Trauma-focused therapies (eg, trauma-focused cognitive-behavioral therapy, narrative exposure, and mindfulness) may be optimal for youth experiencing chronic pain to address the potential for an ongoing stress response.
Keywords: Pediatric pain, Stress response, Psychosocial intervention, Treatment outcomes
Abstract
Nonpharmacological treatments for chronic pain in youth have been identified as first-line treatments over and above medication. Therapies such as cognitive-behavioral therapy and mindfulness-based stress reduction have shown good efficacy in reducing the psychological correlates (eg, anxiety, depression, and stress) and social or behavioral sequelae (eg, limited physical activity and lack of school engagement) associated with pediatric chronic pain. However, minimal research has examined the physiological mechanism(s) of action for these interventions. A recent review (Cunningham, et al., 2019) emphasized the need for objective (ie, physiological) assessment of treatment response in pediatric pain populations. The current review adds to this literature by identifying the physiological stress response as a particular target of interest in interventions for pediatric pain. Research indicates that youth with chronic pain report high rates of psychological stress, posttraumatic stress symptoms, and exposure to adverse childhood experiences (abuse/neglect, etc). In addition, a host of research has shown strong parallels between the neurobiology of pain processing and the neurobiology of stress exposure in both youth and adults. Interventions such as narrative or exposure therapy (eg, trauma-focused cognitive-behavioral therapy) and mindfulness-based or meditation-based therapies have shown particular promise in alleviating the neurobiological impact that stress and pain can have on the body, including reduction in allostatic load and altered connectivity in multiple brain regions. However, no study to date has specifically looked at these factors in the context of pediatric pain treatment. Future research should further explore these constructs to optimize prevention in and treatment of these vulnerable populations.
1. Introduction
The central and peripheral nervous systems have long been identified as primary intervention targets to address pain chronicity in youth.53,112 Many providers immediately turn to pharmacological interventions such as neuropathic pain medication or atypical antidepressants (eg, tricyclics) to modify or tamp down nervous system sensitization associated with pain chronicity.29 However, the efficacy in youth is not well documented in receiver operating characteristic analysis trials.40 Moreover, the engagement of other treatment options has often been shown to be useful. Combining physical therapy and psychosocial intervention (eg, cognitive-behavioral therapy [CBT]) independently or concomitantly with drug therapy has been found to optimize or outperform the pharmacological impact on these outcomes by addressing the psychological and social aspects of pain-related disability, such as decreased pain tolerability, decreased social interaction, and increased functional impairment and fear of pain.22 Emerging research has started to indicate that these nonpharmacological interventions, much like the above noted classes of medication, may directly and indirectly modify nervous system sensitivity.17 However, the literature on the objective (ie, neurobiological) responses to these interventions for youth with chronic pain remains limited. While psychosocial interventions have shown variable engagement due to their required duration of implementation,26,110 pharmacological therapies may also take time and produce side effects. The natural evolution of brain changes through brain–brain interactions induced by these therapies may produce adaptive therapeutic changes. Such approaches need rigorous comparative evaluation akin to the standards used to evaluate new medications,23 alone or in combination.55
Measures of the neurobiological stress response (eg, hypothalamic–pituitary–adrenal [HPA] axis and autonomic nervous system) have been proposed to be unique predictors of pain chronicity in youth76 given the frequent occurrence of psychological stress (herein referred to as “stress”), posttraumatic stress symptoms (PTSS),77,85 and adverse childhood experiences (ACEs; abuse, neglect, violent/conflictual home environment, parent/guardian separation or divorce, etc.28) documented in pediatric pain populations.75,77,79,80 Outside of pain, a large body of research highlights the malleability of the stress response and the ensuing “wear and tear” that stress can impose on the nervous system, most notably in youth (vs. adults).19,44,59,66,67,69 Thus, modification of the stress response may decrease pain chronicity and impairment in vulnerable and affected youth; this hypothesis remains untested. Several interventions exist that address the unique biopsychosocial (BPS) correlates of stress in youth. Some of these interventions have not been performed in pediatric pain populations (eg, trauma-focused cognitive-behavioral therapy [TF-CBT]) but many have, including mindfulness-based stress reduction (MBSR) and biofeedback.8,11,62,64 However, only a few of these studies have specifically examined the efficacy of these interventions in modifying the physiological stress response. Furthermore, evidence suggests that many youth with chronic pain do not respond to treatment (eg, decrease in disability or increase in adaptive coping) after engaging in traditionally used psychosocial therapies (eg, CBT).16,103 One reason for such treatment failure may be that currently accepted interventions for chronic pain do not adequately target the stress response as a major factor in pain maintenance. However, this hypothesis remains untested. Accordingly, the aims of the current review are to (1) propose the stress response as a unique target for psychosocial intervention to alleviate chronic pain and (2) outline new avenues for treatment that may enhance outcomes (eg, disability or pain intensity) through more directly and comprehensively targeting the stress response. Across both aims, we identify salient gaps in the literature and propose avenues for future research.
2. Neurobiological underpinnings of pain and potential targets of intervention
Chronic pain in youth is diagnosed when pain lasts for 3 months or longer in the absence of a primary medical issue.24 The underlying neurobiology of chronic pain is frequently conceptualized as the product of central sensitization, which is the increase in activity and responsiveness in nociceptive pathways in the central and peripheral nervous systems after a painful event.43,53 However, up to 50% of youth with chronic pain fail to report a recognizable inciting incident for their chronic pain50 for a number of reasons, including when minor injuries and unknown or undiagnosed disease processes (eg, rare disease) are involved. The diagnostic conceptualization of pediatric chronic pain is often formulated using the BPS model of pain.4,35 This model asserts that pain chronicity occurs because of the interaction between central sensitization or centralization of pain and HPA axis hyperactivity, in conjunction with psychological (eg, anxiety, depression, pain catastrophizing, and fear of pain) and social (eg, decreased social engagement and school attendance) factors contributing to functional disability and, in turn, pain chronicity.34,35 The pain feedback loop is exacerbated by these sociobiological loads, producing a failure of homeostasis and increased allostatic load (AL), ie, wear and tear on regulatory systems, including the nervous system, in response to repeated or prolonged stress.19,44
Aspects of neurological functioning relevant to both the physiological stress response and pain processing in youth76 are highlighted in Figure 1. The HPA axis (depicted in red) is one of the primary moderators of the neuroendocrine and neurobiological stress response, comprising the hypothalamus, pituitary gland, and adrenal cortex and releasing adrenal hormones (eg, epinephrine/norepinephrine) and corticosteroids (eg, cortisol) in response to stress.32,76 Major brain areas implicated in the stress response and pain processing (depicted in green) include the prefrontal cortex, hippocampus, amygdala, and locus coeruleus (LC).20,76 Finally, constructs representing the broader impact of stress on neurobiology (depicted in blue) include, as mentioned above, increased AL.71,78
Figure 1.
