Abstract
Background:
Breast implant illness (BII) is a term popularized by social media to describe systemic symptoms that patients ascribe to their breast implants. Though the concept of implants as an underlying cause for a systemic illness remains controversial, few studies have delineated the implant characteristics, capsular histology, and outcomes of patients who undergo explantation for BII.
Methods:
We retrospectively reviewed the demographics, presenting symptoms, outcomes, capsular histology, and culture results of all women who presented to the senior author with symptoms attributed to BII and underwent breast implant removal with capsulectomy from August 2016 to February 2020. Chi-square and logistic regression analyses were performed to evaluate association between implant type, composition, and findings of inflammation on capsule pathology.
Results:
Among 248 patients, 111 (23%) capsules demonstrated inflammatory changes on permanent pathology. Capsular inflammation was independently associated with silicone versus saline (right odds ratio [OR] = 2.18 [1.16–4.11], P = 0.016, left OR = 2.35 [1.08–5.12], P = 0.03) and textured versus smooth implants (right OR = 2.18 [1.16–4.11], P = 0.016, left OR = 2.25 [1.17–4.31], P = 0.01). Silicone material was present in the capsules of 12 patients (4.8%). Fourteen patients had positive cultures. There was one pneumothorax (0.4%), three hematomas requiring evacuation (1%), and two DVTs (0.8%). Of 228 patients, 206 (90.4%) reported high satisfaction with the outcome of the procedure.
Conclusions:
In a large cohort of BII patients, we found that capsular inflammation is significantly associated with silicone and textured implants. Implant removal with capsulectomy can be safely performed in patients with BII with a low complication rate and high patient satisfaction.
INTRODUCTION
Breast implant illness (BII) is a novel description for a constellation of symptoms potentially driven by a poorly characterized immune or biochemical response to breast implants.1,2 The name for this disease process has been coined by women who believe they have become ill from their implants rather than by a medical professional society. Awareness of BII is increasingly fueled by the power of social media, with one recent study reporting an online group that reached nearly 110,000 members.3,4 BII symptoms are frequently nonspecific, vary in severity, and can affect nearly all organ systems, characteristics which have been noted to overlap with many somatization disorders.5–7 Despite growing concern among the general public regarding BII, breast augmentation is on the rise, with nearly 330,000 procedures performed in 2018 (a 15% increase from 2014), and national data show ongoing trends favoring implant-based breast reconstruction.8,9 The leading professional societies in plastic surgery have hosted several panels to discuss BII, and continue to offer forums to facilitate dialog among patients, patient advocates, and surgeons.10–12
There is a paucity of knowledge about the possible pathophysiology of BII, and many prior studies of implants and systemic disease have occurred in nonsurgical fields with controversial conclusions.2,6,13–18 Treatment recommendations for this patient group can vary widely, with nearly all surgeons advocating frank and even-handed discussion with patients in light of strong evidence supporting the safety of implants,14,19 but disagreeing whether surgical treatment including explantation and capsulectomy should be offered for symptoms of uncertain etiology.2,4,20 We sought to better characterize the presenting symptomatology, postoperative outcomes, patient satisfaction, and capsular findings of a population of patients who self-identified as having BII and proceeded to undergo removal of their implants combined with excision of the associated implant capsule.
METHODS
This study was conducted after receiving approval from the hospital institutional review board at Abington Hospital-Jefferson Health with a waiver of the need for individual consent (IRB#19-039). We retrospectively reviewed the medical records of all women 18 years of age and older who presented to the senior author from 2016 to 2020 with systemic symptoms that patients ascribed to their breast implants and subsequently underwent total capsulectomy and implant removal after appropriately balanced discussion of expectations, risks, and the current scientific evidence.
Patients underwent explantation via previous inframammary, mastectomy, or periareolar incisions when possible, or through concurrent mastopexy if being performed. Bilateral capsulectomies were performed and cultures were routinely obtained intraoperatively through a capsulotomy made to access the implant pocket. A portion of the capsule was divided and submitted for permanent pathology.
Data obtained from medical records included demographics, indication for initial placement of implants (reconstruction versus cosmetic), medical history, physical examination findings, presenting symptoms, results of any laboratory tests obtained, operative findings at time of surgery, simultaneous procedures, and postoperative follow-up. The senior author obtained cultures from all implant pockets before excision of the capsule. The first four postoperative visit notes were reviewed to determine each patient’s level of satisfaction with the results of the procedure and specific postoperative symptoms when available, with a mean follow-up of 6 months.
Statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, N.C.). Chi-squared analysis was utilized for independent variables, and logistic regression analysis was used to evaluate implant characteristics associated with findings of inflammation on pathology, which was defined as calcification or microcalcifications, histiocytic reaction or abundance of histiocytes, macrophages, or giant cells, presence of sclerosis, lymphoid or lymphocytic infiltration, or the term inflammation otherwise contained in the final pathology report with reference to the capsule.
