Abstract
Computational modeling has contributed to hippocampal research in a wide variety of ways and through a large diversity of approaches, reflecting the many advanced cognitive roles of this brain region. The intensively studied neuron type circuitry of the hippocampus is a particularly conducive substrate for spiking neural models. Here we present an online knowledge base of spiking neural network simulations of hippocampal functions. First, we overview theories involving the hippocampal formation in subjects such as spatial representation, learning, and memory. Then we describe an original literature mining process to organize published reports in various key aspects, including: (i) subject area (e.g., navigation, pattern completion, epilepsy); (ii) level of modeling detail (Hodgkin-Huxley, integrate-and-fire, etc.); and (iii) theoretical framework (attractor dynamics, oscillatory interference, self-organizing maps, and others). Moreover, every peer-reviewed publication is also annotated to indicate the specific neuron types represented in the network simulation, establishing a direct link with the Hippocampome.org portal. The web interface of the knowledge base enables dynamic content browsing and advanced searches, and consistently presents evidence supporting every annotation. Moreover, users are given access to several types of statistical reports about the collection, a selection of which is summarized in this paper. This open access resource thus provides an interactive platform to survey spiking neural network models of hippocampal functions, compare available computational methods, and foster ideas for suitable new directions of research.
Keywords: hippocampus, spiking neural network, computational, modeling, knowledge base
Introduction
The hippocampal formation serves many integral roles in mammalian cognition and has been the focus of a wide range of research. For instance, it has long been considered critical for associative learning (Palm, 2013) and declarative memory (Opitz, 2014). Spatial representation importantly depends on hippocampal processing with neurons such as place, grid, border, and head direction cells (HDC) (O’Keefe & Dostrovsky, 1971; Moser et al., 2008; Giocomo et al., 2011). Early hypotheses on hippocampal involvement in pattern completion and separation (Marr, 1971) anticipated the participation of region cornu ammonis 3 (CA3) in recalling previously learned traces from partial inputs, and of the dentate gyrus (DG) in creating orthogonal representations of similar memories (Hummos et al., 2014).
The interplay between in vivo, in vitro, and in silico experimentation enables the exploration and refinement of theories that can be challenging to test in animals. In addition, modeling studies can help narrow down the set of potential problem solutions, thus facilitating future animal studies. For instance, integrating computational simulations with the in vitro investigation of epilepsy revealed a link between seizure induction and specific combinations of network topology and synaptic strengths (Netoff et al., 2004). As computational technologies continue to improve, neuroscience research could increasingly benefit from the incorporation of computational methods.
Much information exists about the hippocampal circuit, including neuron types and their connections, which can be valuable to include in computational models. A backbone of principal glutamatergic neurons interconnects the main areas of the hippocampal formation, including DG granule cells (GCs), CA3, CA2, CA1, and subiculum (Sub) pyramidal cells (PCs), and entorhinal cortex (EC) stellate cells (SC) and PCs (Bennett & Pereda, 2006; Rowland et al., 2018). This excitatory loop is balanced by a great variety of local GABAergic interneurons through feedforward and feedback inhibition. Such an interplay is crucial to establish oscillatory rhythms including theta, gamma, and sharp wave ripples (SWR) (Buzsáki, 2002; Colgin & Moser, 2010). A major source of input to the hippocampus through the EC (Witter et al., 2000; Kesner & Rolls, 2015) originates in extrahippocampal projections from the postrhinal and perirhinal cortices (Burwell and Amaral, 1998).
This work provides a web-based interactive overview of spiking neural network models of the hippocampal region, highlighting major progress in the field as well as areas upon which future work can expand. The annotations of neuron types and hippocampal subregions represented in each model directly connects this resource to Hippocampome.org, a knowledge base supporting data-driven simulations with a focus on the rodent neuron-type circuit of the hippocampus and entorhinal cortex (Wheeler et al., 2015; Hamilton et al., 2017; Sanchez-Aguilera et al., 2021). This free online resource currently contains over 28,000 pieces of knowledge about a wide range of neural properties, including electrophysiology, morphology, transcriptomics, and connectivity (Komendantov et al., 2019; Moradi & Ascoli, 2020; Rees et al., 2016; Venkadesh, Komendantov, Wheeler, Hamilton, & Ascoli, 2019; White et al., 2020; Tecuatl et al., 2021). The online resource described here is accessible at Hippocampome.org/cognome.
The remainder of this article is organized as the following. The next section surveys models and theories of hippocampal functions, operationally divided into spatial navigation, associative learning and long-term episodic memory, pattern separation/completion, and neurological disorders. The subsequent section describes the knowledge base tool including the graphical user interface, annotation results, and methodology. Lastly, we discuss the findings derived from this effort as well as future work and opportunities for the field.
Models and Theories of Hippocampal Functions
Spatial Navigation.
The involvement of the hippocampal formation in spatial representation and path finding requires several cell types. Grid cells map the subject’s location by firing at periodic positions across the spatial environment organized in a grid-like arrangement. Grid cells are most abundant in layer II of the medial entorhinal cortex (MEC), but also exist in other areas (Sargolini et al., 2006). Several factors have been theorized to influence grid cell operations. The primary population of excitatory neurons in MEC layer II, SCs, influence each other through intermediary connections of inhibitory neurons (Dhillon and Jones, 2000). Continuous attractor networks (CANs) are a major class of models used to explain grid cell activities (Shipston-Sharman et al., 2016). One study that used supercomputing to simulate over 1.5 million synaptic connections (Solanka et al., 2015) found that moderate levels of neural noise may promote grid cell firing and location tracking activity called attractor bumps. The noise level is also theorized to enable a wide range of gamma rhythm frequencies without disrupting grid cell firing.
Place cells are neurons mainly located in CA1, CA2, and CA3 (Mankin et al., 2015) that associate sensory input, e.g., smell, taste, or vision, with physical locations. Some models have theorized feedforward connections between place-to-grid or grid-to-place cells as a means of communicating navigation awareness, e.g., through separate attractor networks (Agmon & Burak, 2020). Balancing excitatory and inhibitory signaling is also important for controlling spatial navigation. Simulation work suggested that intra- and extra-hippocampal inhibition may influence the maintenance of rate- and phase-coding in CA1 place cells (Cutsuridis & Hasselmo, 2012; Cutsuridis, 2017), as also corroborated by experiments with mice (Kaifosh et al., 2013). Another theory posited that feedback inhibition supports the coexistence of theta-nested gamma oscillations with attractor states that generate grid cell firing fields (Pastoll et al., 2013). The theory also specified that temporal codes influenced by theta-nested gamma oscillations are multiplexed in gamma oscillations with rate codes in firing fields. A computational model developed to test this theory used a layer of 68 horizontal by 58 vertical excitatory cells and a layer of 34 horizontal by 30 vertical interneurons uniformly distributed on a twisted torus. The twisted torus shape has been theorized as a three-dimensional physical arrangement in which grid cells exist (Guanella et al., 2007). Both the simulations and optogenetic experiments with mice provided support for the theory. The same research lab also reviewed excitatory-inhibitory interaction-based continuous attractor network models of grid cells (Shipston-Sharman et al., 2016).
Models can vary in the number of cognitive dimensions they represent. Some models simulate recognition of two- or three-dimensional spatial environment (Nolan, 2018; Laptev & Burgess, 2019). Grid cell maps have been theorized to exist in modules that represent unique places but can contain different physical location scales (Edvardsen, 2019; Zeng et al., 2019). A computational simulation of higher-dimensional encoding in grid cells hypothesized that, while grid cell activity is inherently two-dimensional, animals can project an arbitrary number of N-dimensional variables into two-dimensional modules (Klukas et al., 2019).
Another major class of network-level navigation models are those that simulate the theory of oscillatory interference (OI). These models store spatially relevant information in the rhythmic activity resulting from the intersection between theta rhythms. OI has been theorized to include oscillatory activities controlled in part by velocity perception (Bush & Schmidt-Hieber, 2018). The phase of such a velocity-controlled oscillator (VCO) relative to a baseline theta oscillation tracks the displacement away from a preferred direction (Burgess & Burgess, 2014). Models combining CAN and OI have been developed to address criticisms of pure CAN or OI models (Bush & Burgess, 2014). For example, OI models have been criticized for relying on precisely timed oscillations, which contrasts with evidence of oscillations that are temporally stable and spatially defined in theta-phase precession (Dodson et al., 2011; Jeewajee et al., 2014). A critique of CAN grid cell models is that they typically do not account for theta modulation and phase precession (Bush & Schmidt-Hieber, 2018). Theta phase precession is the occurrence of spiking by individual neurons in association with the phase of neural oscillations in surrounding cells (O’Keefe & Recce, 1993). These neural oscillations, found for instance in CA1, are theorized to contribute important information for place cells (Kang & DeWeese, 2019).
Several other components have been included in spatial navigation models to increase biological authenticity. One such element is a diverse family of boundary or border cells, which fire at specific distances and directions from environmental boundaries (Bush et al., 2014; Bicanski & Burgess, 2020). Cells coding for boundaries have been identified in Sub, parasubiculum, and MEC (Barry et al., 2006; Savelli et al., 2008; Solstad et al., 2008; Lever et al., 2009). Another component is path integration (or dead reckoning), which is the process of an animal updating the understanding of its position by monitoring its trajectory through space relative to a starting point (Fortin, 2008). Path integration leverages information internal to an animal’s body, in contrast to landmark navigation that uses external cues. Furthermore, speed cells located in the MEC correlate their firing rates with the running speed of an animal (Dannenberg et al., 2019). These cells have been included in models to assist with speed code information for path integration activities. Finally, HDC are hypothesized to help navigation by providing head orientation information.
Both forward and reverse activity replay during sleep or after rewards plays an essential role in the creation of many spatial (and non-spatial) memories (Schapiro et al., 2018). Hippocampal neurons fire asynchronously during memory encoding. During sleep or rest, in contrast, the firing occurs in synchronous bursts including SWR. In SWR, short periods of strongly enhanced activity (sharp-waves; ~50–100 ms) occur along with highly synchronous spiking (ripples; ~120–200 Hz) (Jahnke et al., 2015). During SWR, previously stored memories are replayed with a quicker timescale (Vanderwolf, 1969; Buzsáki, 1986, 1989, 2002, 2015; Wilson and McNaughton, 1994; Diekelmann and Born, 2010). A theory of the neural dynamics involved in spatial-temporal memory sequences includes one-shot learning, synfire chains, and global inhibition and disinhibition (Matheus Gauy et al., 2018). In this theory, global inhibition ensures that the same sequence ensemble remains active regardless of the time an animal spends at a location. This is done by preventing a location state cognition change until disinhibition occurs. Corresponding simulations of spatial navigation with a virtual mouse explored the hypothesis that the neural network alternates between an asynchronous mode for memory encoding and a synchronous mode for memory replaying. The theory also includes a new functional cell type, sequence cells, which effectively store sequence information. The observation that acetylcholine (ACh) levels are significantly reduced in periods of quiescence (Kametani and Kawamura, 1990; Marrosu et al., 1995; Hasselmo, 1999) suggested that this neuromodulator may regulate the encoding and consolidation dynamics during exploration and quiescence (Hasselmo, 2006). The aforementioned theory of global inhibition and disinhibition incorporates lowering levels of ACh during reverse replays of memories. These reduced levels cause an increase in network excitability, in turn generating faster replay speed. Similar to replay, preplay, which predicts possible navigation paths, has been also modeled as influencing navigation decisions and theorized to involve hippocampal cells (Azizi et al., 2013).
