Abstract
Background
Circulating levels of the endogenous inhibitor of nitric oxide synthase, asymmetric dimethylarginine (ADMA), are positively associated with the prevalence of metabolic syndrome (MetS) in cross-sectional investigations. It is unclear if circulating ADMA and other methylarginines are associated with incident MetS prospectively.
Methods
We related circulating ADMA, symmetric dimethylarginine (SDMA), L-arginine (ARG) concentrations (measured with a validated tandem mass spectrometry assay) and the ARG/ADMA ratio to MetS and its components in 2914 (cross-sectional analysis, logistic regression; mean age 58 years, 55% women) and 1656 (prospective analysis, Cox regression; mean age 56 years, 59% women) individuals from the Framingham Offspring Study who attended a routine examination.
Results
Adjusting for age, sex, smoking, and eGFR, we observed significant associations of ADMA (direct) and ARG/ADMA (inverse) with odds of MetS (N = 1461 prevalent cases; Odds Ratio [OR] per SD increment 1.13, 95%CI 1.04–1.22; and 0.89, 95%CI 0.82–0.97 for ADMA and ARG/ADMA, respectively). Upon further adjustment for waist circumference, systolic and diastolic blood pressure, glucose, high-density lipoprotein cholesterol, and triglycerides, we observed a positive relation between SDMA and MetS (OR per SD increment 1.15, 95% CI 1.01–1.30) but the other associations were rendered statistically non-significant. We did not observe statistically significant associations between any of the methylarginines and the risk of new-onset MetS (752 incident events) over a median follow-up of 11 years.
Conclusion
It is unclear whether dimethylarginines play an important role in the incidence of cardiometabolic risk in the community, notwithstanding cross-sectional associations. Further studies of larger samples are needed to replicate our findings.
Introduction
Insulin resistance (IR) is a key component of the metabolic syndrome (MetS), which is characterized by abdominal obesity, impairment of fasting glucose, dyslipidemia, and hypertension [1, 2]. Individuals with MetS are at increased risk of developing type 2 diabetes and cardiovascular disease [3–6], presumably because these people have IR and a higher burden of subclinical atherosclerosis [7]. It is well established that impaired endothelial nitric oxide (NO) production, often a mediator of endothelial dysfunction, is an early step during the development of atherosclerosis and vascular disease [8, 9]. In this context, the endogenous inhibitor of nitric oxide synthase (NOS), asymmetric dimethylarginine (ADMA), has emerged as an independent predictor for vascular disease and mortality [10]. Endothelial dysfunction and ADMA have also been reported to be associated with IR in hypertensive patients [11, 12], and each of the individual components of the MetS has been associated with impaired endothelial function [5, 6, 13]. Consistent with these observations, mice with a genetic disruption of endothelial NOS display hyperlipidemia, hypertension, and IR, whereas mice overexpressing human dimethylarginine dimethylaminohydrolase 1 (DDAH1), the enzyme mainly responsible for degrading ADMA, show increased insulin sensitivity [14, 15]. Therefore, we hypothesized that ADMA-mediated NOS inhibition might be involved in the pathogenesis of cardiometabolic risk. However, although individuals with MetS have higher circulating ADMA levels compared to individuals without MetS in some cross-sectional studies [16–18], to date, no prior investigation has examined the association between ADMA and the incidence of MetS prospectively. Furthermore, very little is known about the association of symmetrical dimethylarginine (SDMA) with cardiometabolic risk because SDMA (which does not directly inhibit NOS) was thought to be biologically inert. Recently, studies have shown SDMA exerts its biological effects by inhibiting cationic amino acid transport into cells leading to limited cellular L-arginine (ARG) uptake [19–21]. Nonetheless, it was shown that SDMA is inversely related to IR in healthy individuals, and with glycemic control in patients with diabetes [22–26]. In order to further elucidate the relations between dimethylarginines and the development of metabolic disease, we investigated the associations of circulating ADMA, SDMA, ARG, and the ARG/ADMA ratio with prevalent and incident MetS in the large, community-based Framingham Offspring Study sample. We hypothesized that higher and lower plasma concentrations of ADMA and ARG, respectively, are associated with higher odds of MetS cross-sectionally and with a higher risk of MetS prospectively.
Methods
Study sample
The design and enrollment criteria of the Framingham Offspring Study have been described in detail elsewhere [27]. The present investigation evaluated two samples of the Offspring cohort obtained from attendees at their sixth quadrennial examination cycle (1995–1998): one sample for the cross-sectional analysis of dimethylarginines with prevalent MetS, and another sample for the prospective relations of dimethylarginines and incident MetS.