RESULTS
A total of 248 patients underwent bilateral implant removal with capsulectomy performed by the senior author from August 2016 to February 2020. Two hundred and twenty-six patients (93%) had implants placed for cosmetic purposes. The median patient age at presentation was 45 years (range: 22–72 y), median age at placement of first implants was 29.5 and average BMI was 24. On physical examination, 130 patients (55%) exhibited Baker II and 95 patients (39%) exhibited Baker III/IV capsular contracture at initial presentation. Patient characteristics are summarized in Table 1.
Table 1.
Patient Characteristics
| Average age at presentation (y) | 44 |
| Average age at placement of breast implants (y) | 31 |
| Average BMI | 24 |
| Reason for implant placement | |
| Cosmetic | 226 (93%) |
| Reconstructive | 18 (7%) |
| Current smoker | 19 (8.4%) |
| Diabetes | 7 (2.8%) |
| Grade of capsular contracture | |
| I | 11 (4.7%) |
| II | 122 (52%) |
| III | 60 (25%) |
| IV | 43 (18%) |
| Autoimmune diagnosis | |
| Arthritis | 67 (27%) |
| Chronic inflammatory response syndrome (CIRS) | 3 (1.2%) |
| Lupus | 10 (3%) |
| Sjogren’s syndrome | 3 (1.2%) |
| Raynaud’s sydrome | 10 (4%) |
| Graves disease | 2 (0.8%) |
| Hashimoto’s thyroiditis | 20 (8.1%) |
| Scleroderma | 1 (0.4%) |
| Multiple sclerosis | 1 (0.4%) |
| Ulcerative colitis | 1 (0.4%) |
| Crohn’s disease | 1 (0.4%) |
| History of breast cancer | 10 (4%) |
| History of other cancer | 17 (6.9%) |
| Anxiety | 79 (32%) |
| History of panic attacks | 10 (4%) |
| Depression | 38 (15%) |
| Suicidal ideation | 1 (0.4%) |
| Fibromyalgia | 17 (6.9%) |
| Irritable bowel syndrome | 23 (9.3%) |
| Mild anemia (hemoglobin 11–11.9 g/dL) | 2 (0.8%) |
| Moderate anemia (hemoglobin 8.0–10.9 g/dL) | 2 (0.8%) |
| Leukopenia (WBC < 4.5 × 109/L) | 7 (3%) |
| Elevated alkaline phosphatase (>130 U/L) | 3 (1.2%) |
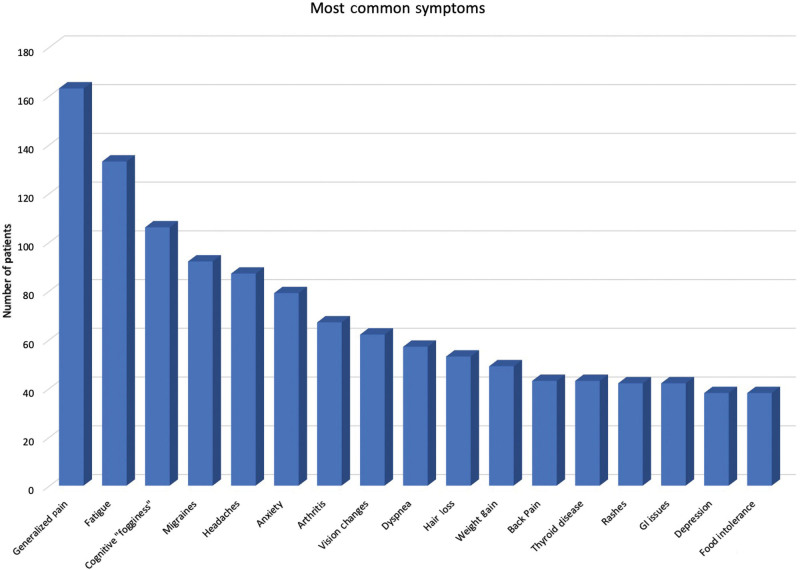
The most common symptoms mentioned at time of initial evaluation included generalized pain, fatigue, cognitive “fogginess,” migraines, headaches, anxiety, arthritis, vision changes, dyspnea, hair loss, weight gain, back pain, rashes, generalized gastrointestinal issues, and depression. The number of complaints did not vary significantly between types of implants. Symptoms are summarized in Figure 1.
Fig. 1.
Most common complaints reported by patients on initial evaluation.