A noteworthy part of the navigation process is simultaneous localization and mapping (SLAM). A computational model known as RatSLAM was used to test the theory that the representations of multiple possible positions compete with each other to create the correct understanding (Milford et al., 2004, 2010). Conjunctive grid cells in the dorsocaudal MEC were hypothesized to represents those positions. In this context, conjunctive refers to the joining of more than one neural function in a single cell, specifically, information about multiple potential positions. This model can potentially uncover how animals can navigate in perceptually ambiguous environments, e.g., poor visibility. Another computational model also inspired by hippocampal neurons aimed to help robots navigate under uncertain conditions including partial or total loss of visual input (Tang & Michmizos, 2018).
Associative Learning and Long-Term Episodic Memory.
Neural network analytics applied to brain imaging data supported the notion of the hippocampus as a mnemonic convergence zone associating distributed information into episodic memory (Backus et al., 2016). Moreover, networking of discrete associative memories would support learning of conceptual knowledge, meant as abstractions that capture the shared meaning of related representations (Kumaran et al., 2009). Alpha and theta rhythms have been theorized to serve complementary roles: during episodic memory processing, synchronization of hippocampal activity to theta mediates the binding of concepts, while desynchronization of neocortical activity with alpha waves identifies concepts as becoming active for additional processing (Parish et al., 2018). This theory and associated model predict that a phase shift forward in neural activity that is synchronizing with theta is an index of successful learning and is responsible for associative memory formation. While these three theories focused on humans, rodents serve as important models.
An influential computational study explored the concept that associative memory encoding and retrieval are two functionally independent sub-cycles of theta rhythms (Hasselmo et al., 2002). Additional simulations expanded on that research by investigating the theory that those theta half-cycles rely on theta-modulated neural inhibition for their storage and retrieval actions (Cutsuridis et al., 2008, 2010; Cutsuridis, 2017), as experimentally verified in mice (Siegle & Wilson, 2014). This same model by Cutsuridis et al. also predicts that theta-modulated distal dendritic inhibition in CA1 removes interference from spurious memories during recall.
Spiking neural network simulations also explored specific mechanisms relating DG neurogenesis to memory processes. A computational study with over 8,000 neurons from EC, DG, and CA3 found that neurogenesis that used three classes of synaptic plasticity notably outperformed other simpler models in terms of encoding and retrieval of associative memories (Chua & Tan, 2017a). Those classes are short term plasticity, spike-timing dependent plasticity (STDP) for excitatory synapses, and STDP for inhibitory synapses impinging on excitatory neurons (Tsodyks et al., 2000; Vogels et al., 2011; Zenke et al., 2015). Interestingly, the signal-to-noise ratio of memory retrieval was adversely affected at high learning rates because spiking activities from the memory encoding phase persisted into the retrieval phase.
Projections between the hippocampal formation and the neocortex provide a key architectural substrate for long-term memory storage. A popular theory of information flow into and out of the hippocampus states that whole episodes are transferred from CA3 to CA1 (Kesner & Rolls, 2015). CA1 neurons would then communicate that information through PCs in the deep layers of the EC. Back-projections in EC layer 5 then relay the information to the neocortical areas that initially provided the inputs to the hippocampus (Lavenex and Amaral, 2000; Witter et al., 2000; Markov et al., 2014). Those back-projections recalling previous episodic events could provide relevant information to the neocortex, consolidating new long-term memory representations (Rolls, 1989a–c, 1990a,b, 2008).
Similar to their above-described role in spatial navigation, SWR are theorized to play a significant part in memory consolidation by aiding re-encoding during memory replay (Buzsáki, 1989; O’Neill et al., 2010). A computational study proposed a neurophysiological mechanism for SWRs based on dendritic sodium spikes (Jahnke et al., 2015), consistent with experimental evidence that dendritic spikes mediate nonlinear amplification of synchronous neural input (Ariav et al., 2003; Gasparini et al., 2004; Polsky et al., 2004; Gasparini and Magee, 2006). This theory provides hypothesized oscillation frequency and waveform characteristics of SWRs, and proposes that replay and SWRs support and generate each other.
Novelty detection is useful for determining if a memory already exists or if, instead, a new one should be made. Animal behavior links CA1 to the processing of novelty signals (Nitz & McNaughton, 2004; Larkin, Lykken, Tye, Wickelgren, & Frank, 2014). In a simulation connecting a novelty detection network in CA1 with auto-associative declarative storage in the neocortex, new input received in parallel by both systems caused a comparison between preexisting and novel recognition (Agerskov, 2016). The model was able to produce a large long-term memory (LTM) recognition signal in the absence of novelty and no LTM signal when a novel input was presented. This study showed that it is generally less economical to implement a dot-product computation (comparing a new input to a LTM in the form of mathematical vectors) in one neuronal ensemble than in a more component-wise fashion. Identification of novel input is also important in managing storage among semantically similar memories to prevent the loss of previously acquired information when new stimuli are presented due to catastrophic interference (French, 1999).
Pattern Separation and Completion.
The hippocampus is a crucial information processing substrate for pattern completion and separation (Goode et al., 2020). The sparse firing of neurons in the DG is thought to assist separation by reducing interference among patterns (Treves & Rolls, 1992; Aimone et al., 2011). In particular, GC dendrites may be key contributors in this activity (Chavlis et al., 2017). Pattern separation is hampered in schizophrenia and Alzheimer’s disease (AD) and both conditions are characterized by decreased dendritic length and spine loss in DG GCs (Ally et al., 2013; Einstein et al., 1994; Jain et al., 2012). Moreover, pattern separation improves with dendritic growth in GC and hippocampal neurogenesis in active compared to sedentary animals (Bolz et al., 2015). Additionally, active GCs during spatial tasks have more complex dendrites (Diamantaki et al., 2016). Chavlis et al.’s theory predicts that dendrites contribute to pattern separation efficiency by affecting the sparsity of firing activity. The computational model exploring the theory also demonstrated the effect of GC inhibition on pattern separation by perisomatic basket cells, dendritic-targeting hilar perforant path associated cells (HIPP), and indirect communication through GABAergic neurons activated by mossy cells (MC).
Another in silico and associated in vivo study offered insights into the mechanisms of pattern separation and completion (Hunt et al., 2018) focusing on the communication between DG mossy fibers (GC axons) and CA3 PCs (Henze et al., 2000; Amaral et al., 2007; Rebola et al., 2017). This research identified for the first time two distinct types of PCs in CA3: one characterized by the classic postsynaptic specializations of mossy-fiber synapses (thorny excrescences), and a new ‘athorny’ type lacking mossy-fiber input. The computational model suggested that athorny cells assist in the occurrence of sharp-wave (SW) synchronization events associated with the replay of neural activity contributing to memory processing. Another function theorized for the athorny cells is to support the creation of transient network attractor states that indicate pattern completion computations have been executed. Other studies also implicated recurrent circuitry and attractor dynamics in the re-instantiation of previously stored representations from incomplete input (Colgin et al., 2010; Knierim & Zhang, 2012; Rennó-Costa et al., 2014; Knierim & Neunuebel, 2016).
A computational study of episodic memory retrieval applied a theory to efficiently find a group of contextual details related to the target episode, uniquely identifying it for recall (Samsonovich & Ascoli, 2005). Computer simulations demonstrated that the process is effective in finding the shortest route in a spatial navigation task that includes a familiar environment for the test subject. Thus, the same pathfinding mechanism works in a spatial maze and in an abstract graph of contexts that combine to distinctly identify an episodic memory. The study predicts that retrieving episodic memories cannot solely rely on information pointers as proposed by the memory indexing theory (Teyler & DiScenna, 1985, 1986; Nadel & Moscovitch, 2001), but it may also require the pathfinding process to re-instate the relevant contextual details.
Neurological Disorders and Other Subjects.
Pathological and neuroimaging studies indicate that the hippocampal formation is the earliest brain region targeted by AD (Gomez-Isla et al., 1996; Moreno et al., 2007; Whitwell et al., 2007; Braak and Del Tredici, 2012). These observations are consistent with the pivotal involvement of the hippocampus in episodic memory, a cognitive function prominently depleted by this disease (Fjell et al., 2014). A computational model and associated in vivo experiment investigated the influence of AD-specific proteins on neural excitability in EC and subsequent circuit effects (Angulo et al., 2017). The in vivo research showed that mutant human amyloid precursor protein (hAPP) expression increased excitability and tau protein (hTau) expression decreased excitability. Mice expressing hAPP were also found to have synaptic downscaling in subicular neurons. Simulation modeling of DG, CA3, CA1, EC, and Sub suggested that EC interneuron pruning can account for both increased excitability in EC and synaptic downscaling in Sub in hAPP-expressing mice, the latter being a compensatory response to the former.
Another neurological disorder commonly associated with hippocampal dysfunction is temporal lobe epilepsy. Scientists have long noted that the frequency of epileptic seizures is not uniformly distributed during the day. Long-term electroencephalographic recordings in rats suggested that locally impaired circadian input in status epilepticus could cause a persistent ~12-hour phase shift in the rate of spontaneous hippocampal spikes (Stanley et al., 2013). Corresponding spiking neural network simulations and parallel evidence from magnetic resonance imaging provided a possible causal nexus between damages in the medial septum and the observed phenomena.
Among the psychiatric diseases implicating the hippocampal formation, schizophrenia is associated with abnormal oscillations. A computational simulation of area CA3 explored the effects of the NMDA receptor antagonist ketamine on the disruption of theta rhythm modulation of gamma rhythms (Neymotin et al., 2011). This study modeled pyramidal, perisomatic (basket), and dendritic-targeting (oriens/lacunosum-moleculare) cells as well as extrinsic medial septal inputs. One of multiple testable predictions resulting from this work, namely the potential functional specificity of ketamine for particular cell types, is relevant to a possible treatment for cognitive deficits of schizophrenia.
Time perception is a fundamental element of normal cognition and identifying its underlying neural processes could produce insights into a wide range of disorders. A computational study proposed that time is first encoded in the hippocampal formation by ramp-shaped firing patterns in lateral EC neurons (Rolls & Mills, 2019). Simulations including integrate-and-fire neurons in an attractor network model demonstrated that these EC signals can be then converted into time cells in other hippocampal regions, e.g., CA1, CA3, or DG. This computational model also showed how time cells can generate replay of neuronal ensemble activities as well as reverse-replay, thus providing a link between time and episodic memories.
Last but not least, integrating principles of neural operations in the hippocampus into robotic applications provides the opportunity for testing the computational models in an embodied environment. A simulation containing CA3 and DG produced visual recognition performance comparable to that of the latest convolutional neural networks (CNN) at the time of that publication, slightly outperforming CNN when noise was included (Zhang et al., 2016).
The Hippocampome.org Cognome Knowledge Base
Function of the tool.
