Abstract
Death receptor Fas transduces cell death signaling upon stimulation by Fas ligand, and this death signaling is mediated by caspase. Recently, we reported that the cell cycle regulator p21 interacts with procaspase 3 to resist Fas-mediated cell death. In the present study, the molecular characterization and functional region of the procaspase 3-p21 complex was further investigated. We observed the p21 expression in the mitochondrial fraction of HepG2 cells and detected Fas-mediated cell death only in the presence of actinomycin D. However, mitochondrial-DNA-lacking HepG2 (MDLH) cells showed this effect even in the absence of actinomycin D. Both p21 and procaspase 3 were expressed in MDLH cells, but the procaspase 3-p21 complex formation was not observed. Interestingly, the resistance to Fas-mediated cell death in the MDLH cells without actinomycin D was recovered after microinjection of HepG2-derived mitochondria into the MDLH cells. We conclude that mitochondria are necessary for procaspase 3-p21 complex formation and propose that the mitochondrial role during cell death is not only death induction but also death suppression.
Cell death is an essential phenomenon for cell homeostasis, as well as cell growth, and its occurrence during embryonic and postembryonic development has been well documented (20, 39). There are two distinct processes leading to cell death: apoptotic cell death and necrotic cell death (39). Apoptotic cell death is accompanied by the condensation and/or fragmentation of nuclei, as well as apoptotic body formation and chromosomal DNA fragmentation into 180-bp oligomers (39). Multiple studies have demonstrated the important role of apoptotic cell death in various disease states and physiological cell death (21), and many factors involved with the death signaling have been identified.
Fas, a transmembrane protein belonging to the tumor necrosis factor/nerve growth factor receptor family (21), transduces the death signaling upon stimulation by Fas ligand or an agonistic Fas antibody, such as the CH-11 clone (41). The molecular mechanism of Fas-mediated apoptosis has been extensively investigated. Caspase is the term used for the interleukin-1β converting enzyme (ICE)/CED-3 cysteine proteinase family (1). During death induction, the sequential activation of the ICE and CPP32 subfamilies has been reported (6, 27, 29, 31, 33), and this phenomenon is known as the “ICE cascade.” At present, 10 genes have been identified as part of the caspase family, and the CPP32 subfamily, including caspase 3 (CPP32/Yama/Apopain [7, 23]) and caspase 8 (FLICE/MACH [2, 19]), in particular acts as the dominant regulator in the death signaling. Therefore, the regulation of CPP32 subfamily activation is an especially important focus for cell death research.
Among the members of the CPP32 subfamily, caspase 3 is especially important in the understanding of apoptotic cell death because of its variant substrate specificity. Cytoplasmic serine proteinase (32), caspase 8 (38), and/or cytotoxic-T-lymphocyte-derived granzyme B (4) proteolyses caspase 3 for its activation, and activated caspase 3 proteolyses and/or activates poly(ADP-ribose) polymerase (37), lamin (14), and/or DFF (16) to induce apoptotic cell death.
Recently, we reported that the cell cycle regulator p21 (Sdi1/CIP1/WAF1) and the IAP gene family ILP act as inactivators of caspase 3 (35, 36). p21 is especially unique in that it interacts with only procaspase 3 by each N-terminal sequence and suppresses its activation by the masking of the cytoplasmic serine proteinase-cleaving site (35, 36). Thus, the activation of caspase 3 is regulated by p21, and procaspase 3-p21 complex formation is an essential system for the cell death since cell survival is a result of cell death suppression (35). In the present study, we further characterized the death suppression machinery by procaspase 3-p21 complex formation. Our results suggest that mitochondria play key role in procaspase 3-p21 complex formation.
MATERIALS AND METHODS
Cell line and culture.
Human hepatoma HepG2 cells were supplied by Yoshihide Tsujimoto (10) and were maintained in RPMI 1640 medium (GIBCO-BRL) supplemented with 10% heat-inactivated fetal calf serum (FCS; GIBCO-BRL) in a humidified atmosphere of 5% CO2 and 95% air.
Preparation of HepG2 cells lacking mitochondrial DNA.
Preparation of the HepG2 cells lacking mitochondrial DNA (MDLH) was done as previously described (5, 11, 13). HepG2 cells were cultured in RPMI 1640 medium containing ethidium bromide (0.4 μg/ml) for about 2 months. The loss of mitochondrial DNA was determined by use of Southern blotting analysis, cell cycle arrest in conditioned medium, and cell growth recovery in uridine-containing medium.
