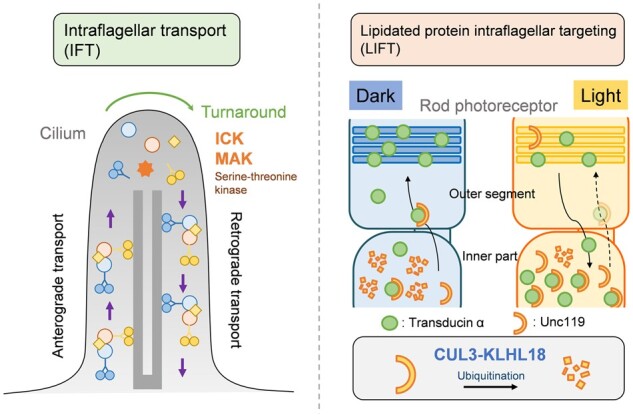
Abstract
Primary cilia are evolutionarily conserved microtubule-based organelles that protrude from the surface of almost all cell types and decode a variety of extracellular stimuli. Ciliary dysfunction causes human diseases named ciliopathies, which span a wide range of symptoms, such as developmental and sensory abnormalities. The assembly, disassembly, maintenance and function of cilia rely on protein transport systems including intraflagellar transport (IFT) and lipidated protein intraflagellar targeting (LIFT). IFT is coordinated by three multisubunit protein complexes with molecular motors along the ciliary axoneme, while LIFT is mediated by specific chaperones that directly recognize lipid chains. Recently, it has become clear that several post-translational modification enzymes play crucial roles in the regulation of IFT and LIFT. Here, we review our current understanding of the roles of these post-translational modification enzymes in the regulation of ciliary protein trafficking as well as their regulatory mechanisms, physiological significance and involvement in human diseases.
Keywords: ICK/CILK1, kinase, MAK, retina, ubiquitin
Graphical Abstract
Fig. 1.
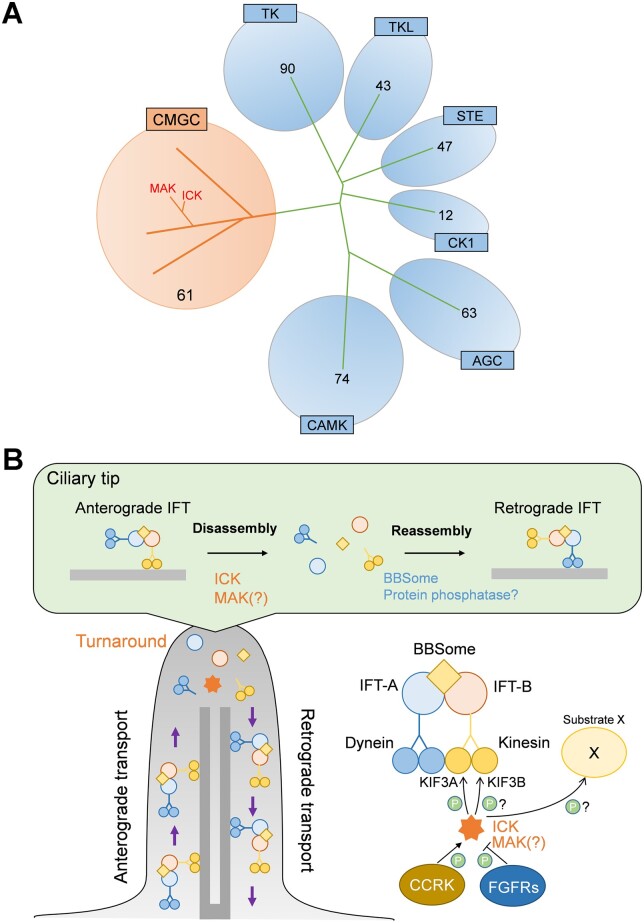
The serine-threonine kinases MAK and ICK in the regulation of IFT. (A) Phylogenetic distribution of protein kinases in humans. MAK and ICK are classified into the CMGC kinase family. TK, Tyrosine kinase; TKL, Tyrosine kinase-like; STE, Homologs of yeast Sterile 7, Sterile 11, Sterile 20 kinases; CK1, Casein kinase 1; AGC, Containing PKA, PKG, PKC families; CAMK, Calcium/calmodulin-dependent protein kinase; CMGC, Containing CDK, MAPK, GSK3, CLK families. Numbers of kinases classified into each family are indicated (110). (B) Proposed model of the regulation of IFT turnaround at the ciliary tip by MAK and ICK.
The primary cilium is a hair-like microtubule-based structure that protrudes from the surface of nearly every cell and performs a wide range of sensory functions across species (1, 2). The ciliary membrane and interior are distinguished from the plasma membrane and the cytoplasm, respectively, by the presence of the transition zone and the transition fibres (3). A variety of signalling receptors localize to primary cilia and receive extracellular stimuli including light, odorants and Hedgehog morphogens. For example, retinal photoreceptor cells develop the outer segment, a light-sensory structure containing components of the phototransduction cascade including photopigments, which are formed initially from the primary cilia in photoreceptor precursors (4). Therefore, cilia function as a signalling centre for multiple transduction pathways. Ciliary dysfunction leads to human diseases termed ‘ciliopathies’, which are characterized by a broad spectrum of symptoms, including polydactyly, cranio-facial abnormalities, brain malformation, obesity, polycystic kidney, anosmia, hearing loss and retinal degeneration (5–7).
Intraflagellar Transport
The biogenesis and maintenance of cilia depend on intraflagellar transport (IFT), the bidirectional protein trafficking system coordinated by three large protein complexes, IFT-B, IFT-A and the BBSome, with molecular motors along the axonemal microtubules of cilia. They assemble into linear arrays, known as IFT trains, which mediate ciliary protein transport along the axoneme in both anterograde and retrograde directions (8). In addition, the three IFT complexes function in ciliary import and export of proteins (9, 10). IFT-B, a 16-subunit complex, mediates anterograde transport from the base to the tip driven by the kinesin-2 motor, whereas IFT-A, a 6-subunit complex, mediates retrograde transport from the tip back to the base driven by the cytoplasmic dynein-2 motor (9, 11). The BBSome, an 8-subunit complex, functions as a coat adaptor for IFT-B and mediates the exit of membrane proteins including GPCRs from cilia (9, 12, 13). In addition, it also assists the assembly of IFT trains at the ciliary base (14). At the ciliary tip, IFT trains are disassembled and then reassembled for turnaround and retrograde transport (15). Mutations in some genes encoding components of IFT trains are known to be associated with ciliopathies including Bardet-Biedl syndrome (BBS) and Joubert syndrome (16).