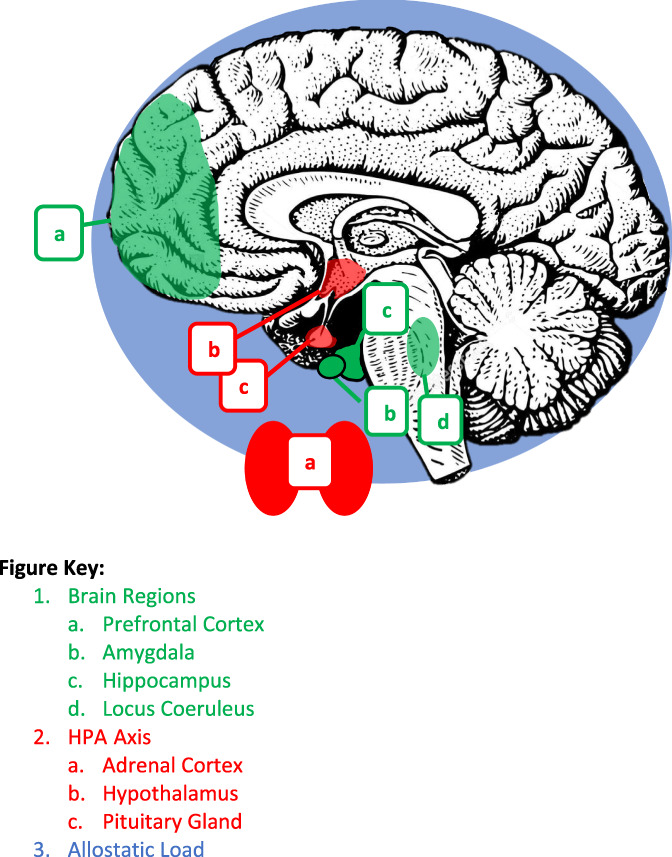
Neurobiological areas that are affected by stress, pain, and psychosocial intervention.
3. Stress—the great arbiter of homeostasis or allostatic load
Stress has been well established as an inciting factor of homeostatic disruption and neurobiological dysfunction in youth and adults.5,13,66 Primary neurobiological processes involved in the stress response also have been concretely identified and involve several aspects of the neurological system, including the HPA axis,32 brain structures that help regulate the HPA axis and manage emotional aspects and interpretation of stressful events,9,20 and, more globally, AL.19,44 For a comprehensive review of the neurobiological impact of stress, refer to the study by Nelson, et al., 2020.76 In short, stress exposure effects include changes in brain structures in conjunction with dysregulation of the HPA axis. The HPA axis is a primary stress response system involving the hypothalamus, pituitary gland, and adrenal glands20 and is a system commonly measured as part of the larger construct of AL (see below). Key biomarkers reflecting HPA axis functioning are levels of glucocorticoids such as cortisol and dehydroepiandrosterone (DHEA).9 Regulation of the HPA axis is, in part, controlled by the hippocampus.32 Results from several studies indicate that prolonged glucocorticoid release in response to stress exposure can lead to decreased hippocampal volume.9,32,67 Other areas affected by prolonged or significant stress and pain include (but are not limited to) the amygdala, prefrontal cortex (PFC), and LC. The amygdala is part of the fear network and shares connectivity across brain regions.72 Research indicates that in response to stress, dendrites in the amygdala expand, which causes increased activity in the area.70,72 In the PFC, endocannabinoids are partially responsible for dampening or blocking the stress response in the HPA axis.39 Remodeling can occur in the PFC in response to stress but can be alleviated after the source of stress is removed.69,70,72 Finally, chronic sources of stress have been shown to induce changes in the physiology and function of the LC, such as dendritic growth by exposure to corticotropin-releasing factor after HPA axis activation.6
Across these processes, the experience of varying degrees and types of stress (eg, psychological stress, abuse, or toxic stress) has been associated with a host of health-related issues in adulthood, including obesity, cancer, diabetes, and chronic pain.2,9,12,29,59,65 However, research on the impact of stress on health-related functioning and disease susceptibility in youth is limited, although ongoing. One of the more prominent health conditions being proposed as related to or exacerbated by stress in pediatric populations is chronic pain.1,56,76,78 The first conceptual framework published on the potential relation between stressful experiences and chronic pain in youth proposed AL as a unique contributor to pain chronicity.78 A more recent review76 expanded on this framework by identifying other shared neurobiological mechanisms across stress and pain, including biomarkers of AL such as maladaptive levels of glucocorticoids (eg, flattened cortisol and increased DHEA—representative of HPA axis functioning19,111), catecholamines (eg, increased epinephrine [E] or norepinephrine [NE]—representative of sympathetic nervous system functioning106,111), and inflammatory cytokines (eg, increased C-reactive protein92,111), and altered connectivity in brain structures implicated in the stress response (as described above).76 The experience of chronic pain may, in fact, fall under the category of “toxic stress” or significant or prolonged exposure to stress, with accompanying activation of the neurobiological stress response,102 given the lengthy average duration of experienced pain and functional disability in these youth.1,45,75 That is, chronic pain may not only be initiated or exacerbated by stress exposures but also be itself a form of toxic stress with consequent neurobiological effects. Exposure in childhood to toxic stress, such as trauma or ACEs, has been linked to lifelong consequences for physical and mental health through the neurobiological correlates described above (eg, AL and HPA axis dysfunction)101,102 and is an important consideration in the context of long-term outcomes in youth with chronic pain.
