Abstract
The detection of tumor-derived circulating nucleic acids in patients with cancer, known as the “liquid biopsy,” has expanded from use in plasma to other bodily fluids in an increasing number of malignancies. Circulating nucleic acids could be of particular use in central nervous system tumors as biopsy carries a 5–7 % risk of major morbidity. This application presents unique challenges that have limited the use of cell-free DNA and RNA in the diagnosis and monitoring of CNS tumors. Recent work suggests that cerebrospinal fluid may be a useful source of CNS tumor-derived circulating nucleic acids. In this review, we discuss the available data and future outlook on the use of the liquid biopsy for CNS tumors.
Keywords: cfDNA, miRNA, Brain tumor, Liquid biopsy, Circulating tumor cells
Introduction
A “liquid biopsy,” sampling of a brain tumor’s genetic profile through access to blood, urine, or cerebral spinal fluid (CSF), would obviate the need for a percutaneous or open biopsy of certain central nervous system (CNS) tumors. A reliable test identifying a specific tumor’s genetic signature could spare many patients an invasive and potentially morbid CNS procedure. Over the past decade, a wide variety of “liquid biopsies” have made their way from the laboratory into the clinical setting: the detection of viral DNA in CSF for the diagnosis of herpes encephalitis in 1990, circulating tumor cells (CTCs) for cancer patients in 2004, and fetal DNA aneuploidy screening for pregnant women in 2011 [1–3]. These techniques may have additional advantages over the traditional biopsy, including the ability to obtain multiple, sequential samples for earlier detection of tumor alterations conferring treatment resistance. Further, liquid biopsies may even be a more sensitive and quantitative assessment of disease progression than clinical imaging. Increasing evidence suggests that single biopsies may not be an adequate representation of the heterogeneous genetic landscape of tumors [4], whereas, a liquid biopsy may offer a more “averaged” view of the tumor environment.
Extending the use of the liquid biopsy to CNS tumors has proven to be more challenging than cancers of other organ systems. One major limitation has been the extremely low concentrations of CTCs and cell-free nucleic acids in the serum of patients with primary CNS tumors. In the last 5 years, however, a number of publications have demonstrated the ability to detect and sequence the low concentrations of nucleic acids found in the serum and CSF of these patients [5•, 6•, 7, 8•, 9–11]. These techniques hold a great deal of promise, but their ultimate role in the diagnosis and treatment of patients with CNS tumors has yet to be defined. In this article, we review how the liquid biopsy has evolved over time (Fig. 1), its current applications, and future directions in the care of patients with CNS tumors.