Serine–Threonine Kinases MAK and ICK in the Regulation of Ciliary Protein Trafficking
Various serine–threonine kinases play crucial roles in many aspects of cilia assembly, disassembly, maintenance and function. Two serine-threonine kinases from a branch of CDK/MAPK/GSK3/CLK (CMGC) kinases, male germ cell-associated kinase (MAK) and intestinal cell kinase (ICK), also known as ciliogenesis-associated kinase 1 (CILK1), are proposed to serve as regulators of IFT turnaround at the ciliary tip and ciliary length in mammalian cells (Fig. 1). Since IFT plays a key role in ciliary length control, regulation of IFT is thought to be important for the ciliary length regulation (17). As the names imply, Mak and Ick were originally identified in male germ cells and intestinal crypt cells, respectively (18, 19). MAK and ICK are evolutionarily conserved mitogen-activating protein kinase-like kinases that exhibit high homology, especially in their catalytic domains (19–21). Mak is predominantly expressed in the retina and testis whereas Ick is ubiquitously expressed among tissues (22). In contrast to the distinct tissue distribution patterns, these protein kinases show a similar subcellular localization. MAK localizes to the distal region of ciliary axonemes in retinal photoreceptor cells (23). ICK localizes mainly to the ciliary tip, which is mediated through the anterograde trafficking by IFT-B (24, 25). Caenorhabditis elegans DYF-5, an orthologue of MAK and ICK, is an IFT cargo molecule transported to the distal segments of sensory cilia (26). In addition, ICK is enriched in ciliary vesicles released from the tip of cilia (27). Mak-deficient mice exhibit elongated photoreceptor ciliary axonemes with the accumulation of IFT88, an IFT-B component, at the distal portion (23). Loss-of-function of ICK causes shortened or elongated cilia, impaired Hedgehog signalling and accumulation of IFT-B, IFT-A and BBSome components at ciliary tips, while ICK overexpression induces accumulation of IFT-B, but not IFT-A and BBSome components at the tip of cilia, suggesting roles of ICK in disassembly of IFT trains in the turnaround process (24, 25, 28–32). Chlamydomonas reinhardtii (Chlamydomonas) LF4, Tetrahymena LF4A, Leishmania mexicana LmxMPK9, and C. elegans DYF-5, orthologues of MAK and ICK, are also involved in the regulation of IFT as well as cilia/flagella length and formation (26, 33–37).
Fig. 1.
The serine-threonine kinases MAK and ICK in the regulation of IFT. (A) Phylogenetic distribution of protein kinases in humans. MAK and ICK are classified into the CMGC kinase family. TK, Tyrosine kinase; TKL, Tyrosine kinase-like; STE, Homologs of yeast Sterile 7, Sterile 11, Sterile 20 kinases; CK1, Casein kinase 1; AGC, Containing PKA, PKG, PKC families; CAMK, Calcium/calmodulin-dependent protein kinase; CMGC, Containing CDK, MAPK, GSK3, CLK families. Numbers of kinases classified into each family are indicated (110). (B) Proposed model of the regulation of IFT turnaround at the ciliary tip by MAK and ICK.
ICK phosphorylates the C-terminal portion of KIF3A, a subunit of kinesin-2, including Thr674 (24, 38). KIF3A Thr674 is positioned in a consensus amino acid sequence for MAK and ICK phosphorylation that is evolutionarily conserved (39). Localization of KIF3A phosphorylated at Thr674 to ciliary tips is observed in mouse embryonic fibroblasts, which is attenuated by Ick deficiency. Inhibition of phosphorylation on serine/threonine residues including Thr674 at the KIF3A C-terminal portion perturbs cilia formation in cultured cells and zebrafish (24). Mouse embryonic fibroblasts carrying a Thr-to-Ala mutation at residue 674 on KIF3A (KIF3A T674A) show slightly elongated cilia without affecting ciliary localization of IFT88 (40). These observations suggest that ICK phosphorylates other substrate protein(s) in addition to the KIF3A C-terminal portion including Thr674 to regulate IFT and ciliary length. In Chlamydomonas, phosphorylation of the kinesin-2 motor subunit FLA8, an orthologue of KIF3B, at Ser663 is required for IFT turnaround at the flagellar tip (41). Intriguingly, FLA8 Ser663 is located in a consensus amino acid sequence for phosphorylation by MAK and ICK that is evolutionarily conserved among species, suggesting that IFT turnaround at the ciliary tip is mediated by phosphorylation of KIF3B in addition to KIF3A by MAK and ICK in mammals. C. elegans hypomorphic mutants of bbs-1, a gene encoding a BBSome component, show accumulation of IFT-B but not IFT-A components at the ciliary tip, as similarly observed in ICK overexpressing cells (14). Given that the BBSome assembles IFT trains at the ciliary base, disassembly and reassembly of IFT trains at the ciliary tip may be mediated by ICK, as well as probably MAK, and the BBSome, respectively.
MAK and ICK in Development, Physiology and Disease
Mak-deficient mice are viable and fertile without obvious developmental abnormalities, but exhibit progressive retinal photoreceptor degeneration (23). Interestingly, mutations in human MAK gene were discovered in patients with autosomal recessive retinitis pigmentosa (RP), a retinal degenerative disease (42, 43). In contrast, Ick-deficient mice exhibit neonatal lethality accompanied with developmental abnormalities observed in multiple organ systems including the bone, lung, kidney, brain, retina, and inner ear (44). In humans, two homozygous loss-of-function mutations in ICK gene, R272Q and G120C, are associated with endocrine-cerebro-osteodysplasia (ECO) syndrome, an autosomal recessive ciliopathy characterized by neonatal lethality with multiple defects involving the endocrine, cerebral, and skeletal systems (31, 45). Another homozygous loss-of-function mutation in human ICK gene, E80K, is associated with short rib-polydactyly syndrome (SRPS), an autosomal recessive ciliopathy showing perinatal lethality with short ribs, shortened and hypoplastic long bones, polydactyly and multiorgan system abnormalities (30). In addition, heterozygous variants in human ICK gene are linked to juvenile myoclonic epilepsy (46). Of them, four strongly linked variants, K220E, K305T, A615T and R632X, affect ICK functions in cilia formation and impair mitosis, cell-cycle exit and radial neuroblast migration while promoting apoptosis in the cerebral cortex (46, 47). In contrast to Ick-deficient mice, homozygous KIF3A T674A knock-in mice exhibit mildly reduced alveolar airspace in the lung, but are viable without gross abnormalities in the bone and brain, suggesting that other substrate(s) in addition to KIF3A Thr674 are phosphorylated by ICK in vivo.
Regulatory Mechanisms of MAK and ICK Activities
MAK and ICK are activated by phosphorylation at the TDY motif in their kinase domain by cell cycle-related kinase (CCRK) in vitro (39). ICK phosphorylation by CCRK negatively regulates ciliogenesis in cultured cells (48). Similar to loss of Mak or Ick, Ccrk deficiency causes dysregulation of cilia length and accumulation of IFT88 at ciliary tips (49). Ccrk-deficient mice show multiple developmental abnormalities associated with dysregulation of Hedgehog signaling, including polydactyly, neural tube patterning defects and malformation of the lung and eye (49–51). CCRK orthologues, Chlamydomonas LF2 and C. elegans DYF-18, also participates in the IFT regulation as well as cilia/flagella length and formation control (26, 36, 52, 53). LF4 phosphorylation at the TDY motif is diminished in the Chlamydomonas lf-2 mutant, suggesting that the LF2-LF4 signaling axis is evolutionarily conserved among species (54). In contrast to CCRK, fibroblast growth factor (FGF) signalling is thought to negatively regulate the ICK activity. Inactivation of Fgf receptor 1 (Fgfr1) or its ligands results in shortened cilia in zebrafish and Xenopus (55). On the other hand, FGFR3 activation shortened cilia and perturbed the localization of IFT20, an IFT-B component, to cilia in mammals (56, 57). Biochemical experiments show that FGFRs interact with, phosphorylate and inactivate ICK. In cultured cells, FGF treatment modulates cilia length through ICK (58).