We introduce a new component of the Hippocampome.org knowledge base, termed “Cognome”, to allow interactive exploration of the literature describing spiking neural networks of hippocampal function in the context of the neuron type circuit of this brain region. This resource thus constitutes an online dynamic extension of this article (cf. also Samsonovich, 2010). The Cognome section of Hippocampome.org provides systematic annotations about the content of 105 articles selected as representative of the field (see Methods section below for literature mining procedure and inclusion criteria). The annotations span a number of dimensions including the main subject(s) explored in an article's simulation, theories, the level of detail of the model (from Hodgkin-Huxley to binary activity), the network scale (from ten to millions of neurons), and the hippocampal subregions and identified neuron types included in the simulation. All dimension annotations are accompanied by supporting evidence in the form of text excerpts and/or annotator notes (except for keywords, which were deemed non-essential). These annotations allow useful search options, intuitive browsing, and statistical summaries of the content.
The graphical user interface of the Cognome (Fig. 1) offers a menu of pages to explore the site. The Main page that first appears on visiting is designed to allow efficient filtering by subject (such as spatial navigation), sorting of the annotated articles by any dimension of interest, and a choice of annotations and bibliographic details to use in sorting (Fig. 1A). The Advanced Search page allows additional query options, for example, only returning articles matching a particular selection in a second dimension (e.g., attractor dynamics as theory) in addition to the article subject (Fig. 1B). The results can further be sorted by a different dimension and bibliographic detail. If users wish to search the full text of each article using manually chosen query terms, they can instead navigate to the Custom Search page (Fig. 1C).
Figure 1.
Types of searches and illustration of representative options. (A) Main page of the Cognome site. (B) Advanced Search. (C) Custom Search.
The Browse Articles page presents the full citation list and links to all annotated literature, with the option of delimiting the results by author last name. Relevant information is returned in a structure suitable for in depth exploration. The top section provides general details about the article of interest, including bibliographic data (journal, year, and authors) as well as abstract (Fig. 2A). The bottom section reports the annotation for all dimensions (Fig. 2B). An optional toggle allows users to display the supporting evidence for each annotated dimension individually (Fig. 2C).
Figure 2.
Main display elements of the Browse Articles page. (A) General article details. (B) Annotations of selected dimension. (C) Evidence supporting annotations.
Additional pages offer more in-depth descriptions of the site’s resources. The Help and Details page provides specific definitions of terms used on the site, including each dimension and items therein. The Site Stats page reports data about the annotation quantities of subjects and other dimensions, along with the counts of dimensions within each subject, revealing both popular research trends and potentially under-explored areas. Accompanying the fully annotated 105 articles of the core collection, the Cognome also includes an extended collection with more than 300 additional articles that still are relevant to spiking neural network models of hippocampal function but are not required to meet the strict selection criteria described in the Methods and are not all fully annotated. Users can toggle between core and extended collections by selecting the appropriate option on the Site Stats page.
Annotation Results.
This section analyzes the annotations of 105 articles (the core collection) fitting inclusion criteria pertinent to the topic of this article as described in the methods section. Complete statistics can be found at hippocampome.org/php/cognome/reporting.php. Subjects, anatomical regions, theories or network algorithms (hereafter abbreviated as theories), neuron types, and keywords could be annotated with multiple entries, while the other dimensions were only permitted to only have one annotation selection per article. For example, an article could describe a computational study of spatial navigation and long-term consolidation including both entorhinal cortex and areas CA3/CA1; however, the type of neuronal model and the number of simulated neurons were typically unique for each publication.
Spatial navigation or memory (hereafter simply termed navigation), episodic memory, and other learning or memory types were collectively the most common subjects (Fig. 3). The group of other learning or memory types consisted of working or short-term memory (n=7), semantic memory (n=4), associative memory (n=11), and reinforcement learning (n=7). The neurological disorders group contained epilepsy (n=24), schizophrenia (n=4), and AD (n=2). The spatial navigation and episodic memory group (n=35) included non-exclusive annotations for spatial navigation (n=32) or episodic memory (n=8), which were often interrelated and overlapping.
Figure 3.
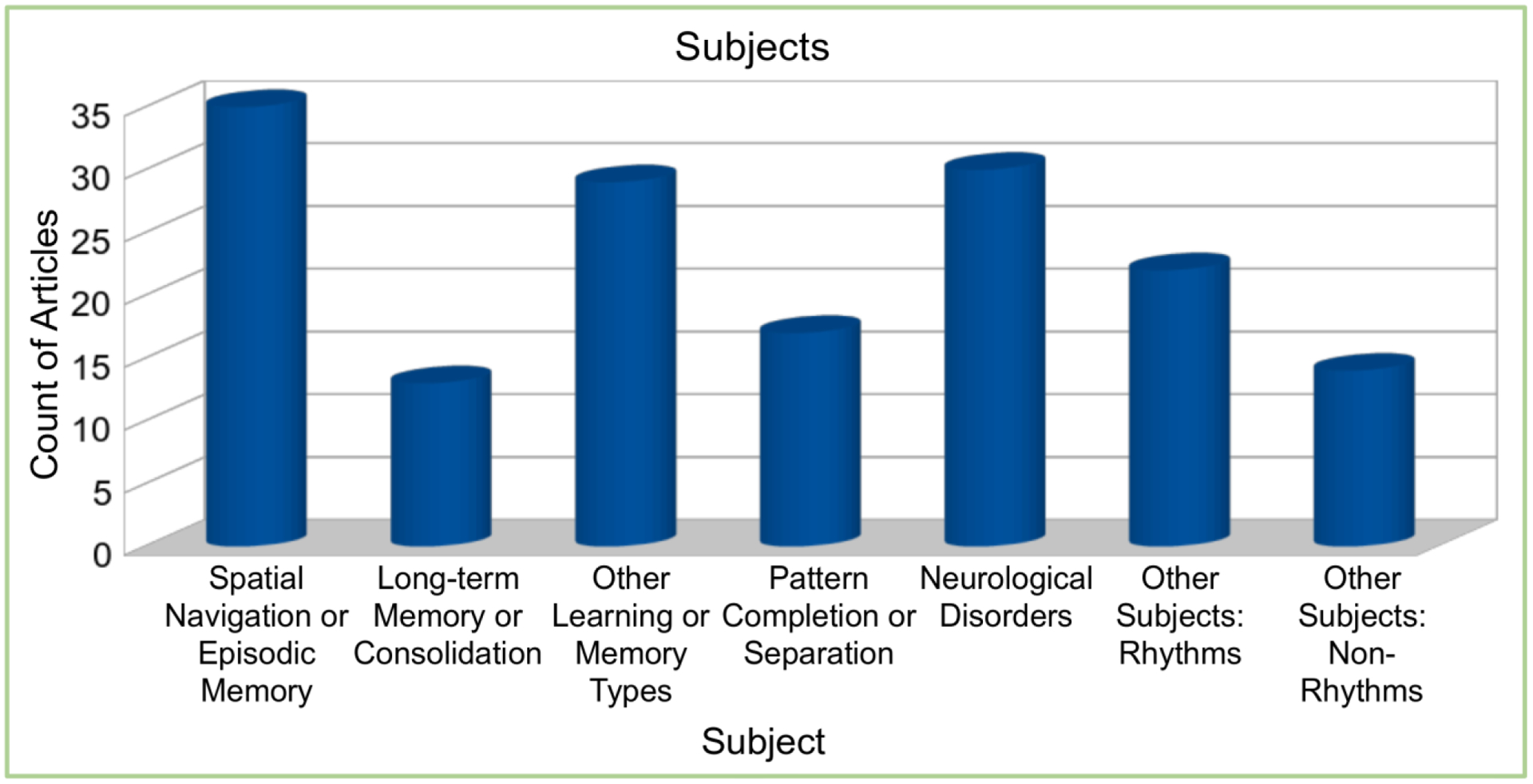
Annotations of subject groups within the Cognome literature.
The most common neuron model found was the Hodgkin-Huxley type (Fig. 4A). The second most common neuron model, integrate-and-fire, was used in 17% more publications than the third most common, the binary model. These three top model types accounted for 88% of the annotated articles. In terms of hippocampal subregions, the most commonly modeled were EC, CA3, and CA1 (Fig. 4B), while DG, Sub, and cornu ammonis 2 (CA2) appeared in fewer studies. Neural networks differed substantially in scale, that is, number of simulated neurons. The most common sizes included less than 500 or between 999 and 100,000 neurons, together accounting for 77% of studies (Fig. 4C). The most frequently found theories were attractor dynamics and theta phase precession (Fig. 4D).
Figure 4.
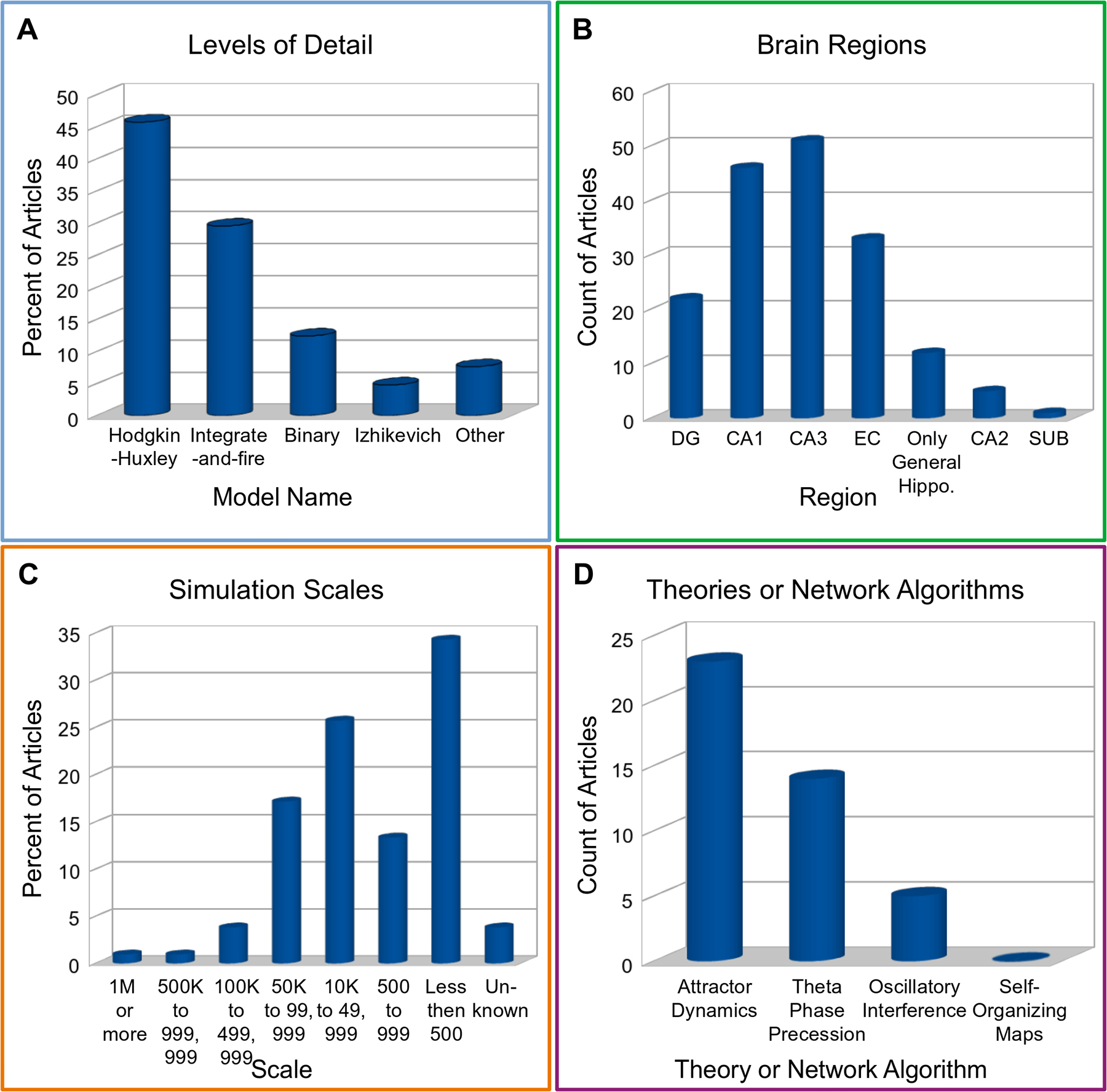
Annotations of selected dimensions within literature. (A) Levels of detail. (B) Anatomical regions. (C) Simulation scales. (D) Theories.