Immunofluorescence analysis of p21.
Cellular localization of p21 was investigated by immunofluorescence. HepG2 cells were fixed with cold fix solution (95% ethyl hydroxide, 5% acetyl hydroxide) for 30 min and then blocked with mouse serum for 2 h. Anti-human p21 antibody (Santa Cruz Biotechnology) was diluted 1/20 with phosphate-buffered saline (PBS) and incubated with the fixed HepG2 cells for 18 h. Cellular localization of p21 was detected by fluorescein isothiocyanate (FITC)-labeled anti-mouse immunoglobulin G IgG by confocal fluorescence microscopy (Leica Japan, Tokyo, Japan). In the present study, nuclei and mitochondria were also detected with propidium iodide (PI) and MitoTracker RED (Molecular Probe, Inc.).
Preparation of fractionated proteins.
Cells were collected, washed with ice-cold PBS, and then suspended in buffer I (2 mM EDTA, 10 mM Tris-HCl; pH 7.5). After incubation on ice for 10 min, an equal volume of buffer II (0.5 M sucrose, 0.1 M KCl, 10 mM MgCl2, 2 mM CaCl2, 2 mM EDTA, 10 mM Tris-HCl; pH 7.5) was added. The nuclear-rich fraction was pelleted by centrifugation (2,700 rpm for 10 min). The supernatant was removed and placed in a separate tube and again centrifuged (43,000 rpm for 90 min). The supernatant was collected as the cytosol-rich fraction, and the pellet was dissolved in buffer III (8 mM CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} 150 mM NaCl, 0.1 M sucrose, 2 mM EDTA, 10 mM Tris-HCl; pH 7.5) and incubated at 4°C for 2 h. The membrane-rich fraction was then collected by centrifugation (43,000 rpm for 60 min). Mitochondria were collected as previously described (8), and the proteins were extracted with 1% Nonidet P40-containing PBS. Each fractionation was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 5 to 20% gels, and p21-expression was detected by immunoblotting analysis with anti-human p21 antibody and the ECL detection system (Amersham).
Immunoblotting analysis.
Sample proteins separated by SDS-PAGE were transferred onto nitrocellulose membranes with a semidry blotting system. The membranes were blocked with PBS containing 5% (wt/vol) skim milk at room temperature for 1 h, washed with a mixture of PBS and 0.05% Tween 20 (Tween-PBS; Sigma), and then incubated overnight at room temperature with each antibody diluted with PBS. After being washed with Tween-PBS, the membranes were incubated with 1,000-fold-diluted biotinylated anti-mouse IgG antibody (BioSource), washed with Tween-PBS, and then incubated with avidin-horseradish peroxidase (Vector Laboratories) at room temperature for 1 h. The membranes were washed with Tween-PBS and then developed with the ECL system.
Immunoprecipitation analysis.
Anti-caspase 3 antibody (Transduction Laboratory) or anti-p21 antibody (Santa Cruz) was mixed with 1 mg of protein from each cell extract for 30 min. Protein A-Sepharose was then added, and the mixture was incubated for 2 h. The immunoprecipitates were heated in SDS sample buffer and separated on 5 to 20% polyacrylamide gels. After transfer, the membranes were immunoblotted, washed with Tween-PBS, and developed by using the ECL system (Amersham).
RESULTS
Cellular localization of p21.
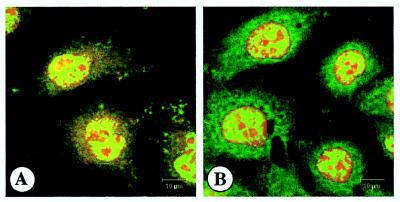
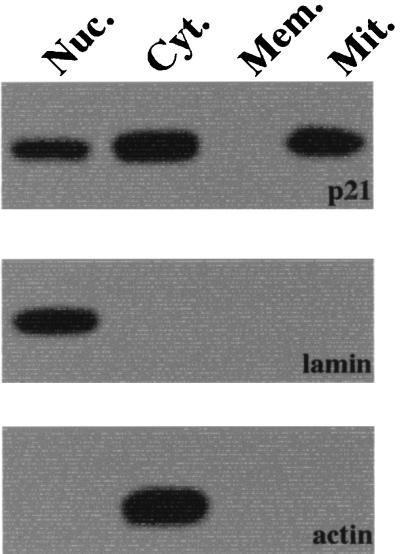
Immunofluorescence analysis was performed to localize the p21 in HepG2 cells. When cells were stained with anti-human p21 antibody, p21 expression was detected in the nuclei and cytoplasm (Fig. 1). In the present study, HepG2 cells were stained with PI (nuclear marker) or MitoTracker Red (dominant marker for mitochondria). To further localize the p21, immunoblotting analysis of cellular fractionations was performed. As shown in Fig. 2, p21 expression was detected in nuclear, cytoplasmic, and mitochondrial proteins but not in the membrane proteins.