Operative details and findings are shown in Table 2. Simultaneous procedures at time of implant removal and total capsulectomy included mastopexy in 53 patients (21%), scar revision in 12 patients (4.9%), breast reconstruction in five patients (2.0%), and abdominoplasty in one patient (0.4%). One patient requested implant replacement of her silicone implants with saline implants combined with capsulectomy (0.4%). Implant rupture at time of explantation was noted in 20 patients on the right (8.2%), and 18 patients on the left (7.4%). Two hundred forty-four patients (98.3%) underwent total capsulectomy on the right and 245 patients (98.7%) had total capsulectomy on the left. Two patients had a partial excision of the capsules bilaterally, and one patient had no capsulectomy performed. The capsules were removed intact in 27 patients (10.8%) on the right and 28 patients (11.2%) on the left. There were six major complications, which consisted of one pneumothorax that required hospital admission for observation, three breast hematomas that required evacuation in the odds ratio (OR), and two deep vein thromboses that were managed with anticoagulation. Minor complications consisted of five delayed seromas and three liquified hematomas which were treated by aspiration. Three patients who underwent simultaneous mastopexies had a suture infection which was treated with antibiotics.
Table 2.
Operative Details
| Incision Type | |
|---|---|
| Previous mastectomy | 15 (6%) |
| Inframammary | 173 (70%) |
| Mastopexy | 57 (23%) |
| Periareolar | 3 (1.2%) |
| Additional procedures performed | |
| Mastopexy | 53 (21%) |
| Scar revision | 12 (4.8%) |
| Implant rupture | |
| Right | 20 (8.2%) |
| Left | 18 (7.38%) |
| Total capsule excision | |
| Right | 244 (98.3%) |
| Left | 245 (98.7%) |
| Capsule removed intact | |
| Right | 27 (10.8%) |
| Left | 28 (11.2%) |
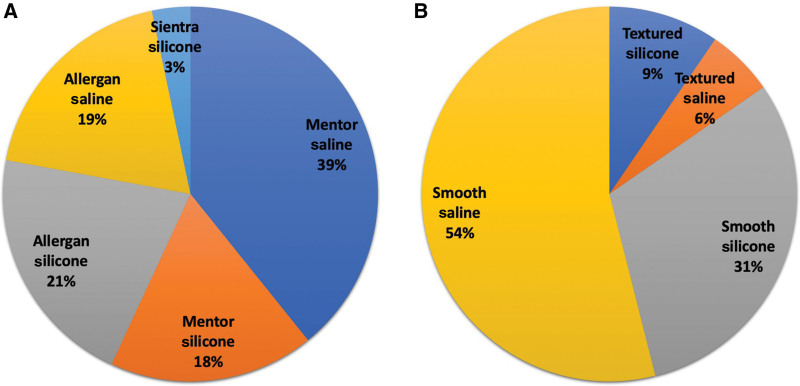
Ninety-eight patients (40.2%) had silicone implants, and 146 (59.8%) had saline implants. Silicone implants included 38 Allergan silicone (21%), 32 Mentor silicone (18%), and six Sientra silicone (3%). Saline implants included 71 Mentor saline (39%), 34 Allergan saline (19%), and one IDEAL saline (0.6%). In 63 patients, the brand of implant could not be determined. Two hundred and seven patients (85%) had smooth implants, and 37 (15%) had textured implants (Fig. 2).
Fig. 2.
Characteristics of removed implants. A, Make and model. B, Texture and fill.
All capsules were submitted to permanent pathology, and 111 (23%) of the capsules were found to have evidence of acute or chronic inflammation. One capsule did have atypical lymphocytic infiltration but was CD30 negative in testing for breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Positive cultures were noted in fourteen patients, eight (3.28%) from right breast pockets, and nine (3.69%) from left breast pockets. The most common organisms from cultures included several strains of Staphylococcus as shown in Table 3. One patient had cultures positive for Candida albicans from both breast pockets and underwent a 2-week course of fluconazole after consultation with an infectious disease specialist but had an otherwise uneventful postoperative course. Twelve patients (4.9%) had capsular findings of “refractile/nonpolarizable foreign material or silicone.” Of these patients, three had bilateral implant rupture, four had one ruptured and one unruptured implant, and five had no evidence of rupture. All of these patients had histiocytic reactive changes, macrophages, or multinucleated cells associated with the refractile material on histology. Four of these patients had saline implants and eight had silicone implants at time of explantation (Table 4).
Table 3.
Classification of Culture Results
| Organism | No. Positive Cultures |
|---|---|
| Staphylococcus epidermidis | 2 |
| Staphylococcus capitis | 3 |
| Staphylococcus lugdunensis | 1 |
| Unspecified: coagulase (−) Staphylococcus | 2 |
| Unspecified: gram (+) cocci | 1 |
| Unspecified: Bacillus sp. | 2 |
| Unspecified: Propionibacterium sp. | 1 |
| Unspecified: few mixed skin flora | 1 |
| Cutibacterium acnes | 1 |
| Candida albicans | 2 |
| Unspecified: gram (+) rods | 1 |
Table 4.