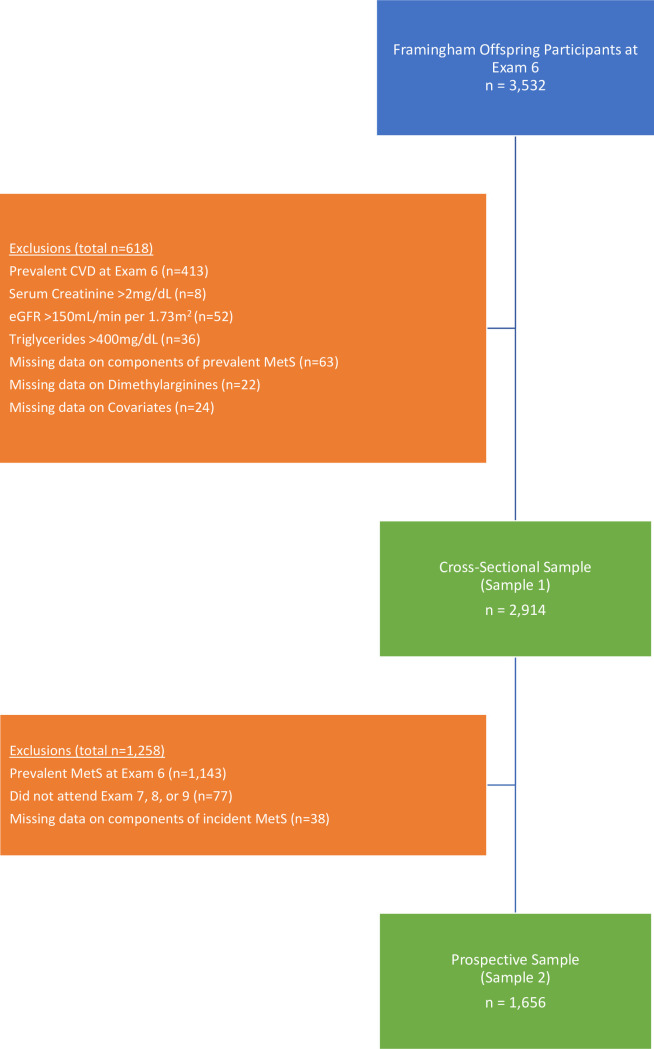
For the cross-sectional analysis, 3532 participants were eligible and 618 individuals were excluded for the following reasons: prevalent cardiovascular disease (coronary heart disease, cerebrovascular disease, intermittent claudication or congestive heart failure; n = 413), serum creatinine >2mg/dL (n = 8), eGFR >150mL/min per 1.73 m2 (n = 52), triglycerides > 400 mg/dL (n = 36), missing data on components of MetS (n = 63), missing data on dimethylarginines (n = 22), missing data on covariates (n = 24), resulting in a sample size of 2914 participants (Sample 1) for the cross-sectional analyses (Fig 1).
Fig 1. Sample exclusion diagram.
Sample 2, which was used for prospective analyses, was nested within Sample 1. From the 2914 individuals in Sample 1, we additionally excluded participants with prevalent MetS at exam 6 (n = 1143), those who did not attend at least one of the subsequent examinations 7 (1998–2001), 8 (2005–2008) or 9 (2011–2014) (n = 77), and those who were missing components of MetS upon follow-up (n = 38). After exclusions, the final sample consisted of 1656 participants for the prospective analysis (Sample 2; Fig 1). The study protocol was approved by the Institutional Review Board at Boston University Medical Center and all participants provided written informed consent.
Assessment of clinical variables and biomarkers of interest
Participants underwent standardized examinations at the Framingham Heart Study research center during which a medical history, a targeted physical examination, anthropometric measurements, and laboratory assessment of cardiovascular risk factors were conducted. Fasting blood samples were obtained from the participants after five to ten minutes rest in a supine position. Plasma samples from the sixth examination cycle, which had been stored without freeze-thaw cycles at -80°C, were used for the measurement of dimethylarginines (ADMA, SDMA) and ARG by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using a stable isotope dilution assay as previously described [28–30].
Assessment of metabolic syndrome
Prevalent and incident MetS were defined as binary variables according to the modified definition of the National Cholesterol Education Program Adult Treatment Panel guidelines [1]. Participants were classified as having MetS if ≥3 of the following criteria were met: abdominal obesity (waist circumference in men ≥102 cm, in women ≥89 cm), elevated blood pressure (systolic ≥130 mmHg or diastolic ≥85 mmHg) or use of antihypertensive medication; elevated fasting glucose (≥100 mg/dl) or use of anti-hyperglycemic medication; elevated triglycerides (≥150 mg/dl) or use of lipid-lowering treatment; or low HDL cholesterol (men <40 mg/dl, women <50 mg/dl).
