sclera | IOL | Morcher 67B explantation, tube shunt revision |
| 6 | 8.5/9.0 | CZ70BD | 17.5 | Artificial iris | Sclera | AV, trimming of the remaining iris (vitrector), IOL explantation |
| 7 | 7.5/8 | CZ70BD | 14 | Artificial iris | Sclera | CE (open sky), AV |
| 8 | 8.5/9.0 | CZ70BD | 11.5 | Artificial iris | Sclera | CE (pars plana lensectomy), temporary Kpro, PPV |
| 9 | 8.0/8.5 | CZ70BD | 20.5 | Artificial iris | Sclera | Removal of retrocorneal membrane, iridoplasty, revision of tube shunt, AV |
| 10 | 8.5/9.0 | CZ70BD | 12 | Artificial iris | Sclera | AV, trimming of the remaining iris (vitrector), IOL explantation |
| 11 | 8.0/8.5 | CZ70BD | 10 | Artificial iris | Sclera | None |
| 12 | 7.75/8.25 | CZ70BD | 18 | Artificial iris | Sclera | AV, trimming of the remaining iris and fibrotic capsule (scissors) |
| 13 | 7.75/8.5 | PCB00 | 12 | Artificial iris | Sclera | AV |
| 14 | 7.75/8.25 | CZ70BD | 7 | Artificial iris | Sclera | AV, trimming of the remaining iris (vitrector) |
AV, anterior vitrectomy; CE, cataract extraction; ID, patient identity; IOL, intraocular lens; Kpro, keratoprosthesis (Landers 7.2 mm).
Clinical Examples
Three study patients are described in detail to demonstrate typical preoperative comorbidities and surgical outcomes after this combined procedure. Subject 6 was 60 years old at the time of the study procedure. Before years, she underwent cosmetic artificial iris (BrightOcular; Stellar Devices LLC) implantation in both eyes in Mexico. Shortly afterward, her right eye developed elevated IOP necessitating tube shunt implantation and antimetabolite agents. She underwent cataract extraction with IOL implantation in both eyes. The cosmetic iris in the left eye was explanted at the time of cataract surgery. Cataract surgery in the right eye was performed through the iris device by enlarging the pupillary aperture. Shortly thereafter, she underwent removal of the iris device in her right eye combined with tube shunt revision and a placement of a scleral patch graft for scleral reinforcement. Finally, she underwent PK for corneal decompensation on the right eye. Figure 1A shows her preoperative appearance. Under our care, she underwent repeat PK, posterior chamber IOL removal, and artificial iris and IOL implantation. Her postoperative course was complicated by elevated IOP requiring additional glaucoma tube shunt implantation 1 month after surgery and 2 revisions. She presented with a mild iritis 6 months after surgery, which was treated medically. One year after surgery, her CDVA was 20/80, and her daytime and nighttime glare symptoms were improved, as was her esthetic appearance score. She later underwent strabismus surgery, blepharoptosis repair, and blepharoplasty. Figure 1B shows her postoperative appearance 2 years after surgery. Her final CDVA 2 years after surgery was 20/40, and her final IOP was 18 mm Hg.
FIGURE 1.
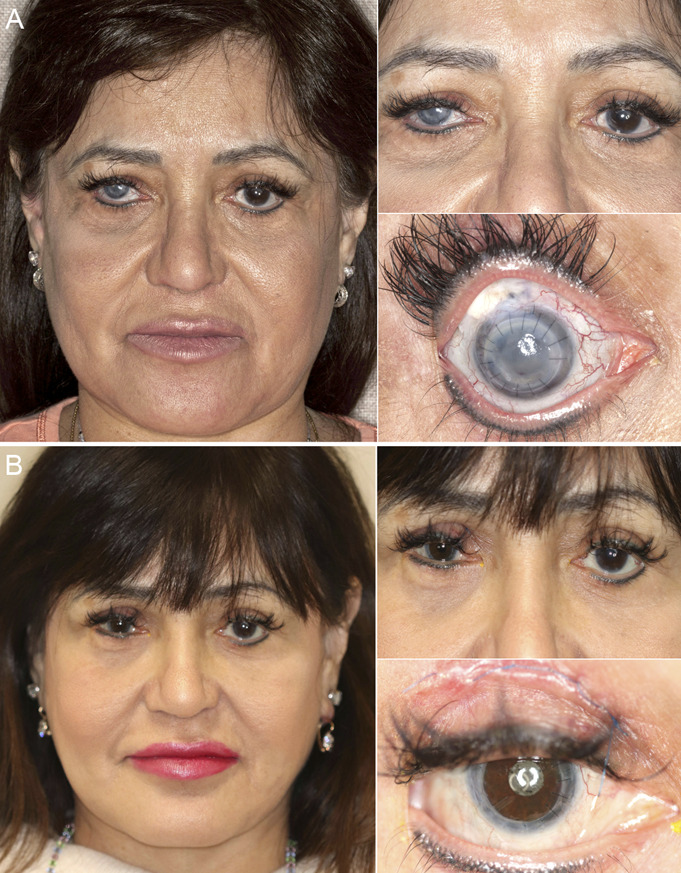
Composite photographs of the preoperative appearance (A) and 2-year postoperative appearance (B) of subject 6. She experienced chronic mydriasis, glaucoma, and corneal decompensation after cosmetic BrightOcular artificial iris implantation.