Linking the annotations of hippocampal models to Hippocampome.org presents a special opportunity to map the simulated neurons in each spiking neural network onto identified neuron types. Hippocampome.org mainly classifies neurons based on the neurotransmitter they release (glutamate or GABA) and axonal-dendritic patterns (and thus potential connectivity). On top of this primary characterization, all Hippocampome.org neuron types are associated with known molecular and electrophysiological properties. When computational models describe the neurons included in the circuit with sufficient details, it is typically possible to match them unambiguously to a Hippocampome.org type. More abstract spiking neural network simulations occasionally only provide general characteristics that identify a set of neuron types. For example, ‘parvalbumin-positive interneurons in CA1’ can correspond to cells such as basket, axo-axonic, horizontal basket, or horizontal axo-axonic cells. In these cases, all possible matches are included in the Cognome with the label of “fuzzy” annotation. Overall, EC had the most neuron type annotations, and DG, CA1, and CA3 had the next highest counts (Fig. 5). The most commonly found types were pyramidal, basket, and stellate cells, followed by axo-axonic, granule, and mossy cells and multiple interneuron types such as bistratified and O-LM cells. The groups with “Other” in their description include all neuron type annotations in that subregion aside from those listed explicitly elsewhere on the graph.
Figure 5.
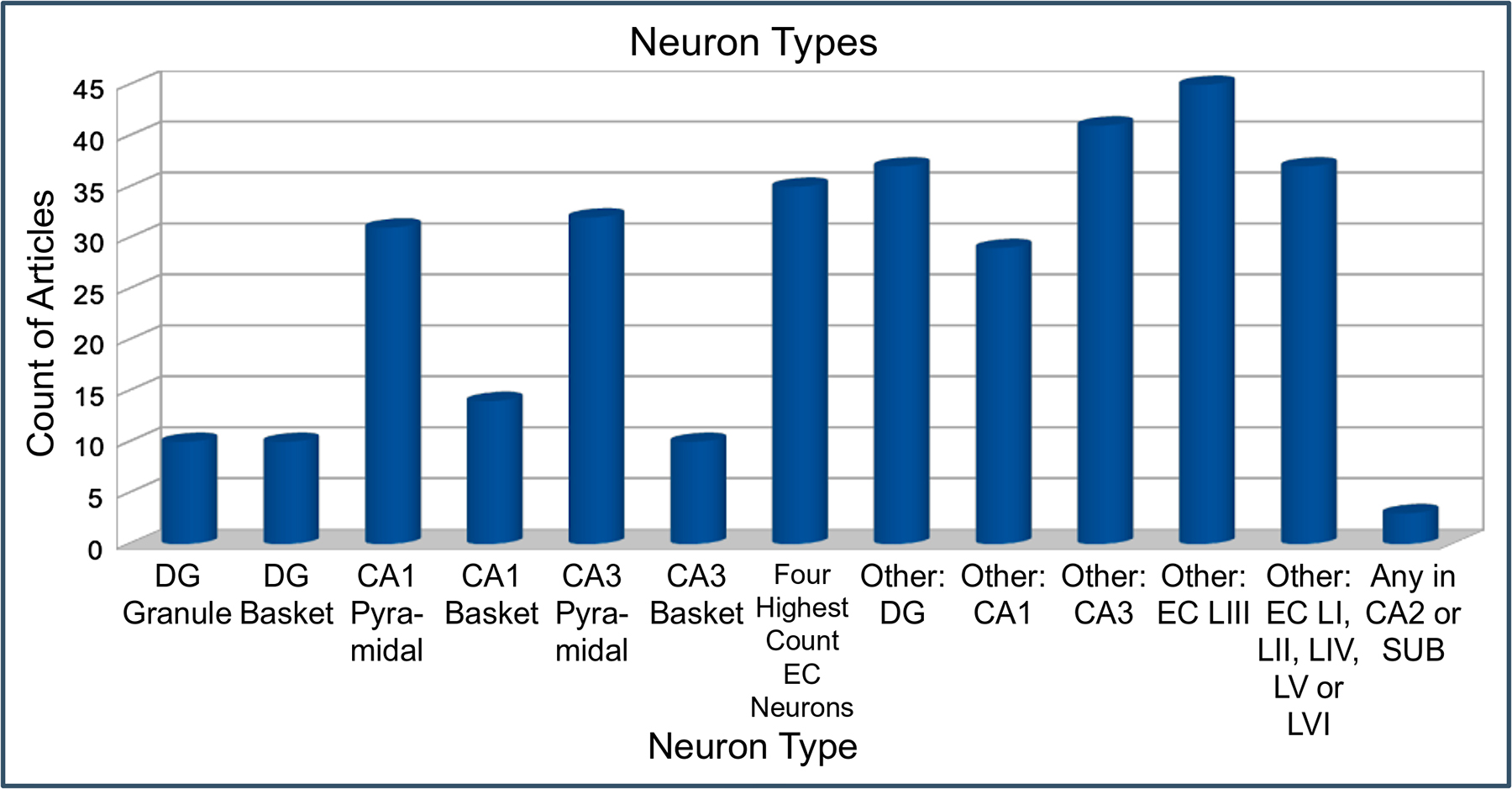
Annotations of neuron types. The four highest count EC neuron types are MEC LII-III pyramidal-multiform, MEC LII stellate, EC LII-III pyramidal-tripolar, and EC LIII pyramidal. The article counts include fuzzy annotations.
Methods.
The core collection consists of original research articles meeting specific inclusion criteria: they must describe a spiking simulation of (a part of) the hippocampal formation at the circuit- or network-level containing at least ten neurons modeled so as to capture individual spike times. Moreover, we set a minimum qualification of ten citations per year (as retrieved from Google Scholar) in order to prioritize the articles with highest impact in the scientific community. We chose this particular threshold to balance a desirable collection size with the time needed for annotations. Additional criteria specifications are listed on the Help page (hippocampome.org/php/cognome/help.php). The extended collection includes articles that do not need to meet the inclusion criteria such as review articles.
Articles were reviewed for qualifications needed for inclusion in the core collection starting from a large bibliography obtained from literature queries that consisted of sets of keywords representing regions, neural activities, and computational models. An objective for the construction of the core collection was to avoid bias in the selection of modeling articles. Therefore, the chosen sets of keywords strived for a balanced assortment not favoring any specific dimension entity over another. For example, when specific anatomical areas were named in queries, at least one keyword representing each hippocampal subregion (including synonyms) was included to give all of them equal retrieval chance.
Optimization software was built to ensure the effectiveness of queries. Specifically, we started from a preliminary collection of 200 potentially relevant articles. The algorithm created shuffled combinations of keywords (while retaining the above-described balance) and ran semi-automated queries with PubMed and Google Scholar. The program tested approximately one hundred thousand query variations, and two hundred million article titles in search results were automatically processed for relevance, requiring approximately one week of computational time. The software automatically counted title matches to the preliminary set and top scoring results were manually verified. The final selection of queries was based on a balance of relevant matches and number of articles retrieved: the best performing queries resulted in a 70% match to example articles and 4574 unique article titles. The list of keywords the software considered, the example articles used, and the final selection of queries are all available on the Cognome Help page.
Software automatically parsed the title, year, and citation count from the query results downloaded from PubMed and Google Scholar. The results were manually reviewed, and any incorrect values were corrected. A program was built to automatically extract Google Scholar citation counts based on PubMed titles using the Python module scholarly (Silva, n.d.). Annotation software was created that automatically retrieved abstracts based on article titles from PubMed, if possible, or else supplied a link to a Google Scholar search for the title, so that the abstract could be reviewed. If an abstract indicated the potential relevance of an article, then that full text was reviewed for inclusion.
Multiple computational technologies were used to create the site. The site was built using a LAMP (Linux, Apache, MySQL, and PHP) software stack. PubMed’s Entrez Programming Utilities were used to automatically import article details (Sayers, 2010). The source code for the work is publicly available at github.com/Hippocampome-Org/php.
Discussion
Interpretation of Site Findings.
Building a simulation involves a strategic choice of which computational components to focus resources on. The Hodgkin-Huxley model is the most commonly found neuron model, indicating its popularity in network-scale simulations. Its utility in capturing key biological detail evidently justifies the relatively heavier computational requirements, which become progressively less prohibitive with the continuous advancement of computer hardware. For example, a Hodgkin-Huxley model of neural entrainment in theta rhythms simulated over 160,000 neurons through use of supercomputing (Navas-Olive et al., 2020). The study comprised realistic morphologies of CA1 pyramidal cells including ion channels, seven types of interneurons, calcium dynamics, and synaptic signaling, thus combining a large neuron count with detailed neurophysiology.
The other side of the computational coin relative to the modeling level of detail is the number of simulated neurons. Large scales can allow observing aggregate effects of large neural populations, while small scales can facilitate deeper analyses of specific physiological characteristics of individual neurons. A model with more than a million rat DG neurons and 470 million synapses (Schneider et al., 2012) eliminated scaling adjustments required in an earlier, smaller simulation of the same neural system with ‘only’ 50,000 neurons (Dyhrfjeld-Johnsen et al., 2007). In particular, connection probabilities had to be increased fivefold in the older (but not in the newer) model to account for the reduced network scale. The larger model also can enable a more straightforward mapping of actual neurons to simulated ones which could facilitate a more direct comparison to results of animal studies. Towards the other end of the network scale spectrum, a model of pattern completion and separation and seizure-like activities with 527 neurons reproduced experimental synaptic current dynamics, short-term synaptic plasticity, and network spatial connectivity patterns (Hummos et al., 2014). The relatively smaller scale helped researchers to focus on a specific neurotransmitter, ACh, including its effects on neural excitation while controlling plasticity levels and progressively increasing levels of activity in EC to CA3 projections across 30 trials.