4. Psychosocial intervention and chronic pain—what is the current evidence?
The Centers for Disease Control and Prevention released a report in 2016 that outlined best practices for chronic pain treatment.22 In this report, nonpharmacological treatments, most notably psychosocial interventions such as CBT, were identified as first-line treatments for chronic pain in youth.22 Cognitive-behavioral therapy has been found to be beneficial for chronic pain through targeting decreased levels of activity in relation to emotional distress and functional disability (eg, behavioral activation) and addressing altered cognitions surrounding the pain experience (eg, reframing catastrophizing pain thoughts).26 More recently, mindfulness-based interventions such as MBSR and acceptance and commitment therapy have shown promise in addressing cognitive and behavioral aspects of the pain experience11,52,64,93 by focusing on observing one's present state in a nonjudgmental manner.37 Although there is societal demand for nonpharmacological treatments, their fidelity and quality still need to be defined.
Multiple articles have reviewed the efficacy of psychotherapy in the treatment of pediatric pain (eg, Palermo, et al., 2010; Fisher, et al., 2014; and Eccleston, et al., 2014), with heaviest focus on randomized clinical trials using CBT interventions, biofeedback, and relaxation training.30,31,89 Results of these systematic reviews and meta-analyses generally reveal a significant positive effect of interventions on pain intensity and functional disability. Individual studies on the efficacy of psychosocial interventions also have shown a strong short-term positive impact on psychological (eg, anxiety, depression, fear of pain, and pain catastrophizing) and social (eg, peer engagement and school attendance) factors commonly observed in pediatric pain patients.16,25,48,57,60,103 Efforts to examine the impact of combining physical training with psychosocial intervention have also begun, with promising results.47,49 However, research indicates that a significant subset of youth fails to respond to traditional psychosocial interventions geared towards alleviating chronic pain.15,103,105 Initial factors identified as barriers to successful treatment include patient anxiety and other psychological symptoms and parent factors, such as pain history and pain catastrophizing.14,15 However, few studies have objectively examined changes (or lack thereof) that may occur in aspects of neurobiological pain processing (eg, HPA axis dysregulation: cortisol/DHEA and autonomic nervous system function: E/NE) in response to psychosocial intervention. Consequently, data are limited as to whether traditional targets of intervention, including psychological impairment (eg, anxiety and depression), disability, and pain intensity, contribute to or are representative of physiological or neurobiological changes in the individual. A recent review emphasized the need for the objective assessment of pain treatment outcomes17 and identified several brain mechanisms to consider, including those involved in the attention network, cognitive control (eg, prefrontal cortex), nociceptive processing (eg, thalamus and insula), and the fear network and emotional processing (eg, amygdala and hippocampus).17 Research assessing the responsiveness of these areas, including areas intrinsic to the physiological stress response, to available psychosocial interventions for pain remains limited.
5. Psychosocial and narrative-based interventions focused on stress or trauma exposure
Several evidence-based interventions exist for youth with a history of trauma or posttraumatic stress disorder (PTSD) that may be relevant to consider in the context of treating youth with chronic pain. One of the most robust interventions for children and adolescents is TF-CBT.61,74 Trauma-focused cognitive-behavioral therapy operates under the framework “PRACTICE,” which represents several common tenets across typical CBT therapies, such as psychoeducation and parenting skills (P), relaxation training (R), affective modulation (A), and cognitive coping (C). Trauma-focused cognitive-behavioral therapy also includes elements of intervention specifically catered to trauma, including gradual emotional and cognitive exposure to the trauma (eg, asking the child to name the trauma directly: “when I was abused” vs “when that thing happened to me”), trauma narrative construction and processing (T), and in vivo mastery of the trauma (I). The goal of engaging trauma-exposed children and adolescents in therapy with emotional exposure and narrative work is to desensitize them to the fear or distress that they associate with memories or situations connected to the trauma (eg, seeing people or going places that remind them of the traumatic event).61,74 Talking through specific details of the event in the form of a narrative can also help correct negative or inaccurate thoughts or beliefs about the traumatic experience (eg, “I could have done something to stop it”) that may be perpetuating the fear or distress.21,74 Evidence indicates that pediatric interventions that include a trauma narrative lead to greater reductions in trauma-related fear and general anxiety as well as parent-related distress relative to treatments that do not include a trauma narrative component.21 Recent studies among traumatized youth also indicate that narrative therapy, whether as part of TF-CBT or independently, produces more robust and stable treatment outcomes, including reductions in PTSD symptoms and improvements in sleep, when compared with treatment as usual.91
5.1. Mindfulness
One of the other major categories of intervention geared towards addressing the impact of stress and trauma is mindfulness-based interventions. As mentioned above, the basic tenet of mindfulness is teaching individuals to observe their experience in a nonjudgmental manner37 and has been used successfully in the context of chronic pain. In the context of trauma, similar to TF-CBT and narrative or exposure work, mindfulness practice can lead individuals to view their traumas or stress with kindness and nonjudgment rather than fear or avoidance.7 This is exemplified in one of the tenets of MBSR that teaches “stress + resistance = suffering.”36 Most often studied in traumatized or highly stressed adults, an abundance of evidence suggests that mindfulness can lead to sustained improvement in mood and PTSS and increases in resilience.33,51 Preliminary work in traumatized or at-risk pediatric populations also indicates that MBSR leads to greater feelings of competence with stress management and enhanced self-awareness.42
6. Neurobiological perspectives on stress-focused or trauma-focused interventions
Given that the neurobiological areas of interest described above (eg, HPA axis dysregulation: cortisol/DHEA, sympathetic nervous system dysregulation: E/NE, and altered brain connectivity) are proposed mechanisms of pain,76,78,107 they may also be considered as intervention outcomes to be assessed through objective measurement (eg, saliva assay, urine analysis, and functional magnetic resonance imaging) over the course of treatment. The functioning of the HPA axis is already studied in the context of psychosocial intervention. Generally, HPA axis dysfunction can manifest as dysregulated glucocorticoid release, sleep disruption, blood pressure issues, and immunosuppression.19,32 Admittedly, evidence has been inconsistent on the ability of psychosocial interventions to target HPA axis dysregulation through normalized cortisol or DHEA levels. In the context of PTSD, a recent review indicated that certain trauma-focused interventions, including TF-CBT, prolonged exposure (PE), and eye-movement desensitization reprocessing, performed on adults with PTSD, are effective in mitigating dysregulation in basal cortisol levels and the cortisol awakening response in certain subsamples; however, more broad results from these studies remain mixed.86,99,100 In youth particularly, evidence suggests that certain aspects of HPA axis functioning (eg, basal cortisol levels) may be indicative of who may be more or less responsive to TF-CBT113 and MBSR63 and, moreover, may be a more reliable measure of treatment response than subjective measures such as psychological questionnaires.63 This latter finding supports the rationality of efforts to objectively measure treatment response through biological measures such as saliva assay rather than rely on traditional pain measures such as (self-reported) disability and pain intensity.