Subject 9 was 48 years old at the time of the triple procedure. He had keratoconus in both eyes, complicated by hydrops in the left eye, which was treated by PK. He experienced multiples secondary graft failures (SGFs), endophthalmitis 2 years before the triple procedure, and elevated IOP, which was treated by tube shunt implantation. Figure 2A shows his preoperative appearance. Under our care, he underwent repeat PK, tube shunt revision, removal of a dislocated posterior chamber IOL, AV, and artificial iris and IOL implantation. One year after study surgery, his CDVA was 20/60, and his esthetic appearance score improved. His daytime and nighttime glare symptom score worsened from 1 to 3. Figure 2B shows his postoperative appearance 3 months after surgery. His postoperative course was complicated by RD that was repaired 13 months after surgery by pars plana vitrectomy (PPV) and C3F8 13% injection. Eighteen months after surgery, he experienced endothelial rejection that led to SGF. This was addressed by another PK 19 months after the triple procedure. His final CDVA, 26 months after the triple procedure, was 20/25, and his final IOP was 5 mm Hg.
FIGURE 2.
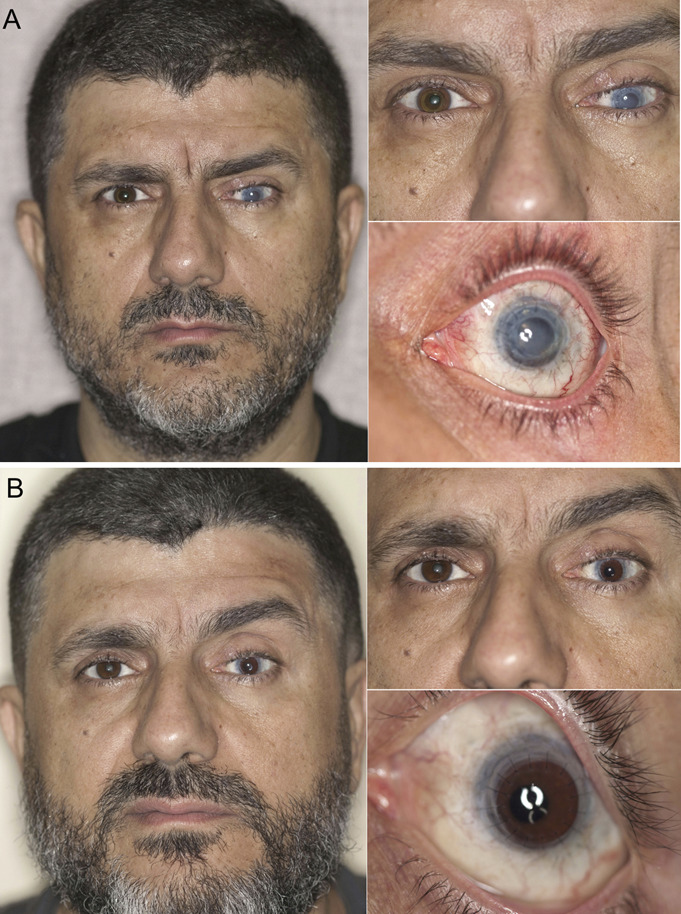
Composite photographs of the preoperative appearance (A) and 3-month postoperative appearance (B) of subject 9. He had chronic mydriasis, glaucoma, and SGF from repeat penetrating keratoplasties. The initial indication was for keratoconus complicated by acute hydrops.
Subject 14 was 55 years old at the time of the study procedure. Ten years before, he was injured at work in an accident that occurred without eye protection. A pressurized chamber with fused silica quartz walls exploded, sending fragments of glass into both of his eyes. A large glass shard penetrated the left cornea, rupturing his globe, causing iris tissue to prolapse, and producing a vitreous hemorrhage. Smaller glass fragments produced nonpenetrating lacerations into the right cornea and several eyelid lacerations. After initial surgical exploration of the left eye and repair of the rupture and eyelid lacerations, he underwent 2 PPVs to evacuate vitreous hemorrhage and repair RD. Pars plana lensectomy was performed during the initial PPV. After these surgeries, he was aphakic and largely aniridic. Figure 3A shows his preoperative appearance. To correct the aphakia, aniridia, and corneal failure, he underwent the triple procedure. Figure 3B shows his 3 months postoperative appearance. His postoperative course was complicated by IOP elevation 1 month after surgery, which was successfully managed by applying additional glaucoma medications. Five months after surgery, he presented with an exposed Gore-Tex suture that had to be covered with a patch graft. At 1 year, his CDVA was 20/63, and his daytime and nighttime glare symptoms improved both from 5 to 2. His final CDVA at 1 year was 20/63, and his final IOP was 26 mm Hg.
FIGURE 3.