Fig. 1.
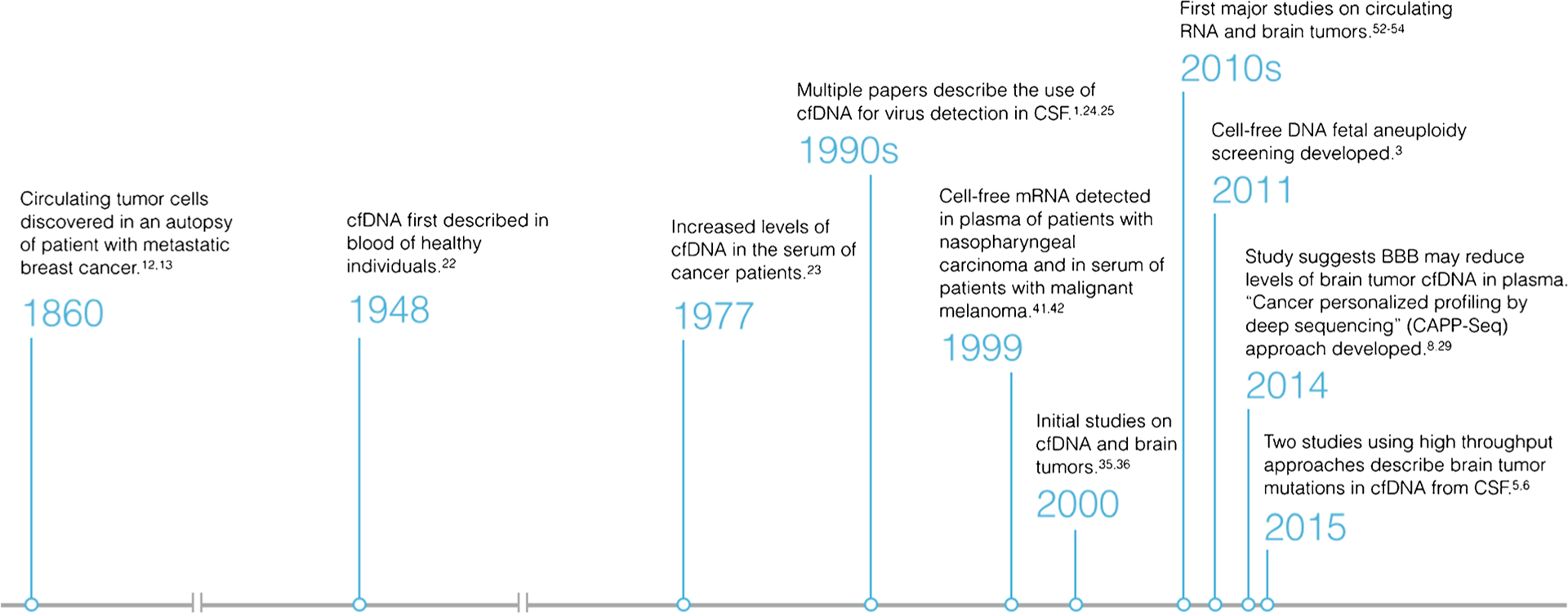
Timeline depicting how the liquid biopsy has evolved
CTCs: the First Liquid Biopsy
Perhaps owing to their larger size, CTCs can be considered one of the first major frontiers related to the liquid biopsy. The first description of these cells is widely attributed to Ashworth in 1869, who documented cells that appeared like tumor in the blood of a metastatic breast cancer patient at the time of autopsy [12, 13]. These cells, which are thought to migrate or shed into the circulation from a primary or metastatic tumor, are found in extremely low concentrations, making them difficult to detect in the background of native blood cells (Fig. 2) [14]. Almost a century later, the development of immunomagnetic assays, which attach specific antibodies to a magnetic bead, allowed for single cells to be detected in 1 ml of blood [15]. It was not until 2004 that Cristofanilli et al. clearly demonstrated the prognostic value of CTCs in metastatic breast cancer patients in a prospective trial [2]. The presence of CTCs in peripheral blood has now been described in many common carcinomas and in CNS metastases. Its prognostic value has been demonstrated in breast, prostate, and colorectal cancer [2, 16, 17]. In 2004, the FDA approved the Cell-Search system (Viridex) for CTC enrichment and detection in the blood of breast cancer patients. This technology can now be used for patients with metastatic breast, colorectal, and prostate cancers [18].
Fig. 2.
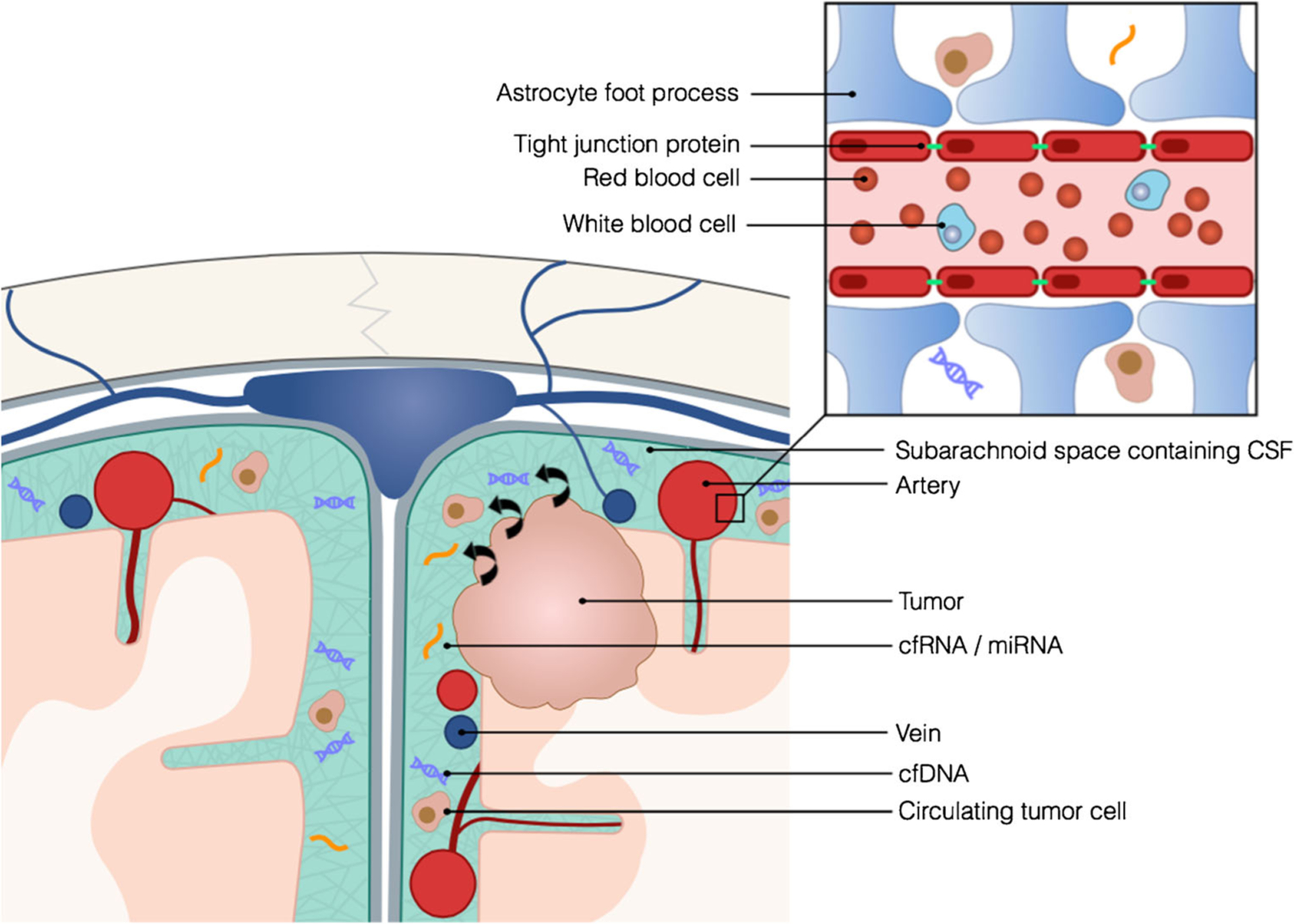
CSF may be a more optimal source of tumor-derived circulating nucleic acids than blood which may have poorer signal-to-noise ratios due to the presence of the BBB and native blood cells
Unfortunately, many of the techniques used to isolate CTCs from blood rely on epithelial markers, such as EpCAM, which are not present on cells from primary brain tumors. To overcome these limitations, researchers have used a combination of physical separation techniques and immunofluorescence for more CNS specific markers, such as GFAP and Nestin, to identify CTCs in glioma patients [9, 19, 20]. Although it has been speculated that the blood-brain barrier (BBB) plays a role in the dissemination of CTCs, it appears that CTCs still retain some ability to spread even in the presence of an undisrupted BBB [9]. Primary CNS tumors rarely metastasize outside of the CNS, so the application of plasma CTCs is unclear. The use of CTCs for patients with primary brain tumors may ultimately lag behind circulating nucleic acid techniques due to the technical challenges of cell capture.