Statistical analysis
Using sample 1, we examined the cross-sectional associations of ADMA, SDMA, ARG, and the ARG/ADMA ratio (independent variables, separate model for each) with the presence of MetS (dependent variable) using two multivariable logistic regression models. The first model was adjusted for age, sex, smoking, and eGFR. The second model additionally included waist circumference, systolic and diastolic blood pressure, fasting glucose, high-density lipoprotein cholesterol, and triglycerides. To evaluate potential nonlinear associations between each of the biomarkers and prevalence of the MetS, we created restricted cubic spline plots with 3 knots at the 10th, 50th, and 90th percentiles [31].
Next, we evaluated the associations of ADMA, SDMA, ARG, and ARG/ADMA (independent variables, separate model for each) with the incidence of new-onset MetS (absence of MetS at examination cycle 6 and presence of MetS at any of examination cycles 7, 8, or 9) using Cox regression models with discrete time intervals, with examination cycle 6 serving as the baseline, adjusting for the same covariates listed above. We created restricted cubic splines to evaluate potential nonlinear associations between each biomarker and incident MetS. Statistical significance was assessed based on a Bonferroni-adjusted two-sided p-value of <0.0125 (0.05 divided by 4 [= number of methylarginine biomarkers evaluated]). The SAS Software version 9.4 (Cary, NC) was used for all analyses. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Baseline characteristics
The baseline characteristics of the cross-sectional and prospective study samples (samples 1 and 2, respectively) are displayed in Table 1. More than half of the participants of the cross-sectional sample displayed abdominal obesity or elevated blood pressure.
Table 1. Characteristics of study participants at the baseline examination for the cross-sectional and prospective analyses.
| Cross-sectional sample | Prospective sample | |||
|---|---|---|---|---|
| Men (n = 1310) | Women (n = 1604) | Men (n = 683) | Women (n = 973) | |
| Clinical characteristics | ||||
| Age, years | 58 ± 10 | 58 ± 10 | 56 ± 10 | 56 ± 9 |
| Smoking, N (%) | 185 (14) | 237 (15) | 97 (14) | 141 (15) |
| Hypertension, N (%) | 534 (41) | 565 (35) | 167 (24) | 183 (19) |
| Diabetes, N (%) | 115 (9) | 100 (6) | 17 (2) | 5 (0.5) |
| Metabolic Syndrome, N (%) | 568 (43) | 575 (36) | N/A | N/A |
| Abdominal obesity, N (%) | 607 (46) | 961 (60) | 180 (26) | 401 (41) |
| Elevated BP, N (%) | 766 (58) | 768 (48) | 268 (39) | 277 (28) |
| Elevated triglycerides, N (%) | 475 (36) | 515 (32) | 89 (13) | 113 (12) |
| Low HDL cholesterol, N (%) | 493 (38) | 483 (30) | 112 (16) | 133 (14) |
| Elevated fasting glucose, N (%) | 653 (50) | 493 (31) | 212 (31) | 96 (10) |
| Laboratory Values | ||||
| BMI, kg/m2 | 28.5 ± 4.4 | 27.2 ± 5.6 | 27.0 ± 3.7 | 25.2 ± 4.2 |
| Waist circumference, cm | 101 ± 11 | 94 ± 15 | 97 ± 9 | 88 ± 12 |
| SBP, mmHg | 130 ± 17 | 126 ± 20 | 124.4 ± 16.0 | 120 ± 17 |
| DBP, mmHg | 78 ± 9 | 74 ± 9 | 75.9 ± 8.6 | 72 ± 9 |
| Fasting glucose, mg/dL | 105 ± 24 | 99 ± 23 | 98.6 ± 17.3 | 91 ± 9 |
| Triglycerides, mg/dL | 131 ± 68 | 125 ± 64 | 104 ± 48 | 100 ± 48 |
| Total cholesterol, mg/dL | 200 ± 35 | 211 ± 38 | 200 ± 34 | 207 ± 38 |
| HDL cholesterol, mg/dL | 45 ± 12 | 59 ± 16 | 49 ± 12 | 64 ± 15 |
| eGFR, mL/min | 87 ± 17 | 85 ± 19 | 87 ± 16 | 87 ± 18 |
| ADMA, μmol/L | 0.55 ± 0.12 | 0.54 ± 0.13 | 0.54 ± 0.12 | 0.53 ± 0.13 |
| SDMA, μmol/L | 0.40 ± 0.10 | 0.39 ± 0.09 | 0.40 ± 0.09 | 0.38 ± 0.09 |
| L-arginine, μmol/L | 79.8 ± 21.1 | 77.9 ± 20.4 | 79.5 ± 20.6 | 78.4 ± 21.2 |
| Arg/ADMA | 150.6 ± 45.3 | 149.2 ± 43.8 | 152.4 ± 44.7 | 152.9 ± 44.0 |
Data are displayed as means ± standard deviation or frequency and percent (parentheses). Hypertension is defined as SBP/DBP of ≥140/90 or use of anti-hypertension medication. Abdominal obesity is defined among men as a waist circumference ≥102 cm, and among women as a waist circumference ≥89 cm. Elevated BP is defined as SBP/DBP ≥ 130/85 or use of anti-hypertension medication. Elevated triglycerides are defined as ≥150 mg/dL or use of lipid-lowering medication. Low HDL is defined as <40mg/dL for men and <50mg/dL for women. Elevated fasting glucose is defined as fasting glucose ≥100mg/dL or use of anti-hyperglycemic medication.