Lipidated Protein Intraflagellar Targeting in Retinal Photoreceptor Cells
In parallel to IFT, lipidated protein intraflagellar targeting (LIFT) plays crucial roles in establishing a dynamic ciliary signaling compartment (59). Transport of some lipidated proteins into cilia relies on specific chaperones uncoordinated 119 (UNC119) and phosphodiesterase 6δ (PDE6δ), which directly recognize lipid chains in the cytoplasmic region and unload their cargoes inside cilia upon interacting with the small ARF-like GTPase protein ARL3 bound to GTP (60–63). ARL3 is converted from its inactive GDP-bound state to the active GTP-bound state inside cilia by ARL13B, a guanine nucleotide exchange factor (GEF) constitutively localized to cilia (64). In contrast, retinitis pigmentosa 2 (RP2), a GTPase-activating protein (GAP) localized at the base of cilia, is predicted to keep ARL3 in its GDP-bound state outside cilia (65, 66).
Molecular mechanisms and physiological roles of LIFT have been well studied in retinal rod and cone photoreceptor cells. Rod photoreceptor cells are sensitive to light and are responsible for low light vision, while cone photoreceptor cells operate at a brighter range of light intensities and are responsible for high-resolution daylight and colour vision. Subcellular localization of rod transducin, a heterotrimeric G protein that is a mediator of the phototransduction cascade, changes responding to ambient light (67–71). The inner part and outer segment of the photoreceptor cell are connected by a connecting cilium. Rod transducin is transported to the outer segment from the inner part through the connecting cilium, and then concentrated in the outer segment under dark-adapted conditions. After light reception, rod transducin translocates from the outer segment to the inner part through the connecting cilium. This light- and dark-dependent translocation of transducin modulates photosensitivity in rod photoreceptors, thereby contributing to light and dark adaptation. Localization of the α-subunit of rod transducin (rTα) to the outer segment is required for normal light sensitivity of rod photoreceptors (72). In contrast, light-dependent rTα translocation from the outer segment to the inner part protects the retina from the light-induced damage (73–76). UNC119 interacts with the acylated N termini of rTα and suppresses rhodopsin-mediated transducin activation (63, 77). The dark-triggered transport of rTα to the outer segment is inhibited in the Unc119-deficient mouse retina (63). Deletion of unc-119 in C. elegans also blocks G protein trafficking to cilia, suggesting an evolutionarily conserved role of UNC119 (63). Unc119-deficient mice exhibit a slowly progressive retinal degeneration (78). A heterozygous stop codon (K57X) was found in the human UNC119 gene of an individual with late-onset dominant cone dystrophy (79). Transgenic mice carrying the identical mutation show mitochondrial ANT-1-mediated retinal degeneration (79, 80). On the other hand, PDE6δ, encoded by Pde6d, is a prenyl-binding protein that promotes translocation of the βγ-subunit of rod transducin (rTβγ) from the inner part to the outer segment. In the Pde6d−/− mouse retina, rTγ, known to be farnesylated, mislocalizes to the inner part (81, 82). In addition to rTγ, several components of the phototransduction cascade are also prenylated in rod and cone photoreceptors. PDE6α and PDE6β, rod cGMP phosphodiesterase catalytic subunits, PDE6α’, a cone cGMP phosphodiesterase catalytic subunit and rhodopsin kinase (GRK1) are farnesylated or geranylgeranylated (83–85). Reduced localization of GRK1 and PDE6α’ to the outer segment as well as mislocalization of rod PDE6 subunits to the inner part are observed in Pde6d−/− rod and cone photoreceptors (82). As a consequence, loss of Pde6d results in altered electrophysiological properties of photoreceptor cells and a slowly progressing retinal degeneration (82). Consistent with Unc119 and Pde6d deficiency, Arl3 conditional knockout mice show trafficking defects of lipidated proteins including rTα, rTγ, rod PDE6 and GRK1 to outer segments in rod photoreceptors and subsequent retinal degeneration (86). A mutation in the human ARL3 gene is linked to autosomal dominant RP (87). Deletion of Arl13b in the mouse retina leads to mislocalization of rTα and rod PDE6 subunits but faster retinal degeneration than Arl3 deficiency does, suggesting additional functions of ARL13B other than a GEF for ARL3 in rod photoreceptor cells (88, 89). In humans, mutations in the ARL13B gene are associated with Joubert syndrome, an autosomal recessive ciliopathy characterized by multiple symptoms including retinal degeneration (90, 91). Rp2-deficient mice exhibit defects in trafficking of GRK1 as well as rod and cone PDE6 to the outer segment, and subsequent slowly progressing retinal degeneration (92). Mutations in the human RP2 gene are associated with X-linked RP, macular atrophy and cone-rod dystrophy (93–95).
Regulation of Transducin Translocation During Light-Dark Adaptation by the CUL3-KLHL18 Ubiquitin Ligase
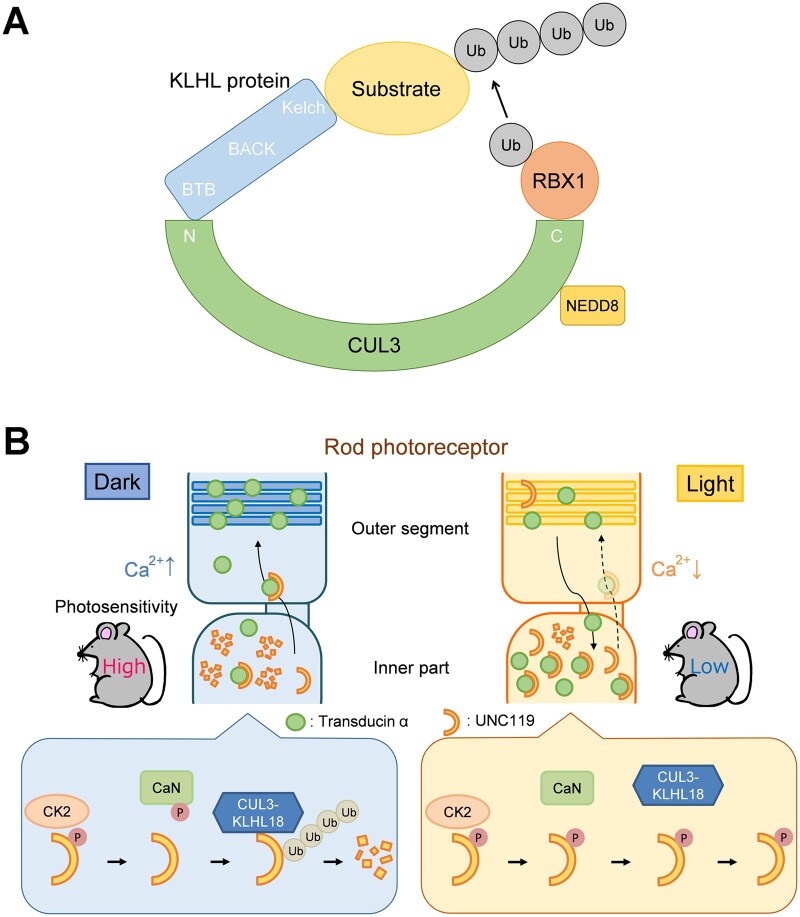
The ubiquitin proteasome system is one of the fundamental regulatory tools used by eukaryotic cells. Cullin-RING (really interesting new gene) ubiquitin ligases (CRLs) form one of the largest groups of ubiquitin E3 ligases that regulate diverse cellular pathways (96). Cullin-3 (CUL3) bridges the interaction between the RING protein RBX1 and substrate adaptors which deliver targets for ubiquitination and proteasomal degradation (97). Covalent attachment of the ubiquitin-like protein NEDD8 to Cullin family proteins is required for the activation of the Cullin-based ubiquitin E3 ligases (98). The N-terminus of CUL3 interacts with the Broad Complex, Tramtrack, Bric-a-Brac (BTB) domain of substrate adaptors including the Kelch-like (KLHL) family proteins, which contain one BTB domain, one BTB and C-terminal kelch (BACK) domain, and five to six Kelch repeats (Fig. 2A) (99). The HUGO Gene Nomenclature Committee (HGNC) presently defines 42 KLHL genes.