As discussed above, changes in brain structures, such as the hippocampus, amygdala, PFC, and LC, have been shown to take place in conjunction with exposure to stress and the ensuing dysregulation of the HPA axis.6,39,69,70,72 However, few studies have directly measured functional connectivity in these brain regions preintervention and postintervention. Findings from the few relevant studies, mostly in adults, suggest that certain exposure-based trauma interventions (eg, PE) enhance connectivity in the amygdala and hippocampus.114 These enhanced connections may improve an individual's inhibition, memory encoding and retrieval, and ability to re-evaluate threats.114 Some adult studies have also observed volumetric changes in the amygdala54 and decreased activation of the PFC3 after certain psychotherapies (eg, eye-movement desensitization reprocessing and exposure-based interventions). Evidence in the context of stress-related disorders generally indicates that trauma-focused interventions are more effective at producing brain changes than basic cognitive-behavioral or supportive psychotherapy without an exposure component.3
More global outcomes of stress on the body that may be influenced by interventions include AL. As described above, AL is a construct that represents the multisystem wear and tear and long-term vulnerability to illness or disease that repeated or prolonged stress can impose.66 Once present, AL is maintained through continued exposure to stress and consequent health-related behaviors, such as disrupted sleep, poor nutrition, and sedentary behaviors or reduced physical exercise.66,68,70 Several studies, predominantly in adults, highlight the responsivity of AL, measured through a multifactorial composite of individual mechanisms, to psychosocial intervention. For example, preliminary evidence suggests that MBSR or CBT can help mitigate AL after significant or prolonged stress exposure.87,97 Complementary interventions such as yoga or tai chi also have shown preliminary effectiveness in their ability to mitigate AL.18,97 However, our current understanding on how psychosocial interventions and AL may interact is lacking, and more research in both pediatric and adult populations is needed.
7. Implications for future pediatric pain interventions
A stress-focused or trauma-focused approach to intervention may be the logical next step in optimizing psychosocial intervention for these youth. This argument is further strengthened by preliminary evidence suggesting that youth with chronic pain and PTSD experience decreased response to non–trauma-focused CBT intervention.77 Applying a trauma-focused lens to chronic pain intervention may include adapted elements of trauma therapies (eg, TF-CBT and narrative exposure) or streamlining care to mindfulness-based strategies. A strong body of research indicates that the nature of pain memories is highly predictive of long-term pain outcomes in youth.81–84 Accordingly, youth with chronic pain may benefit from the general framework of TF-CBT adapted to apply to the context of pain. This could include psychoeducation about BPS aspects of stress or trauma and chronic pain (P), relaxation training (R) and affective modulation (A) put into the context of pain and pain-related distress, and cognitive coping (C) applied to pain catastrophizing and fear of pain. Trauma-focused aspects of the intervention, including trauma narrative construction and processing (T) and in vivo mastery of the trauma (I), may involve constructing and processing a narrative surrounding a particularly stressful or intense pain memory. Working with youth, with or without a history of other stressful experiences, to deconstruct or build a narrative surrounding their memory of pain (eg, examine cognitive biases and emotions surrounding the event) as one would a traumatic memory may contribute to desensitizing the strong emotional or fear-based response to pain that many youth experience.104 In this context, regulation of the HPA axis through trauma-focused or narrative work may optimize overall health and sleep patterns, which could then mitigate AL,97 alter connectivity in various brain areas affected by stress,76 and decrease the associated pain response.38,90 Longitudinally, these interventions may also protect the individual from known effects of chronic toxic stress, including AL and increased risk for poor health and early death.96,101,102 More research in pediatric pain populations is needed that implements objective measures (eg, functional magnetic resonance imaging and saliva assay), in conjunction with traditionally measured pain outcomes (eg, disability and pain intensity), to assess the neurobiological effects of interventions on the stress response and on pain.
Although mindfulness-based approaches have been studied in adult chronic pain populations and show promise in the treatment of chronic pain,62,64,94,98,108 these types of interventions have been examined only recently in pediatric pain populations.11,52,58,109 Minimal research has specifically examined the ability of mindfulness practice to directly affect the neurobiological processes (eg, altered functional connectivity and AL) common to the experiences of both stress and pain in youth. Based on the strong evidence in adults that mindfulness-based practice can have a host of protective effects on psychosocial and neurobiological functioning,7,27,33,36 this intervention may be an important choice to optimize treatment in youth with chronic pain who report stress or trauma exposure. In fact, in light of the proposed categorization of chronic pain as an instigator or form of toxic stress, mindfulness-based approaches may be a more effective intervention choice than basic CBT approaches for chronic pain rehabilitation in youth with or without a trauma history. However, to date, no study has directly examined this nor the potential implications of combining aspects of mindfulness within the CBT framework for pediatric pain management.10 In these contexts, more research is needed, particularly treatment studies that incorporate neurobiological mechanism and outcome measurements, to determine the most effective interventions for these vulnerable youth.
8. Conclusions
A host of research has identified strong overlap between neurobiological correlates of stress and of pain.76,78 In addition, evidence suggests that youth with chronic pain report high rates of ACEs,41,79,80 PTSS,77,85 and general psychological stress56,75 when compared with the general population or to matched nonpain peers. However, research remains limited on how a history or ongoing experiences of stress or trauma may influence the manifestation of chronic pain (eg, neurobiology) in youth or the course of and response to treatment. In parallel, evidence indicates that a subset of youth with chronic pain fail to respond to traditional psychotherapy for pain management (eg, CBT).46,103 Given that stress-exposed youth are at increased risk for altered brain connectivity, HPA axis activation, and AL, trauma-focused therapies, such as TF-CBT, narrative exposure, or mindfulness-based interventions, may be more appropriate interventions for youth experiencing chronic pain than current therapies. Specifically, these intervention approaches, above and beyond traditional modalities, may better interrupt any ongoing stress response and directly target and desensitize the emotional response, memories, and neurobiological mechanisms surrounding the experience of stress and maintenance of pain.