Cell-Free DNA (cfDNA) and Cancer
Originally described in healthy individuals, cell-free DNA (cfDNA) has evolved into a promising diagnostic modality. Its clinical application areas have expanded over time, as it has continued to be described in various bodily fluids and in various clinical applications. cfDNA consists of short 70–200 base pair fragments of genomic DNA (both coding and non-coding) and mitochondrial DNA found extracellularly in bodily fluids [21]. cfDNAwas first described in 1948 by Mandel et al. in blood samples collected from healthy individuals [22]. It was not until 30 years later that Leon et al. discovered increased levels of cfDNA in serum of cancer patients using an antibody-based detection system [23]. This technique did not clearly identify the cfDNA as tumor-derived, but did suggest a correlation between cfDNA quantity and disease metastases. The authors then aptly predicted that testing serum for cfDNA would play a role in tracking treatment response [23].
Several key advances have facilitated the investigation of cfDNA for the liquid biopsy. The development of polymerase chain reaction in 1983 allowed scientists to precisely amplify and map the genetic mutations in DNA. This boon to cancer research also led to the rapid development of “liquid biopsies” for infectious diseases. In the 1990s, several groups reported the presence of herpes simplex virus and varicella zoster virus DNA in the CSF of symptomatic patients [1, 24, 25] and PCR for the viral DNA quickly replaced viral culture and brain biopsy. Early PCR techniques could easily detect the foreign viral genome, but could not reliably distinguish mutant cancer DNA signatures from the background noise of normal DNA in plasma. Despite these challenges, groups began to recognize genetic features of cancer in cfDNA, such as mutations in oncogenes, tumor suppressors, and microsatellite repeats [26, 27].
Next generation sequencing technology refers to a multitude of techniques that increase the sensitivity and speed of nucleic acid sequencing. Additional methods that have developed around sequencing workflows have made it easier to detect tiny fractions of abnormal cfDNA and cfRNA in bodily fluids. For example, in droplet digital PCR (ddPCR), DNA templates are diluted into thousands of droplets (one DNA template or less per picoliter droplet) in which independent PCR reactions occur [28]. Compartmentalization of the reaction reduces background DNA and contamination, creating an increased signal-to-noise ratio. BEAMing (Beads, Emulsions, Amplification, Magnetics), a similar digital PCR-based method, utilizes magnetic beads and flow cytometry to quantify small amounts of nucleic acids. Introduced in 2005, this method involves mixing products from an initial PCR reaction with primer-coated magnetic beads [28]. After running a second PCR reaction on the beads, products are hybridized to fluorescent probes and measured. Pan et al. described two approaches to interrogate brain tumor cfDNA mutations in CSF using these techniques [6•]. They first utilized ddPCR and targeted amplicon sequencing to search for known tumor driver mutations, then globally characterized the mutations using cancer panel sequencing. Another promising technique known as “cancer personalized profiling by deep sequencing,” or CAPP-Seq, used optimized library preparation methods to capture genomic regions that frequently contain recurrent mutations [29•]. Once captured, these regions underwent deep-sequencing, which allows for the ultra sensitive detection of low mutant fractions of plasma cfDNA. An advantage of this approach is its scalability since it does not require patient specific customization [29•]. Although only implemented for non-small-cell lung carcinoma in the study, this approach should be applicable to other cancers.