Fig. 2.
The CUL3-KLHL18 ubiquitin ligase in the regulation of LIFT. (A) Schematic diagram of CUL3-KLHL ubiquitin ligases. (B) Proposed model of the α-subunit of rod transducin (rTα) translocation during light and dark adaptation in rod photoreceptor cells. In rod photoreceptors, intracellular Ca2+ concentration under dark conditions is higher than that under light conditions. In darkness, CK2-phosphorylated UNC119 is dephosphorylated by calcineurin, a Ca2+-dependent phosphatase. CUL3-KLHL18 efficiently ubiquitinates and degrades the dephosphorylated UNC119, thereby facilitating rTα transport from the inner part to the outer segment. In the light, calcineurin-mediated dephosphorylation of CK2-phosphorylated UNC119 is suppressed due to low Ca2+ concentration. Inefficient ubiquitination and degradation of the phosphorylated UNC119 by CUL3-KLHL18 results in increased amounts of UNC119 and subsequent inhibition of rTα translocation to the outer segment. CaN, calcineurin.
Recently, Kelch-like 18 (Klhl18), one of the Klhl genes, was found to be predominantly expressed in retinal photoreceptor cells. Klhl18-deficient mice exhibit decreased light responses in rod photoreceptors and rTα mislocalization from the outer segment to the inner part. Loss of Klhl18 or treatment of MLN4924, a small molecule inhibitor of the NEDD8-activating enzyme (NAE) (100), suppresses light-induced retinal degeneration. The CUL3-KLHL18 ubiquitin ligase ubiquitinates and degrades UNC119 in rod photoreceptor cells, preferentially under dark conditions. UNC119 overexpression phenocopies the rTα mislocalization observed in the Klhl18−/− mouse retina. These observations suggest that CUL3-KLHL18 modulates rTα translocation during light and dark adaptation through UNC119 ubiquitination and degradation. Furthermore, regulatory mechanisms underlying the CUL3-KLHL18-UNC119 axis were investigated. The phosphorylation level of UNC119 is elevated under light conditions compared with that under dark conditions. UNC119 is phosphorylated by casein kinase 2 (CK2), which is a serine/threonine kinase expressed in retinal photoreceptor cells (101). In contrast, UNC119 is dephosphorylated by Calcineurin, a Ca2+- and calmodulin-dependent serine/threonine protein phosphatase (102, 103). UNC119 degradation by CUL3-KLHL18 is suppressed and facilitated by UNC119 phosphorylation and dephosphorylation, respectively. Inhibition of CK2 decreases the amount of UNC119 in the retina, while inhibition of Calcineurin increases the retinal expression level of UNC119, causes rTα mislocalization to the inner part of photoreceptors, and protects the retina from light-induced damage. Collectively, these results suggest that CUL3-KLHL18 promotes UNC119 ubiquitination and degradation depending on phosphorylation, thereby regulating light- and dark-dependent rTα translocation in the retina (Fig. 2B) (104). Given that light exposure is a suspected risk factor for the progression of age-related macular degeneration and RP (105–108), inhibition of this signaling pathway may be a potential therapeutic target.
Concluding Remarks
It has recently become clear that several post-translational modification enzymes play key roles in the regulation of ciliary protein trafficking. Cumulative evidence of the functional mechanisms of MAK and ICK has unravelled many aspects of the physiological significance of the coordinated IFT turnaround at the ciliary tip and its involvement in human ciliopathies. Identification and functional characterization of the CUL3-KLHL18 ubiquitin ligase in retinal photoreceptor cells put forward our knowledge of a series of regulatory mechanisms from photoreception to light-dark adaptation. However, these findings raise new questions. For example, assuming that IFT trains are disassembled by MAK and ICK at the ciliary tip, how are they reassembled for retrograde IFT? In parallel to the BBSome as mentioned above, serine-threonine phosphatase(s) may also contribute to reassembly of IFT trains at the ciliary tip through dephosphorylating KIF3A and other substrate(s). In addition, although rTβγ also shows light- and dark-dependent translocation between the outer segment and the inner part of retinal photoreceptor cells, which modulates rod light responses (109), rTβγ translocation is independent of CUL3-KLHL18. PDE6δ ubiquitination and degradation by an unknown E3 ubiquitin ligase may underpin rTβγ translocation during light and dark adaptation. Further analyses are needed to unveil comprehensive regulatory mechanisms underlying ciliary protein trafficking, whose understanding would contribute to develop therapeutic strategies to treat human diseases.
Funding
This work was supported by Grant-in-Aid for Scientific Research (18H02593, 20K07326) from the Japan Society for the Promotion of Science, The Cell Science Research Foundation, Suzuken Memorial Foundation, The Uehara Memorial Foundation, and The Takeda Science Foundation.
Conflict of interest
None declared.
Abbreviations
- BBS
Bardet-Biedl syndrome
- CK2
casein kinase 2
- FGF
fibroblast growth factor
- IFT
intraflagellar transport
- LIFT
lipidated protein intraflagellar targeting.