The recurring presence of certain theories reflects at least in part the experimental support for their explanatory power. For instance, an attractor network theory predicts that, in darkness or other conditions causing HDC drift errors, individual HDC should shift off course of the preferred position by the same amount and direction (Angelaki & Laurens, 2020). Experiments have verified that prediction by observing coherent HDC drifting in rats (Butler et al., 2017; Bassett et al., 2018). Activity observed in mice during slow-wave sleep (SWS) and rapid eye movements (REM) sleep also corroborated the idea that attractor dynamics occur with HDCs (Peyrache et al., 2015). The research investigated the theory that HDCs are arranged in a conceptual ring and increase firing when an animal is in a cell’s preferred head direction. In the study, HDCs with similar preferred head directions displayed positive temporal correlations with firing, whereas HDCs with opposite preferred directions fired in an anti-correlated manner. At the same time, our inclusion criteria requiring simulations to represent individual spike times also affected the presence of certain theories. For instance, OI and self-organizing maps could be modeled by mean firing rates. Several articles containing those theories can be found in the extended collection, although the annotations in that case are not assured to be as complete.
The relative representation of hippocampal subregions in models relates to their putative involvement in specific cognitive functions, such as grid cells in EC and place cells in CA1 and CA3 (Fortin, 2008). Models of auto-associative memory have hypothesized that CA3 stores engrams in recurrent connections and receives input representing old memories from the EC through the perforant path (Cerasti & Treves, 2010; Chua & Tan, 2017b; McNaughton & Morris, 1987; Pilly et al., 2018; Rolls, 1989b). Pattern separation has long been theorized to be supported by DG and its mossy fiber output to CA3 (Piatti et al., 2013; Rolls, 1990b; Treves & Rolls, 1994), possibly through the incorporation of adult-born neurogenesis (Aimone et al., 2009; Noguès et al., 2012). Work toward a full-scale DG simulation has aimed to pinpoint pathological changes in epilepsy on specific neuron types (Schneider et al., 2012). Another model investigated seizure-associated spiking phase shifts in CA3 with connections to CA1 (Stanley et al., 2013). Experiments in vitro reported CA3 hypoactivity that is hypothesized to affect the ability of the hippocampal outputs to control epileptiform synchronization in EC (Biagini et al., 2005).
Many different neuron types were found in simulations in the core collection. Including a diversity of neuron types can enhance the authenticity of modeled neural activities compared to real subjects. A full-scale simulation of rodent CA1 included a pyramidal cell type and a variety of interneuron types to investigate the dynamic interactions between oscillatory rhythms and circuit architecture (Bezaire et al., 2016). Properties of neuron type connectivity, including synaptic weights and time constants, were derived (when available) from experimental paired recordings. The simulation produced complex activities such as emergent theta and gamma oscillations as well as theta phase preferential firing, generating insights on some needed levels of excitation for those activities. The effects of each neuron type’s signaling on rhythmic behaviors was also studied.
Potential Future Work on the Cognome Knowledge Base.
Currently, new articles can be added to the site only by users with private access. As more literature is published over time, enabling public submissions of article content could help keep the knowledge base up to date. Another known limitation is that, while articles with more than 10 citations per year are likely to have high interest in their research communities, articles with fewer citations may have been overlooked but still contain valuable insights. Many articles with lower citation count are present in the extended collection. Along with adding more articles, literature could be further reviewed to identify more theories and subjects to add as annotation options. Moreover, increased instructions and user-friendly methods could be added to tools released in the open-source code of this project to improve adoption and reusability.
Future Work Opportunities in the Field.
A goal of this literature survey is to help identify gaps in theories that may be amenable for computational investigation. An interesting problem is the potential inefficiency of the cognitive processes associated with spatial replay (Matheus Gauy et al., 2018). In particular, episodic memories relying on place cells that have navigation loops (e.g., a circular path ending where it starts) could be corrupted by confusion about the sequential order of visits. A long enough episodic memory would in principle include the sequence of visits, but shorter memory segments representing individual loop paths need to be connected appropriately. Spiking neural network modeling may help elucidate the mechanism of hippocampal replays enabling animals to correctly choose a direction in a forked path upon multiple visit occurrences (Wu & Foster, 2014). Even if no loops are present, the same locations can be visited many times. The sequence of each route traveled may matter especially if the animal adopts the strategy of trying paths in a certain order. Replays using only recurrent connectivity among place cells, where activation of a place cell could trigger the memory of any path regardless of order of occurrence, could result in ineffective planning. A rat study of spatial navigation found that previously traversed trajectories are reflected in some, but not all, replays (Gupta et al., 2010). The occurrence of trajectories in replays indicates that the animal remembered the sequence of paths that were traveled. What are the neurophysiological mechanisms representing the sequence of paths traveled? One possibility is the presence of a dedicated functional neuron type, sequence cells, supplying this information (Gupta et al., 2010). An alternative theory posits time cells can coordinate sequences with episodic and spatial memory (Rolls & Mills, 2019). Yet another possibility is that of associative memories connecting time points and traveled paths together in an overarching timeline. New spiking network simulations could help investigate the neural activity responsible for such cognitive functions.
Computational research on spatial memory has proposed that attractor networks in MEC and hippocampal subregions such as CA1 cooperate in navigation, and future research might explore the validity of that idea (Agmon & Burak, 2020). When an animal position can only be estimated through idiothetic path integration, the model predicts a lag in the CA1 representation of position relative to EC. To test the model predictions experimentally, recording technology may be needed to simultaneously record from MEC and CA1 with sufficiently high throughput to capture population activity. Another testable theory is that reciprocal connections between EC grid cells and place cells in other hippocampal areas create an error-correction process to avoid drift between distinct grid cell modules that would cause progressive location misinterpretation. Data-driven simulations could explore the extent of activity pairing among MEC grid cell modules in order to quantify their communication of drift levels. A proposed mechanism to coordinate grid cell module activities based on synaptic connectivity dynamics within MEC is independent of hippocampal subregions outside of EC (Mosheiff & Burak, 2019). In vivo tests could deliver sensory input of places that are poor in quality while measuring the drift in different modules. If blocking hippocampal inputs outside of EC prevents the coordination of drift between modules, one could conclude that those hippocampal areas are needed to help manage the drift. The model also predicts that synaptic connections between place cells and grid cells create variability in spiking rates of grid cells across their firing fields. Testing that prediction could require disabling hippocampal inputs outside of EC to observe if firing rates lose variability across firing fields.
The Cognome knowledge base can be used to direct areas of future work. Chronologically inspecting the publications related to a theory may help infer the temporal evolution of the underlying conceptual framework. Statistics on the most common methods used for an area of research can be informative about approaches to perform such studies in additional work. Using the author search, one can find the latest modeling articles from accomplished researchers in their fields. The design of the Cognome search pages aimed to provide multiple analysis opportunities for researchers. Literature including specific models and their usage statistics can provide information about competing models and associated theories. Future simulation directions can potentially be inferred from the literature (or absence thereof) in certain subjects and by remaining problems listed in those articles. A high number of publications in a subject area can indicate popular trends of research and substantial existing knowledge about underlying methods, and thus opportunities for future work.
Concluding Remarks.
The hippocampal formation contributes to many unique but also interrelated cognitive functions. For instance, spatial navigation shares many general attributes with episodic memory while at the same time presenting specific neural activities such as those of grid cells. The landscape of hippocampal models ranging from abstract to fine-detailed allows researchers to select from a rich diversity of properties to include in their work. The statistical reporting on annotations reported in this work can help investigators effectively survey content in a variety of articles related to spiking neural network simulations. Moreover, open-source release of the knowledge base and underlying code can facilitate future review work on different neural systems as well as the continuous expansion of this one. Continual advancements in computer hardware, model algorithms, and neural stimulation and recording technologies make data-driven computational approaches increasingly important in research. The Cognome can thus foster the symbiotic relationship between modelers and experimentalists toward producing more discoveries about the hippocampal formation and its multifarious neural dynamics.
Acknowledgments.
The authors thank Dr. Diek Wheeler, Jeffrey Kopsick, and Dr. David Hamilton for their valuable research and writing feedback. We also thank Dr. Keivan Moradi for his very helpful research feedback. This work was supported in part by National Institutes of Health grants R01NS39600 and U01MH114829.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agerskov C (2016). Vector Symbolic Spiking Neural Network Model of Hippocampal Subarea CA1 Novelty Detection Functionality. Neural Computation, 28(4), 613–628. 10.1162/NECO_a_00826 [DOI] [PubMed] [Google Scholar]