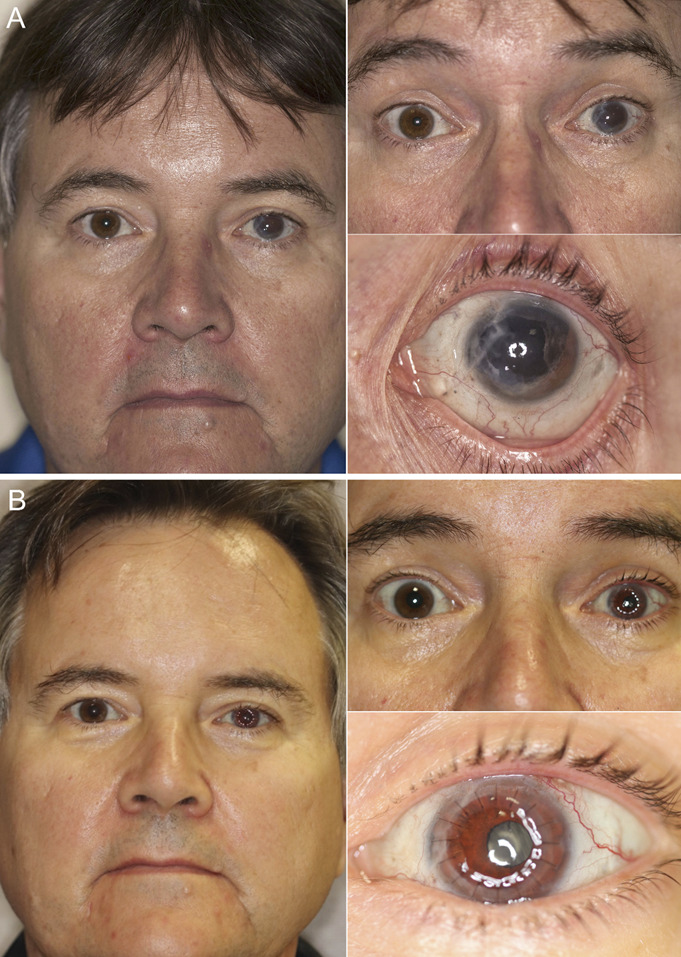
Composite photographs of the preoperative appearance (A) and 3-month postoperative appearance (B) of subject 14. He had a large iris defect, aphakia, glaucoma, corneal scarring, and corneal decompensation from expulsive blunt trauma and multiple previous surgeries.
Safety Outcomes
Safety outcomes are tabulated in Table 3. The median preoperative CDVA was 2.0 logMAR (range 0.9–2.3 logMAR), improving significantly to 0.7 logMAR (range 0.2–2.6 logMAR) at the last follow-up examination (P = 0.02). Visual acuity data are graphed in Figure 4. Eleven eyes (78.5%) had a spherical equivalent ≤3 D, and 3 eyes (21.4%) had a spherical equivalent >3 D. Two eyes (14.3%) lost vision, including 1 eye that experienced a second globe rupture from postoperative bunt trauma. One eye ended up with no light perception (subject 4). At the time of her initial presentation, her visual potential was limited, and her CDVA was hand motion. Her IOP was in the low range despite a silicone oil fill (9 mm Hg). She was aware of the risks of surgery, given the high complexity of her eye, but expressed a desire to proceed for cosmetic reasons. One month after surgery, her visual acuity dropped to light perception and her IOP decreased to a prephthisical level of 1 mm Hg. At the 1-year examination, she had chronic hypotony, and her daytime and nighttime glare symptoms scores were decreased. Despite these issues, her quality of life improved, particularly her esthetic appearance.
TABLE 3.
Safety Outcomes of Sutured Artificial Iris and IOL Implantation Combined With Penetrating Keratoplasty
| ID | Length of Follow up (Mo) | CDVA (Snellen) Baseline–Final | Spherical Equivalent Refractive Error (D) | IOP (mm Hg) Baseline/Final | Postoperative Complications | Graft Rejection/Time From Surgery (Mo) | Secondary Graft Failure/Time From Surgery (Mo) | Secondary IOP Elevation (n Medications*)/Time From Surgery (Mo) | Secondary Surgical Interventions | Donor ECC (Cells/mm2) | Final ECC (Cells/mm2)† | Change in ECC (%) |
| 1 | 54.9 | CF–20/80 | 5.1 | 19/11 | Iritis (mild), self-cessation of topical corticosteroid | Yes/31.6 | Yes/35.3 | No | DSEK | 2950 | <500 | −83.1 |
| 2 | 44.6 | HM–20/80 | 1.1 | 20/16 | Iritis (mild) | No | No | Yes (5)/0.9 | Tube shunt implantation | 3106 | 1759 | −43.4 |
| 3 | 41.7 | 20/400–HM | 4.6 | 21/2 | CME, iritis (mild), band keratopathy, globe rupture with artificial iris and uveal tissue extrusion, graft dehiscence, RD, CD | No | Yes/37.7 | Yes (2)/0.9 | Tube shunt implantation, emergency globe closure, PPV with silicone oil injection plus PK | 3295 | <500 | −84.8 |
| 4 | 25.0 | HM–NLP | 1.3 | 9/2 | Iritis (mild), phthisis bulbi | Yes/11.5 | No | No | No | 2933 | 2020 | −31.1 |
| 5 | 33.9 | HM–20/30 | 0.3 | 13/8 | CME, iritis (moderate), RD | Yes/11.4 | Yes/19.4 | No | DSEK, PPV, scleral buckle | 3002 | <500 | −83.3 |
| 6 | 24.6 | LP–20/40 | 5.5 | 12/18 | Iritis (severe) | No | No | Yes (5)/0.9 | Tube shunt implantation, tube shunt revision ×2, strabismus repair, blepharoptosis repair, blepharoplasty | 2980 | 1832 | −38.5 |
| 7 | 13.0 | CF–20/63 | 0.0 | 11/29 | Iritis (mild) | Yes/11.6 | Yes/11.6 | Yes (2)/1.4 | DSEK | 3457 | <500 | −85.5 |
| 8 | 18.9 | HM–HM | 1.0 | 8/13 | Iritis (moderate) | Yes/0.5 | No | No | No | 3100 | 1300 | −58.1 |
| 9 | 19.2 | CF–20/25 | 0.0 | 23/5 | Iritis (moderate), RD | Yes/19.2 | Yes/19.2 | No | PK, tube shunt ligature, intravitreal corticosteroid injection, PPV, C3F8 gas injection | 2918 | 993 | −66.0 |
| 10 | 17.3 | 20/160–20/40 | 0.9 | 10/6 | Iritis (mild) | No | No | No | No | 3003 | 1549 | −48.4 |
| 11 | 19.1 | CF–20/125 | 1.5 | 12/6 | Iritis (mild), fungal keratitis, corneal perforation | Yes/3.3 | Yes/15.6 | No | Corneal patch graft, DSEK | 2959 | <500 | −83.1 |
| 12 | 13.2 | CF–20/125 | 1.3 | 21/9 | Iritis (mild), graft-host dehiscence (broken suture) | No | No | Yes (2)/0.5 | Wound revision | 2857 | 1767 | −38.2 |
| 13 | 8.0 | 20/200–20/25 | 2.0 | 15/16 | Iritis (severe), hyphema, vitreous hemorrhage | No | Yes/3.1 | No | AC washout, PK | 2865 | 2457 | −14.2 |
| 14 | 6.0 | CF–20/63 | 0.5 | 12/26 | CME, iritis (mild), exposed Gore-Tex suture | No | No | Yes (2)/1.3 | Scleral patch graft | 2865 | 1469 | −48.7 |
At the time of IOP elevation.