References
- 1.Gerdes J.M., Davis E.E., Katsanis N. (2009) The vertebrate primary cilium in development, homeostasis, and disease. Cell 137, 32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malicki J.J., Johnson C.A. (2017) The cilium: cellular antenna and central processing unit. Trends Cell Biol. 27, 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Gonzalo F.R., Reiter J.F. (2017) Open sesame: how transition fibers and the transition zone control ciliary composition. Cold Spring Harb. Perspect. Biol. 9, a028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tokuyasu K., Yamada E. (1959) The fine structure of the retina studied with the electron microscope. IV. Morphogenesis of outer segments of retinal rods. J. Biophys. Biochem. Cytol. 6, 225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fliegauf M., Benzing T., Omran H. (2007) When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8, 880–893 [DOI] [PubMed] [Google Scholar]
- 6.Nigg E.A., Raff J.W. (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139, 663–678 [DOI] [PubMed] [Google Scholar]
- 7.Anvarian Z., Mykytyn K., Mukhopadhyay S., Pedersen L.B., Christensen S.T. (2019) Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 15, 199–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechtreck K.F. (2015) IFT-cargo interactions and protein transport in cilia. Trends Biochem. Sci. 40, 765–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachury M.V. (2018) The molecular machines that traffic signaling receptors into and out of cilia. Curr. Opin. Cell Biol. 51, 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama K., Katoh Y. (2018) Ciliary protein trafficking mediated by IFT and BBSome complexes with the aid of kinesin-2 and dynein-2 motors. J. Biochem. 163, 155–164 [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum J.L., Witman G.B. (2002) Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3, 813–825 [DOI] [PubMed] [Google Scholar]
- 12.Wingfield J.L., Lechtreck K.F., Lorentzen E. (2018) Trafficking of ciliary membrane proteins by the intraflagellar transport/BBSome machinery. Essays Biochem. 62, 753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye F., Nager A.R., Nachury M.V. (2018) BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J. Cell Biol. 217, 1847–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Q., Zhang Y., Li Y., Zhang Q., Ling K., Hu J. (2012) The BBSome controls IFT assembly and turnaround in cilia. Nat. Cell Biol. 14, 950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chien A., Shih S.M., Bower R., Tritschler D., Porter M.E., Yildiz A. (2017) Dynamics of the IFT machinery at the ciliary tip. Elife 6, e28606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiter J.F., Leroux M.R. (2017) Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18, 533–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa H., Marshall W.F. (2011) Ciliogenesis: building the cell's antenna. Nat. Rev. Mol. Cell Biol. 12, 222–234 [DOI] [PubMed] [Google Scholar]
- 18.Matsushime H., Jinno A., Takagi N., Shibuya M. (1990) A novel mammalian protein kinase gene (mak) is highly expressed in testicular germ cells at and after meiosis. Mol. Cell. Biol. 10, 2261–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Togawa K., Yan Y.X., Inomoto T., Slaugenhaupt S., Rustgi A.K. (2000) Intestinal cell kinase (ICK) localizes to the crypt region and requires a dual phosphorylation site found in map kinases. J. Cell. Physiol. 183, 129–139 [DOI] [PubMed] [Google Scholar]
- 20.Miyata Y., Nishida E. (1999) Distantly related cousins of MAP kinase: biochemical properties and possible physiological functions. Biochem. Biophys. Res. Commun. 266, 291–295 [DOI] [PubMed] [Google Scholar]
- 21.Shinkai Y., Satoh H., Takeda N., Fukuda M., Chiba E., Kato T., Kuramochi T., Araki Y. (2002) A testicular germ cell-associated serine-threonine kinase, MAK, is dispensable for sperm formation. Mol. Cell. Biol. 22, 3276–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsutsumi R., Chaya T., Furukawa T. (2018) Enriched expression of the ciliopathy gene Ick in cell proliferating regions of adult mice. Gene Expr. Patterns. 29, 18–23 [DOI] [PubMed] [Google Scholar]
- 23.Omori Y., Chaya T., Katoh K., Kajimura N., Sato S., Muraoka K., Ueno S., Koyasu T., Kondo M., Furukawa T. (2010) Negative regulation of ciliary length by ciliary male germ cell-associated kinase (Mak) is required for retinal photoreceptor survival. Proc. Natl. Acad. Sci. USA. 107, 22671–22676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaya T., Omori Y., Kuwahara R., Furukawa T. (2014) ICK is essential for cell type-specific ciliogenesis and the regulation of ciliary transport. Embo J. 33, 1227–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura K., Noguchi T., Takahara M., Omori Y., Furukawa T., Katoh Y., Nakayama K. (2020) Anterograde trafficking of ciliary MAP kinase-like ICK/CILK1 by the intraflagellar transport machinery is required for intraciliary retrograde protein trafficking. J. Biol. Chem. 295, 13363–13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi P., Xie C., Ou G. (2018) The kinases male germ cell-associated kinase and cell cycle-related kinase regulate kinesin-2 motility in Caenorhabditis elegans neuronal cilia. Traffic 19, 522–535 [DOI] [PubMed] [Google Scholar]
- 27.Phua S.C., Chiba S., Suzuki M., Su E., Roberson E.C., Pusapati G.V., Schurmans S., Setou M., Rohatgi R., Reiter J.F., Ikegami K., Inoue T. (2017) Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell 168, 264–279 e215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon H., Song J., Shin J.O., Lee H., Kim H.K., Eggenschwiller J.T., Bok J., Ko H.W. (2014) Intestinal cell kinase, a protein associated with endocrine-cerebro-osteodysplasia syndrome, is a key regulator of cilia length and Hedgehog signaling. Proc. Natl. Acad. Sci. USA. 111, 8541–8546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broekhuis J.R., Verhey K.J., Jansen G. (2014) Regulation of cilium length and intraflagellar transport by the RCK-kinases ICK and MOK in renal epithelial cells. PLoS One 9, e108470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paige Taylor S., Kunova Bosakova M., Varecha M., Balek L., Barta T., Trantirek L., Jelinkova I., Duran I., Vesela I., Forlenza K.N., Martin J.H., Hampl A.University of Washington Center for Mendelian GenomicsBamshad M., Nickerson D., Jaworski M.L., Song J., Ko H.W., Cohn D.H., Krakow D., Krejci P. (2016) An inactivating mutation in intestinal cell kinase, ICK, impairs hedgehog signalling and causes short rib-polydactyly syndrome. Hum. Mol. Genet. 25, 3998–4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oud M.M., Bonnard C., Mans D.A., Altunoglu U., Tohari S., Ng A.Y.J., Eskin A., Lee H., Rupar C.A., de Wagenaar N.P., Wu K.M., Lahiry P., Pazour G.J., Nelson S.F., Hegele R.A., Roepman R., Kayserili H., Venkatesh B., Siu V.M., Reversade B., Arts H.H. (2016) A novel ICK mutation causes ciliary disruption and lethal endocrine-cerebro-osteodysplasia syndrome. Cilia 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto S., Chaya T., Omori Y., Kuwahara R., Kubo S., Sakaguchi H., Furukawa T. (2017) Ick ciliary kinase is essential for planar cell polarity formation in inner ear hair cells and hearing function. J. Neurosci. 37, 2073–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berman S.A., Wilson N.F., Haas N.A., Lefebvre P.A. (2003) A novel MAP kinase regulates flagellar length in Chlamydomonas. Curr. Biol. 13, 1145–1149 [DOI] [PubMed] [Google Scholar]