FIG. 1.
Immunofluorescence analysis of p21. HepG2 cells were stained with FITC-labeled anti-human p21 antibody (green staining in panels A and B). Cells were also stained with MitoTracker Red (a dominant marker for mitochondria [red in panel A]) and PI (nuclear marker [red in panel B]). After the staining, cells were examined by confocal fluorescence microscopy.
FIG. 2.
Immunoblotting analysis of p21. Nuclear (Nuc.), cytoplasmic (Cyt.), membrane (Mem.), and mitochondrial (Mit.) proteins were collected from HepG2 cells and then separated by SDS-PAGE. After the SDS-PAGE separation, the expression of p21 (top panel), lamin (middle panel, nuclear protein marker), and actin (bottom panel, cytoplasmic protein marker) were examined by immunoblotting.
Fas-mediated cell death in MDLH cells.
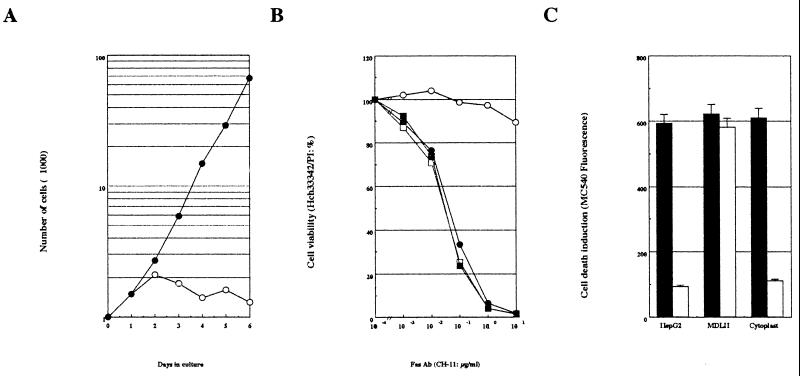
The mitochondrial localization of p21 in HepG2 cells was of particular interest, and the role of mitochondrial p21 was further examined. We prepared mitochondrial-DNA-lacking HepG2 (MDLH) cells by a previously described method (5, 11, 13). As shown in Fig. 3A, ethidium bromide treatment (for about 2 months) downregulated cell growth in the HepG2-conditioned medium (RPMI 1640, 10% FCS), and cell growth was restored by the addition of uridine. These results are consistent with previous observations (5, 11, 13). In addition, Southern blotting analysis revealed that cellular DNA extracted from the MDLH cells did not contain mitochondrial DNA (data not shown). Thus, we established MDLH cells as mitochondrial-DNA-lacking HepG2 cells.
FIG. 3.
Effect of mitochondrial DNA in Fas-mediated cell death. (A) Preparation of MDLH cells. HepG2 cells were treated with 0.4 μg of ethidium bromide for about 2 months. After this treatment, cell growth in the conditioned medium (RPMI 1640, 10% FCS) with (●) or without (○) 50 μg of uridine per ml was measured by using trypan blue. (B) Fas-mediated cell death in HepG2 and MDLH cells. HepG2 (circles) and MDLH (squares) cells were treated with various concentrations of agonistic Fas antibody (CH-11 clone) in the presence (solid symbols) or absence (open symbols) of 0.5 μg of actinomycin D per ml for 24 h. After treatment, cell viability was measured with Hoechst 33342-PI staining. (C) Fas-mediated cell death in HepG2 and MDLH cells, and HepG2 cytoplast. HepG2 and MDLH cells and HepG2 cytoplasts were treated with 1 μg of CH-11 clone per ml in the presence (solid column) or absence (open column) of 0.5 μg of actinomycin D per ml for 24 h. After treatment, cell death induction was measured by using Merocyanine 540.