In case of repeat corneal transplantation, the last ECC available before repeat transplantation was used. When the ECC was <500 cells/mm2, 500 cells/mm2 was used to estimate the percentage change.
CD, choroidal detachment; CF, counting fingers; CME, cystoid macular edema; DSEK, Descemet-stripping endothelial keratoplasty; ECC, endothelial cell count; HM, hand motion; ID, patient identity; LP, light perception; NLP, no light perception; PK, penetrating keratoplasty; RD, retinal detachment.
FIGURE 4.
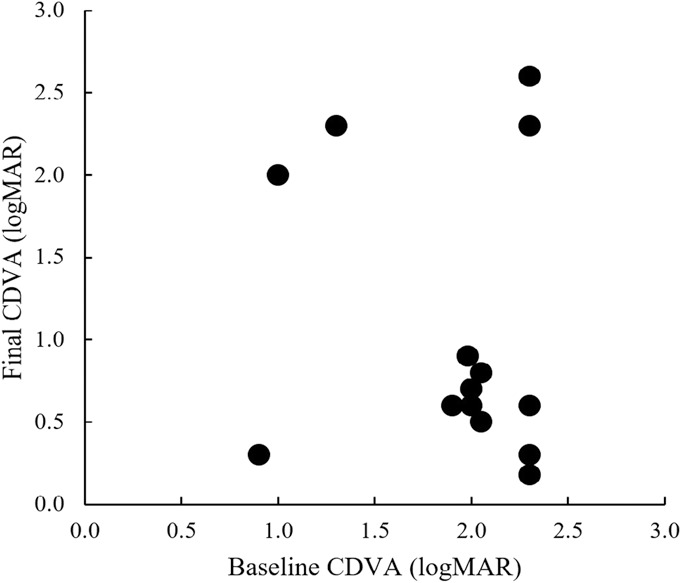
Visual outcomes of sutured custom artificial iris and IOL implantation combined with penetrating keratoplasty. P = 0.02; Wilcoxon paired test.
Postoperative iritis developed in 13 eyes (92.9%). It was mild to moderate in severity in 12 of 14 eyes (85.7%), including 1 patient with a history of uveitis (subject 9) and 1 patient who self-discontinued his topical corticosteroid drops (subject 1). In the severe case (subject 13), the iritis was associated with a hyphema that resolved after an AC washout. All cases were medically controlled by increasing the topical corticosteroid regimen. Three eyes (21.4%) presented with postoperative RDs including 1 patient (subject 3) with a history of previous RD repair who redetached after repeat globe rupture. Three eyes (21.4%) developed cystoid macular edema. Other complications occurred rarely or were related to previous comorbidities of the eyes but not to the artificial iris device itself. One eye (7.1%) developed a fungal keratitis that led to corneal perforation (subject 11). He had history of repeat bacterial and Acanthamoeba keratitis in previous corneal transplants. One patient (7.1%) presented with a graft–host dehiscence secondary to a broken suture, and 1 eye (7.1%) presented with an exposed Gore-Tex suture, as mentioned previously.
Average IOP dropped from 14 ± 5 mm Hg at baseline to 12 ± 8 mm Hg at the final follow-up examination (P = 0.33). Although all eyes had preoperative glaucoma, secondary IOP elevations developed in 6 eyes (42.9%) at an average of 1.0 ± 0.3 months after surgery, necessitating glaucoma surgery in 3 eyes (21.4%), including 1 eye with a history of tube shunt implantation (subject 6).
Graft rejection occurred in 7 eyes and SGF occurred in 7 eyes (50.0%), including 5 eyes (35.7%) with tube shunts, at a median interval of 19.2 months (range 3.1–37.7 months) after the triple procedure. There was considerable overlap between these 2 groups, of course. Of the 7 eyes with endothelial rejections, 2 (14.3%) were diagnosed with concomitant SGF, and 3 (21.4%) subsequently developed SGF. Average donor ECC was 3020 ± 172 cells/mm2 before transplantation, decreasing to 1122 ± 608 cells/mm2 by the final follow-up examination, representing a change of −57.6% (P < 0.01). The SGFs were treated by 4 Descemet-stripping endothelial keratoplasty procedures and 3 PKs.