Though we now have many techniques to identify cfDNA, we do not yet understand exactly how and why concentrations of nucleic acids vary in various bodily fluids. cfDNA may arise from apoptotic or necrotic cells [30], rapidly dividing cells [31] or CTCs. A recent review by Crowley et al. suggests that low numbers of circulating tumor cells cannot account for the quantity of cfDNA typically seen in blood [32]. Some authors have suggested that macrophages may actively release cfDNA into the serum [33, 34].
cfDNA and Primary CNS Tumors
Naturally, research on cfDNA and the liquid biopsy has expanded into the realm of neuro-oncology as the CNS represents a high-risk area for biopsy. In 2003, one of the first studies focusing exclusively on patients with glioblastoma (GBM) used serum cfDNA to detect promoter hypermethylation of specific genes. In this study, Balaña et al. [35] used methylation specific PCR to interrogate the promoter methylation status of MGMT, p16, DAPK, and RASSF1A in circulating serum DNA from GBM patients. Promoter methylation of MGMT, p16, DAPK, and RASSF1A was detected in 11 of 28 (39.3 %), 15 of 27 (53.6 %), 9 of 26 (34.3 %), and 13 of 26 (50 %) serum samples, respectively. The methylation status of serum cfDNA corresponded well with the methylation status of the matched tumor tissue samples. Their findings also corroborated other studies of methylation status in GBM tissue; MGMT methylation status was predictive of response and time to progression in BCNU-treated patients.
Balaña’s work was followed shortly by a study focusing on mitochondrial DNA mutations (mtDNA) in matched samples of tumor tissue, blood, and CSF in patients with medulloblastoma [36]. While mtDNA mutations in seven of eight CSF samples were detected, in only one of these cases did the CSF mtDNA mutations exactly match those in the corresponding tumor. In this patient, the CSF sample was obtained one month after the completion of radiation therapy. Though there was no sign of disease on the patient’s magnetic resonance imaging at the time, this patient developed recurrent disease 5 months later. The authors suggested this may be due to the presence of a small number of therapy resistant cells whose presence was otherwise undetectable via conventional methods such as magnetic resonance imaging and cytology. Exactly why there was disparity between tumor and CSF mtDNA mutations in the majority of patients, however, remains unclear.
The ideal source of cfDNA has been a topic of much debate. In 2010, Lavon et al. published a paper supporting Balaña’s earlier work, re-demonstrating the high specificity of serum cfDNA promoter methylation patterns and allelic chromosomal losses such as 1p19q deletions in gliomas of various grades [7]. Despite nearly 100 % specificity, the authors noted that the rate of detection of deletions in the serum was between 50–60 %, making the test relatively insensitive [7]. Liu et al. also reported nearly 100 % specificity detecting aberrant promoter methylation patterns in matched tumor, serum, and CSF samples of patients with malignant gliomas [37]. In addition, their techniques demonstrated differences in tumor, serum, and CSF detection rates. While neither paper directly addresses the concern, together they suggest that broader use of serum cfDNA for primary brain tumor mutation detection may be limited, due either to thresholds in technique sensitivity, or permeability of these molecules through the BBB or blood-tumor barrier (Fig. 2). The use of CSF rather than serum might circumvent this problem. Liu et al. highlighted this possibility with an example of a patient in their cohort who eventually developed leptomeningeal involvement after negative cytologic testing but positive EGFR mutation status in cfDNA isolated from CSF. The notion that CSF is a better source of circulating nucleic acids for primary brain tumors was further supported by Bettegowda and colleagues in 2014 [8•]. Their study, which included 640 patients of various cancer types, included 41 patients with primary brain tumors. Less than 50 % of patients with medulloblastomas or metastatic cancers of the kidney, thyroid, or prostate had detectable levels of tumor-derived cfDNA in plasma. Additionally, tumor derived cfDNA was found in plasma in less than 10 % of patients with gliomas. The authors hypothesized that these results could be explained by barriers such as the BBB or organs coated with mucin impairing the release of cfDNA into the circulation [8•].
In the past year, two papers have demonstrated a high throughput sequencing approach for the detection of brain tumor-derived cfDNA in CSF. A study by our group applied both ddPCR with targeted amplicon sequencing and cancer panel sequencing to search for cancer mutations in cfDNA of CSF of primary and metastatic brain tumor patients [6•]. Cancer panel sequencing specifically targets regions previously identified as containing genes of interest and several sequencing kits are available commercially. Using this technique, we were able to identify seven SNV mutations in the CSF of a patient with leptomeningeal disease from non-small-cell lung cancer that were concordant with the primary lung tumor. For the remainder of the patients, mutations of interest were first identified through exome sequencing of normal and brain tumor DNA, and then were compared to CSF and serum samples. Tumor mutations were detected in the CSF of 6 of 7 patients. The only patient who did not have detectable mutations in the CSF had a vestibular schwannoma, a benign tumor encased in arachnoid layers. Interestingly, the ratio of CSF to plasma mutant concentration of cfDNA corresponded with burden of CNS versus systemic disease. Therefore, in patients with quiescent systemic disease, but active brain disease, CSF may be a more reliable source of brain tumor-derived cfDNA than plasma.
Our paper was quickly followed by a study by Wang et al. which investigated the presence of brain and spinal cord tumor mutations in CSF [5•]. They found detectable levels of tumor-derived cfDNA in 26 of 35 cases (74 %). In cases where no tumor cfDNA was detected, the tumors were either low-grade or not directly adjacent to a large CSF reservoir. Prospective, longitudinal studies that correlate cfDNAwith disease activity and treatment response will ultimately be required before CSF or serum cfDNA can be used clinically as a liquid biopsy for primary brain tumors.