9. Future directions
Research is only just beginning to elucidate links between stress and pain in youth. Currently, we do not have evidence demonstrating the extent to which the overlap in neurobiological mechanisms of stress and pain contributes to one another, particularly in youth. Studies are needed to confirm the existence of these associations. However, given the preliminary evidence that stress-based neurological processes may be implicated in pain chronicity,76,78 modifying existing psychosocial treatments to acknowledge and actively address stress histories and their sequelae is critical. For example, randomized clinical trials performed with CBT vs TF-CBT or MBSR in these youth are an important next step. Preassessments and postassessments with brain imaging and neuroendocrine biomarkers (eg, salivary cortisol) should be conducted to confirm the impact of these interventions at the biological level. Evidence is also currently unclear on which stress-based neurobiological mechanism(s) (eg, AL or altered brain connectivity) may be the optimal intervention target(s), especially given their strong associations and bidirectional effects on each other. Future research should pursue this line of inquiry because such information will inform the development of therapeutic approaches that maximize effectiveness and enhance decision-making around best methods for assessing treatment outcomes. Alternative and complementary measures of physiological effects of stress, such as telomere attrition and DNA methylation,73,88,95 also should be explored in this context. The results from these studies may inform the development of new research and treatment avenues with the goal of optimizing care for these vulnerable youth.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgments
National Center for Complementary and Integrative Health—NCCIH 1K23AT010643-01A1 (S.N.).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
David Borsook, Email: dbblue700@gmail.com.
Michelle Bosquet Enlow, Email: michelle.bosquet@childrens.harvard.edu.
References
- [1].Abdallah CG, Geha P. Chronic pain and chronic stress: two sides of the same coin? Chronic Stress 2017;1:2470547017704763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anda R, Tietjen G, Schulman E, Felitti V, Croft J. Adverse childhood experiences and frequent headaches in adults. Headache 2010;50:1473–81. [DOI] [PubMed] [Google Scholar]
- [3].Barsaglini A, Sartori G, Benetti S, Pettersson-Yeo W, Mechelli A. The effects of psychotherapy on brain function: a systematic and critical review. Prog Neurobiol 2014;114:1–14. [DOI] [PubMed] [Google Scholar]
- [4].Basch MC, Chow ET, Logan DE, Schechter NL, Simons LE. Perspectives on the clinical significance of functional pain syndromes in children. J Pain Res 2015;8:675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bijlsma R, Loeschcke V. Environmental stress, adaptation and evolution: an overview. J Evol Biol 2005;18:744–9. [DOI] [PubMed] [Google Scholar]
- [6].Borodovitsyna O, Joshi N, Chandler D. Persistent stress-induced neuroplastic changes in the locus coeruleus/norepinephrine system. Neural plasticity 2018;2018:1892570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Boyd JE, Lanius RA, McKinnon MC. Mindfulness-based treatments for posttraumatic stress disorder: a review of the treatment literature and neurobiological evidence. J Psychiatry Neurosci 2018;43:7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Breuner C, Bouchard M, Ahrens K, Bompadre V, Abelon R. Biofeedback for musculoskeletal pain—does it work and what factors are associated with successful outcomes? J Adolesc Health 2018;62:S96. [Google Scholar]
- [9].Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics 2007;119:509–16. [DOI] [PubMed] [Google Scholar]
- [10].Cayoun BA. Mindfulness-integrated CBT: Principles and practice. Hoboken, NJ: John Wiley & Sons, 2011. [Google Scholar]
- [11].Chadi N, McMahon A, Vadnais M, Malboeuf-Hurtubise C, Djemli A, Dobkin PL, Lacroix J, Luu TM, Haley N. Mindfulness-based intervention for female adolescents with chronic pain: a pilot randomized trial. J Can Acad Child Adolesc Psychiatry 2016;25:159. [PMC free article] [PubMed] [Google Scholar]
- [12].Charbonneau AM, Mezulis AH, Hyde JS. Stress and emotional reactivity as explanations for gender differences in adolescents' depressive symptoms. J Youth Adolescence 2009;38:1050–8. [DOI] [PubMed] [Google Scholar]
- [13].Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Horm Res Paediatrics 2003;59:161–79. [DOI] [PubMed] [Google Scholar]
- [14].Chow ET, Otis JD, Simons LE. The longitudinal impact of parent distress and behavior on functional outcomes among youth with chronic pain. J Pain 2016;17:729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cunningham NR, Jagpal A, Tran ST, Kashikar-Zuck S, Goldschneider KR, Coghill RC, Lynch-Jordan AM. Anxiety adversely impacts response to cognitive behavioral therapy in children with chronic pain. J Pediatr 2016;171:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cunningham NR, Kashikar-Zuck S. Nonpharmacological treatment of pain in rheumatic diseases and other musculoskeletal pain conditions. Curr Rheumatol Rep 2013;15:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cunningham NR, Kashikar-Zuck S, Coghill RC. Brain mechanisms impacted by psychological therapies for pain: identifying targets for optimization of treatment effects. Pain Rep 2019;4:e767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].D'Alessio L, Korman GP, Sarudiansky M, Guelman LR, Scévola L, Pastore A, Obregón A, Roldán EJ. Reducing allostatic load in depression and anxiety disorders: physical activity and yoga practice as add-on therapies. Front Psychiatry 2020;11:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav 2012;106:29–39. [DOI] [PubMed] [Google Scholar]
- [20].De Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005;6:463. [DOI] [PubMed] [Google Scholar]
- [21].Deblinger E, Mannarino AP, Cohen JA, Runyon MK, Steer RA. Trauma‐focused cognitive behavioral therapy for children: impact of the trauma narrative and treatment length. Depress anxiety 2011;28:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. Jama 2016;315:1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel therapeutic agents. Jama 2014;311:368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dworkin RH, Bruehl S, Fillingim RB, Loeser JD, Terman GW, Turk DC. Multidimensional diagnostic criteria for chronic pain: introduction to the ACTTION–American pain society pain taxonomy (AAPT). J Pain 2016;17:T1–9. [DOI] [PubMed] [Google Scholar]