One patient (subject 3) returned 3 years after surgery having recently experienced graft dehiscence complicated by artificial iris extrusion. The graft dehiscence was repaired emergently elsewhere but resulted in SGF, chronic hypotony with choroidal detachment, and RD. He underwent PPV with silicone oil injection and repeat PK and was left aphakic. At the time of this report, we are hoping to be able to return him to the operating room 1 day for another IOL and artificial iris.
Efficacy Outcomes
Efficacy outcomes at 1 year are summarized in Table 4. The mean preoperative VFQ-25 score was 72 ± 10, and the mean postoperative score was 77 ± 9 (P < 0.01). The average daytime glare symptom score improved from 3.3 ± 1.8 before surgery to 1.9 ± 1.1 after surgery (P = 0.08). Individual daytime glare scores improved in 8 eyes (57.1%), were stable in 4 eyes (28.6%), and worsened in 2 eyes (14.2%). The average nighttime glare symptom score improved from 2.8 ± 2.0 at baseline to 1.9 ± 1.2 at 1-year (P = 0.26). Nighttime glare scores improved in 7 eyes (50.0%), were stable in 3 eyes (21.4%), and worsened in 4 eyes (28.6%). There was no significant change in the average daytime light sensitivity from baseline (3.3 ± 1.7) to 1-year (2.1 ± 1.1; P = 0.07). Daytime light sensitivity scores improved in 8 eyes (57.1%), were stable in 4 eyes (28.6%), and worsened in 2 eyes (14.2%). The average nighttime light sensitivity score remained stable at 2.3 ± 1.4 before surgery and 1.5 ± 1.1 after surgery (P = 0.12). Nighttime light sensitivity score improved in 5 eyes (35.7%), were stable in 8 eyes (57.1%), and worsened in 1 eye (7.1%). The global esthetic score changed significantly, improving to “very much improved” in 13 of 14 eyes (92.9%; P < 0.01). The artificial iris was centered in 4 eyes (28.6%), 1 mm decentered in 8 eyes (57.1%), and 2 mm decentered in 2 eyes (14.2%).
TABLE 4.
Efficacy Outcomes 1 Year After Sutured Artificial Iris and IOL Implantation Combined With Penetrating Keratoplasty
| ID | VFQ-25 | Daytime Glare Symptom Score | Nighttime Glare Symptom Score | Daytime Light Sensitivity Score | Nighttime Light Sensitivity Score | Global Aesthetic Improvement (Final) | Artificial Iris Centered?* | |||||
| Baseline | 1 Year | Baseline | 1 Year | Baseline | 1 Year | Baseline | 1 Year | Baseline | 1 Year | |||
| 1 | 77 | 93 | 1 | 1 | 1 | 2 | 2 | 1 | 3 | 1 | Very much improved | No, 1 mm inferiorly |
| 2 | 62 | 65 | 5 | 1 | 5 | 1 | 2 | 2 | 2 | 1 | Very much improved | No, 1 mm superiorly |
| 3 | 91 | 88 | 5 | 1 | 5 | 1 | 1 | 1 | 1 | 1 | Very much improved | Yes |
| 4 | 74 | 77 | 1 | 5 | 1 | 5 | 1 | 5 | 1 | 5 | Very much improved | No, 1 mm superiorly |
| 5 | 62 | 67 | 4 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | Very much improved | Yes |
| 6 | 93 | 93 | 5 | 2 | 4 | 3 | 2 | 2 | 2 | 2 | Much improved | Yes |
| 7 | 79 | 82 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | Improved | No, 1 mm inferotemporally |
| 8 | 88 | 97 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Improved | No, 2 mm nasally |
| 9 | 53 | 58 | 1 | 3 | 1 | 3 | 3 | 3 | 2 | 2 | Much improved | Yes |
| 10 | 86 | 84 | 3 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | No change | No, 2 mm inferiorly |
| 11 | 79 | 93 | 5 | 1 | 5 | 1 | 2 | 1 | 1 | 1 | Much improved | No, 1 mm inferiorly |
| 12 | 72 | 75 | 3 | 3 | 1 | 3 | 2 | 3 | 2 | 2 | Much improved | No, 1 mm inferiorly |
| 13 | 59 | 59 | 5 | 2 | 5 | 2 | 3 | 2 | 2 | 1 | Improved | No, 1 mm temporally |
| 14 | 75 | 85 | 5 | 2 | 5 | 2 | 2 | 2 | 2 | 1 | Very much improved | No, 1 mm temporally |
| Average | 72 | 77 | 3.3 | 1.9 | 2.8 | 1.9 | 3.3 | 2.1 | 2.3 | 1.5 | 13/14 (92.9%) improved to very much improved | 4/14 (28.6%) centered |
| SD | 10 | 9 | 1.8 | 1.1 | 2.0 | 1.2 | 1.7 | 1.1 | 1.4 | 1.1 | ||
| P Value | <0.01 | 0.08 | 0.26 | 0.07 | 0.12 | |||||||
At final examination.
ID, patient identity.
DISCUSSION
We report the safety and efficacy outcomes of a cohort of patients with large iris defects who were implanted with sutured custom silicone artificial irises and IOLs at the time of PK and followed up prospectively. We demonstrate that the safety of the procedure is marginal, due in large part to the preoperative complexity of these eyes. Mild to moderate postoperative inflammation was the most frequent complication, followed by IOP elevation during the first postoperative month. SGF was common as a late occurrence. The procedure was very effective at improving CDVA, cosmesis, and quality of life.