Circulating RNA and Brain Tumors
Circulating RNAs have also been a topic of much investigation. However, this paradigm has matured rather recently due to the complex and transient nature of RNA expression. Circulating RNAs consist of messenger RNA and noncoding micro RNA (miRNA). These exist in surprisingly stable form in plasma, perhaps due to association with subcellular particles or exosome packaging [38, 39]. The majority of mRNA found in plasma consists of short fragments [40]. Lo et al. were the first to describe the detection of tumor-derived cell-free mRNA in patients with nasopharyngeal carcinoma in 1999 [41]. This was followed shortly by another study describing the detection of tumor-derived cell-free mRNA in the serum of patients with malignant melanoma [42]. Since then, a variety of other tumor-derived cell-free mRNA products have been found including TERT, Her2/neu, and CCND1 [43–45].
Despite these promising studies on circulating mRNA, the cancer research community has gravitated toward miRNAs. This may be due to the high level of variability seen in circulating mRNA levels. miRNA fragments, usually 19–25 base pairs in length, are thought to be derived from 70–100 base pair hairpin precursor molecules [21]. They regulate post transcriptional gene expression and are involved in a variety of cell processes such as apoptosis, proliferation, and differentiation [21]. Surprisingly, the use of miRNA as a liquid biopsy has made rapid progress since 2008, when circulating miRNAs were detected for the first time in the blood of diffuse large B-cell lymphoma patients and prostate cancer patients [46, 47]. In 2010, Hu et al. demonstrated that, like cfDNA, a panel of specific miRNA in blood can predict survival in patients with non-small-cell lung carcinoma [48]. Circulating miRNA has subsequently been found in a number of other body fluids including urine, saliva, fecal material, and CSF [49–52].
Research on cell-free RNA has recently expanded into tumors of the CNS. One of the first papers on circulating RNA and brain tumors was published in 2011 by Baraniskin et al. [52]. In this study, the authors identified miRNAs characteristic for primary diffuse large B-cell lymphoma of the CNS in CSF. This was quickly followed by a similar study in gliomas by the same group, which showed characteristic overexpression of miR-15b and miR-21 [53]. Together, these markers were able to distinguish patients with gliomas from patients with primary central nervous system lymphomas, brain metastases, or leptomeningeal carcinomas. Tepyluk et al. corroborated this finding, demonstrating that the CSF of patients with GBM was enriched for miR-10b and miR-21 [54]. Patients with brain metastases were distinguished by high expression of members of the miR-200 family. In the short time since these studies, a number of authors have used next-generation sequencing related techniques to increase the sensitivity of miRNA detection in plasma, serum, and CSF [10, 11]. For example, Chen and colleagues used BEAMing and ddPCR to detect IDH1 glioma mutations in mRNA transcripts isolated from CSF [11]. In this study, mutated transcripts were not reliably detected in serum.
While it appears that cfDNA in CSF may be more ample and may have less background noise than serum cfDNA, there is less agreement on the optimal source of brain tumor-derived miRNA. Studies have reported widely varying sensitivity and specificity. A meta-analysis conducted in 2014 included 23 studies from six articles that profiled miRNA in serum, blood, or CSF for a variety of diseases of the CNS [55]. The authors concluded that CSF-based assays produced more accurate results than those of blood; pooled sensitivity increased from 0.83 to 0.86, specificity increased from 0.81 to 0.88 in blood versus CSF. A second meta-analysis concluded that panels including miR-21, regardless of source, may be more specific for glioma, with sensitivity and specificity of 0.82 (95 % CI 0.72–0.89) and 0.94 (95 % CI 0.85–0.98), respectively [56]. These studies suggest a role for miRNA in the diagnosis and treatment of brain tumors, but like cfDNA, prospective, longitudinal studies are required to clearly define the utility of miRNA.
Future Directions
The utility of circulating nucleic acids for the diagnosis and monitoring of brain tumors is certainly promising. While sequencing techniques have been available for decades, the ability to detect, differentiate, and sequence low concentrations of tumor-derived nucleic acids efficiently is a relatively recent innovation. As a result, there is no consensus on which nucleic acid (DNA or RNA), which bodily fluid (serum or CSF), or which technique (targeted panel or whole exome sequencing) is best suited for use as a liquid biopsy in brain tumor patients. As many of the authors included in this review have concluded, larger prospective trials are needed to solidify the role of circulating nucleic acids in brain tumor patient care.
While there are a number of small exploratory studies underway, an ideal study would prospectively enroll patients, possibly those already enrolling in larger therapeutic trials, to have samples of cfDNA and RNA from CSF and serum collected prior to and at the time of surgical resection for correlation with tumor tissue. As Wang et al. have suggested, tumors close to CSF reservoirs should be prioritized. By comparing cfDNA and RNA sequencing to surgical pathology, the current gold standard, the sensitivity and specificity can be accurately determined. Though repeat CSF sampling may not be an option for most patients, repeat serum and CSF testing at specific time points during treatment should also be performed, which would ultimately allow for correlation with survival and with treatment efficacy. Including patients who are undergoing re-operation for progression versus pseudo-progression should also be prioritized. If cfDNA or RNA techniques can demonstrate clear separation in these patients, this could dramatically alter their treatment courses. We look forward to seeing the data from Gaur and Fadul’s prospective study of miRNA-10b in glioma patients, estimated to close accrual to 200 patients in May 2018 [57].