- 34.Bengs F., Scholz A., Kuhn D., Wiese M. (2005) LmxMPK9, a mitogen-activated protein kinase homologue affects flagellar length in Leishmania mexicana. Mol. Microbiol. 55, 1606–1615 [DOI] [PubMed] [Google Scholar]
- 35.Burghoorn J., Dekkers M.P., Rademakers S., de Jong T., Willemsen R., Jansen G. (2007) Mutation of the MAP kinase DYF-5 affects docking and undocking of kinesin-2 motors and reduces their speed in the cilia of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 104, 7157–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurya A.K., Rogers T., Sengupta P. (2019) A CCRK and a MAK kinase modulate cilia branching and length via regulation of axonemal microtubule dynamics in Caenorhabditis elegans. Curr. Biol. 29, 1286–1300 e1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Y.Y., Maier W., Baumeister R., Minevich G., Joachimiak E., Wloga D., Ruan Z., Kannan N., Bocarro S., Bahraini A., Vasudevan K.K., Lechtreck K., Orias E., Gaertig J. (2019) LF4/MOK and a CDK-related kinase regulate the number and length of cilia in tetrahymena. PLoS Genet. 15, e1008099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh Y.S., Wang E.J., Gailey C.D., Brautigan D.L., Allen B.L., Fu Z. (2019) Ciliopathy-associated protein kinase ICK requires its non-catalytic carboxyl-terminal domain for regulation of ciliogenesis. Cells 8, 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu Z., Larson K.A., Chitta R.K., Parker S.A., Turk B.E., Lawrence M.W., Kaldis P., Galaktionov K., Cohn S.M., Shabanowitz J., Hunt D.F., Sturgill T.W. (2006) Identification of yin-yang regulators and a phosphorylation consensus for male germ cell-associated kinase (MAK)-related kinase. Mol. Cell. Biol. 26, 8639–8654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gailey C.D., Wang E.J., Jin L., Ahmadi S., Brautigan D.L., Li X., Xu W., Scott M.M., Fu Z. (2021) Phosphosite T674A mutation in kinesin family member 3A fails to reproduce tissue and ciliary defects characteristic of CILK1 loss of function. Dev. Dyn. 250, 263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang Y., Pang Y., Wu Q., Hu Z., Han X., Xu Y., Deng H., Pan J. (2014) FLA8/KIF3B phosphorylation regulates kinesin-II interaction with IFT-B to control IFT entry and turnaround. Dev. Cell. 30, 585–597 [DOI] [PubMed] [Google Scholar]
- 42.Ozgul R.K., Siemiatkowska A.M., Yucel D., Myers C.A., Collin R.W., Zonneveld M.N., Beryozkin A., Banin E., Hoyng C.B., van den Born L.I., European Retinal Disease C., Bose R., Shen W., Sharon D., Cremers F.P., Klevering B.J., den Hollander A.I., Corbo J.C, European Retinal Disease Consortium (2011) Exome sequencing and cis-regulatory mapping identify mutations in MAK, a gene encoding a regulator of ciliary length, as a cause of retinitis pigmentosa. Am. J. Hum. Genet. 89, 253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tucker B.A., Scheetz T.E., Mullins R.F., DeLuca A.P., Hoffmann J.M., Johnston R.M., Jacobson S.G., Sheffield V.C., Stone E.M. (2011) Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germ cell-associated kinase (MAK) as a cause of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 108, E569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Z., Gailey C.D., Wang E.J., Brautigan D.L. (2019) Ciliogenesis associated kinase 1: targets and functions in various organ systems. FEBS Lett. 593, 2990–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lahiry P., Wang J., Robinson J.F., Turowec J.P., Litchfield D.W., Lanktree M.B., Gloor G.B., Puffenberger E.G., Strauss K.A., Martens M.B., Ramsay D.A., Rupar C.A., Siu V., Hegele R.A. (2009) A multiplex human syndrome implicates a key role for intestinal cell kinase in development of central nervous, skeletal, and endocrine systems. Am. J. Hum. Genet. 84, 134–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey J.N., de Nijs L., Bai D., Suzuki T., Miyamoto H., Tanaka M., Patterson C., Lin Y.C., Medina M.T., Alonso M.E., Serratosa J.M., Duron R.M., Nguyen V.H., Wight J.E., Martinez-Juarez I.E., Ochoa A., Jara-Prado A., Guilhoto L., Molina Y., Yacubian E.M., Lopez-Ruiz M., Inoue Y., Kaneko S., Hirose S., Osawa M., Oguni H., Fujimoto S., Grisar T.M., Stern J.M., Yamakawa K., Lakaye B., Delgado-Escueta A.V. (2018) Variant intestinal-cell kinase in juvenile myoclonic epilepsy. N Engl. J. Med. 378, 1018–1028 [DOI] [PubMed] [Google Scholar]
- 47.Wang E.J., Gailey C.D., Brautigan D.L., Fu Z. (2020) Functional alterations in ciliogenesis-associated kinase 1 (CILK1) that result from mutations linked to juvenile myoclonic epilepsy. Cells 9, 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y., Roine N., Makela T.P. (2013) CCRK depletion inhibits glioblastoma cell proliferation in a cilium-dependent manner. EMBO Rep. 14, 741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snouffer A., Brown D., Lee H., Walsh J., Lupu F., Norman R., Lechtreck K., Ko H.W., Eggenschwiler J. (2017) Cell cycle-related kinase (CCRK) regulates ciliogenesis and Hedgehog signaling in mice. PLoS Genet. 13, e1006912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lupu F.I., Burnett J.B., Eggenschwiler J.T. (2018) Cell cycle-related kinase regulates mammalian eye development through positive and negative regulation of the Hedgehog pathway. Dev. Biol. 434, 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee H., Ko H.W. (2020) Cell cycle-related kinase is a crucial regulator for ciliogenesis and Hedgehog signaling in embryonic mouse lung development. BMB Rep. 53, 367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tam L.W., Wilson N.F., Lefebvre P.A. (2007) A CDK-related kinase regulates the length and assembly of flagella in Chlamydomonas. J. Cell Biol. 176, 819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phirke P., Efimenko E., Mohan S., Burghoorn J., Crona F., Bakhoum M.W., Trieb M., Schuske K., Jorgensen E.M., Piasecki B.P., Leroux M.R., Swoboda P. (2011) Transcriptional profiling of C. elegans DAF-19 uncovers a ciliary base-associated protein and a CDK/CCRK/LF2p-related kinase required for intraflagellar transport. Dev. Biol. 357, 235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y., Ren Y., Pan J. (2019) Regulation of flagellar assembly and length in Chlamydomonas by LF4, a MAPK-related kinase. Faseb J. 33, 6431–6441 [DOI] [PubMed] [Google Scholar]
- 55.Neugebauer J.M., Amack J.D., Peterson A.G., Bisgrove B.W., Yost H.J. (2009) FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature 458, 651–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin L., Kaci N., Estibals V., Goudin N., Garfa-Traore M., Benoist-Lasselin C., Dambroise E., Legeai-Mallet L. (2018) Constitutively-active FGFR3 disrupts primary cilium length and IFT20 trafficking in various chondrocyte models of achondroplasia. Hum. Mol. Genet. 27, 1–13 [DOI] [PubMed] [Google Scholar]