Fas-mediated cell death in the HepG2 and MDLH cells, as well as in HepG2 cytoplasts, was investigated (Fig. 3B and C). HepG2 cells showed Fas-mediated cell death only in the presence of the de novo protein synthesis inhibitor (transcriptional inhibitor) actinomycin D, a finding consistent with previous reports (10, 35). Interestingly, however, MDLH cells showed the Fas-mediated cell death both in the presence and in the absence of actinomycin D. In contrast, cells lacking nuclear DNA (HepG2 cytoplasts prepared by a previously described method [12]) showed the same trend as the HepG2 cells. These results suggest an involvement of mitochondrial DNA in the resistance to Fas-mediated cell death of the HepG2 cell.
Involvement of p21 on mitochondria in the resistance to Fas-mediated cell death.
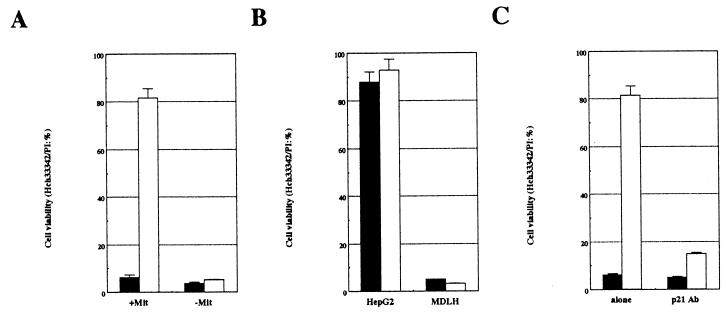
Our results strongly suggested an involvement of the mitochondria in the resistance to the Fas-mediated cell death of HepG2 cells. To further examine the physiological role of mitochondria during Fas-mediated cell death, HepG2-derived mitochondria were microinjected into MDLH cells. Under these conditions, Fas-mediated cell death was encountered only in the presence of actinomycin D (Fig. 4A). We also demonstrated that microinjection of p21 rescued HepG2 cells from Fas-mediated cell death even in the presence of actinomycin D. However, its injection into MDLH cells did not rescue them from Fas-mediated cell death (Fig. 4B). Further, mitochondria microinjected into MDLH cells suppressed Fas-mediated cell death, but mitochondria treated with p21 monoclonal antibody did not (Fig. 4C). These results suggest that mitochondria are essential for the p21-induced death suppression.
FIG. 4.
Effect of HepG2-derived mitochondrion microinjection into MDLH cells. (A) MDLH cells were microinjected with (+Mit) or without (−Mit) HepG2-derived mitochondria. (B) p21 was microinjected into HepG2 or MDLH cells. (C) HepG2-derived mitochondria were treated with p21 antibody (p21 Ab) or without it (alone) for 4 h on ice and then were microinjected into MDLH cells. After each microinjection, cells were treated with 1 μg of CH-11 clone per ml in the presence (solid columns) or absence (open columns) of 0.5 μg of actinomycin D per ml for 24 h, and then cell viability was measured by the Hoechst 33342-PI staining procedure.
Mitochondria are essential for procaspase 3-p21 complex formation.
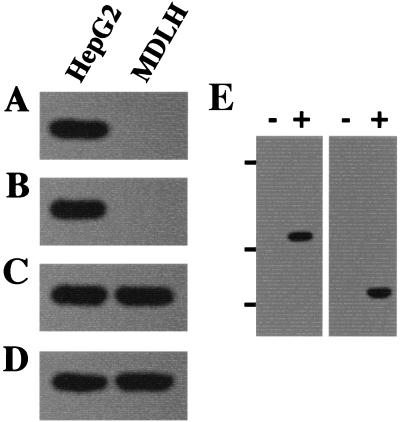
These results demonstrate that mitochondria are involved in the resistance to Fas-mediated cell death in HepG2 cells. We had previously reported that procaspase 3-p21 complex formation contributes to the resistance to Fas-mediated cell death (35), and p21 expression was encountered in the mitochondrial fraction of HepG2 cells in the current study (Fig. 1 and 2). Therefore, we investigated the effect of mitochondria in procaspase 3-p21 complex formation. Both HepG2 and MDLH cells showed expression of p21 and procaspase 3, but procaspase 3-p21 complex formation was encountered only in the HepG2 cells and not in the MDLH cells (Fig. 5A to D). However, MDLH cells showed procaspase 3-p21 complex formation when cells were microinjected with HepG2-derived mitochondria (Fig. 5E). These results strongly suggest that mitochondria are essential for procaspase 3-p21 complex formation to resist Fas-mediated cell death.
FIG. 5.