- Agmon H, & Burak Y (2020). A theory of joint attractor dynamics in the hippocampus and the entorhinal cortex accounts for artificial hippocampal remapping and individual grid cell field-to-field variability. BioRxiv, 2020.03.02.974253. 10.1101/2020.03.02.974253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Deng W, & Gage FH (2011). Resolving new memories: A critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron, 70(4), 589–596. 10.1016/j.neuron.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, & Gage FH (2009). Computational Influence of Adult Neurogenesis on Memory Encoding. Neuron, 61(2), 187–202. 10.1016/j.neuron.2008.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Hussey EP, Ko PC, & Molitor RJ (2013). Pattern separation and pattern completion in Alzheimer’s disease: Evidence of rapid forgetting in amnestic mild cognitive impairment. Hippocampus, 23(12), 1246–1258. 10.1002/hipo.22162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Scharfman HE, & Lavenex P (2007). The dentate gyrus: Fundamental neuroanatomical organization (dentate gyrus for dummies). In Scharfman HE (Ed.), Progress in Brain Research (Vol. 163, pp. 3–790). Elsevier. 10.1016/S0079-6123(07)63001-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelaki DE, & Laurens J (2020). The head direction cell network: Attractor dynamics, integration within the navigation system, and three-dimensional properties. Current Opinion in Neurobiology, 60, 136–144. 10.1016/j.conb.2019.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo SL, Orman R, Neymotin SA, Liu L, Buitrago L, Cepeda-Prado E, Stefanov D, Lytton WW, Stewart M, Small SA, Duff KE, & Moreno H (2017). Tau and amyloid-related pathologies in the entorhinal cortex have divergent effects in the hippocampal circuit. Neurobiology of Disease, 108, 261–276. 10.1016/j.nbd.2017.08.015 [DOI] [PubMed] [Google Scholar]
- Ariav G, Polsky A, & Schiller J (2003). Submillisecond Precision of the Input-Output Transformation Function Mediated by Fast Sodium Dendritic Spikes in Basal Dendrites of CA1 Pyramidal Neurons. Journal of Neuroscience, 23(21), 7750–7758. 10.1523/JNEUROSCI.23-21-07750.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi AH, Wiskott L, & Cheng S (2013). A computational model for preplay in the hippocampus. Frontiers in Computational Neuroscience, 7. 10.3389/fncom.2013.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus AR, Bosch SE, Ekman M, Grabovetsky AV, & Doeller CF (2016). Mnemonic convergence in the human hippocampus. Nature Communications, 7(1), 11991. 10.1038/ncomms11991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry C, Lever C, Hayman R, Hartley T, Burton S, O’Keefe J, Jeffery K, & Burgess N (2006). The boundary vector cell model of place cell firing and spatial memory. Reviews in the Neurosciences, 17(1–2), 71–97. https://pubmed.ncbi.nlm.nih.gov/16703944/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JP, Wills TJ, & Cacucci F (2018). Self-Organized Attractor Dynamics in the Developing Head Direction Circuit. Current Biology, 28(4), 609–615.e3. 10.1016/j.cub.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M, & Pereda A (2006). Pyramid power: Principal cells of the hippocampus unite! Brain Cell Biology, 35(1), 5–11. 10.1007/s11068-006-9004-x [DOI] [PubMed] [Google Scholar]
- Bezaire MJ, Raikov I, Burk K, Vyas D, & Soltesz I (2016). Interneuronal mechanisms of hippocampal theta oscillations in a full-scale model of the rodent CA1 circuit. ELife, 5, e18566. 10.7554/eLife.18566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini G, D’Arcangelo G, Baldelli E, D’Antuono M, Tancredi V, & Avoli M (2005). Impaired activation of CA3 pyramidal neurons in the epileptic hippocampus. NeuroMolecular Medicine, 7(4), 325–342. 10.1385/NMM:7:4:325 [DOI] [PubMed] [Google Scholar]
- Bicanski A,, & Burgess N (2020). Neuronal vector coding in spatial cognition. Nat Rev Neurosci. 21(9):453–470. doi: 10.1038/s41583-020-0336-9 [DOI] [PubMed] [Google Scholar]
- Bolz L, Heigele S, & Bischofberger J (2015). Running Improves Pattern Separation during Novel Object Recognition. Brain Plasticity, 1(1), 129–141. 10.3233/BPL-150010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, & Del Tredici K (2012). Alzheimer’s disease: Pathogenesis and prevention. Alzheimer’s & Dementia, 8(3), 227–233. 10.1016/j.jalz.2012.01.011 [DOI] [PubMed] [Google Scholar]
- Burgess CP, & Burgess N (2014). Controlling Phase Noise in Oscillatory Interference Models of Grid Cell Firing. Journal of Neuroscience, 34(18), 6224–6232. 10.1523/JNEUROSCI.2540-12.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, & Amaral DG (1998). Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 391(3):293–321. [DOI] [PubMed] [Google Scholar]
- Bush D, Barry C, & Burgess N (2014). What do grid cells contribute to place cell firing? Trends in Neurosciences, 37(3), 136–145. 10.1016/j.tins.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D, & Burgess N (2014). A Hybrid Oscillatory Interference/Continuous Attractor Network Model of Grid Cell Firing. Journal of Neuroscience, 34(14), 5065–5079. 10.1523/JNEUROSCI.4017-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D, & Schmidt-Hieber C (2018). Computational Models of Grid Cell Firing. In Cutsuridis V, Graham BP, Cobb S, & Vida I (Eds.), Hippocampal Microcircuits: A Computational Modeler’s Resource Book (pp. 585–613). Springer International Publishing. 10.1007/978-3-319-99103-0_16 [DOI] [Google Scholar]
- Butler WN, Smith KS, van der Meer MAA, & Taube JS (2017). The Head-Direction Signal Plays a Functional Role as a Neural Compass during Navigation. Current Biology, 27(9), 1259–1267. 10.1016/j.cub.2017.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G (1989). Two-stage model of memory trace formation: A role for “noisy” brain states. Neuroscience, 31(3), 551–570. 10.1016/0306-4522(89)90423-5 [DOI] [PubMed] [Google Scholar]
- Buzsáki György. (1986). Hippocampal sharp waves: Their origin and significance. Brain Research, 398(2), 242–252. 10.1016/0006-8993(86)91483-6 [DOI] [PubMed] [Google Scholar]
- Buzsáki György. (2002). Theta Oscillations in the Hippocampus. Neuron, 33(3), 325–340. 10.1016/S0896-6273(02)00586-X [DOI] [PubMed] [Google Scholar]
- Buzsáki György. (2015). Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus, 25(10), 1073–1188. 10.1002/hipo.22488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasti E, & Treves A (2010). How Informative Are Spatial CA3 Representations Established by the Dentate Gyrus? PLoS Computational Biology, 6(4). 10.1371/journal.pcbi.1000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavlis S, Petrantonakis PC, & Poirazi P (2017). Dendrites of dentate gyrus granule cells contribute to pattern separation by controlling sparsity. Hippocampus, 27(1), 89–110. 10.1002/hipo.22675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua Y, & Tan C (2017a). Multiple Plasticity Mechanisms Enhance Associative Memory Retrieval in a Spiking Network Model of the Hippocampus. 2017 AAAI Spring Symposium Series. 2017 AAAI Spring Symposium Series. https://www.aaai.org/ocs/index.php/SSS/SSS17/paper/view/15342 [Google Scholar]
- Chua Y, & Tan C (2017b). Neurogenesis and multiple plasticity mechanisms enhance associative memory retrieval in a spiking network model of the hippocampus. ArXiv:1704.07526 [q-Bio]. http://arxiv.org/abs/1704.07526 [Google Scholar]
- Colgin LL, Leutgeb S, Jezek K, Leutgeb JK, Moser EI, McNaughton BL, & Moser M-B (2010). Attractor-Map Versus Autoassociation Based Attractor Dynamics in the Hippocampal Network. Journal of Neurophysiology, 104(1), 35–50. 10.1152/jn.00202.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, & Moser EI (2010). Gamma oscillations in the hippocampus. Physiology (Bethesda, Md.), 25(5), 319–329. 10.1152/physiol.00021.2010 [DOI] [PubMed] [Google Scholar]
- Cutsuridis V (2017). Computational Models of Memory Formation in Healthy and Diseased Microcircuits of the Hippocampus. In Computational Models of Brain and Behavior (pp. 333–344). John Wiley & Sons, Ltd. 10.1002/9781119159193.ch24 [DOI] [Google Scholar]
- Cutsuridis V, Cobb S, & Graham BP (2010). Encoding and retrieval in a model of the hippocampal CA1 microcircuit. Hippocampus, 20(3), 423–446. 10.1002/hipo.20661 [DOI] [PubMed] [Google Scholar]
- Cutsuridis V, Cobb S, & Graham BP (2008). Encoding and Retrieval in a CA1 Microcircuit Model of the Hippocampus. In Kůrková V, Neruda R, & Koutník J(Eds.), Artificial Neural Networks—ICANN 2008 (pp. 238–247). Springer. 10.1007/978-3-540-87559-8_25 [DOI] [Google Scholar]
- Cutsuridis V, & Hasselmo M (2012). GABAergic contributions to gating, timing, and phase precession of hippocampal neuronal activity during theta oscillations. Hippocampus, 22(7), 1597–1621. 10.1002/hipo.21002 [DOI] [PubMed] [Google Scholar]
- Dannenberg H, Kelley C, Hoyland A, Monaghan CK, & Hasselmo ME (2019). The Firing Rate Speed Code of Entorhinal Speed Cells Differs across Behaviorally Relevant Time Scales and Does Not Depend on Medial Septum Inputs. Journal of Neuroscience, 39(18), 3434–3453. 10.1523/JNEUROSCI.1450-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon A, & Jones RSG (2000). Laminar differences in recurrent excitatory transmission in the rat entorhinal cortex in vitro. Neuroscience, 99(3), 413–422. 10.1016/S0306-4522(00)00225-6 [DOI] [PubMed] [Google Scholar]
- Diamantaki M, Frey M, Berens P, Preston-Ferrer P, & Burgalossi A (2016). Sparse activity of identified dentate granule cells during spatial exploration. ELife, 5, e20252. 10.7554/eLife.20252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, & Born J (2010). Slow-wave sleep takes the leading role in memory reorganization. Nature Reviews Neuroscience, 11(3), 218–218. 10.1038/nrn2762-c2 [DOI] [Google Scholar]
- Dodson PD, Pastoll H, & Nolan MF (2011). Dorsal–ventral organization of theta-like activity intrinsic to entorhinal stellate neurons is mediated by differences in stochastic current fluctuations. The Journal of Physiology, 589(12), 2993–3008. 10.1113/jphysiol.2011.205021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J, Santhakumar V, Morgan RJ, Huerta R, Tsimring L, & Soltesz I (2007). Topological Determinants of Epileptogenesis in Large-Scale Structural and Functional Models of the Dentate Gyrus Derived From Experimental Data. Journal of Neurophysiology, 97(2), 1566–1587. 10.1152/jn.00950.2006 [DOI] [PubMed] [Google Scholar]
- Edvardsen V (2019). Goal-directed navigation based on path integration and decoding of grid cells in an artificial neural network. Natural Computing, 18(1), 13–27. 10.1007/s11047-016-9575-0 [DOI] [Google Scholar]
- Einstein G, Buranosky R, & Crain BJ (1994). Dendritic pathology of granule cells in Alzheimer’s disease is unrelated to neuritic plaques. Journal of Neuroscience, 14(8), 5077–5088. 10.1523/JNEUROSCI.14-08-05077.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, & Walhovd KB (2014). What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Progress in Neurobiology, 117, 20–40. 10.1016/j.pneurobio.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin N (2008). 1.21—Navigation and Episodic-Like Memory in Mammals. In Byrne JH (Ed.), Learning and Memory: A Comprehensive Reference (pp. 385–417). Academic Press. 10.1016/B978-012370509-9.00091-7 [DOI] [Google Scholar]
- French RM (1999). Catastrophic forgetting in connectionist networks. Trends in Cognitive Sciences, 3(4), 128–135. 10.1016/S1364-6613(99)01294-2 [DOI] [PubMed] [Google Scholar]
- Gasparini S, & Magee JC (2006). State-Dependent Dendritic Computation in Hippocampal CA1 Pyramidal Neurons. Journal of Neuroscience, 26(7), 2088–2100. 10.1523/JNEUROSCI.4428-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini S, Migliore M, & Magee JC (2004). On the Initiation and Propagation of Dendritic Spikes in CA1 Pyramidal Neurons. Journal of Neuroscience, 24(49), 11046–11056. 10.1523/JNEUROSCI.2520-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giocomo LM, Moser M-B, & Moser EI (2011). Computational Models of Grid Cells. Neuron, 71(4), 589–603. 10.1016/j.neuron.2011.07.023 [DOI] [PubMed] [Google Scholar]