We also believe that these techniques may be used to help diagnose and treat patients with metastatic disease to the CNS. A recently published paper by Brastianos et al. used whole-exome sequencing in 86 matched samples of brain metastases and primary tumors and demonstrated that although separate brain metastases in the same patient were relatively genetically homogenous, their genomic profiles were quite distinct from the primary tumor or extracranial metastases [58]. The vast majority of patients do not undergo surgery or biopsy for brain metastases. CSF sampling could detect clinically actionable mutations in the CNS that would otherwise not be known and reduce the number of patients undergoing surgery for brain metastases of unknown etiology. This is particularly relevant for EGFR-mutated lung cancer patients, who may have the option for second or third generation drugs that target resistance mutations. For patients with suspected leptomeningeal disease, it is also possible that cfDNA or RNA may be more sensitive than the current gold standard, CSF cytology. One potential pitfall, however, is that we do not currently have a technique that can differentiate between cfDNA and RNA from a brain parenchymal versus leptomeningeal metastases. Because many physicians make the diagnosis clinically and radiographically, a study to confirm this would require multiple sites with a special interest in leptomeningeal metastases.
Conclusion
Circulating nucleic acids have been detected in a variety of bodily fluids and their diagnostic applications have continued to expand. A great amount of interest has been generated around their use for a “liquid biopsy” for cancer. This approach may be particularly beneficial for brain tumors since biopsies are often unable to be performed or repeated. The ability to successfully utilize circulating nucleic acids to better diagnose and monitor tumors of the CNS and to select targeted therapeutics would most certainly improve our ability to care for this patient population. The studies we have reviewed here confirm that circulating DNA and RNA are present and reliably detectable in the fluids of patients with primary and metastatic brain tumors. The optimal use of these tests as diagnostic and therapeutic tools, however, has yet to be defined. We are eager to see these techniques integrated into clinical trial protocols so that prognostic and predictive values can be determined. Only with these parameters defined can the liquid biopsy approach its ultimate goal, obviating the need for an invasive surgical procedure to diagnose and treat cancer patients.
Financial Support
The authors have no financial interests to declare in the preparation of this work.
Abbreviations
- BBB
Blood-brain barrier
- miRNA
micro RNA
- GBM
Glioblastoma
- CNS
Central nervous system
- CSF
Cerebral spinal fluid
- cfDNA
Cell-free DNA
- CTCs
Circulating tumor cells
- ddPCR
Digital droplet PCR
- mtDNA
Mitochondrial DNA
Footnotes
Conflict of Interest Ian D. Connolly, Yingmei Li, Melanie Hayden Gephart, and Seema Nagpal declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Rowley AH, Whitley RJ, Lakeman FD, Wolinksy SM. Rapid detection of herpes-simplex-virus DNA in cerebrospinal fluid of patients with herpes simplex encephalitis method of making. Lancet. 1990;1:440–1. [DOI] [PubMed] [Google Scholar]
- 2.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. [DOI] [PubMed] [Google Scholar]
- 3.Committee Opinion Summary No. 640 [Internet]. Obstet. Gynecol 2015. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006250-201509000-00045 [DOI] [PubMed]
- 4.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. [Internet].2012;366:883–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22397650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.•.Wang Y, Springer S, Zhang M, McMahon KW, Kinde I, Dobbyn L, et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci. 2015;112(31):9704–9. doi: 10.1073/pnas.1511694112. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang et al. investigated the use of CSF as a reservior for the detection of cfDNA brain and spinal tumor mutations using a high throughput sequencing approach. In a large cohort of 35 primary CNS tumors, the authors demonstrated that cfDNA tumor mutations can be detected in CSF when the tumor interfaces with a CSF reservior.
- 6.•.Pan W, Gu W, Nagpal S, Gephart MH, Quake SR. Brain tumor mutations detected in cerebral spinal fluid. Clin Chem. 2015;61: 514–22. doi: 10.1373/clinchem.2014.235457. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pan et al. is one of the first studies to primarily focus on the detection of brain tumor mutations in CSF using a high throughput sequencing approach. The authors concluded that CSF may be a superior reservior for brain tumor cfDNA when the systemic disease burden is low.
- 7.Lavon I, Refael M, Zelikovitch B, Shalom E, Siegal T. Serum DNA can define tumor-specific genetic and epigenetic markers in gliomas of various grades. Neuro-Oncol. 2010;12:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.•.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med 2014;6:224ra24. Available from: http://stm.sciencemag.org/content/6/224/224ra24.short. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bettegowda et al. investigated levels of mutant cfDNA in a large cohort of 640 patients with a variety of cancers. Interestingly, the authors observed that CNS tumors and tumors with mucinous features were detected less reliably in plasma. They hypothesized that the blood–brain barrier and mucinous layers may interfere with the shedding of tumor cfDNA into the circulation.