Cross-sectional association of ADMA, SDMA, ARG, and ARG/ADMA with prevalent metabolic syndrome
Higher ADMA and lower ARG/ADMA concentrations were associated with higher odds of prevalent MetS, adjusting for age, sex, smoking, and eGFR, but further adjustment for waist circumference, systolic and diastolic blood pressure, glucose, high-density lipoprotein (HDL) cholesterol, and triglycerides rendered the associations statistically non-significant. SDMA was not associated with MetS when adjusting for age, sex, smoking, and eGFR, but further adjustment for the covariates listed above rendered the association statistically significant (Table 2). Examination of restricted cubic spline plots did not show significant non-linear associations (S1 Fig).
Table 2. Association of biomarkers with prevalent metabolic syndrome.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Biomarker | OR (95% CI) | p-value | OR (95% CI) | p-value |
| ADMA | 1.13 (1.04, 1.22) | 0.002 | 1.08 (0.96, 1.22) | 0.19 |
| SDMA | 0.94 (0.87, 1.02) | 0.16 | 1.15 (1.01, 1.30) | 0.032 |
| L-Arginine | 0.97 (0.90, 1.05) | 0.45 | 1.05 (0.94, 1.17) | 0.39 |
| ARG/ADMA | 0.89 (0.82, 0.97) | 0.004 | 0.99 (0.89, 1.11) | 0.92 |
Data are displayed as odds ratios (95% confidence intervals) per 1 standard deviation increase in the respective biomarker.
Model 1 is adjusted for age, sex, smoking, and eGFR.
Model 2 includes the adjustment variables in Model 1 plus waist circumference, SBP, DBP, glucose, HDL cholesterol, and triglycerides.
Prospective association of ADMA, SDMA, ARG, and ARG/ADMA with incident metabolic syndrome
During a median follow-up of 11 years, 752 individuals developed new-onset MetS (Table 3). We did not observe a statistically significant association between any of the methylarginine biomarkers and risk of developing new-onset MetS. As with cross-sectional analyses, examination of restricted cubic spline plots did not show significant non-linear associations between biomarkers and incident MetS (S2 Fig).
Table 3. Association of biomarkers with incident metabolic syndrome.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Biomarker | HR (95% CI) | p-value | HR (95% CI) | p-value |
| ADMA | 1.02 (0.94, 1.11) | 0.61 | 1.02 (0.94, 1.12) | 0.63 |
| SDMA | 0.94 (0.86, 1.02) | 0.14 | 1.04 (0.95, 1.14) | 0.37 |
| L-Arginine | 1.01 (0.93, 1.10) | 0.80 | 1.02 (0.94, 1.11) | 0.63 |
| ARG/ADMA | 1.00 (0.92, 1.08) | 0.94 | 1.02 (0.93, 1.10) | 0.73 |
Data are displayed as hazard ratios (95% confidence intervals) per 1 standard deviation increase in the respective biomarker.
Model 1 is adjusted for age, sex, smoking, and eGFR.
Model 2 includes the adjustment variables in Model 1 plus waist circumference, SBP, DBP, glucose, HDL cholesterol, and triglycerides.
Discussion
Principal findings
Cross-sectionally, higher ADMA and lower ARG/ADMA were associated with higher odds of prevalent MetS adjusting for age, sex, smoking, and eGFR, but further adjustment for additional covariates rendered these associations statistically non-significant. Of note, SDMA was not associated with odds of MetS in minimally-adjusted models (adjusting for age, sex, smoking, and eGFR), but the association became significant in fully-adjusted models.
Experimental evidence for NOS-inhibition in metabolic disease
Experimental evidence connects impairment of endothelial NO production with metabolic disturbances. Apart from the associations of the genetic models of the eNOS knockout mice and the DDAH1 transgenic mice with insulin sensitivity noted earlier [14, 15], it has been shown that infusion of the NOS-inhibitor N-monomethyl L-arginine (L-NMMA) in rats induced hypertension and insulin-resistance [32]. Moreover, infusion of ADMA into C57BL/6 and apolipoproteinE (ApoE) knockout mice increased plasma triglycerides [33]. Furthermore, there are additional experimental data supporting a causal relation between NOS inhibition and IR. In an experimental setting it was observed that insulin-mediated glucose uptake is closely connected to insulin-mediated, NOS-dependent vasodilation and microvascular recruitment, which in turn are attenuated by NOS-inhibition [34–36]. Moreover, a non-obese IR rat model fed with fructose showed an elevation of circulating and aortic ADMA concentrations, as well as reduced DDAH aortic content and increased aortic arginase activity in IR. Likewise, ARG supplementation and arginase inhibition improve endothelial vasodilatation in IR rats providing further functional corroboration [2].