- [25].Eccleston C, Morley S, Williams A, Yorke L, Mastroyannopoulou K. Systematic review of randomised controlled trials of psychological therapy for chronic pain in children and adolescents, with a subset meta-analysis of pain relief. PAIN 2002;99:157–65. [DOI] [PubMed] [Google Scholar]
- [26].Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol 2014;69:153. [DOI] [PubMed] [Google Scholar]
- [27].Epel E, Daubenmier J, Moskowitz JT, Folkman S, Blackburn E. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann New York Acad Sci 2009;1172:34–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998;14:245–58. [DOI] [PubMed] [Google Scholar]
- [29].Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. PAIN 2010;150:573–81. [DOI] [PubMed] [Google Scholar]
- [30].Fisher E, Heathcote L, Palermo TM, de C Williams AC, Lau J, Eccleston C. Systematic review and meta-analysis of psychological therapies for children with chronic pain. J Pediatr Psychol 2014;39:763–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fisher E, Law E, Dudeney J, Palermo TM, Stewart G, Eccleston C. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database of Systematic Reviews 2018, Issue 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Frodl T, O'Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis 2013;52:24–37. [DOI] [PubMed] [Google Scholar]
- [33].Gallegos AM, Lytle MC, Moynihan JA, Talbot NL. Mindfulness-based stress reduction to enhance psychological functioning and improve inflammatory biomarkers in trauma-exposed women: a pilot study. Psychol Trauma Theor Res Pract Pol 2015;7:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gatchel RJ. Comorbidity of chronic pain and mental health disorders: the biopsychosocial perspective. Am Psychol 2004;59:795–805. [DOI] [PubMed] [Google Scholar]
- [35].Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581–624. [DOI] [PubMed] [Google Scholar]
- [36].Greenberg M. The stress-proof brain: Master your emotional response to stress using mindfulness and neuroplasticity. Oakland, CA:New Harbinger Publications, 2017. [Google Scholar]
- [37].Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits: a meta-analysis. J psychosomatic Res 2004;57:35–43. [DOI] [PubMed] [Google Scholar]
- [38].Harrison L, Wilson S, Munafò MR. Pain-related and psychological symptoms in adolescents with musculoskeletal and sleep problems. Clin J Pain 2016;32:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TTY, Karatsoreos IN, Mackie K, Viau V, Pickel VM. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci 2011;31:10506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hirschfeld G, Wager J, Schmidt P, Zernikow B. Minimally clinically significant differences for adolescents with chronic pain—variability of ROC-based cut points. J Pain 2014;15:32–9. [DOI] [PubMed] [Google Scholar]
- [41].Huffhines L, Jackson Y. Child maltreatment, chronic pain, and other chronic health conditions in youth in foster care. J Child Adolesc Trauma 2019;23:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jee SH, Couderc JP, Swanson D, Gallegos A, Hilliard C, Blumkin A, Cunningham K, Heinert S. A pilot randomized trial teaching mindfulness-based stress reduction to traumatized youth in foster care. Complement Ther Clin Pract 2015;21:201–9. [DOI] [PubMed] [Google Scholar]
- [43].Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018;129:343–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehavioral Rev 2010;35:2–16. [DOI] [PubMed] [Google Scholar]
- [45].Kashikar-Zuck S, Cunningham N, Peugh J, Black WR, Nelson S, Lynch-Jordan AM, Pfeiffer M, Tran ST, Ting TV, Arnold LM, Carle A, Noll J, Powers SW, Lovell DJ. Long-term outcomes of adolescents with juvenile-onset fibromyalgia into adulthood and impact of depressive symptoms on functioning over time. PAIN 2019;160:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kashikar-Zuck S, Cunningham N, Sil S, Bromberg MH, Lynch-Jordan AM, Strotman D, Peugh J, Noll J, Ting TV, Powers SW, Lovell DJ, Arnold LM. Long-term outcomes of adolescents with juvenile-onset fibromyalgia in early adulthood. Pediatrics 2014;133:e592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kashikar-Zuck S, Flowers SR, Strotman D, Sil S, Ting TV, Schikler KN. Physical activity monitoring in adolescents with juvenile fibromyalgia: findings from a clinical trial of cognitive-behavioral therapy. Arthritis Care Res 2013;65:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kashikar-Zuck S, Sil S, Lynch-Jordan AM, Ting TV, Peugh J, Schikler KN, Hashkes PJ, Arnold LM, Passo M, Richards-Mauze MM, Powers SW, Lovell DJ. Changes in pain coping, catastrophizing, and coping efficacy after cognitive-behavioral therapy in children and adolescents with juvenile fibromyalgia. J Pain 2013;14:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kashikar-Zuck S, Tran ST, Barnett K, Bromberg MH, Strotman D, Sil S, Thomas SM, Joffe N, Ting TV, Williams SE, Myer GD. A qualitative examination of a new combined cognitive-behavioral and neuromuscular training intervention for juvenile fibromyalgia. Clin J Pain 2016;32:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. PAIN 2011;152:2729–38. [DOI] [PubMed] [Google Scholar]
- [51].Klatt M, Steinberg B, Duchemin AM. Mindfulness in Motion (MIM): an onsite mindfulness based intervention (MBI) for chronically high stress work environments to increase resiliency and work engagement. J visualized experiments: JoVE 2015;101:e52359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ladwig RJ, Weisman SJ. A randomized, controlled pilot study of mindfulness-based stress reduction for pediatric chronic pain. Altern therapies Health Med 2013;19:8. [PubMed] [Google Scholar]
- [53].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Laugharne J, Kullack C, Lee CW, McGuire T, Brockman S, Drummond PD, Starkstein S. Amygdala volumetric change following psychotherapy for posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci 2016;28:312–18. [DOI] [PubMed] [Google Scholar]
- [55].Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systemic review and meta‐analyses to determine first‐line treatments. Depress Anxiety 2016;33:792–806. [DOI] [PubMed] [Google Scholar]