- 9.Sullivan JP, Nahed BV, Madden MW, Oliveira SM, Springer S, Bhere D, et al. Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discov. 2014;4:1299–309. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4221467&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgos KL, Javaherian A, Bomprezzi R, Ghaffari L, Rhodes S, Courtright A, et al. Identification of extracellular miRNA in human cerebrospinal fluid by next-generation sequencing. RNA. 2013;19: 712–22. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3677285&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen WW, Balaj L, Liau LM, Samuels ML, Kotsopoulos SK, Maguire CA, et al. BEAMing and droplet digital PCR analysis of mutant IDH1 mRNA in glioma patient serum and cerebrospinal fluid extracellular vesicles. Mol Ther Nucleic-Acids. 2013;2:e109. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3732870&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4:650–61. AACR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen WW, Balaj L, Liau LM, Samuels ML, Kotsopoulos SK, Maguire CA, et al. BEAMing and droplet digital PCR analysis of mutant idh1 mrna in glioma patient serum and cerebrospinal fluid extracellular vesicles. Mol Ther Nucleic-Acids. 2013;2:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alix-Panabieres C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110–8. [DOI] [PubMed] [Google Scholar]
- 15.Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, Terstappen LW, et al. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci U S A. 1998;95:4589–94. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=22534&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scher HI, Heller G, Molina A, Attard G, Danila DC, Jia X, et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J Clin Oncol Am Soc Clin Oncol. 2015;33:1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol Am Soc Clinl Oncol. 2008;26:3213–21. [DOI] [PubMed] [Google Scholar]
- 18.De Mattos-Arruda L, Cortes J, Santarpia L, Vivancos A, Tabernero J, Reis-Filho JS, et al. Circulating tumour cells and cell-free DNA as tools for managing breast cancer. Nat Rev Clin Oncol. 2013;10: 377–89. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- 19.Müller C, Holtschmidt J, Auer M, Heitzer E, Lamszus K, Schulte A, et al. Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med. 2014;6:247ra101. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25080476. [DOI] [PubMed] [Google Scholar]
- 20.Macarthur KM, Kao GD, Chandrasekaran S, Alonso-Basanta M, Chapman C, Lustig RA, et al. Detection of brain tumor cells in the peripheral blood by a telomerase promoter-based assay. Cancer Res. 2014;74:2152–9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4144786&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–37. [DOI] [PubMed] [Google Scholar]
- 22.Mandel P. Les acides nucleiques du plasma sanguin chez l’homme. CR Acad Sci Paris. 1948;142:241–3. [PubMed] [Google Scholar]
- 23.Leon SA, Shapirio B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646–50. [PubMed] [Google Scholar]
- 24.Puchhammer-Stöckl E, Popow-Kraupp T, Heinz FX, Mandl CW, Kunz C. Detection of varicella-zoster virus DNA by polymerase chain reaction in the cerebrospinal fluid of patients suffering from neurological complications associated with chicken pox or herpes zoster. J Clin Microbiol Am Soc Microbiol. 1991;29:1513–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1995;171:857–63. [DOI] [PubMed] [Google Scholar]
- 26.Wang J-Y, Hsieh J-S, Chang M-Y, Huang T-J, Chen F-M, Cheng TL, et al. Molecular detection of APC, K-ras, and p53 mutations in the serum of colorectal cancer patients as circulating biomarkers. World J Surg. 2004;28:721–6. [DOI] [PubMed] [Google Scholar]
- 27.Shaw J a, Smith BM, Walsh T, Johnson S, Primrose L, Slade MJ, et al. Microsatellite alterations plasma DNA of primary breast cancer patients. Clin Cancer Res. 2000;6:1119–24. [PubMed] [Google Scholar]
- 28.Benesova L, Belsanova B, Suchanek S, Kopeckova M, Minarikova P, Lipska L, et al. Mutation-based detection and monitoring of cell-free tumor DNA in peripheral blood of cancer patients. Anal Biochem. 2013;433:227–34. doi: 10.1016/j.ab.2012.06.018.Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 29.•.Newman AM, Bratman SV, To J, Wynne JF, Eclov NCW, Modlin L a, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. Nature Publishing Group; 2014;20:548–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24705333. [DOI] [PMC free article] [PubMed] [Google Scholar]; Newman et al. describeed an innovative approach, termed CAPP-Seq, that uses bioinformatic methods to identify common areas of recurrent mutations followed by interrogation of these areas using deep sequencing. Although described for lung cancer, this approach could have broad implications for the implementation of the liquid biopsy for other cancers since it is not patient specific.