Comparison to the literature
ADMA and MetS
Several cross-sectional investigations have analyzed ADMA plasma levels in people with MetS while others evaluated the associations of ADMA plasma levels with individual components of MetS. Recent prospective studies have related plasma dimethylarginines to the risk of developing MetS but their findings are not consistent [24–26, 37–39]. Several studies reported that plasma ADMA was not significantly higher in people with MetS [19–21, 40], although the literature has not been entirely consistent in this regard [16–18]. Furthermore, plasma ADMA concentrations have also been directly related to measures of IR such as the homeostasis model assessment (HOMA), insulin suppression test or hyperinsulinemic, and euglycemic clamp in non-diabetic individuals, including healthy people as well as obese, elderly and hypertensive individuals [2, 41–43]. Clinical studies also have reported higher ADMA plasma concentrations with higher values of anthropometric measures of excess adiposity such as body mass index (BMI), waist circumference, body fat mass, and body weight in healthy individuals [44, 45]. This relation between ADMA, obesity, and IR is further supported by interventional studies, which have shown that weight loss was associated with a lowering of circulating ADMA levels in obese individuals, which in turn was accompanied by an increase in insulin sensitivity and NO production [46, 47]. Contrary to these findings, our prospective investigation showed no statistically significant association between ADMA and risk of developing new-onset MetS. Furthermore, the attenuation of the cross-sectional association we observed between ADMA and presence of MetS could be due to the fact that we adjusted the model for variables that are components of the MetS.
SDMA and MetS
SDMA has been less well investigated, but there is increased focus with regards to its relation with cardiometabolic diseases. SDMA was inversely associated with the HOMA index in a sample of healthy white individuals [22]. In another report evaluating patients with type 2 diabetes, plasma SDMA levels were inversely associated with glycemic control [23]. Marliss and colleagues reported that, similar to ADMA, plasma SDMA levels were positively related with IR and triglycerides, and inversely related with HDL cholesterol [42]. In our investigation, we observed a direct association of SDMA with prevalent but not incident MetS, which is consistent with some prior reports [19–21, 25, 26]. Moreover, SDMA was related to MetS cross-sectionally in a fully-adjusted model but not a minimally adjusted model, perhaps suggesting the presence of reverse confounding by HDL [48, 49].
The mechanisms by which SDMA exerts its biological effects is by inhibiting cationic amino acid transport into cells leading to limited cellular l-arginine uptake [19–21]. Additionally, there are some experimental studies connecting SDMA to inflammation and oxidative stress [50, 51], but additional prospective studies are needed to elucidate how SDMA may be directly related to the development of metabolic disturbances.
Strengths and limitations
The strengths of our investigation include its design with both cross-sectional and prospective components, and the large community-based and well-phenotyped sample. Furthermore, the routine assessment of clinical and laboratory data, including validated assays for dimethylarginines, enabled us to perform multivariable analysis adjusting for relevant covariates. However, our sample consisted predominantly of white, middle-aged individuals, so our results may not be generalizable to other races and ethnicities. Moreover, it is possible that dimethylarginine concentrations fluctuated between exam 6 and the time when assessment of incident metabolic syndrome was performed; however, dimethylarginines were only measured at examination cycle 6, therefore we are not able to account for such fluctuations.
Conclusions
In our investigation of a large prospective, community-based sample, it is not clear whether dimethylarginines play an important role in the pathogenesis of cardiometabolic risk in the community.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The excellent technical assistance of Anna Steenpass and Mariola Kastner is gratefully acknowledged. Drs. Moser, Duncan, and Xanthakis are the guarantors of this work and, as such, had full access to all the data in the investigation and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability
All data can be found here: https://biolincc.nhlbi.nih.gov/studies/framoffspring/.