Procaspase 3-p21 complex formation in HepG2 and MDLH cells. Coimmunoprecipitation (A and B) and immunoblotting (C and D) analysis were performed to investigate the effect of mitochondrial DNA in procaspase 3-p21 complex formation. p21 (A) or caspase 3 (B) immunoprecipitates collected from HepG2 or MDLH cells were separated by SDS-PAGE. Immunoblotting with antibodies to caspase 3 (A) or p21 (B) was then performed. Each cell extract was also separated by SDS-PAGE and then immunoblotted with antibodies to caspase 3 (C) or p21 (D). (E) MDLH cells were microinjected with (+) or without (−) HepG2-derived mitochondria, and then p21 (left) or caspase 3 (right) immunoprecipitates were separated by SDS-PAGE. After SDS-PAGE, an immunoblotting procedure was performed with antibody to caspase 3 (left) or p21 (right) to examine procaspase-p21 complex formation. Bars on the left side show the positions of the protein markers: 45, 30, and 20.1 kDa from top to bottom.
DISCUSSION
Death receptor Fas transduces cell death signaling into cells upon stimulation by Fas ligand and is involved with various physiological cell death and disease states (21, 30, 34). Recent studies revealed various death-associated factors. Caspase, a member of the ICE/CED-3 cysteine proteinase family (1), is an essential factor in Fas-mediated cell death signaling (20). There are three subfamilies known as the ICE, CPP32, and ICH-1 subfamilies. Among the members of the CPP32 subfamily of caspase, caspase 3 (CPP32/Yama/Apopain) is important in cell death induction. Caspase 3 proteolyses and/or activates poly(ADP-ribose) polymerase (37), lamin (14), and/or DFF (16), and its activation is triggered by cytoplasmic serine proteinase (32), cytotoxic-T-lymphocyte-derived granzyme B (4), and/or caspase 8 (FLICE/MACH [38]). Recently, we reported that activation of caspase 3 during Fas-mediated cell death was regulated by the cell cycle regulator p21 and the IAP gene family ILP (35). The N-terminal region (16- to 33-amino-acid sequence) binds to the p3 region of procaspase 3, and ILP binds to the caspase 3-p17 region, including the active site (36). The inactivation mechanism of p21 is especially unique that p21 directly interacts with only procaspase 3 and not with activated caspase 3 (35). In addition, p21 protects procaspase 3 from caspase 3-activating cytoplasmic serine proteinase (32, 36). Therefore, we further investigated the inactivation of caspase 3 by p21 in the present study.
We examined the HepG2 cellular localization of p21 by using immunofluorescence and immunoblotting methods and observed p21 expression in nuclear, cytoplasmic, and mitochondrial fraction. p21 was identified originally as a cyclin-dependent-kinase (Cdk) inhibitor. It interacts with Cdk and proliferating cell nuclear antigen (PCNA) to induce cell cycle arrest (3, 17, 22). Therefore, the nuclear p21 is most likely complexed with Cdk and PCNA, whereas the cytoplasmic p21 remains unbound. As described above, we demonstrated p21 expression in the mitochondrial fraction of HepG2 cells. This is notable since characterization of mitochondrial p21 has not been reported and recent cell death investigations indicate that mitochondria play an important role in cell death signaling (9).
To investigate the effect of mitochondrial p21 in Fas-mediated cell death, we prepared MDLH cells. HepG2 cells show Fas-mediated cell death upon stimulation by the agonistic anti-human Fas antibody CH-11 clone (41) only in the presence of the de novo protein synthesis inhibitor (transcriptional inhibitor) actinomycin D (10, 35). This resistance to Fas-mediated cell death in the absence of actinomycin D is due to caspase 3 inactivation by p21 and ILP (35). In the present study, however, Fas-mediated cell death in the absence of actinomycin D was encountered in the MDLH cells. In contrast, nuclear-DNA-lacking HepG2 cells (HepG2 cytoplasts) also showed Fas-mediated cell death only in the presence of actinomycin D. On the basis of these results, we suggest that mitochondria are necessary for the resistance to Fas-mediated cell death of HepG2 cells and that the mitochondrial regulation of cell death involves not only death induction but also death suppression. The role of mitochondria during cell death is death induction by caspase 3 activation. This activation is induced by mitochondrial factors, such as cytochrome c and AIF (9, 15, 42), and Bcl-2 suppresses this step (26, 28, 40). However, a mitochondrial factor(s) is not required for caspase 3 activation in all cases of cell death, since caspase 8 (FLICE/MACH) and cytotoxic-T-lymphocyte-derived granzyme B can directly activate caspase 3 without the influence of mitochondria (4, 38). Therefore, we also suggest that Fas-mediated cell death induction in HepG2 cells does not require the presence of mitochondria and that caspase 3 activation in HepG2 cells is triggered by a cytoplasmic serine proteinase (32) and caspase 8.