Patients with Findings of Nonpolarizable Refractile Material or Silicone on Pathology
| Laterality of Capsular Pathology | Laterality of Implant Rupture | Implant Make | Implant Model | Implant Composition | Capsular Culture |
|---|---|---|---|---|---|
| Right | Bilateral | Allergan | Saline | Textured | Negative |
| Right | Right | Allergan | Silicone | Smooth | Negative |
| Right | N/A | Allergan | Saline | Textured | Negative |
| Bilateral | Right | N/A | Silicone | Smooth | Negative |
| Bilateral | N/A | Mentor | Saline | Smooth | Negative |
| Bilateral | N/A | Allergan | Saline | Smooth | Negative |
| Bilateral | N/A | Allergan | Silicone | Textured | Negative |
| Bilateral | Bilateral | N/A | Silicone | Textured | Negative |
| Bilateral | Bilateral | N/A | Silicone | Textured | Negative |
| Bilateral | Left | N/A | Silicone | Textured | Negative |
| Bilateral | Left | N/A | Silicone | Textured | Negative |
| Bilateral | N/A | Mentor | Silicone | Textured | Negative |
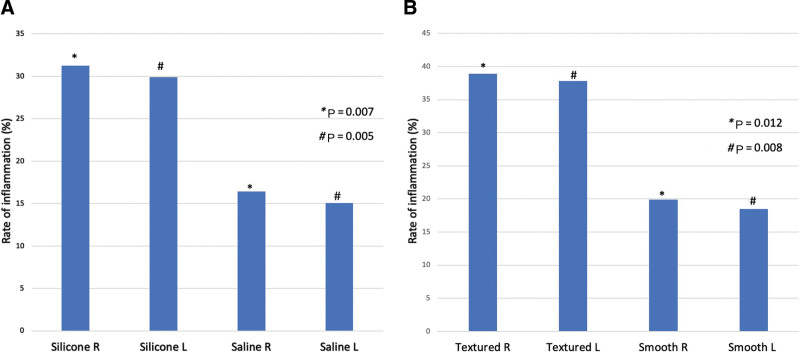
Capsular inflammation was significantly associated with silicone implants vs. saline implants (right: 31.3% silicone versus saline 16.4%, P = 0.007; left: 29.9% silicone versus 15.1% saline, P = 0.005). Additionally, inflammation was significantly associated with textured implants versus smooth implants (right: 38.9% textured versus 19.9% smooth, P = 0.01; left: 37.8% textured versus 18.5% smooth, P = 0.008). Figure 3 shows rates of inflammation by implant type.
Fig. 3.
Rates of inflammation by implant type. A, implant fill. B, implant texture.
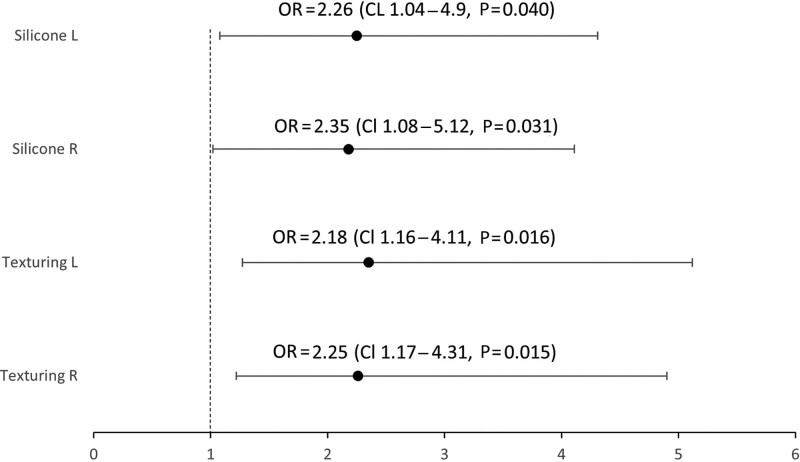
On logistic regression modeling, capsular inflammation was independently associated with silicone versus saline (right: OR = 2.18 [1.16–4.11], P = 0.016, Left: OR = 2.25 [1.17–4.31], P = 0.015) and textured versus smooth implants (right: OR = 2.26 [1.04–4.9], P = 0.040, left: OR = 2.35 [1.08–5.12], P = 0.031) (Fig. 4). Textured and silicone characteristics independently increased inflammation when present together to approximately 51% but had an additive rather than synergistic effect on increasing inflammation.
Fig. 4.
Logistic regression modeling for ORs with regards to texturing and implant fill.
The average number of follow-up visits was 3.9 ± 2.1, with a duration of 1.8–6 months. Postoperative visit notes addressed specific symptoms in 46 patients, and of these, 44 (96%) reported a decrease in the number of symptoms after surgery. Of 228 patients reporting their level of satisfaction with the procedure at first postoperative visit, 206 (90.4%) reported they were satisfied with the results and had no or minor complaints. In subsequent follow-up visits, 125 of 153 (81.7%) patients reported high satisfaction at their second postoperative visit, 59 of 75 (78.7%) reported high satisfaction at their third postoperative visits, and 30 of 39 (77%) reported high satisfaction at the fourth postoperative visit.