Funding Statement
This study was partially supported by a research grant Bo1431/4-1 (Dr. Böger) by the Deutsche Forschungsgemeinschaft, by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contracts N01-HC-25195, HHSN268201500001I and 75N92019D00031) and by NHLBI 5T32HL125232 (T32 Multidisciplinary Training Program in Cardiovascular Epidemiology). Dr. Vasan is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 2.El Assar M, Angulo J, Santos-Ruiz M, Ruiz de Adana JC, Pindado ML, Sánchez-Ferrer A, et al. Asymmetric dimethylarginine (ADMA) elevation and arginase up-regulation contribute to endothelial dysfunction related to insulin resistance in rats and morbidly obese humans. J Physiol. 2016Jun1;594(11):3045–60. doi: 10.1113/JP271836 Epub 2016 Mar 4. ; PMCID: PMC4887698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–14. doi: 10.1016/j.jacc.2006.09.032 [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Schulze MB, Pischon T, Bergmann MM, Joost HG, Boeing H. Metabolic syndrome and risk of incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam Study. Cardiovasc Diabetol. 2008;7:35. doi: 10.1186/1475-2840-7-35 PMCID: PMC2627822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sciacqua A, Grillo N, Quero M, Sesti G, Perticone F. Asymmetric dimethylarginine plasma levels and endothelial function in newly diagnosed type 2 diabetic patients. Int J Mol Sci. 2012Oct24;13(11):13804–15. doi: 10.3390/ijms131113804 ; PMCID: PMC3509551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Li C, Chen W, He K, Ma H, Ma B, et al. Relationship between Serum Asymmetric Dimethylarginine Level and Microvascular Complications in Diabetes Mellitus: A Meta-Analysis. Biomed Res Int. 2019Feb25;2019:2941861. doi: 10.1155/2019/2941861; PMCID: PMC6413490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingelsson E, Sullivan LM, Murabito JM, Fox CS, Benjamin EJ, Polak JF, et al. Prevalence and prognostic impact of subclinical cardiovascular disease in individuals with the metabolic syndrome and diabetes. Diabetes. 2007;56:1718–26. doi: 10.2337/db07-0078 [DOI] [PubMed] [Google Scholar]
- 8.Ganz P, Vita JA. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation. 2003; 108: 2049–2053. doi: 10.1161/01.CIR.0000089507.19675.F9 [DOI] [PubMed] [Google Scholar]
- 9.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation 2005;111: 363–368. doi: 10.1161/01.CIR.0000153339.27064.14 [DOI] [PubMed] [Google Scholar]
- 10.Böger RH, Maas R, Schulze F, Schwedhelm E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality—an update on patient populations with a wide range of cardiovascular risk. Pharmacol Res. 2009a;60:481–7. doi: 10.1016/j.phrs.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 11.Perticone F, Sciacqua A, Maio R, Perticone M, Galiano Leone G, Bruni R, et al. Endothelial dysfunction, ADMA and insulin resistance in essential hypertension. Int J Cardiol. 2010;142:236–41. doi: 10.1016/j.ijcard.2008.12.131 [DOI] [PubMed] [Google Scholar]
- 12.Gamil S, Erdmann J, Schwedhelm E, Bakheit KH, Abdalrahman IBB, Mohamed AO. Increased Serum Levels of Asymmetric Dimethylarginine and Symmetric Dimethylarginine and Decreased Levels of Arginine in Sudanese Patients with Essential Hypertension. Kidney Blood Press Res. 2020Aug19:1–10. doi: 10.1159/000508695 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial dysfunction in metabolic syndrome: prevalence, pathogenesis and management. Nutr Metab Cardiovasc Dis. 2010;20:140–6. doi: 10.1016/j.numecd.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 14.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342–5. doi: 10.1161/01.cir.104.3.342 [DOI] [PubMed] [Google Scholar]
- 15.Sydow K, Mondon CE, Schrader J, Konishi H, Cooke JP. Dimethylarginine dimethylaminohydrolase overexpression enhances insulin sensitivity. Arterioscler Thromb Vasc Biol. 2008;28:692–7. doi: 10.1161/ATVBAHA.108.162073 PMCID: PMC3165027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siervo M, Bluck LJ. In vivo nitric oxide synthesis, insulin sensitivity, and asymmetric dimethylarginine in obese subjects without and with metabolic syndrome. Metabolism. 2012;61:680–8. doi: 10.1016/j.metabol.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 17.Palomo I, Contreras A, Alarcón LM, Leiva E, Guzmán L, Mujica V, et al. Elevated concentration of asymmetric dimethylarginine (ADMA) in individuals with metabolic syndrome. Nitric Oxide. 2011;24:224–8. doi: 10.1016/j.niox.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 18.Rentoukas E, Tsarouhas K, Kaplanis I, Korou E, Nikolaou M, Marathonitis G, et al. Connection between telomerase activity in PBMC and markers of inflammation and endothelial dysfunction in patients with metabolic syndrome. PLoS One. 2012;7(4):e35739. doi: 10.1371/journal.pone.0035739 PMCID: PMC3338458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gore MO, Lüneburg N, Schwedhelm E, Ayers CR, Anderssohn M, Khera A, et al. Symmetrical dimethylarginine predicts mortality in the general population: observations from the Dallas heart study. Arterioscler Thromb Vasc Biol. 2013Nov;33(11):2682–8. doi: 10.1161/ATVBAHA.113.301219 Epub 2013 Sep 5. . [DOI] [PubMed] [Google Scholar]