We also performed microinjection of mitochondria to further investigate their role in resistance to Fas-mediated cell death. As described above, MDLH cells showed the Fas-mediated cell death both in the presence and in the absence of actinomycin D. In contrast, intact HepG2 cells showed this only in the presence of actinomycin D. When intact HepG2-derived mitochondria were microinjected into MDLH cells, Fas-mediated cell death in MDLH cells was induced only in the presence of actinomycin D. Thus, MDLH cells reacquired the resistance due to exogenous intact mitochondria. These results strongly suggest that the resistance to Fas-mediated cell death encountered in HepG2 cells is regulated by mitochondria. In addition, p21 suppressed Fas-mediated cell death in HepG2 cells but not in MDLH cells, suggesting that p21-induced death suppression requires mitochondria. Actually, p21 which interacted with mitochondria suppressed Fas-mediated cell death, since exogenous mitochondria did not rescue MDLH cells from Fas-mediated cell death when p21 on exogenous mitochondria was masked with its antibody. These results strongly suggest that p21, especially expressed on mitochondria, is important for the suppression of Fas-mediated cell death as a result of caspase 3 inactivation.
In our previous study (35) and in the present study, we demonstrated that an important factor for the caspase 3 inactivation, p21, was detected in the mitochondrial fraction. Cells lacking mitochondrial DNA did not show the resistance to Fas-mediated cell death, but resistance occurred after the microinjection of intact HepG2-derived mitochondria. These results led us to the possibility that mitochondria play an essential role in procaspase 3-p21 complex formation. As shown in Fig. 5, we demonstrated that MDLH cells could not form the procaspase 3-p21 complex. Since expression of both p21 and procaspase 3 was detected in MDLH cells, the interruption of procaspase 3-p21 complex formation in MDLH cells is most likely not due to the absence of either protein, whereas MDLH cells showed procaspase 3-p21 complex formation only when HepG2-derived mitochondria were microinjected into MDLH cells. We therefore suggest that the procaspase 3-p21 complex formation to resist Fas-mediated cell death is induced directly by mitochondria.
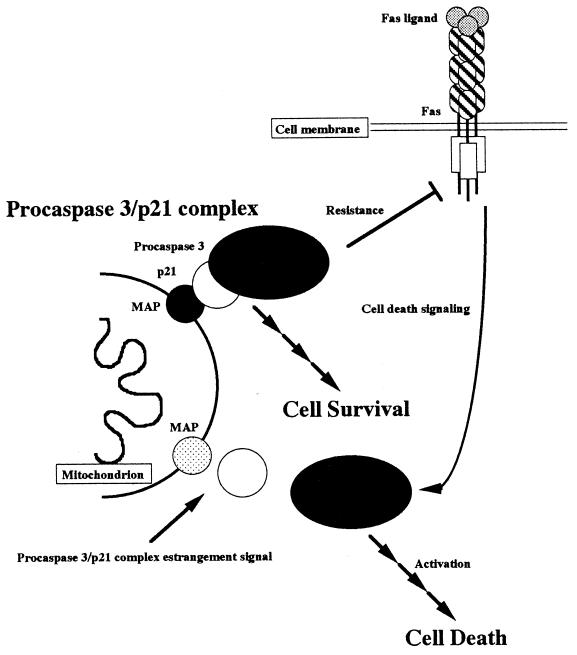
The mitochondrion is an organelle essential for cell growth and energy supply (5). Recently, mitochondria were additionally reported to be a cell-death-inducing organelle. During cell death signaling, mitochondrial damage, such as the decreased mitochondrial membrane potential, occurs at an early step (24, 28), and the release of death inducers such as cytochrome c and AIF (26, 40) can activate caspase. This early mitochondrial damage during cell death initiation is intriguing, partly because mitochondrial parasitism during evolution was first suggested some time ago (18). Is the role of mitochondria death induction and/or energy supply? Some viruses operate the death suppression system when they infect host cells, such as the death suppressor expression induced by the cowpox crmA virus (25). Do mitochondria also alter the death suppression system? Our conclusions regarding these possibilities are addressed schematically in Fig. 6. p21 is an essential factor for caspase 3 inactivation and interacts with mitochondria via an unknown mitochondrial protein which acts as the adapter. Cells lacking mitochondrial DNA showed Fas-mediated cell death even in the absence of actinomycin D, and procaspase 3-p21 complex formation does not occur. p21 does not contain a membrane-associated region in its sequence, so a physical interaction between mitochondria and p21 may be mediated by a mitochondrial protein since procaspase 3-p21 complex formation did not occur in the absence of mitochondrial DNA. We suggest that the death suppression (caspase 3 inactivation) machinery, i.e., the procaspase 3-p21 complex formation, occurs at the mitochondria and that the mitochondrial regulation of cell death is not only death induction but also death suppression. In addition, we propose that mitochondria are involved with cell survival due to the support of the procaspase 3-p21 complex formation to resist Fas-mediated cell death.