- 30.Schwarzenbach H, Alix-Panabières C, Müller I, Letang N, Vendrell J-P, Rebillard X, et al. Cell-free tumor DNA in blood plasma as a marker for circulating tumor cells in prostate cancer. Clin Cancer Res. 2009;15:1032–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19188176. [DOI] [PubMed] [Google Scholar]
- 31.Van Der Vaart M, Pretorius PJ. Circulating DNA: its origin and fluctuation. Ann N Y Acad Sci. 2008;1137:18–26. [DOI] [PubMed] [Google Scholar]
- 32.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nature. 2013;10: 472–84. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- 33.Choi J-J, Reich CF, Pisetsky DS. The role of macrophages in the in vitro generation of extracellular DNA from apoptotic and necrotic cells. Immunology. 2005;115:55–62. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1782131&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diehl F, Li M, Dressman D, He Y, Shen D, Szabo S, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci U S A. 2005;102:16368–73. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1283450&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balaña C, Ramirez JL, Taron M, Multiforme G, Roussos Y, Ariza A, et al. O 6-methyl-guanine-DNA methyltransferase methylation in serum and tumor dna predicts response to temozolamide plus cisplatin in glioblastoma multiforme o 6 -methyl-guanine-DNA methyltransferase methylation in serum and tumor DNA predicts response to 1. 2003;9:1461–8. [PubMed] [Google Scholar]
- 36.Wong LC, Lueth M, Li X, Lau CC, Vogel H. Detection of mitochondrial dna mutations in the tumor and cerebrospinal fluid of medulloblastoma patients detection of mitochondrial dna mutations in the tumor and cerebrospinal fluid of medulloblastoma patients 1. 2003;3866–71. [PubMed] [Google Scholar]
- 37.Liu B-L, Cheng J-X, Zhang W, Zhang X, Wang R, Lin H, et al. Quantitative detection of multiple gene promoter hypermethylation in tumor tissue, serum, and cerebrospinal fluid predicts prognosis of malignant gliomas. Neuro-Oncol. 2010;12:540–8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3:e3694. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2577891&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez-Mercado M, Manterola L, Larrea E, Goicoechea I, Arestin M, Armesto M, et al. The circulating transcriptome as a source of non-invasive cancer biomarkers: concepts and controversies of non-coding and coding RNA in body fluids. J. Cell. Mol. Med 2015;XX:n/a – n/a.Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Hefnawy T, Raja S, Kelly L, Bigbee WL, Kirkwood JM, Luketich JD, et al. Characterization of amplifiable, circulating RNA in plasma and its potential as a tool for cancer diagnostics. Clin Chem Am Assoc Clin Chem. 2004;50:564–73. [DOI] [PubMed] [Google Scholar]
- 41.Lo KW, Lo YM, Leung SF, Tsang YS, Chan LY, Johnson PJ, et al. Analysis of cell-free Epstein-Barr virus associated RNA in the plasma of patients with nasopharyngeal carcinoma. Clin Chem. 1999;45:1292–4. [PubMed] [Google Scholar]
- 42.Kopreski MS, Benko FA, Kwak LW, Gocke CD. Detection of tumor messenger RNA in the serum of patients with malignant melanoma. Clin Cancer Res. 1999;5:1961–5. [PubMed] [Google Scholar]
- 43.Chen XQ, Bonnefoi H, Pelte MF, Lyautey J, Lederrey C, Movarekhi S, et al. Telomerase RNA as a detection marker in the serum of breast cancer patients. Clin Cancer Res. 2000;6:3823–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11051224. [PubMed] [Google Scholar]
- 44.Fleischhacker M, Beinert T, Ermitsch M, Seferi D, Possinger K, Engelmann C, et al. Detection of amplifiable messenger RNA in the serum of patients with lung cancer. Ann N Y Acad Sci. 2001;945: 179–88. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11708476. [DOI] [PubMed] [Google Scholar]
- 45.García V, García JM, Peña C, Silva J, Domínguez G, Lorenzo Y, et al. Free circulating mRNA in plasma from breast cancer patients and clinical outcome. Cancer Lett. 2008;263:312–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18280643. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672–5. Wiley Online Library. [DOI] [PubMed] [Google Scholar]
- 48.Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu Y, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol. 2010;28:1721–6. [DOI] [PubMed] [Google Scholar]
- 49.Millholland JM, Li S, Fernandez CA, Shuber AP. Detection of low frequency FGFR3 mutations in the urine of bladder cancer patients using next-generation deep sequencing. Res Rep Urol. 2012;4:33. Dove Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Zhou X, John MARS, Wong DTW. RNA profiling of cell-free saliva using microarray technology. J Dent Res. 2004;83:199–203. SAGE Publications. [DOI] [PubMed] [Google Scholar]
- 51.Ahmed FE, Jeffries CD, Vos PW, Flake G, Nuovo GJ, Sinar DR, et al. Diagnostic microRNA markers for screening sporadic human colon cancer and active ulcerative colitis in stool and tissue. Cancer Genomics Proteomics. 2009;6:281–95. International Institute of Anticancer Research. [PubMed] [Google Scholar]
- 52.Baraniskin A, Kuhnhenn J, Schlegel U, Chan A, Deckert M, Gold R, et al. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood. 2011;117:3140–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21200023. [DOI] [PubMed] [Google Scholar]
- 53.Baraniskin A, Kuhnhenn J, Schlegel U, Maghnouj A, Zöllner H, Schmiegel W, et al. Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro-Oncol. 2012;14:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teplyuk NM, Mollenhauer B, Gabriely G, Giese A, Kim E, Smolsky M, et al. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro-Oncol. 2012;14:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei D, Wan Q, Li L, Jin H, Liu Y, Wang Y, et al. MicroRNAs as potential biomarkers for diagnosing cancers of central nervous system: a meta-analysis. Mol Neurobiol. 2014;51(3):1452–61. [DOI] [PubMed] [Google Scholar]
- 56.Qu S, Guan J, Liu Y. Identification of microRNAs as novel biomarkers for glioma detection: a meta-analysis based on 11 articles. J Neurol Sci. 2015;348:181–7. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022510X14007655. [DOI] [PubMed] [Google Scholar]
- 57.Evaluating the expression levels of microRNA-10b in patients with gliomas. Available from: https://clinicaltrials.gov/ct2/show/NCT01849952
- 58.Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A., Jones RT, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]