- 20.Schwedhelm E, Wallaschofski H, Atzler D, Dörr M, Nauck M, Völker U, et al. Incidence of all-cause and cardiovascular mortality predicted by symmetric dimethylarginine in the population-based study of health in pomerania. PLoS One. 2014May12;9(5):e96875. doi: 10.1371/journal.pone.0096875; PMCID: PMC4018357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meinitzer A, Kielstein JT, Pilz S, Drechsler C, Ritz E, Boehm BO, et al. Symmetrical and asymmetrical dimethylarginine as predictors for mortality in patients referred for coronary angiography: the Ludwigshafen Risk and Cardiovascular Health study. Clin Chem. 2011Jan;57(1):112–21. doi: 10.1373/clinchem.2010.150854 Epub 2010 Oct 29. . [DOI] [PubMed] [Google Scholar]
- 22.Schutte AE, Schutte R, Huisman HW, van Rooyen JM, Fourie CM, Malan L, et al. Dimethylarginines: their vascular and metabolic roles in Africans and Caucasians. Eur J Endocrinol. 2010;162:525–33. doi: 10.1530/EJE-09-0865 [DOI] [PubMed] [Google Scholar]
- 23.Can A, Bekpinar S, Gurdol F, Tutuncu Y, Unlucerci Y, Dinccag N. Dimethylarginines in patients with type 2 diabetes mellitus: relation with the glycaemic control. Diabetes Res Clin Pract. 2011;94:e61–4. doi: 10.1016/j.diabres.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 24.Tain YL, Hsu CN. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins (Basel). 2017Mar6;9(3):92. doi: 10.3390/toxins9030092; PMCID: PMC5371847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bode-Böger SM, Scalera F, Kielstein JT, Martens-Lobenhoffer J, Breithardt G, Fobker M, et al. Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol. 2006Apr;17(4):1128–34. doi: 10.1681/ASN.2005101119 Epub 2006 Feb 15. . [DOI] [PubMed] [Google Scholar]
- 26.Emrich IE, Zawada AM, Martens-Lobenhoffer J, Fliser D, Wagenpfeil S, Heine GH, et al. Symmetric dimethylarginine (SDMA) outperforms asymmetric dimethylarginine (ADMA) and other methylarginines as predictor of renal and cardiovascular outcome in non-dialysis chronic kidney disease. Clin Res Cardiol. 2018Mar;107(3):201–213. doi: 10.1007/s00392-017-1172-4 Epub 2017 Nov 3. . [DOI] [PubMed] [Google Scholar]
- 27.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813 [DOI] [PubMed] [Google Scholar]
- 28.Böger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in thecommunity. Circulation. 2009b;119:1592–600. doi: 10.1161/CIRCULATIONAHA.108.838268 PMCID: PMC2742491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwedhelm E, Tan-Andresen J, Maas R, Riederer U, Schulze F, Böger RH. Liquid chromatography-tandem mass spectrometry method for the analysis of asymmetric dimethylarginine in human plasma. Clin Chem 2005;51:1268–71. doi: 10.1373/clinchem.2004.046037 [DOI] [PubMed] [Google Scholar]
- 30.Schwedhelm E, Maas R, Tan-Andresen J, Schulze F, Riederer U, Böger RH. High-throughput liquid chromatographic-tandem mass spectrometric determination of arginine and dimethylated arginine derivatives in human and mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2007;851:211–9. doi: 10.1016/j.jchromb.2006.11.052 [DOI] [PubMed] [Google Scholar]
- 31.Harrell, F.E. (2010) Regression Modeling Strategies: with applications to linear models, logistic regression, and survival analyses. Springer-Verlag New York, Inc. New York, USA
- 32.Baron AD, Zhu JS, Marshall S, Irsula O, Brechtel G, Keech C. Insulin resistance after hypertension induced by the nitric oxide synthesis inhibitor L-NMMA in rats. Am J Physiol. 1995Oct;269(4 Pt 1):E709–15. doi: 10.1152/ajpendo.1995.269.4.E709 [DOI] [PubMed] [Google Scholar]
- 33.Xiao HB, Yang ZC, Jia SJ, Li NS, Jiang DJ, Zhang XH, et al. Effect of asymmetric dimethylarginine on atherogenesis and erythrocyte deformability in apolipoprotein E deficient mice. Life Sci. 2007;81:1–7. doi: 10.1016/j.lfs.2007.04.019 [DOI] [PubMed] [Google Scholar]