- [56].Lindfors P, Folkesson Hellstadius L, Östberg V. Perceived stress, recurrent pain, and aggregate salivary cortisol measures in mid‐adolescent girls and boys. Scand J Psychol 2017;58:36–42. [DOI] [PubMed] [Google Scholar]
- [57].Logan DE, Carpino EA, Chiang G, Condon M, Firn E, Gaughan VJ, Hogan M, Leslie DS, Olson K, Sager S, Sethna N, Simons LE, Zurakowski D, Berde CB. A day-hospital approach to treatment of pediatric complex regional pain syndrome: initial functional outcomes. Clin J Pain 2012;28:766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lovas DA, Pajer K, Chorney JM, Vo DX, Howlett M, Doyle A, Huber A. Mindfulness for adolescent chronic pain: a pilot feasibility study. J Child Adolesc Ment Health 2017;29:129–36. [DOI] [PubMed] [Google Scholar]
- [59].Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009;10:434. [DOI] [PubMed] [Google Scholar]
- [60].Lynch-Jordan AM, Sil S, Peugh J, Cunningham N, Kashikar-Zuck S, Goldschneider KR. Differential changes in functional disability and pain intensity over the course of psychological treatment for children with chronic pain. PAIN 2014;155:1955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mannarino AP, Cohen JA, Deblinger E. Trauma-focused cognitive-behavioral therapy. Evidence-based approaches for the treatment of maltreated children. New York, NY: Springer, 2014. pp.165–85. [Google Scholar]
- [62].Marchand WR. Mindfulness-based stress reduction, mindfulness-based cognitive therapy, and Zen meditation for depression, anxiety, pain, and psychological distress. J Psychiatr Practice 2012;18:233–52. [DOI] [PubMed] [Google Scholar]
- [63].Matousek RH, Dobkin PL, Pruessner J. Cortisol as a marker for improvement in mindfulness-based stress reduction. Complement Therapies Clin Pract 2010;16:13–19. [DOI] [PubMed] [Google Scholar]
- [64].McCracken LM, Vowles KE. Acceptance and commitment therapy and mindfulness for chronic pain: model, process, and progress. Am Psychol 2014;69:178. [DOI] [PubMed] [Google Scholar]
- [65].McEwen BS. Protective and damaging effects of stress mediators. New Engl J Med 1998;338:171–9. [DOI] [PubMed] [Google Scholar]
- [66].McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann New York Acad Sci 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- [67].McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann New York Acad Sci 2001;933:265–77. [DOI] [PubMed] [Google Scholar]
- [68].McEwen BS. Biomarkers for assessing population and individual health and disease related to stress and adaptation. Metabolism 2015;64:S2–S10. [DOI] [PubMed] [Google Scholar]
- [69].McEwen BS. In pursuit of resilience: stress, epigenetics, and brain plasticity. Ann New York Acad Sci 2016;1373:56–64. [DOI] [PubMed] [Google Scholar]
- [70].McEwen BS. Neurobiological and systemic effects of chronic stress. Chronic stress 2017;1:2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism 2010;59(suppl 1):S9–15. [DOI] [PubMed] [Google Scholar]
- [72].McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 2016;41:3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Morath J, Moreno-Villanueva M, Hamuni G, Kolassa S, Ruf-Leuschner M, Schauer M, Elbert T, Bürkle A, Kolassa I-T. Effects of psychotherapy on DNA strand break accumulation originating from traumatic stress. Psychotherapy Psychosomatics 2014;83:289–97. [DOI] [PubMed] [Google Scholar]
- [74].Murray LK, Cohen JA, Mannarino AP. Trauma-focused cognitive behavioral therapy for youth who experience continuous traumatic exposure. J Peace Psychol 2013;19:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Nelson S, Burns M, Logan D. The clinical utility of a brief psychological stress measure (Patient-Reported outcomes measurement information system) in youth with chronic pain. Pain Med 2021;22:91–99. [DOI] [PubMed] [Google Scholar]
- [76].Nelson S, Burns M, McEwen B, Borsook D. Stressful experiences in youth:“set-up” for diminished resilience to chronic pain. Brain Behav Immunity Health 2020;5:100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nelson S, Cunningham N. The impact of posttraumatic stress disorder on clinical presentation and psychosocial treatment response in youth with functional abdominal pain disorders: an exploratory study. Children 2020;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nelson S, Cunningham N, Kashikar-Zuck S. A conceptual framework for understanding the role of adverse childhood experiences in pediatric chronic pain. Clin J Pain 2017;33:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Nelson S, Cunningham N, Peugh J, Jagpal A, Arnold L, Lynch-Jordan A, Kashikar-Zuck S. Clinical profiles of young adults with juvenile-onset fibromyalgia with and without a history of trauma. Arthritis Care Res 2017;69:1636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nelson S, Simons L, Logan D. The incidence of adverse childhood experiences (ACEs) and their association with pain-related and psychosocial impairment in youth with chronic pain. Clin J Pain 2017. [DOI] [PubMed] [Google Scholar]
- [81].Noel M, Chambers CT, McGrath PJ, Klein RM, Stewart SH. The influence of children's pain memories on subsequent pain experience. PAIN 2012;153:1563–72. [DOI] [PubMed] [Google Scholar]
- [82].Noel M, Palermo TM, Chambers CT, Taddio A, Hermann C. Remembering the pain of childhood: applying a developmental perspective to the study of pain memories. PAIN 2015;156:31–4. [DOI] [PubMed] [Google Scholar]
- [83].Noel M, Pavlova M, Lund T, Jordan A, Chorney J, Rasic N, Brookes J, Hoy M, Yunker WK, Graham S. The role of narrative in the development of children's pain memories: influences of father–and mother–child reminiscing on children's recall of pain. PAIN 2019;160:1866–75. [DOI] [PubMed] [Google Scholar]
- [84].Noel M, Rabbitts JA, Fales J, Chorney J, Palermo TM. The influence of pain memories on children's and adolescents' post-surgical pain experience: a longitudinal dyadic analysis. Health Psychol 2017;36:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Noel M, Wilson AC, Holley AL, Durkin L, Patton M, Palermo TM. Posttraumatic stress disorder symptoms in youth with vs without chronic pain. PAIN 2016;157:2277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Olff M, de Vries G-J, Güzelcan Y, Assies J, Gersons BP. Changes in cortisol and DHEA plasma levels after psychotherapy for PTSD. Psychoneuroendocrinol 2007;32:619–26. [DOI] [PubMed] [Google Scholar]