- 57.Kunova Bosakova M., Varecha M., Hampl M., Duran I., Nita A., Buchtova M., Dosedelova H., Machat R., Xie Y., Ni Z., Martin J.H., Chen L., Jansen G., Krakow D., Krejci P. (2018) Regulation of ciliary function by fibroblast growth factor signaling identifies FGFR3-related disorders achondroplasia and thanatophoric dysplasia as ciliopathies. Hum. Mol. Genet. 27, 1093–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kunova Bosakova M., Nita A., Gregor T., Varecha M., Gudernova I., Fafilek B., Barta T., Basheer N., Abraham S.P., Balek L., Tomanova M., Fialova Kucerova J., Bosak J., Potesil D., Zieba J., Song J., Konik P., Park S., Duran I., Zdrahal Z., Smajs D., Jansen G., Fu Z., Ko H.W., Hampl A., Trantirek L., Krakow D., Krejci P. (2019) Fibroblast growth factor receptor influences primary cilium length through an interaction with intestinal cell kinase. Proc. Natl. Acad. Sci. USA 116, 4316–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jensen V.L., Leroux M.R. (2017) Gates for soluble and membrane proteins, and two trafficking systems (IFT and LIFT), establish a dynamic ciliary signaling compartment. Curr. Opin. Cell Biol. 47, 83–91 [DOI] [PubMed] [Google Scholar]
- 60.Ismail S.A., Chen Y.X., Rusinova A., Chandra A., Bierbaum M., Gremer L., Triola G., Waldmann H., Bastiaens P.I., Wittinghofer A. (2011) Arl2-GTP and Arl3-GTP regulate a GDI-like transport system for farnesylated cargo. Nat. Chem. Biol. 7, 942–949 [DOI] [PubMed] [Google Scholar]
- 61.Ismail S.A., Chen Y.X., Miertzschke M., Vetter I.R., Koerner C., Wittinghofer A. (2012) Structural basis for Arl3-specific release of myristoylated ciliary cargo from UNC119. Embo J. 31, 4085–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright K.J., Baye L.M., Olivier-Mason A., Mukhopadhyay S., Sang L., Kwong M., Wang W., Pretorius P.R., Sheffield V.C., Sengupta P., Slusarski D.C., Jackson P.K. (2011) An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev. 25, 2347–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang H., Constantine R., Vorobiev S., Chen Y., Seetharaman J., Huang Y.J., Xiao R., Montelione G.T., Gerstner C.D., Davis M.W., Inana G., Whitby F.G., Jorgensen E.M., Hill C.P., Tong L., Baehr W. (2011) UNC119 is required for G protein trafficking in sensory neurons. Nat. Neurosci. 14, 874–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gotthardt K., Lokaj M., Koerner C., Falk N., Gießl A., Wittinghofer A. (2015) A G-protein activation cascade from Arl13B to Arl3 and implications for ciliary targeting of lipidated proteins. Elife 4, e11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Veltel S., Gasper R., Eisenacher E., Wittinghofer A. (2008) The retinitis pigmentosa 2 gene product is a GTPase-activating protein for Arf-like 3. Nat. Struct. Mol. Biol. 15, 373–380 [DOI] [PubMed] [Google Scholar]
- 66.Evans R.J., Schwarz N., Nagel-Wolfrum K., Wolfrum U., Hardcastle A.J., Cheetham M.E. (2010) The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum. Mol. Genet. 19, 1358–1367 [DOI] [PubMed] [Google Scholar]
- 67.Brann M.R., Cohen L.V. (1987) Diurnal expression of transducin mRNA and translocation of transducin in rods of rat retina. Science 235, 585–587 [DOI] [PubMed] [Google Scholar]
- 68.Philp N.J., Chang W., Long K. (1987) Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett. 225, 127–132 [DOI] [PubMed] [Google Scholar]
- 69.Whelan J.P., McGinnis J.F. (1988) Light-dependent subcellular movement of photoreceptor proteins. J. Neurosci. Res. 20, 263–270 [DOI] [PubMed] [Google Scholar]
- 70.Organisciak D.T., Xie A., Wang H.M., Jiang Y.L., Darrow R.M., Donoso L.A. (1991) Adaptive changes in visual cell transduction protein levels: effect of light. Exp. Eye Res. 53, 773–779 [DOI] [PubMed] [Google Scholar]
- 71.Sokolov M., Lyubarsky A.L., Strissel K.J., Savchenko A.B., Govardovskii V.I., Pugh E.N. Jr.,, Arshavsky V.Y. (2002) Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron 34, 95–106. [DOI] [PubMed] [Google Scholar]
- 72.Kerov V., Rubin W.W., Natochin M., Melling N.A., Burns M.E., Artemyev N.O. (2007) N-terminal fatty acylation of transducin profoundly influences its localization and the kinetics of photoresponse in rods. J. Neurosci. 27, 10270–10277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peng Y.W., Zallocchi M., Wang W.M., Delimont D., Cosgrove D. (2011) Moderate light-induced degeneration of rod photoreceptors with delayed transducin translocation in shaker1 mice. Invest. Ophthalmol. Vis. Sci. 52, 6421–6427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian M., Wang W., Delimont D., Cheung L., Zallocchi M., Cosgrove D., Peng Y.W. (2014) Photoreceptors in whirler mice show defective transducin translocation and are susceptible to short-term light/dark changes-induced degeneration. Exp. Eye Res. 118, 145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kerov V., Artemyev N.O. (2011) Diffusion and light-dependent compartmentalization of transducin. Mol. Cell. Neurosci. 46, 340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Majumder A., Pahlberg J., Boyd K.K., Kerov V., Kolandaivelu S., Ramamurthy V., Sampath A.P., Artemyev N.O. (2013) Transducin translocation contributes to rod survival and enhances synaptic transmission from rods to rod bipolar cells. Proc. Natl. Acad. Sci. USA 110, 12468–12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gopalakrishna K.N., Doddapuneni K., Boyd K.K., Masuho I., Martemyanov K.A., Artemyev N.O. (2011) Interaction of transducin with uncoordinated 119 protein (UNC119): implications for the model of transducin trafficking in rod photoreceptors. J. Biol. Chem. 286, 28954–28962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ishiba Y., Higashide T., Mori N., Kobayashi A., Kubota S., McLaren M.J., Satoh H., Wong F., Inana G. (2007) Targeted inactivation of synaptic HRG4 (UNC119) causes dysfunction in the distal photoreceptor and slow retinal degeneration, revealing a new function. Exp. Eye Res. 84, 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi A., Higashide T., Hamasaki D., Kubota S., Sakuma H., An W., Fujimaki T., McLaren M.J., Weleber R.G., Inana G. (2000) HRG4 (UNC119) mutation found in cone-rod dystrophy causes retinal degeneration in a transgenic model. Invest. Ophthalmol. Vis. Sci. 41, 3268–3277 [PubMed] [Google Scholar]
- 80.Mori N., Ishiba Y., Kubota S., Kobayashi A., Higashide T., McLaren M.J., Inana G. (2006) Truncation mutation in HRG4 (UNC119) leads to mitochondrial ANT-1-mediated photoreceptor synaptic and retinal degeneration by apoptosis. Invest. Ophthalmol. Vis. Sci. 47, 1281–1292 [DOI] [PubMed] [Google Scholar]
- 81.Hagiwara K., Wada A., Katadae M., Ito M., Ohya Y., Casey P.J., Fukada Y. (2004) Analysis of the molecular interaction of the farnesyl moiety of transducin through the use of a photoreactive farnesyl analogue. Biochemistry 43, 300–309 [DOI] [PubMed] [Google Scholar]
- 82.Zhang H., Li S., Doan T., Rieke F., Detwiler P.B., Frederick J.M., Baehr W. (2007) Deletion of PrBP/delta impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc. Natl. Acad. Sci. USA 104, 8857–8862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anant J.S., Ong O.C., Xie H.Y., Clarke S., O'Brien P.J., Fung B.K. (1992) In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J. Biol. Chem. 267, 687–690 [PubMed] [Google Scholar]