At the time of this report, an extensive literature review was conducted, which identified 3 retrospective studies describing this triple procedure. They are summarized in Table 5 and represent 12 eyes in total.11,13,18 Detailed outcomes of the combined procedure were reported by Yoeruek and Bartz-Schmidt13 (5 eyes, mean follow-up of 24 months). Using a knotless Z-suture technique to secure the artificial iris and IOL to the sclera, they described good visual improvement, no new glaucoma, a well-centered iris in all cases, and only 1 patient with graft failure and elevated IOP.13 Two additional retrospective studies described the outcomes of the triple procedure performed with the other artificial implants.19,20 Similar to our results, CDVA was improved but postoperative complications, including secondary glaucoma, intraocular inflammation, graft rejection, and secondary failure, were common. In comparison with these 3 studies, our study is prospective and includes more eyes than the other 3 studies combined.
TABLE 5.
Published Retrospective Studies of Sutured Custom Silicone Artificial Iris and IOL Implantation Combined With Penetrating Keratoplasty
| Journal, Year | First Author | N Eyes/N Combined With IOL and PK | Mean Follow Up (Mo) | Artificial Iris Trimming and Placement | Artificial Iris Fixation | IOL Fixation | Other Procedures | Safety Findings | Efficacy Findings |
| Graefes Arch exp ophthalmol, 2013 | Forlini | 4/2 | 6.0 (maximum) | Trimmed to 11 mm in combined cases, placed in the sulcus | Sutured to the sclera with 10-0 prolene, in patient 1 with 2 sutures and in patient 2 with 4 sutures | Sutured to the anterior face of the artificial iris in 1 patient and to the back face of the artificial iris in the other | Failed attempt to clip an iris claw IOL to both the posterior and the anterior faces of the artificial iris in 1 patient, mini-glaucoma tube shunt implantation in the other | Not mentioned | Artificial iris stable and centered in both eyes |
| Acta ophthalmol, 2016 | Spitzer | 34/5 | 24.0 | Not trimmed, placed in the sulcus | To the sclera with Z-sutures | IOL implantation performed in 13 eyes and PK in 5 eyes; of the 13 eyes receiving an IOL, it was sutured to the artificial iris in 7 eyes, sutured to the sclera in 3 eyes, placed in the ciliary sulcus in 2 eyes, and placed within the capsular bag in 1 eye | Peripheral iridectomy of the artificial iris in 31 eyes | CDVA worsening in 9 eyes, glaucoma and hypotony in 3 eyes each, persistent intraocular inflammation or CME in 7 eyes each, endothelial decompensation requiring additional corneal transplantation in 6 eyes, artificial iris repositioning in 4 eyes | Outcomes of the 5 PKs not mentioned specifically, improved CDVA in 25 eyes, improved glare sensitivity in 15 eyes |
| Eye, 2018 | Yoeruek | 5/5 | 24.6 | Horizontal CD minus 1 mm, placed in the sulcus | To the sclera with Z-sutures | Sutured to the back face of the artificial iris | Anterior vitrectomy in 2 eyes | Graft failure and uncontrolled IOP developed in 1 eye | Improved CDVA in 4 eyes, glare reduction in 2 eyes, artificial iris centered in all eyes |
CD, corneal diameter; CDVA, corrected distance visual acuity; CME, cystoid macular edema; IOL, intraocular lens; PK, penetrating keratoplasty.
Several techniques to fixate the IOL to the sclera have been described, such as the previously mentioned knotless Z-suture technique or the use of scleral flaps or its variant with pockets of Hoffman et al.17,21 The main differences concern the number of points of fixation to the sclera (2 or 4), the type of suture used (9-0 or 10-0 Prolene or CV-8 Gore-Tex), and the presence of knots on or within the sclera.14,15,22 We first chose to suture our IOLs to the sclera with 9-0 Prolene at essentially 2 points of fixation and later switched to suturing the artificial iris to the sclera with CV-8 Gore-Tex suture at 4 points of fixation because we believe that this technique provides better stabilization of the artificial iris implant over the long-term. This is particularly important in cases of combined PK, where repeat corneal transplantation might be necessary. We also prefer suturing the iris implant, even if the knotless Z-suture technique as described has good initial outcomes.15 The downside of our approach is the risk for scleral thinning and suture exposure, which occurred in 1 eye in our series, necessitating the placement of a scleral patch graft.
Reduced CDVA was our primary safety endpoint. There was a statistically significant improvement in median CDVA at the last follow-up examination. Two patients experienced a drop in CDVA, related in the first case to repeat globe rupture and in the second to chronic hypotony. In the latter case, the vision decline was related to a progression of preoperative comorbidities or the surgical trauma necessary for device implantation but unlikely to the device itself. We found that the triple procedure, including our IOL power calculation method, achieved satisfactory visual outcomes, enabling patients to perform daily activities at a higher level of function.23 The choice of an appropriate IOL power is critical, so careful attention should be given to the keratometry values.
Surgical complications and secondary surgical interventions were additional safety endpoints. Postoperative iritis was the most frequent complication. It was found to be more frequent than in a previous series of stand-alone artificial iris implantations.8 Prolonged surgery times and concomitant procedures such as AV or trimming of the remnant iris might have played a role in the high rate of complications we observed. In parallel, IOP increased in 6 eyes, including 3 that required additional glaucoma surgery. Trabecular inflammation and pigment dispersion from iritis might be the mechanisms; however, corticosteroid treatment was increased in several cases to manage iritis, and that might have played a role as well. Other possible mechanisms of iritis and IOP elevation might include uveal touch by the artificial iris or sutures.