DISCUSSION
Brief History of the BII Controversy
The association of breast implants with autoimmune or systemic symptoms is an ongoing, heavily debated topic. Despite early reports of patients with silicone implants developing an immunoadjuvant disease,21–23 large retrospective studies comparing incidence of autoimmune diseases in women with silicone implants found no association, a finding confirmed by a special committee of the Institute of Medicine in 1999.19,24–27 This ultimately resulted in lifting the FDA moratorium on silicone implants but has by no means put an end to the controversy surrounding implant-related systemic illness. In recent years, an increasingly large number of women with prominent social media presence are seeking implant removal for a constellation of nonspecific systemic symptoms referred to as BII. A recent review by Magnusson et al2 suggests that efforts at scientific investigation of an underlying pathophysiology for these symptoms have unfortunately been hampered by misrepresentation in the media and an excessive focus on litigation. The pathogenesis of an immunoadjuvant disease process associated with breast implants has been contested in the literature for decades, with several rheumatology studies stipulating a direct effect of silicone in biochemically altering metabolic or cellular processes,13,22,28,29 whereas others argue that the constellation of somatic symptoms ascribed to implants may be the result of disrupted pain processing pathways leading to psychological distress in a manner similar to disorders like fibromyalgia.5,30 The relation of either these hypotheses to BII remains unclear at the present time; however, an important question to address is whether implant removal and excision of the associated capsule as many BII patients specifically request is associated with consistent symptom improvement and postoperative satisfaction. To this end, we sought to characterize the presenting symptoms, demographics, outcomes, and implant and capsular findings of a large cohort of BII patients who presented to the senior author and ultimately elected to undergo implant removal with total capsulectomy.
Presenting Symptoms, Postoperative Outcomes, and Patient Satisfaction
In our cohort of patients, we found that preoperatively the most common presenting symptoms were nonspecific somatic complaints such as generalized pain (163 patients, 67%) and fatigue (133 patients, 55%). This characterizes the difficulty of defining BII as an entity, as complaints are frequently nonspecific and highly subjective in nature, a theme which is shared with reports of immunoadjuvant disease related to silicone implants in the past. We found that in 46 patients who had postoperative follow-up addressing specific symptoms, 44 patients (96%) reported overall improvement. Previous explantation studies have noted substantial symptomatic improvement in patients who did not meet laboratory or diagnostic criteria for known autoimmune disorders, such as the study by De Boer et al30 which reviewed 23 published case series and reports from 1960 to 2016 and found that nearly 75% of patients reported symptomatic improvement after removal of their silicone implants. Rohrich et al31 noted that a higher number of musculoskeletal complaints were associated with higher likelihood of improvement in 38 patients with silicone implants who underwent explantation. In the largest retrospective study of explantation in BII patients to date, Wee et al32 found sustained improvement across 11 symptom domains, which encompassed cognitive, musculoskeletal, and systemic symptoms in 752 patients which was maintained after 30 postoperative days. Interestingly, the authors of this recent study found similar symptom improvement with removal of both silicone and saline implants, and did not observe a difference in patient self-reported outcomes between patients with textured or smooth implants.32 One of the difficulties in monitoring symptom improvement in BII patients is the duration of follow-up, as many patients are frequently self-referred over a potentially large geographic area and have limited follow-up with their surgeon unless postoperative complications arise. Therefore, it is difficult to address the frequency of symptom recurrence or the success of implant removal in the long term, and previous studies of BII have largely been limited to studying outcomes in the first 6 months.32,33 In two older explantation studies for patients who complained of systemic symptoms, an initial period of symptom improvement was followed by recurrence when longer duration follow-up was available. Slavin and Goldwyn34 found that in eight patients who underwent implant removal with systemic complaints, only one of eight patients had sustained improvement after 2.5 years of follow-up. The study was notably limited by the relatively small number of patients with symptoms that fit the pattern of BII, with the majority of patients requesting explantation either from fear of harmful consequences or aesthetic reasons. Godfrey and Godfrey20 found that in 37 women who underwent explantation followed by autologous breast reconstruction, although 33 had initial improvement 1 month postoperatively, 21 patients had relapse of symptoms by 6 months, and only seven patients reported improvement by 12-month follow-up. Although these prior explantation studies included a subset of women with ostensibly systemic symptoms, evaluation of outcomes was limited by including patients who underwent implant removal due to local symptoms related to contracture, anxiety about implants due to the silicone controversy of the 1990s, or explantation for older generation implants that had a higher rate of rupture and leakage.20,31,35–38 Additionally, previous studies largely focused on patients with silicone implants in light of the FDA moratorium, whereas the majority (59.8%) of patients in our study had saline implants.