- Gómez-Isla T, Price JL, Jr DWM, Morris JC, Growdon JH, & Hyman BT (1996). Profound Loss of Layer II Entorhinal Cortex Neurons Occurs in Very Mild Alzheimer’s Disease. Journal of Neuroscience, 16(14), 4491–4500. 10.1523/JNEUROSCI.16-14-04491.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Tanaka KZ, Sahay A, & McHugh TJ (2020). An Integrated Index: Engrams, Place Cells, and Hippocampal Memory. Neuron, 107(5), 805–820. 10.1016/j.neuron.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guanella A, Kiper D, & Verschure P (2007). A model of grid cells based on a twisted torus topology. International Journal of Neural Systems, 17(04), 231–240. 10.1142/S0129065707001093 [DOI] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MAA, Touretzky DS, & Redish AD (2010). Hippocampal Replay Is Not a Simple Function of Experience. Neuron, 65(5), 695–705. 10.1016/j.neuron.2010.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DJ, White CM, Rees CL, Wheeler DW, & Ascoli GA (2017). Molecular fingerprinting of principal neurons in the rodent hippocampus: A neuroinformatics approach. Journal of Pharmaceutical and Biomedical Analysis, 144, 269–278. 10.1016/j.jpba.2017.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME (1999). Neuromodulation: Acetylcholine and memory consolidation. Trends in Cognitive Sciences, 3(9), 351–359. 10.1016/S1364-6613(99)01365-0 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME (2006). The role of acetylcholine in learning and memory. Current Opinion in Neurobiology, 16(6), 710–715. 10.1016/j.conb.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelón C, & Wyble BP (2002). A proposed function for hippocampal theta rhythm: Separate phases of encoding and retrieval enhance reversal of prior learning. Neural Computation, 14(4), 793–817. 10.1162/089976602317318965 [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, & Barrionuevo G (2000). The multifarious hippocampal mossy fiber pathway: A review. Neuroscience, 98(3), 407–427. 10.1016/S0306-4522(00)00146-9 [DOI] [PubMed] [Google Scholar]
- Hummos A, Franklin CC, & Nair SS (2014). Intrinsic mechanisms stabilize encoding and retrieval circuits differentially in a hippocampal network model. Hippocampus, 24(12), 1430–1448. 10.1002/hipo.22324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt DL, Linaro D, Si B, Romani S, & Spruston N (2018). A novel pyramidal cell type promotes sharp-wave synchronization in the hippocampus. Nature Neuroscience, 21(7), 985–995. 10.1038/s41593-018-0172-7 [DOI] [PubMed] [Google Scholar]
- Jahnke S, Timme M, & Memmesheimer R-M (2015). A Unified Dynamic Model for Learning, Replay, and Sharp-Wave/Ripples. Journal of Neuroscience, 35(49), 16236–16258. 10.1523/JNEUROSCI.3977-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Yoon SY, Zhu L, Brodbeck J, Dai J, Walker D, & Huang Y (2012). Arf4 Determines Dentate Gyrus-Mediated Pattern Separation by Regulating Dendritic Spine Development. PLOS ONE, 7(9), e46340. 10.1371/journal.pone.0046340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeewajee A, Barry C, Douchamps V, Manson D, Lever C, & Burgess N (2014). Theta phase precession of grid and place cell firing in open environments. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1635), 20120532. 10.1098/rstb.2012.0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaifosh P, Lovett-Barron M, Turi GF, Reardon TR, & Losonczy A (2013). Septo-hippocampal GABAergic signaling across multiple modalities in awake mice. Nature Neuroscience, 16(9), 1182–1184. 10.1038/nn.3482 [DOI] [PubMed] [Google Scholar]
- Kametani H, & Kawamura H (1990). Alterations in acetylcholine release in the rat hippocampus during sleep-wakefulness detected by intracerebral dialysis. Life Sciences, 47(5), 421–426. 10.1016/0024-3205(90)90300-G [DOI] [PubMed] [Google Scholar]
- Kang L, & DeWeese MR (2019). Replay as wavefronts and theta sequences as bump oscillations in a grid cell attractor network. ELife, 8, e46351. 10.7554/eLife.46351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, & Rolls ET (2015). A computational theory of hippocampal function, and tests of the theory: New developments. Neuroscience & Biobehavioral Reviews, 48, 92–147. 10.1016/j.neubiorev.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Klukas M, Lewis M, & Fiete I (2019). Flexible representation of higher-dimensional cognitive variables with grid cells. BioRxiv, 578641. 10.1101/578641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, & Neunuebel JP (2016). Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics. Neurobiology of Learning and Memory, 129, 38–49. 10.1016/j.nlm.2015.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, & Zhang K (2012). Attractor Dynamics of Spatially Correlated Neural Activity in the Limbic System. Annual Review of Neuroscience, 35(1), 267–285. 10.1146/annurev-neuro-062111-150351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komendantov AO, Venkadesh S, Rees CL, Wheeler DW, Hamilton DJ, & Ascoli GA (2019). Quantitative firing pattern phenotyping of hippocampal neuron types. Scientific Reports, 9(1), 17915. 10.1038/s41598-019-52611-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Summerfield JJ, Hassabis D, & Maguire EA (2009). Tracking the emergence of conceptual knowledge during human decision making. Neuron, 63(6), 889–901. 10.1016/j.neuron.2009.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laptev D, & Burgess N (2019). Neural Dynamics Indicate Parallel Integration of Environmental and Self-Motion Information by Place and Grid Cells. Frontiers in Neural Circuits, 13. 10.3389/fncir.2019.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MC, Lykken C, Tye LD, Wickelgren JG, & Frank LM (2014). Hippocampal output area CA1 broadcasts a generalized novelty signal during an object-place recognition task. Hippocampus, 24(7), 773–783. 10.1002/hipo.22268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, & Amaral DG (2004). Perirhinal and parahippocampal cortices of the macaque monkey: Intrinsic projections and interconnections. Journal of Comparative Neurology, 472(3), 371–394. 10.1002/cne.20079 [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, Jeewajee A, O’Keefe J, & Burgess N (2009). Boundary Vector Cells in the Subiculum of the Hippocampal Formation. Journal of Neuroscience, 29(31), 9771–9777. 10.1523/JNEUROSCI.1319-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Diehl GW, Sparks FT, Leutgeb S, & Leutgeb JK (2015). Hippocampal CA2 activity patterns change over time to a larger extent than between spatial contexts. Neuron, 85(1), 190–201. 10.1016/j.neuron.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov NT, Vezoli J, Chameau P, Falchier A, Quilodran R, Huissoud C, Lamy C, Misery P, Giroud P, Ullman S, Barone P, Dehay C, Knoblauch K, & Kennedy H (2014). Anatomy of hierarchy: Feedforward and feedback pathways in macaque visual cortex. Journal of Comparative Neurology, 522(1), 225–259. 10.1002/cne.23458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D (1971). Simple memory: A theory for archicortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 262(841), 23–81. 10.1098/rstb.1971.0078 [DOI] [PubMed] [Google Scholar]
- Marrosu F, Portas C, Mascia MS, Casu MA, Fà M, Giagheddu M, Imperato A, & Gessa GL (1995). Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Research, 671(2), 329–332. 10.1016/0006-8993(94)01399-3 [DOI] [PubMed] [Google Scholar]
- Matheus Gauy M, Lengler J, Einarsson H, Meier F, Weissenberger F, Yanik MF, & Steger A (2018). A Hippocampal Model for Behavioral Time Acquisition and Fast Bidirectional Replay of Spatio-Temporal Memory Sequences. Frontiers in Neuroscience, 12. 10.3389/fnins.2018.00961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, & Morris RGM (1987). Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences, 10(10), 408–415. 10.1016/0166-2236(87)90011-7 [DOI] [Google Scholar]
- Milford Michael J., Wiles J, & Wyeth GF (2010). Solving Navigational Uncertainty Using Grid Cells on Robots. PLOS Computational Biology, 6(11), e1000995. 10.1371/journal.pcbi.1000995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milford MJ, Wyeth GF, & Prasser D (2004). RatSLAM: A hippocampal model for simultaneous localization and mapping. IEEE International Conference on Robotics and Automation, 2004. Proceedings. ICRA ‘04. 2004, 1, 403–408 Vol.1. 10.1109/ROBOT.2004.1307183 [DOI] [Google Scholar]
- Moradi K, & Ascoli GA (2020). A comprehensive knowledge base of synaptic electrophysiology in the rodent hippocampal formation. Hippocampus, 30(4), 314–331. 10.1002/hipo.23148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno H, Wu WE, Lee T, Brickman A, Mayeux R, Brown TR, & Small SA (2007). Imaging the Aβ-Related Neurotoxicity of Alzheimer Disease. Archives of Neurology, 64(10), 1467–1477. 10.1001/archneur.64.10.1467 [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, & Moser M-B (2008). Place Cells, Grid Cells, and the Brain’s Spatial Representation System. Annual Review of Neuroscience, 31(1), 69–89. 10.1146/annurev.neuro.31.061307.090723 [DOI] [PubMed] [Google Scholar]
- Mosheiff N, & Burak Y (2019). Velocity coupling of grid cell modules enables stable embedding of a low dimensional variable in a high dimensional neural attractor. ELife, 8, e48494. 10.7554/eLife.48494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, & Moscovitch M (2001). The hippocampal complex and long-term memory revisited. Trends in Cognitive Sciences, 5(6), 228–230. 10.1016/S1364-6613(00)01664-8 [DOI] [PubMed] [Google Scholar]
- Navas-Olive A, Valero M, Jurado-Parras T, de Salas-Quiroga A, Averkin RG, Gambino G, Cid E, & de la Prida LM (2020). Multimodal determinants of phase-locked dynamics across deep-superficial hippocampal sublayers during theta oscillations. Nature Communications, 11(1), 2217. 10.1038/s41467-020-15840-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netoff TI, Clewley R, Arno S, Keck T, & White JA (2004). Epilepsy in Small-World Networks. Journal of Neuroscience, 24(37), 8075–8083. 10.1523/JNEUROSCI.1509-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neymotin SA, Lazarewicz MT, Sherif M, Contreras D, Finkel LH, & Lytton WW (2011). Ketamine disrupts θ modulation of γ in a computer model of hippocampus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(32), 11733–11743. 10.1523/JNEUROSCI.0501-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D, & McNaughton B (2004). Differential Modulation of CA1 and Dentate Gyrus Interneurons During Exploration of Novel Environments. Journal of Neurophysiology, 91(2), 863–872. 10.1152/jn.00614.2003 [DOI] [PubMed] [Google Scholar]
- Noguès X, Corsini MM, Marighetto A, & Abrous DN (2012). Functions for adult neurogenesis in memory: An introduction to the neurocomputational approach and to its contribution. Behavioural Brain Research, 227(2), 418–425. 10.1016/j.bbr.2011.08.009 [DOI] [PubMed] [Google Scholar]
- Nolan M (2018). A Model for Grid Firing and Theta-Nested Gamma Oscillations in Layer 2 of the Medial Entorhinal Cortex. In Cutsuridis V, Graham BP, Cobb S, & Vida I(Eds.), Hippocampal Microcircuits: A Computational Modeler’s Resource Book (pp. 567–584). Springer International Publishing. 10.1007/978-3-319-99103-0_15 [DOI] [Google Scholar]
- O’Keefe J, & Dostrovsky J (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research, 34(1), 171–175. 10.1016/0006-8993(71)90358-1 [DOI] [PubMed] [Google Scholar]
- O’Keefe John, & Recce ML (1993). Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus, 3(3), 317–330. 10.1002/hipo.450030307 [DOI] [PubMed] [Google Scholar]
- O’Neill J, Pleydell-Bouverie B, Dupret D, & Csicsvari J (2010). Play it again: Reactivation of waking experience and memory. Trends in Neurosciences, 33(5), 220–229. 10.1016/j.tins.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Opitz B (2014). Memory function and the hippocampus. Frontiers of Neurology and Neuroscience, 34, 51–59. 10.1159/000356422 [DOI] [PubMed] [Google Scholar]