- 84.Inglese J., Glickman J.F., Lorenz W., Caron M.G., Lefkowitz R.J. (1992) Isoprenylation of a protein kinase. Requirement of farnesylation/alpha-carboxyl methylation for full enzymatic activity of rhodopsin kinase. J. Biol. Chem. 267, 1422–1425 [PubMed] [Google Scholar]
- 85.Anant J.S., Fung B.K. (1992) In vivo farnesylation of rat rhodopsin kinase. Biochem. Biophys. Res. Commun. 183, 468–473 [DOI] [PubMed] [Google Scholar]
- 86.Hanke-Gogokhia C., Wu Z., Gerstner C.D., Frederick J.M., Zhang H., Baehr W. (2016) Arf-like Protein 3 (ARL3) regulates protein trafficking and ciliogenesis in mouse photoreceptors. J. Biol. Chem. 291, 7142–7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strom S.P., Clark M.J., Martinez A., Garcia S., Abelazeem A.A., Matynia A., Parikh S., Sullivan L.S., Bowne S.J., Daiger S.P., Gorin M.B. (2016) De novo occurrence of a variant in ARL3 and apparent autosomal dominant transmission of retinitis pigmentosa. PLoS One 11, e0150944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanke-Gogokhia C., Wu Z., Sharif A., Yazigi H., Frederick J.M., Baehr W. (2017) The guanine nucleotide exchange factor Arf-like protein 13b is essential for assembly of the mouse photoreceptor transition zone and outer segment. J. Biol. Chem. 292, 21442–21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dilan T.L., Moye A.R., Salido E.M., Saravanan T., Kolandaivelu S., Goldberg A.F.X., Ramamurthy V. (2019) ARL13B, a Joubert syndrome-associated protein, is critical for retinogenesis and elaboration of mouse photoreceptor outer segments. J. Neurosci. 39, 1347–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cantagrel V., Silhavy J.L., Bielas S.L., Swistun D., Marsh S.E., Bertrand J.Y., Audollent S., Attie-Bitach T., Holden K.R., Dobyns W.B., Traver D., Al-Gazali L., Ali B.R., Lindner T.H., Caspary T., Otto E.A., Hildebrandt F., Glass I.A., Logan C.V., Johnson C.A., Bennett C., Brancati F., International Joubert Syndrome Related Disorders Study G., Valente E.M., Woods C.G., Gleeson J.G, International Joubert Syndrome Related Disorders Study Group (2008) Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am. J. Hum. Genet. 83, 170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas S., Cantagrel V., Mariani L., Serre V., Lee J.-E., Elkhartoufi N., de Lonlay P., Desguerre I., Munnich A., Boddaert N., Lyonnet S., Vekemans M., Lisgo S.N., Caspary T., Gleeson J., Attié-Bitach T. (2015) Identification of a novel ARL13B variant in a Joubert syndrome-affected patient with retinal impairment and obesity. Eur. J. Hum. Genet. 23, 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang H., Hanke-Gogokhia C., Jiang L., Li X., Wang P., Gerstner C.D., Frederick J.M., Yang Z., Baehr W. (2015) Mistrafficking of prenylated proteins causes retinitis pigmentosa 2. FASEB J. 29, 932–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwahn U., Lenzner S., Dong J., Feil S., Hinzmann B., van Duijnhoven G., Kirschner R., Hemberger M., Bergen A.A., Rosenberg T., Pinckers A.J., Fundele R., Rosenthal A., Cremers F.P., Ropers H.H., Berger W. (1998) Positional cloning of the gene for X-linked retinitis pigmentosa 2. Nat. Genet. 19, 327–332 [DOI] [PubMed] [Google Scholar]
- 94.Jayasundera T., Branham K.E., Othman M., Rhoades W.R., Karoukis A.J., Khanna H., Swaroop A., Heckenlively J.R. (2010) RP2 phenotype and pathogenetic correlations in X-linked retinitis pigmentosa. Arch. Ophthalmol. 128, 915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dandekar S.S., Ebenezer N.D., Grayson C., Chapple J.P., Egan C.A., Holder G.E., Jenkins S.A., Fitzke F.W., Cheetham M.E., Webster A.R., Hardcastle A.J. (2004) An atypical phenotype of macular and peripapillary retinal atrophy caused by a mutation in the RP2 gene. Br. J. Ophthalmol. 88, 528–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petroski M.D., Deshaies R.J. (2005) Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6, 9–20 [DOI] [PubMed] [Google Scholar]
- 97.Sarikas A., Hartmann T., Pan Z.Q. (2011) The cullin protein family. Genome Biol. 12, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pan Z.Q., Kentsis A., Dias D.C., Yamoah K., Wu K. (2004) Nedd8 on cullin: building an expressway to protein destruction. Oncogene 23, 1985–1997 [DOI] [PubMed] [Google Scholar]
- 99.Dhanoa B.S., Cogliati T., Satish A.G., Bruford E.A., Friedman J.S. (2013) Update on the Kelch-like (KLHL) gene family. Hum. Genomics 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soucy T.A., Smith P.G., Milhollen M.A., Berger A.J., Gavin J.M., Adhikari S., Brownell J.E., Burke K.E., Cardin D.P., Critchley S., Cullis C.A., Doucette A., Garnsey J.J., Gaulin J.L., Gershman R.E., Lublinsky A.R., McDonald A., Mizutani H., Narayanan U., Olhava E.J., Peluso S., Rezaei M., Sintchak M.D., Talreja T., Thomas M.P., Traore T., Vyskocil S., Weatherhead G.S., Yu J., Zhang J., Dick L.R., Claiborne C.F., Rolfe M., Bolen J.B., Langston S.P. (2009) An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458, 732–736 [DOI] [PubMed] [Google Scholar]
- 101.Trojan P., Rausch S., Giessl A., Klemm C., Krause E., Pulvermuller A., Wolfrum U. (2008) Light-dependent CK2-mediated phosphorylation of centrins regulates complex formation with visual G-protein. Biochim. Biophys. Acta 1783, 1248–1260 [DOI] [PubMed] [Google Scholar]
- 102.Klee C.B., Crouch T.H., Krinks M.H. (1979) Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc. Natl. Acad. Sci. USA 76, 6270–6273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Klee C.B., Ren H., Wang X. (1998) Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 273, 13367–13370 [DOI] [PubMed] [Google Scholar]
- 104.Chaya T., Tsutsumi R., Varner L.R., Maeda Y., Yoshida S., Furukawa T. (2019) Cul3-Klhl18 ubiquitin ligase modulates rod transducin translocation during light-dark adaptation. Embo J. 38, e101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parmeggiani F., Sato G., De Nadai K., Romano M.R., Binotto A., Costagliola C. (2011) Clinical and rehabilitative management of retinitis pigmentosa: up-to-date. Curr. Genomics 12, 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schick T., Ersoy L., Lechanteur Y.T., Saksens N.T., Hoyng C.B., den Hollander A.I., Kirchhof B., Fauser S. (2016) History of sunlight exposure is a risk factor for age-related macular degeneration. Retina 36, 787–790 [DOI] [PubMed] [Google Scholar]
- 107.Mitchell P., Liew G., Gopinath B., Wong T.Y. (2018) Age-related macular degeneration. Lancet 392, 1147–1159 [DOI] [PubMed] [Google Scholar]
- 108.Marquioni-Ramella M.D., Suburo A.M. (2015) Photo-damage, photo-protection and age-related macular degeneration. Photochem. Photobiol. Sci. 14, 1560–1577 [DOI] [PubMed] [Google Scholar]
- 109.Kassai H., Aiba A., Nakao K., Nakamura K., Katsuki M., Xiong W.H., Yau K.W., Imai H., Shichida Y., Satomi Y., Takao T., Okano T., Fukada Y. (2005) Farnesylation of retinal transducin underlies its translocation during light adaptation. Neuron 47, 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. (2002) The protein kinase complement of the human genome. Science 298, 1912–1934 [DOI] [PubMed] [Google Scholar]