We observed a relatively low complication rate in our practice of implant removal and capsulectomy, with six of 248 (2.4%) patients having a major complication defined as pneumothorax, hematoma requiring evacuation, or DVT, and eight of 248 (4.4%) patients having a minor complication defined as seroma, liquefied hematoma, or wound infection. Other than the singular complication of pneumothorax, it is difficult to ascribe any particular complication to addition of capsulectomy to the procedure. Though the addition of capsulectomy is controversial for asymptomatic patients undergoing removal of textured implants for future concern of BII or BIA-ALCL,15 a large number of BII patients including our cohort also have a high rate of capsular contracture, and addition of capsulectomy may lead to a more substantial symptom improvement of local musculoskeletal symptoms.32 A prior small retrospective controlled study by Kappel and Pruijn39 found a more pronounced improvement in systemic symptoms when capsulectomy was added to the implant removal procedure.
Inflammation on Capsular Histology
We found that acute or chronic inflammation was present in 111 (23%) of capsules on permanent pathology, and there was a significant association with silicone and textured implants. Chronic inflammation in the form of calcification surrounding implants has been found to correlate with implant shell thickness, duration after placement, and integrity of the shell in prior studies, and although more frequently associated with older generation silicone implants, has been associated with the elastomer shell of saline implants as well.40–42 Additionally, small amounts of silicone in the capsule outside an otherwise intact implant shell have been found to induce chronic inflammation by uptake into macrophages, subsequently triggering cytokine production and fibroblast activity.43,44 Though we found evidence of this “silicone bleed” phenomenon in five patients with unruptured implants, the clinical relation to BII is currently not understood, as only a small subset of patients in our study demonstrated capsular inflammation on histology or findings of silicone material. The pathogenesis of BII remains largely hypothetical, as no consistent rheumatologic, histologic, or microbiological finding has substantiated a clear underlying pathophysiology for the condition. In light of some promising recent studies such as that by Lee et al,33 we speculate that textured implants may be associated with more inflammation due to increased propensity for biofilm formation, which may be difficult to detect by routine bacterial cultures. Moreover, Wee et al32 found that patients with capsular contracture had a significantly greater self-reported improvement after explantation. Though this could partially attest to the mechanical nature of some symptoms such as chest wall restriction, the association with improvement in more nebulous symptoms such as fatigue and cognitive problems could also suggest a shared inflammatory pathogenesis between capsular contractures and BII.
Culture Results
Fourteen patients in our cohort had positive culture results, including eight patients with positive cultures of the right breast pocket and nine with positive cultures of the left breast pocket (3.69%). The most common organisms were strains of Staphlyococcus (47%), which is consistent with cultures of prior studies of periprosthetic implant colonization such as the study by Peters et al,45 which evaluated the implants and capsules of 100 women who had silicone implants removed between 1992 and 1995. Their group found 42% of the capsules were colonized with bacteria and 25% were heavily calcified suggesting chronic inflammation.45 Though the clinical relevance of these positive cultures to BII is currently unknown, Lee et al33 recently compared microbiological data between 50 patients undergoing explantation and capsulectomy for BII with a control group that underwent implant exchange, finding that the BII group had a six-fold higher rate of positive cultures. The most common organisms they reported were Propionbacterium acnes in 24% of the BII group, followed by Staphylococcus epidermidis in 6%. Cultures were obtained by grinding a portion of the divided capsule sent directly for microbiological analysis as well as part of the implant shell, a method which may have a greater yield for detection of microorganisms within a biofilm structure compared to the routine cultures obtained in our study, and these elaborate methods merit further investigation to elucidate the role of a potential indolent infection as a cause for BII.
Our study is limited by its retrospective nature and lack of standard documentation, without which we were unable to evaluate changes in specific symptoms after explantation or correlate capsular findings on pathology with symptom severity preoperatively. Like prior studies of explantation as a treatment for patients presenting with systemic symptoms, our study is additionally challenged by the subjective bias of defining BII symptoms, lack of a control group, and selection bias as patients were predominantly self-referred to our office for explantation. Follow-up duration was also a mean of 6 months, which limits our ability to predict long-term symptom resolution or recurrence.
Nonetheless, we found that evidence of acute or chronic inflammation was significantly more common in silicone compared to saline and textured compared to smooth implants. This interesting finding potentially suggests an association between a specific implant composition and development of symptoms described as BII. We also found that implant removal with capsulectomy had a low complication rate, and that the majority of patients expressed satisfaction with their postoperative outcomes as well as improvement in their overall symptoms during the follow-up period. Building on the results of our retrospective study, we are currently conducting a prospective study focusing on standardized comparison of preoperative symptoms and postoperative improvement to determine which patients would most likely benefit from implant removal and capsulectomy.
CONCLUSION
Our data suggest that in a subset of patients presenting with BII symptoms, there is an underlying inflammatory response associated with the implant capsule, a response which appears to be more common in silicone versus saline and textured versus smooth implants. This response may be associated with symptoms of BII. More research is necessary to further elucidate the underlying process fueling BII; however, in this study, we have demonstrated that implant removal with total capsulectomy can be safely performed in the BII population with minimal complications and high patient satisfaction.