- Palm G (2013). Neural associative memories and sparse coding. Neural Networks, 37, 165–171. 10.1016/j.neunet.2012.08.013 [DOI] [PubMed] [Google Scholar]
- Parish G, Hanslmayr S, & Bowman H (2018). The Sync/deSync Model: How a Synchronized Hippocampus and a Desynchronized Neocortex Code Memories. Journal of Neuroscience, 38(14), 3428–3440. 10.1523/JNEUROSCI.2561-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoll H, Solanka L, van Rossum MCW, & Nolan MF (2013). Feedback Inhibition Enables Theta-Nested Gamma Oscillations and Grid Firing Fields. Neuron, 77(1), 141–154. 10.1016/j.neuron.2012.11.032 [DOI] [PubMed] [Google Scholar]
- Peyrache A, Lacroix MM, Petersen PC, & Buzsáki G (2015). Internally organized mechanisms of the head direction sense. Nature Neuroscience, 18(4), 569–575. 10.1038/nn.3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti VC, Ewell LA, & Leutgeb JK (2013). Neurogenesis in the dentate gyrus: Carrying the message or dictating the tone. Frontiers in Neuroscience, 7, 50. 10.3389/fnins.2013.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilly PK, Howard MD, & Bhattacharyya R (2018). Modeling Contextual Modulation of Memory Associations in the Hippocampus. Frontiers in Human Neuroscience, 12. 10.3389/fnhum.2018.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polsky A, Mel BW, & Schiller J (2004). Computational subunits in thin dendrites of pyramidal cells. Nature Neuroscience, 7(6), 621–627. 10.1038/nn1253 [DOI] [PubMed] [Google Scholar]
- Rebola N, Carta M, & Mulle C (2017). Operation and plasticity of hippocampal CA3 circuits: Implications for memory encoding. Nature Reviews Neuroscience, 18(4), 208–220. 10.1038/nrn.2017.10 [DOI] [PubMed] [Google Scholar]
- Rees CL, Wheeler DW, Hamilton DJ, White CM, Komendantov AO, & Ascoli GA (2016). Graph Theoretic and Motif Analyses of the Hippocampal Neuron Type Potential Connectome. ENeuro, 3(6). 10.1523/ENEURO.0205-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennó-Costa C, Lisman JE, & Verschure PFMJ (2014). A Signature of Attractor Dynamics in the CA3 Region of the Hippocampus. PLOS Computational Biology, 10(5), e1003641. 10.1371/journal.pcbi.1003641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (1989a). Functions of Neuronal Networks in the Hippocampus and Cerebral Cortex in Memory. In Cotterill RMJ (Ed.), Models of Brain Function (pp. 15–33). Cambridge University Press. [Google Scholar]
- Rolls ET (1989b). Functions of Neuronal Networks in the Hippocampus and Neocortex in Memory. In Byrne JH & Berry WO (Eds.), Neural Models of Plasticity (pp. 240–265). Academic Press. 10.1016/B978-0-12-148955-7.50017-5 [DOI] [Google Scholar]
- Rolls ET (1989c). The representation and storage of information in neural networks in the primate cerebral cortex and hippocampus. In The computing neuron (pp. 125–159). [Google Scholar]
- Rolls ET (1990a). Functions of the primate hippocampus in spatial processing and memory. In Neurobiology of comparative cognition (pp. 339–362). Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Rolls ET (1990b). Theoretical and Neurophysiological Analysis of the Functions of the Primate Hippocampus in Memory. Cold Spring Harbor Symposia on Quantitative Biology, 55, 995–1006. 10.1101/SQB.1990.055.01.095 [DOI] [PubMed] [Google Scholar]
- Rolls ET (2008). Memory, Attention, and Decision-Making: A Unifying Computational Neuroscience Approach. Oxford University Press. [Google Scholar]
- Rolls ET, & Mills P (2019). The Generation of Time in the Hippocampal Memory System. Cell Reports, 28(7), 1649–1658.e6. 10.1016/j.celrep.2019.07.042 [DOI] [PubMed] [Google Scholar]
- Rowland DC, Obenhaus HA, Skytøen ER, Zhang Q, Kentros CG, Moser EI, & Moser M-B (2018). Functional properties of stellate cells in medial entorhinal cortex layer II. ELife, 7, e36664. 10.7554/eLife.36664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonovich AV, & Ascoli GA (2005). A simple neural network model of the hippocampus suggesting its pathfinding role in episodic memory retrieval. Learning & Memory, 12(2), 193–208. 10.1101/lm.85205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonovich AV (2010). Toward a Unified Catalog of Implemented Cognitive Architectures. Frontiers in Artificial Intelligence and Applications. Volume 221: Biologically Inspired Cognitive Architectures, 195–244. 10.3233/978-1-60750-661-4-195 [DOI] [Google Scholar]
- Sanchez-Aguilera A, Wheeler DW, Jurado-Parras T, Valero M, Nokia MS, Cid E, Fernandez-Lamo I, Sutton N, García-Rincón D, de la Prida LM, & Ascoli GA (2021). An update to Hippocampome.org by integrating single-cell phenotypes with circuit function in vivo. PLoS Biology, 19(5), e3001213. 10.1371/journal.pbio.3001213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser M-B, & Moser EI (2006). Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science (New York, N.Y.), 312(5774), 758–762. 10.1126/science.1125572 [DOI] [PubMed] [Google Scholar]
- Savelli F, Yoganarasimha D, & Knierim JJ (2008). Influence of boundary removal on the spatial representations of the medial entorhinal cortex. Hippocampus, 18(12), 1270–1282. 10.1002/hipo.20511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers E (2010). A General Introduction to the E-utilities. In Entrez Programming Utilities Help [Internet]. National Center for Biotechnology Information (US). https://www.ncbi.nlm.nih.gov/books/NBK25497/ [Google Scholar]
- Schapiro AC, McDevitt EA, Rogers TT, Mednick SC, & Norman KA (2018). Human hippocampal replay during rest prioritizes weakly learned information and predicts memory performance. Nature Communications, 9(1), 3920. 10.1038/s41467-018-06213-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CJ, Bezaire M, & Soltesz I (2012). Toward a full-scale computational model of the rat dentate gyrus. Frontiers in Neural Circuits, 6. 10.3389/fncir.2012.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipston-Sharman O, Solanka L, & Nolan MF (2016). Continuous attractor network models of grid cell firing based on excitatory-inhibitory interactions. The Journal of Physiology, 594(22), 6547–6557. 10.1113/JP270630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle JH, & Wilson MA (2014). Enhancement of encoding and retrieval functions through theta phase-specific manipulation of hippocampus. ELife, 3, e03061. 10.7554/eLife.03061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva SAC, Panos Ipeirotis, Victor. (n.d.). scholarly: Simple access to Google Scholar authors and citations (1.2.0) [Python; OS Independent]. RetrievedApril 16, 2021, from https://github.com/scholarly-python-package/scholarly
- Solanka L, van Rossum MC, & Nolan MF (2015). Noise promotes independent control of gamma oscillations and grid firing within recurrent attractor networks. ELife, 4, e06444. 10.7554/eLife.06444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser M-B, & Moser EI (2008). Representation of Geometric Borders in the Entorhinal Cortex. Science, 322(5909), 1865–1868. 10.1126/science.1166466 [DOI] [PubMed] [Google Scholar]
- Stanley DA, Talathi SS, Parekh MB, Cordiner DJ, Zhou J, Mareci TH, Ditto WL, & Carney PR (2013). Phase shift in the 24-hour rhythm of hippocampal EEG spiking activity in a rat model of temporal lobe epilepsy. Journal of Neurophysiology, 110(5), 1070–1086. 10.1152/jn.00911.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, & Michmizos KP (2018). Gridbot: An autonomous robot controlled by a Spiking Neural Network mimicking the brain’s navigational system. Proceedings of the International Conference on Neuromorphic Systems, 1–8. 10.1145/3229884.3229888 [DOI] [Google Scholar]
- Tecuatl C, Wheeler DW, Sutton N, & Ascoli GA (2021). Comprehensive Estimates of Potential Synaptic Connections in Local Circuits of the Rodent Hippocampal Formation by Axonal-Dendritic Overlap. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 41(8), 1665–1683. 10.1523/JNEUROSCI.1193-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, & DiScenna P (1986). The hippocampal memory indexing theory. Behavioral Neuroscience; US: American Psychological Association. 10.1037/0735-7044.100.2.147 [DOI] [PubMed] [Google Scholar]
- Teyler TJ, & DiScenna P (1985). The role of hippocampus in memory: A hypothesis. Neuroscience & Biobehavioral Reviews, 9(3), 377–389. 10.1016/0149-7634(85)90016-8 [DOI] [PubMed] [Google Scholar]
- Treves A, & Rolls ET (1994). Computational analysis of the role of the hippocampus in memory. Hippocampus, 4(3), 374–391. 10.1002/hipo.450040319 [DOI] [PubMed] [Google Scholar]
- Treves A, & Rolls ET (1992). Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus, 2(2), 189–199. 10.1002/hipo.450020209 [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Uziel A, & Markram H (2000). T Synchrony Generation in Recurrent Networks with Frequency-Dependent Synapses. Journal of Neuroscience, 20(1), RC50–RC50. 10.1523/JNEUROSCI.20-01-j0003.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH (1969). Hippocampal electrical activity and voluntary movement in the rat. Electroencephalography and Clinical Neurophysiology, 26(4), 407–418. 10.1016/0013-4694(69)90092-3 [DOI] [PubMed] [Google Scholar]
- Venkadesh S, Komendantov AO, Wheeler DW, Hamilton DJ, & Ascoli GA (2019). Simple models of quantitative firing phenotypes in hippocampal neurons: Comprehensive coverage of intrinsic diversity. PLOS Computational Biology, 15(10), e1007462. 10.1371/journal.pcbi.1007462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Sprekeler H, Zenke F, Clopath C, & Gerstner W (2011). Inhibitory Plasticity Balances Excitation and Inhibition in Sensory Pathways and Memory Networks. Science, 334(6062), 1569–1573. 10.1126/science.1211095 [DOI] [PubMed] [Google Scholar]
- Wheeler DW, White CM, Rees CL, Komendantov AO, Hamilton DJ, & Ascoli GA (2015). Hippocampome.org: A knowledge base of neuron types in the rodent hippocampus. ELife, 4, e09960. 10.7554/eLife.09960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CM, Rees CL, Wheeler DW, Hamilton DJ, & Ascoli GA (2020). Molecular expression profiles of morphologically defined hippocampal neuron types: Empirical evidence and relational inferences. Hippocampus, 30(5), 472–487. 10.1002/hipo.23165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, & Jack CR (2007). 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain, 130(7), 1777–1786. 10.1093/brain/awm112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, & McNaughton BL (1994). Reactivation of hippocampal ensemble memories during sleep. Science, 265(5172), 676–679. 10.1126/science.8036517 [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, Haeften T. van, Machielsen WCM, Rombouts SARB, Barkhof F, Scheltens P, & Silva F. H. L. da. (2000). Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus, 10(4), 398–410. [DOI] [PubMed] [Google Scholar]
- Wu X, & Foster DJ (2014). Hippocampal Replay Captures the Unique Topological Structure of a Novel Environment. Journal of Neuroscience, 34(19), 6459–6469. 10.1523/JNEUROSCI.3414-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng T, Li X, & Si B (2019). Bayesian Integration of Multi-resolutional Grid Codes for Spatial Cognition. ArXiv:1910.05881 [q-Bio]. http://arxiv.org/abs/1910.05881 [Google Scholar]
- Zenke F, Agnes EJ, & Gerstner W (2015). Diverse synaptic plasticity mechanisms orchestrated to form and retrieve memories in spiking neural networks. Nature Communications, 6(1), 6922. 10.1038/ncomms7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zeng Y, Zhao D, Wang L, Zhao Y, & Xu B. (2016). HMSNN: Hippocampus inspired Memory Spiking Neural Network. 2016 IEEE International Conference on Systems, Man, and Cybernetics (SMC), 002301–002306. 10.1109/SMC.2016.7844581 [DOI] [Google Scholar]