Endothelial rejection occurred frequently, leading to SGF in most eyes in which it was diagnosed. Endothelial rejection was more frequent compared with PK performed for optical indications or corneal diseases.24 This is expected because our population had multiples risk factors for rejection.25 Most reports on artificial iris implantation describe ECC loss due to the surgical implantation in these eyes with low ECCs.9 The impact of artificial implantation on the endothelium remains unknown, and the mechanisms of endothelial demise might be multifactorial.26
Compared with outcomes of PK combined with scleral IOL fixation, the triple procedure might present higher rate of postoperative iritis and SGF.27 However, the role of the artificial iris itself remains to be elucidated. Indeed, the presence of a tube shunt is a well-known risk factor for ECC loss after PK, Descemet-stripping endothelial keratoplasty, and Descemet membrane endothelial keratoplasty.28,29 Postoperative iritis might also be involved, triggering a chronic proinflammatory, proapoptotic, and prooxidative state from a breach in the blood–aqueous barrier.26
The need for increased topical corticosteroid treatment to prevent rejection and secondary failure remains to be determined and must be balanced against the risk for IOP increase. Longer follow-up, including larger samples, are required to confirm our results; nevertheless, patients should be counseled accordingly. Future therapeutic options should be considered because the triple procedure led to an increased likelihood of needing repeat corneal transplantation. Options might include topical immunosuppression by cyclosporine or tacrolimus to prevent rejection, and ρ-kinase inhibitors to enhance the endothelial cell function.30,31
The triple procedure was successful at improving quality of life and cosmetic appearance. The VFQ-25 worsened in 2 patients, including subject 3 who presented with a repeat globe rupture. Only 1 patient failed to experience a subjective cosmetic improvement after artificial iris implantation. Longer follow-up will be useful to confirm if these improvements are maintained. There was no significant change in subjective light or glare sensitivity at 1 year. Corneal irregularity from the transplant or early corneal failure might have replaced the light and glare sensitivity caused by the iris defect.32
Perfect centration of the artificial iris was achieved in only one third of patients, lower compared with a previous report of stand-alone artificial iris implantation, although most patients noted an improvement in cosmetic appearance.9 Initial centration was evaluated at the end of surgery after the corneal button was sutured. Correct evaluation of the centration at this time is challenging because of high astigmatism and corneal edema that is present at the end of surgery. Decentration is more understandably more noticeable in eyes with blue irises and less noticeable in eyes with brown irises.
Our findings provide a short-term overview of the feasibility and efficacy of the described triple procedure. Long-term studies are required to confirm its potential clinical benefits. Given the small sample size, the high variability of clinical presentations, and the preoperative ocular comorbidities of patients included, comparisons between patients in this and other series must made cautiously. Careful consideration should be taken before intending to perform this surgery, including systematic attempt to evaluate the visual potential after surgery and appropriate surgeon training to manage complex anterior segments and corneal decompensation.
In conclusion, the artificial iris triple procedure performed by experienced surgeons in appropriate situations can improve visual, quality of life, and cosmetic outcomes; however, postoperative inflammation, IOP elevation, and SGF were common during the first 18 months after surgery.
Footnotes
Supported, in part, by the Amalia Simon Roth endowed research fund at UCLA.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ayliffe W, Groth SL, Sponsel WE. Small-incision insertion of artificial iris prostheses. J Cataract Refract Surg. 2012;38:362–367. [DOI] [PubMed] [Google Scholar]
- 2.Koch KR, Heindl LM, Cursiefen C, et al. Artificial iris devices: benefits, limitations, and management of complications. J Cataract Refract Surg. 2014;40:376–382. [DOI] [PubMed] [Google Scholar]
- 3.Mayer C, Tandogan T, Hoffmann AE, et al. Artificial iris implantation in various iris defects and lens conditions. J Cataract Refract Surg. 2017;43:724–731. [DOI] [PubMed] [Google Scholar]
- 4.Rickmann A, Szurman P, Januschowski K, et al. Long-term results after artificial iris implantation in patients with aniridia. Graefes Arch Clin Exp Ophthalmol. 2016;254:1419–1424. [DOI] [PubMed] [Google Scholar]
- 5.Price MO, Price FW, Jr, Chang DF, et al. Ophtec iris reconstruction lens United States clinical trial phase I. Ophthalmology. 2004;111:1847–1852. [DOI] [PubMed] [Google Scholar]
- 6.Miller KM, Kuo A, Olson MD, et al. Safety and efficacy of black iris diaphragm intraocular lens implantation in eyes with large iris defects: report 4. J Cataract Refract Surg. 2018;44:686–700. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Jung J, Lin SR, et al. Safety and efficacy of colored Iris reconstruction lens implantation. Am J Ophthalmol. 2020;216:174–185. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet C, Miller KM. Safety and efficacy of custom foldable silicone artificial iris implantation: a prospective compassionate-use case series. J Cataract Refract Surg. 2020;46:893–901. [DOI] [PubMed] [Google Scholar]