- [87].Ortiz R, Sibinga EM. The role of mindfulness in reducing the adverse effects of childhood stress and trauma. Children 2017;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Osler M, Bendix L, Rask L, Rod NH. Stressful life events and leucocyte telomere length: do lifestyle factors, somatic and mental health, or low grade inflammation mediate this relationship? Results from a cohort of Danish men born in 1953. Brain Behav Immun 2016;58:248–53. [DOI] [PubMed] [Google Scholar]
- [89].Palermo TM, Eccleston C, Lewandowski AS, Williams ACdC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. PAIN 2010;148:387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Palermo TM, Fonareva I, Janosy NR. Sleep quality and efficiency in adolescents with chronic pain: relationship with activity limitations and health-related quality of life. Behav Sleep Med 2008;6:234–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Park JK, Park J, Elbert T, Kim SJ. Effects of narrative exposure therapy on posttraumatic stress disorder, depression, and insomnia in traumatized North Korean refugee youth. J Traumatic Stress 2020;33:353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Pay JN, Shaw AM. Towards salivary C-reactive protein as a viable biomarker of systemic inflammation. Clin Biochem 2019;68:1–8. [DOI] [PubMed] [Google Scholar]
- [93].Pielech M, Vowles KE, Wicksell R. Acceptance and commitment therapy for pediatric chronic pain: theory and application. Children 2017;4:10. [Google Scholar]
- [94].Reiner K, Tibi L, Lipsitz JD. Do mindfulness-based interventions reduce pain intensity? A critical review of the literature. Pain Med 2013;14:230–42. [DOI] [PubMed] [Google Scholar]
- [95].Ridout KK, Khan M, Ridout SJ. Adverse childhood experiences run deep: toxic early life stress, telomeres, and mitochondrial DNA copy number, the biological markers of cumulative stress. Bioessays 2018;40:1800077. [DOI] [PubMed] [Google Scholar]
- [96].Rogosch FA, Dackis MN, Cicchetti D. Child maltreatment and allostatic load: consequences for physical and mental health in children from low-income families. Development Psychopathol 2011;23:1107–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Rosemberg M-AS, Granner J, Li Y, Seng JS. A scoping review of interventions targeting allostatic load. Stress 2020;23:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D. Mindfulness-based stress reduction for chronic pain conditions: variation in treatment outcomes and role of home meditation practice. J psychosomatic Res 2010;68:29–36. [DOI] [PubMed] [Google Scholar]
- [99].Schubert CF, Schreckenbach M, Kirmeier T, Gall-Kleebach DJ, Wollweber B, Buell DR, Uhr M, Rosner R, Schmidt U. PTSD psychotherapy improves blood pressure but leaves HPA axis feedback sensitivity stable and unaffected: first evidence from a pre-post treatment study. Psychoneuroendocrinology 2019;100:254–63. [DOI] [PubMed] [Google Scholar]
- [100].Schumacher S, Niemeyer H, Engel S, Cwik JC, Knaevelsrud C. Psychotherapeutic treatment and HPA axis regulation in posttraumatic stress disorder: a systematic review and meta-analysis. Psychoneuroendocrinology 2018;98:186–201. [DOI] [PubMed] [Google Scholar]
- [101].Shern DL, Blanch AK, Steverman SM. Toxic stress, behavioral health, and the next major era in public health. Am J Orthopsychiatry 2016;86:109. [DOI] [PubMed] [Google Scholar]
- [102].Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, Pascoe J, Wood DL, Health CoPAoCaF. Committee on Early Childhood A, and Dependent Care. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012;129:e232–46. [DOI] [PubMed] [Google Scholar]
- [103].Sil S, Arnold LM, Lynch-Jordan A, Ting TV, Peugh J, Cunningham N, Powers SW, Lovell DJ, Hashkes PJ, Passo M, Schikler KN, Kashikar-Zuck S. Identifying treatment responders and predictors of improvement after cognitive-behavioral therapy for juvenile fibromyalgia. PAIN 2014;155:1206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Simons LE, Kaczynski KJ. The fear avoidance model of chronic pain: examination for pediatric application. J Pain 2012;13:827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Simons LE, Logan DE, Chastain L, Cerullo M. Engagement in multidisciplinary interventions for pediatric chronic pain: parental expectations, barriers, and child outcomes. Clin J Pain 2010;26:291–9. [DOI] [PubMed] [Google Scholar]
- [106].Theall KP, Drury SS, Shirtcliff EA. Cumulative neighborhood risk of psychosocial stress and allostatic load in adolescents. Am J Epidemiol 2012;176(suppl_7):S164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Timmers I, Quaedflieg CW, Hsu C, Heathcote LC, Rovnaghi CR, Simons LE. The interaction between stress and chronic pain through the lens of threat learning. Neurosci Biobehavioral Rev 2019;107:641–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Veehof M, Trompetter H, Bohlmeijer ET, Schreurs KMG. Acceptance-and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther 2016;45:5–31. [DOI] [PubMed] [Google Scholar]
- [109].Waelde LC, Feinstein AB, Bhandari R, Griffin A, Yoon IA, Golianu B. A pilot study of mindfulness meditation for pediatric chronic pain. Children 2017;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Westmacott R, Hunsley J, Best M, Rumstein-McKean O, Schindler D. Client and therapist views of contextual factors related to termination from psychotherapy: a comparison between unilateral and mutual terminators. Psychotherapy Res 2010;20:423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wiley JF, Gruenewald TL, Karlamangla AS, Seeman TE. Modeling multisystem physiological dysregulation. Psychosomatic Med 2016;78:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. PAIN 2011;152(3 suppl):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zantvoord JB, Ensink JB, op den Kelder R, Wessel AM, Lok A, Lindauer RJ. Pretreatment cortisol predicts trauma-focused psychotherapy response in youth with (partial) posttraumatic stress disorder. Psychoneuroendocrinology 2019;109:104380. [DOI] [PubMed] [Google Scholar]
- [114].Zhu X, Suarez‐Jimenez B, Lazarov A, Helpman L, Papini S, Lowell A, Durosky A, Lindquist MA, Markowitz JC, Schneier F. Exposure‐based therapy changes amygdala and hippocampus resting‐state functional connectivity in patients with posttraumatic stress disorder. Depress anxiety 2018;35:974–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