- 34.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990;85:1844–52. doi: 10.1172/JCI114644 PMCID: PMC296649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–9. doi: 10.1172/JCI117433 PMCID: PMC295191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin.Am J Physiol Endocrinol Metab. 2003;285(1):E123–9. doi: 10.1152/ajpendo.00021.2003 [DOI] [PubMed] [Google Scholar]
- 37.Tsikas D, Bollenbach A, Hanff E, Kayacelebi AA. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine (hArg): the ADMA, SDMA and hArg paradoxes. Cardiovasc Diabetol. 2018Jan4;17(1):1. doi: 10.1186/s12933-017-0656-x; PMCID: PMC5753492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlesinger S, Sonntag SR, Lieb W, Maas R. Asymmetric and Symmetric Dimethylarginine as Risk Markers for Total Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS One. 2016Nov3;11(11):e0165811. doi: 10.1371/journal.pone.0165811; PMCID: PMC5094762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willeit P, Freitag DF, Laukkanen JA, Chowdhury S, Gobin R, Mayr M, et al. Asymmetric dimethylarginine and cardiovascular risk: systematic review and meta-analysis of 22 prospective studies. J Am Heart Assoc. 2015May28;4(6):e001833. doi: 10.1161/JAHA.115.001833; PMCID: PMC4599532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia RG, Perez M, Maas R, Schwedhelm E, Böger RH, López-Jaramillo P. Plasma concentrations of asymmetric dimethylarginine (ADMA) in metabolic syndrome. Int J Cardiol. 2007Nov15;122(2):176–8. doi: 10.1016/j.ijcard.2006.11.058 [DOI] [PubMed] [Google Scholar]
- 41.Stühlinger MC, Abbasi F, Chu JW, Lamendola C, McLaughlin TL, Cooke JP, et al. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA. 2002;287:1420–6. doi: 10.1001/jama.287.11.1420 [DOI] [PubMed] [Google Scholar]
- 42.Marliss EB, Chevalier S, Gougeon R, Morais JA, Lamarche M, Adegoke OA, et al. Elevations of plasma methylarginines in obesity and ageing are related to insulin sensitivity and rates of protein turnover. Diabetologia. 2006Feb;49:351–9. doi: 10.1007/s00125-005-0066-6 [DOI] [PubMed] [Google Scholar]
- 43.Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, et al. Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol. 2005; 46:518–23. doi: 10.1016/j.jacc.2005.04.040 [DOI] [PubMed] [Google Scholar]
- 44.Reimann M, Schutte AE, Malan NT, Schwarz PE, Benndorf RA, Schulze F, et al. Asymmetric dimethylarginine is associated with parameters of glucose metabolism in Caucasian but not in African women from South Africa. Exp Clin Endocrinol Diabetes. 2007;115:600–5. doi: 10.1055/s-2007-981683 [DOI] [PubMed] [Google Scholar]
- 45.Puchau B, Zulet MA, Urtiaga G, Navarro-Blasco I, Martínez JA. Asymmetric dimethylarginine association with antioxidants intake in healthy young adults: a role as an indicator of metabolic syndrome features. Metabolism. 2009;58:1483–8. doi: 10.1016/j.metabol.2009.04.037 [DOI] [PubMed] [Google Scholar]
- 46.McLaughlin T, Stühlinger M, Lamendola C, Abbasi F, Bialek J, Reaven GM, et al. Plasma asymmetric dimethylarginine concentrations are elevated in obese insulin-resistant women and fall with weight loss. J Clin Endocrinol Metab. 2006May;91(5):1896–900. doi: 10.1210/jc.2005-1441 [DOI] [PubMed] [Google Scholar]
- 47.Patle R, Dubb S, Alaghband-Zadeh J, Sherwood RA, Tam F, Frankel A, et al. Improved blood pressure, nitric oxide and asymmetric dimethylarginine are independent after bariatric surgery. Ann Clin Biochem. 2012;49(Pt 6):589–94. doi: 10.1258/acb.2012.012069 [DOI] [PubMed] [Google Scholar]
- 48.Lawlor DA, Hart CL, Hole DJ, Davey Smith G. Reverse causality and confounding and the associations of overweight and obesity with mortality. Obesity (Silver Spring). 2006Dec;14(12):2294–304. doi: 10.1038/oby.2006.269 . [DOI] [PubMed] [Google Scholar]
- 49.Zewinger S, Kleber ME, Rohrer L, Lehmann M, Triem S, Jennings RT, et al. Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur Heart J. 2017May21;38(20):1597–1607. doi: 10.1093/eurheartj/ehx118 . [DOI] [PubMed] [Google Scholar]
- 50.Schepers E, Glorieux G, Dhondt A, Leybaert L, Vanholder R. Role of symmetric dimethylarginine in vascular damage by increasing ROS via store-operated calcium influx in monocytes. Nephrol Dial Transplant. 2009;24(5):1429–35. doi: 10.1093/ndt/gfn670 [DOI] [PubMed] [Google Scholar]
- 51.Schepers E, Barreto DV, Liabeuf S, Glorieux G, Eloot S, Barreto FC, et al. European Uremic Toxin Work Group (EUTox). Symmetric dimethylarginine as a proinflammatory agent in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(10):2374–83. doi: 10.2215/CJN.01720211 PMCID: PMC3359555 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All data can be found here: https://biolincc.nhlbi.nih.gov/studies/framoffspring/.