- 9.Mayer CS, Reznicek L, Hoffmann AE. Pupillary reconstruction and outcome after artificial Iris implantation. Ophthalmology. 2016;123:1011–1018. [DOI] [PubMed] [Google Scholar]
- 10.Wolf A, Shajari M. Slip and slide technique for combined small-incision artificial iris and IOL implantation. J Cataract Refract Surg. 2020. In press. doi: 10.1097/j.jcrs.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 11.Forlini C, Forlini M, Rejdak R, et al. Simultaneous correction of post-traumatic aphakia and aniridia with the use of artificial iris and IOL implantation. Graefes Arch Clin Exp Ophthalmol. 2013;251:667–675. [DOI] [PubMed] [Google Scholar]
- 12.Gooi P, Teichman JC, Ahmed. Sutureless intrascleral fixation of a custom-tailored iris prosthesis with an intraocular lens. J Cataract Refract Surg. 2014;40:1759–1763. [DOI] [PubMed] [Google Scholar]
- 13.Yoeruek E, Bartz-Schmidt KU. A new knotless technique for combined transscleral fixation of artificial iris, posterior chamber intraocular lens, and penetrating keratoplasty. Eye (Lond). 2019;33:358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang S, Baig K, Kalevar A, et al. Novel approach to scleral fixation of a reper intraocular lens and artificial iris complex following pars plana lensectomy and vitrectomy for ectopia lentis and cataract in a patient with aniridia and nystagmus. Retin Cases Brief Rep. 2019;00:1–4. [DOI] [PubMed] [Google Scholar]
- 15.Spitzer MS, Yoeruek E, Leitritz MA, et al. A new technique for treating posttraumatic aniridia with aphakia: first results of haptic fixation of a foldable intraocular lens on a foldable and custom-tailored iris prosthesis. Arch Ophthalmol. 2012;130:771–775. [DOI] [PubMed] [Google Scholar]
- 16.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–1058. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman RS, Fine IH, Packer M. Scleral fixation without conjunctival dissection. J Cataract Refract Surg. 2006;32:1907–1912. [DOI] [PubMed] [Google Scholar]
- 18.Spitzer MS, Nessmann A, Wagner J, et al. Customized humanoptics silicone iris prosthesis in eyes with posttraumatic iris loss: outcomes and complications. Acta Ophthalmol. 2016;94:301–306. [DOI] [PubMed] [Google Scholar]
- 19.Mashor RS, Bahar I, Kaiserman I, et al. Combined penetrating keratoplasty and implantation of iris prosthesis intraocular lenses after ocular trauma. J Cataract Refract Surg. 2011;37:582–587. [DOI] [PubMed] [Google Scholar]
- 20.Miller AR, Olson MD, Miller KM. Functional and cosmetic outcomes of combined penetrating keratoplasty and iris reconstruction lens implantation in eyes with a history of trauma. J Cataract Refract Surg. 2007;33:808–814. [DOI] [PubMed] [Google Scholar]
- 21.Szurman P, Petermeier K, Aisenbrey S, et al. Z-suture: a new knotless technique for transscleral suture fixation of intraocular implants. Br J Ophthalmol. 2010;94:167–169. [DOI] [PubMed] [Google Scholar]
- 22.Das S, Nicholson M, Deshpande K, et al. Scleral fixation of a foldable intraocular lens with polytetrafluoroethylene sutures through a Hoffman pocket. J Cataract Refract Surg. 2016;42:955–960. [DOI] [PubMed] [Google Scholar]
- 23.Higgins KE, Wood J, Tait A. Vision and driving: selective effect of optical blur on different driving tasks. Hum Factors. 1998;40:224–232. [DOI] [PubMed] [Google Scholar]
- 24.Borderie VM, Guilbert E, Touzeau O, et al. Graft rejection and graft failure after anterior lamellar versus penetrating keratoplasty. Am J Ophthalmol. 2011;151:1024–1029.e1. [DOI] [PubMed] [Google Scholar]
- 25.Guilbert E, Bullet J, Sandali O, et al. Long-term rejection incidence and reversibility after penetrating and lamellar keratoplasty. Am J Ophthalmol. 2013;155:560–569.e2. [DOI] [PubMed] [Google Scholar]
- 26.Anshu A, Price MO, Richardson MR, et al. Alterations in the aqueous humor proteome in patients with a glaucoma shunt device. Mol Vis. 2011;17:1891–1900. [PMC free article] [PubMed] [Google Scholar]
- 27.Malta JB, Banitt M, Musch DC, et al. Long-term outcome of combined penetrating keratoplasty with scleral-sutured posterior chamber intraocular lens implantation. Cornea. 2009;28:741–746. [DOI] [PubMed] [Google Scholar]
- 28.Anshu A, Price MO, Price FW. Descemet's stripping endothelial keratoplasty: long-term graft survival and risk factors for failure in eyes with preexisting glaucoma. Ophthalmology. 2012;119:1982–1987. [DOI] [PubMed] [Google Scholar]
- 29.Birbal RS, Tong CM, Dapena I, et al. Clinical outcomes of Descemet membrane endothelial keratoplasty in eyes with a glaucoma drainage device. Am J Ophthalmol. 2019;199:150–158. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita S, Koizumi N, Ueno M, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. New Engl J Med. 2018;378:995–1003. [DOI] [PubMed] [Google Scholar]
- 31.Zhai L, Zhang X, Liu H, et al. Observation of topical tacrolimus on high-risk penetrating keratoplasty patients: a randomized clinical trial study. Eye (Lond). 2020;34:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wacker K, McLaren JW, Amin SR, et al. Corneal high-order aberrations and backscatter in Fuchs' endothelial corneal dystrophy. Ophthalmology. 2015;122:1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]