Footnotes
Published online 7 September 2021.
Presented at the American Society of Plastic Surgeons, Virtual Plastic Surgery Meeting, October 16–18, 2020; and at the Robert H. Ivy Society of Plastic Surgery, Annual Virtual Scientific Meeting, November 7, 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.The American Society of Aesthetic Plastic Surgeons (ASAPS). Breast Implant Illness—Frequently Asked Questions/Talking Points. Available at https://www.surgery.org/sites/default/files/BreastImplantIllness_8-21-2019_FINAL.pdf. Accessed February 16, 2020.
- 2.Magnusson MR, Cooter RD, Rakhorst H, et al. Breast implant illness: a way forward. Plast Reconstr Surg. 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):74S–81S. [DOI] [PubMed] [Google Scholar]
- 3.Healing Breast Implant Illness Facebook Group. Available at https://www.facebook.com/groups/Healingbreastimplantillness/. Accessed February 16th, 2020.
- 4.Tang SYQ, Israel JS, Afifi AM. Breast implant illness: symptoms, patient concerns, and the power of social media. Plast Reconstr Surg. 2017;140:765e–766e. [DOI] [PubMed] [Google Scholar]
- 5.Dush DM. Breast implants and illness: a model of psychological factors. Ann Rheum Dis. 2001;60:653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mcguire PA, Haws MJ, Nahai F. Breast implant illness: how can we help? Aesthet Surg J. 2019;39:1260–1263. [DOI] [PubMed] [Google Scholar]
- 7.Balk EM, Earley A, Avendano EA, et al. Long-term health outcomes in women with silicone gel breast implants: a systematic review. Ann Intern Med. 2016;164:164–175. [DOI] [PubMed] [Google Scholar]
- 8.The Aesthetic Society. Aesthetic Plastic Surgery National Databank Statistics, 2018. Available at https://www.surgery.org/sites/default/files/ASAPS-Stats2018_0.pdf Accessed February 16, 2020.
- 9.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15–23. [DOI] [PubMed] [Google Scholar]
- 10.ASPS & ASPSP Spring Meeting 2020 Program. American Society of Plastic Surgeons. 2020. Accessed February 16, 2020. [Google Scholar]
- 11.Surgeons ASoAP. The Aesthetic Meeting 2020 Program. Accessed February 16, 2020,
- 12.Leonardo J SM. Society leadership discusses breast-implant concerns with patients. Plastic Surgery News.42;2019. [Google Scholar]
- 13.Watad A, Rosenberg V, Tiosano S, et al. Silicone breast implants and the risk of autoimmune/rheumatic disorders: a real-world analysis. Int J Epidemiol. 2018;47:1846–1854. [DOI] [PubMed] [Google Scholar]
- 14.Coroneos CJ, Selber JC, Offodile AC, II, et al. US FDA breast implant postapproval studies: long-term outcomes in 99,993 patients. Ann Surg. 2019;269:30–36. [DOI] [PubMed] [Google Scholar]
- 15.Swanson E. Analysis of US Food and Drug Administration breast implant postapproval studies finding an increased risk of diseases and cancer: why the conclusions are unreliable. Ann Plast Surg. 2019;82:253–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohrich RJ, Kaplan J, Dayan E. Silicone implant illness: science versus myth? Plast Reconstr Surg. 2019;144:98–109. [DOI] [PubMed] [Google Scholar]
- 17.Ashar BS. Assessing the risks of breast implants and FDA’s vision for the National Breast Implant Registry. Ann Surg. 2019;269:37–38. [DOI] [PubMed] [Google Scholar]
- 18.United States Food and Drug Administration Center for Devices and Radiological Health. FDA Update on the Safety of Silicone Gel Filled Breast Implants. June2011. Available at https://www.fda.gov/downloads/medicaldevices/productsandmedicalproce-dures/implantsandprosthetics/breastimplants/ucm260090.pdf. Accessed February 16, 2020.