FIG. 6.
Schematic model of cell death and cell survival regulated by mitochondria. Fas-mediated cell death signaling is suppressed by procaspase 3-p21 complex formation on mitochondria. p21 interacts with mitochondria via a mitochondrial adapter protein (MAP). Due to procaspase 3-p21 complex formation, the cell is protected from death stimulation and can survive (cell survival). After estrangement of the procaspase 3-p21 complex formation (as with actinomycin D treatment), Fas-mediated cell death signaling is transduced and the cell dies (cell death).
In the present study, we demonstrated that procaspase 3-p21 complex formation occurred in mitochondria via a mitochondrial adapter protein and that the estrangement of this complex is necessary for the transduction of cell death signaling. We found that the estrangement of procaspase 3-p21 complex formation was induced by the downregulation of p21 as a result of actinomycin D treatment (35). This evidence is important for the understanding of the molecular machinery of both cell death and cell survival. Therefore, it will be useful to identify the mitochondrial adapter protein and to study further the estrangement signal of the procaspase 3-p21 complex.
ACKNOWLEDGMENTS
We thank Yoshihide Tsujimoto, Osaka University Medical School, for human hepatoma HepG2 cells; Shigeomi Shimizu, Osaka University Medical School, for his valuable advice about the preparation of MDLH cells; Naomi Haga, University of Tokyo, for his comments about experiments with mitochondria; Hiroko Katoh, Leica Co., Ltd., for her technical support of confocal-fluorescence microscopy; and Masayuki Miura, Osaka University of Medical School, and Mitsuru Furusawa, Daiichi Pharmaceutical Co., Ltd., for their valuable discussions.
The preparation of the manuscript was supported by the Idest Inc., Edmond, Okla.
REFERENCES
- 1.Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornbery N A, Wong W W, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 2.Boldin M P, Goncharov T M, Golstev Y V, Wallach D. Involvement of MACH, a novel MORT-1/FADD-interacting protease, in Fas/APO-1 and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Jackson P K, Kirschner M W, Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995;374:386–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- 4.Darmon A J, Nicholson D W, Bleackley R C. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature. 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- 5.Desjardins P, Frost E, Morais R. Ethidium bromide-induced loss of mitochondrial DNA from primary chicken embryo fibroblasts. Mol Cell Biol. 1985;5:1163–1169. doi: 10.1128/mcb.5.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enari M, Talanian R V, Wong W W, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes-Alnemri T, Litwack G, Alnemri E S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1β-converting enzyme. J Biol Chem. 1994;267:30761–30764. [PubMed] [Google Scholar]
- 8.Fleisher S, Kervina M. Subcellular fractionation of rat liver. Methods Enzymol. 1974;31:6–41. doi: 10.1016/0076-6879(74)31005-1. [DOI] [PubMed] [Google Scholar]
- 9.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa J, Kamada S, Kamiike W, Shimizu S, Imazu T, Matsuda H, Tsujimoto Y. Involvement of CPP32/Yama(-like) proteases in Fas-mediated apoptosis. Cancer Res. 1996;56:1713–1718. [PubMed] [Google Scholar]
- 11.Jacobson M D, Burne J F, King M P, Miyashita T, Reed J C, Raff M C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993;361:365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson M D, Burne J F, Raff M C. Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO J. 1994;13:1899–1910. doi: 10.1002/j.1460-2075.1994.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King M P, Attardi G. Human cells lacking mtDNA: Repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 14.Lazebnik Y A, Takahashi A, Moir R D, Goldman R D, Poirier G G, Kaufmann S H, Earnshaw W C. Studies of lamin proteinase reveal multiple parallel biochemical pathways during apoptosis. J Biol Chem. 1995;92:9042–9046. doi: 10.1073/pnas.92.20.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 17.Luo Y, Hurwitz J, Massague J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature. 1995;375:159–161. doi: 10.1038/375159a0. [DOI] [PubMed] [Google Scholar]
- 18.Margulis L. Symbiotic theory of the origin of eukaryotic organelles; criteria for proof. Symp Soc Exp Biol. 1975;29:21–38. [PubMed] [Google Scholar]
- 19.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 20.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 21.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi M, Robetorye R S, Adami G R, Pereira-Smith O M, Smith J R. Identification of the active region of the DNA synthesis inhibitory gene p21Sdi1/CIP1/WAF1. EMBO J. 1995;14:555–563. doi: 10.1002/j.1460-2075.1995.tb07031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson D W, Ali A, Thornbery N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnic Y A, Munday N A, Raju S M, Smulson M E, Yamin T T, Yu V L, Miller D K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 24.Petit P X, Lecoeur H, Zorn E, Dauguet C, Mignotte B, Gougeon M-L. Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J Cell Biol. 1995;130:157–167. doi: 10.1083/jcb.130.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray C A, Black R A, Kronheim S R, Greenstreet T A, Sleath P R, Salvesen G S, Pickup D J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1β converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacronique V, Matsuda H, Tsujimoto Y. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc Natl Acad Sci USA. 1998;95:1455–1459. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu S, Eguchi Y, Kamiike W, Matsuda H, Tsujimoto Y. Bcl-2 expression prevents activation of ICE protease cascade. Oncogene. 1996;12:2251–2257. [PubMed] [Google Scholar]
- 28.Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda H, Tsujimoto Y. Bcl-2 blocks loss of mitochondrial membrane potencial while ICE inhibitors act at a different step during inhibition of death induced by respiratory chain inhibitors. Oncogene. 1996;13:21–29. [PubMed] [Google Scholar]
- 29.Suzuki A. Amyloid β-protein induces necrotic cell death mediated by ICE cascade in PC12 cells. Exp Cell Res. 1997;234:507–511. doi: 10.1006/excr.1997.3639. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki A, Enari M, Eguchi Y, Matsuzawa A, Nagata S, Tsujimoto Y, Iguchi T. Involvement of Fas in regression of vaginal epithelia after ovariectomy and during an estrous cycle. EMBO J. 1996;15:211–215. [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki A, Iwasaki M, Kato M, Wagai N. Sequential operation of ceramide synthesis and ICE cascade in CPT-11-initiated apoptotic death signaling. Exp Cell Res. 1997;233:41–47. doi: 10.1006/excr.1997.3498. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki A, Iwasaki M, Wagai N. Involvement of cytoplasmic serineproteinase and CPP32 subfamily in the molecular machinery of caspase 3 activation during Fas-mediated apoptosis. Exp Cell Res. 1997;233:48–55. doi: 10.1006/excr.1997.3546. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki A, Kawabata T, Kato M. Necessity of interleukin-1β converting enzyme (ICE) cascade in taxotere-initiating death signaling. Eur J Pharmacol. 1998;343:87–92. doi: 10.1016/s0014-2999(97)01520-3. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki A, Matsuzawa A, Iguchi T. Downregulation of Bcl-2 is the first step on Fas-mediated apoptosis of male reproductive tracts. Oncogene. 1996;13:31–37. [PubMed] [Google Scholar]
- 35.Suzuki A, Tsutomi Y, Akahane K, Araki T, Miura M. Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene. 1998;17:931–940. doi: 10.1038/sj.onc.1202021. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki, A., Y. Tsutomi, M. Miura, and K. Akahane. Caspase 3 inactivation to suppress Fas-mediated apoptosis: identification of binding domain with p21 and ILP, and inactivation machinery by p21. Oncogene, in press. [DOI] [PubMed]
- 37.Tewari M, Quan L T, O’Rourke K, Desnoyers S, Zeng Z, Beidler D R, Poirier G G, Salvesen G S, Dixit V M. Yama/CPP32β, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 38.Thornbery N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstorm P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. A combinatorial approach defined specificities of members of the caspase family and granzyme B: functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 39.Wyllie A H, Kerr J F R, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T-I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 41.Yonehara S, Ishii A, Yonehara M. A cell killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]