- 19.Bondurant S, Ernster V, Herdman R. Institute of Medicine (US) Committee on the Safety of Silicone Breast Implants. Washington, D.C.:National Academies Press (US); 1999. [PubMed] [Google Scholar]
- 20.Godfrey PM, Godfrey NV. Response of locoregional and systemic symptoms to breast implant replacement with autologous tissues: experience in 37 consecutive patients. Plast Reconstr Surg. 1996;97:110–116. [DOI] [PubMed] [Google Scholar]
- 21.Lavranos G, Kouma D, Deveros A, et al. Still’s-like disease induced by breast implants in a middle-aged female health professional. Eur J Case Rep Intern Med. 2017;4:000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brozena SJ, Fenske NA, Cruse CW, et al. Human adjuvant disease following augmentation mammoplasty. Arch Dermatol. 1988;124:1383–1386. [PubMed] [Google Scholar]
- 23.Sergott TJ, Limoli JP, Baldwin CM, Jr, et al. Human adjuvant disease, possible autoimmune disease after silicone implantation: a review of the literature, case studies, and speculation for the future. Plast Reconstr Surg. 1986;78:104–114. [DOI] [PubMed] [Google Scholar]
- 24.Gabriel SE, O’Fallon WM, Kurland LT, et al. Risk of connective-tissue diseases and other disorders after breast implantation. N Engl J Med. 1994;330:1697–1702. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez-Guerrero J, Colditz GA, Karlson EW, et al. Silicone breast implants and the risk of connective-tissue diseases and symptoms. N Engl J Med. 1995;332:1666–1670. [DOI] [PubMed] [Google Scholar]
- 26.Janowsky EC, Kupper LL, Hulka BS. Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases. N Engl J Med. 2000;342:781–790. [DOI] [PubMed] [Google Scholar]
- 27.Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum. 2001;44:2477–2484. [DOI] [PubMed] [Google Scholar]
- 28.Brawer AE. Silicon and matrix macromolecules: new research opportunities for old diseases from analysis of potential mechanisms of breast implant toxicity. Med Hypotheses. 1998;51:27–35. [DOI] [PubMed] [Google Scholar]
- 29.Vasey FB, Havice DL, Bocanegra TS, et al. Clinical findings in symptomatic women with silicone breast implants. Semin Arthritis Rheum. 1994;24(1 suppl 1):22–28. [DOI] [PubMed] [Google Scholar]
- 30.de Boer M, Colaris M, van der Hulst RRWJ, et al. Is explantation of silicone breast implants useful in patients with complaints? Immunol Res. 2017;65:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohrich RJ, Kenkel JM, Adams WP, et al. A prospective analysis of patients undergoing silicone breast implant explantation. Plast Reconstr Surg. 2000;105:2529–2537; discussion 2538–2543. [DOI] [PubMed] [Google Scholar]
- 32.Wee CE, Younis J, Isbester K, et al. Understanding breast implant illness, before and after explantation: a patient-reported outcomes study. Ann Plast Surg. 2020;85(S1 Suppl 1):S82–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee M, Ponraja G, McLeod K, et al. Breast implant illness: a biofilm hypothesis. Plast Reconstr Surg Glob Open. 2020;8:e2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slavin SA, Goldwyn RM. Silicone gel implant explantation: reasons, results, and admonitions. Plast Reconstr Surg. 1995;95:63–69. [DOI] [PubMed] [Google Scholar]
- 35.Thomas WO, 3rd, Harper LL, Wong SW, et al. Explantation of silicone breast implants. Am Surg. 1997;63:421–429. [PubMed] [Google Scholar]
- 36.Maijers MC, de Blok CJ, Niessen FB, et al. Women with silicone breast implants and unexplained systemic symptoms: a descriptive cohort study. Neth J Med. 2013;71:534–540. [PubMed] [Google Scholar]
- 37.Melmed EP. A review of explantation in 240 symptomatic women: a description of explantation and capsulectomy with reconstruction using a periareolar technique. Plast Reconstr Surg. 1998;101:1364–1373. [DOI] [PubMed] [Google Scholar]
- 38.Svahn JK, Vastine VL, Landon BN, et al. Outcome of mammary prostheses explantation: a patient perspective. Ann Plast Surg. 1996;36:594–600. [DOI] [PubMed] [Google Scholar]
- 39.Kappel RM, Pruijn GJ. The monobloc hydrogel breast implant, experiences and ideas. Eur J Plast Surg. 2012;35:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters W, Pritzker K, Smith D, et al. Capsular calcification associated with silicone breast implants: incidence, determinants, and characterization. Ann Plast Surg. 1998;41:348–360. [DOI] [PubMed] [Google Scholar]
- 41.Thomsen JL, Christensen L, Nielsen M, et al. Histologic changes and silicone concentrations in human breast tissue surrounding silicone breast prostheses. Plast Reconstr Surg. 1990;85:38–41. [DOI] [PubMed] [Google Scholar]
- 42.Copeland M, Choi M, Bleiweiss IJ. Silicone breakdown and capsular synovial metaplasia in textured-wall saline breast prostheses. Plast Reconstr Surg. 1994;94:628–633; discussion 634. [PubMed] [Google Scholar]
- 43.Narins RS, Beer K. Liquid injectable silicone: a review of its history, immunology, technical considerations, complications, and potential. Plast Reconstr Surg. 2006;118(Suppl 3):77S–84S. [DOI] [PubMed] [Google Scholar]
- 44.Beekman WH, Feitz R, van Diest PJ, et al. Migration of silicone through the fibrous capsules of mammary prostheses. Ann Plast Surg. 1997;38:441–445. [DOI] [PubMed] [Google Scholar]
- 45.Peters W, Smith D, Fornasier V, et al. An outcome analysis of 100 women after explantation of silicone gel breast implants. Ann Plast Surg. 1997;39:9–19. [DOI] [PubMed] [Google Scholar]