Abstract
Kisspeptin, encoded by Kiss1, stimulates gonadotropin-releasing hormone neurons to govern reproduction. In female rodents, estrogen-sensitive kisspeptin neurons in the rostral anteroventral periventricular (AVPV) hypothalamus are thought to mediate estradiol (E2)-induced positive feedback induction of the preovulatory luteinizing hormone (LH) surge. AVPV kisspeptin neurons coexpress estrogen and progesterone receptors (PGRs) and are activated during the LH surge. While E2 effects on kisspeptin neurons have been well studied, progesterone’s regulation of kisspeptin neurons is less understood. Using transgenic mice lacking PGR exclusively in kisspeptin cells (termed KissPRKOs), we previously demonstrated that progesterone action specifically in kisspeptin cells is essential for ovulation and normal fertility. Unlike control females, KissPRKO females did not generate proper LH surges, indicating that PGR signaling in kisspeptin cells is required for positive feedback. However, because PGR was knocked out from all kisspeptin neurons in the brain, that study was unable to determine the specific kisspeptin population mediating PGR action on the LH surge. Here, we used targeted Cre-mediated adeno-associated virus (AAV) technology to reintroduce PGR selectively into AVPV kisspeptin neurons of adult KissPRKO females, and tested whether this rescues occurrence of the LH surge. We found that targeted upregulation of PGR in kisspeptin neurons exclusively in the AVPV is sufficient to restore proper E2-induced LH surges in KissPRKO females, suggesting that this specific kisspeptin population is a key target of the necessary progesterone action for the surge. These findings further highlight the critical importance of progesterone signaling, along with E2 signaling, in the positive feedback induction of LH surges and ovulation.
Keywords: Kisspeptin, Kiss1, GnRH, progesterone, LH surge, positive feedback, ovulation, AVPV, RP3V
In female mammals, ovulation is triggered by an estradiol (E2)-induced preovulatory luteinizing hormone (LH) surge, which is driven by a surge in gonadotropin-releasing hormone (GnRH) secretion from the brain (ie, E2 positive feedback) (1-3). Kisspeptin, encoded by Kiss1, potently stimulates GnRH secretion and is essential for the GnRH/LH surge, as female mice lacking Kiss1 or its receptor, Kiss1r, fail to exhibit LH surges or concurrent GnRH neuron activation (3-5). The hypothalamus contains 2 populations of kisspeptin-synthesizing neurons, one in the arcuate nucleus (ARC) and another rostrally in the continuum spanning the anteroventral periventricular nucleus and neighboring periventricular nucleus (collectively termed here the AVPV; also called the RP3V) (6-8). E2’s positive feedback effects on the LH surge are thought to be mediated specifically by AVPV Kiss1 neurons, because (1) E2 elevates Kiss1 levels in the AVPV (8-11), (2) AVPV Kiss1 neurons express ERα (11), (3) AVPV Kiss1 mRNA and neuronal activation increase in a circadian manner coincident with the LH surge (10, 12-14), and (4) Kiss1 and kisspeptin levels in the AVPV are sexually dimorphic (greater in females), correlating with the LH surge occurring only in female rodents (8, 15).
While most positive feedback studies focus on E2, progesterone (P4), and its receptor (PGR) are also critical contributors to the LH surge. Like ERα knockout (KO) mice, global PR KO female mice are infertile and unable to produce LH surges (16, 17). Converging pieces of evidence indicate that E2-induced local synthesis of P4 in the hypothalamus is critical for the rodent LH surge (18-20). Specifically, E2 appears to act in ERα-expressing astrocytes in the hypothalamic AVPV to induce the synthesis and secretion of P4 (“neuroP”), which then acts paracrinely to promote the LH surge (18-23). However, the specific neural population(s) that neuroP acts in for this process remains unknown. In immortalized cells representing adult female AVPV kisspeptin neurons (mHypoA51), neuroP augmented E2 action on the expression and release of kisspeptin (24, 25). Because AVPV kisspeptin neurons in vivo express PGR (4, 26-28), we previously tested whether PGR signaling in kisspeptin cells is critical for the LH surge. Using transgenic mice with selective KO of PGR specifically in kisspeptin cells (“KissPRKO”), we demonstrated that P4 signaling directly in kisspeptin cells is necessary for the E2-induction of the LH surge and ovulation ((27); also see (29)). Moreover, AVPV Kiss1 cells of E2-treated KissPRKOs had normal Kiss1 mRNA levels but reduced neuronal activation (27), indicating that P4 signaling in Kiss1 cells is critical for activation of AVPV kisspeptin neurons during the LH surge, perhaps due to P4-induced calcium release, as observed in immortalized kisspeptin cells (25).
While our previous studies implicated that PGR signaling in kisspeptin cells is essential for the LH surge, a limitation was that kisspeptin cells are present in several brain regions, including the AVPV, ARC, and extra-hypothalamic areas like the medial amygdala and bed nucleus of the stria terminalis (30-33). KissPRKOs lack PGR in all these kisspeptin populations, precluding a definitive conclusion of the specific population that is the direct target for P4 in the LH surge process. Therefore, we tested here whether selective reintroduction of PGR in just one kisspeptin population, the AVPV, of KissPRKOs could rescue the occurrence of the LH surge even with PGR still absent in all other kisspeptin cells.
Materials and Methods
Animals
The same mouse line was used as in our prior study (27), including both KissCre+ PRfl/fl females (termed “KissPRKO”; PGR knocked out in Kiss1 cells) and KissCre– PRfl/fl controls (termed “WT”; PR still present in all cells, including Kiss1 cells) (Fig. 1). Our prior study confirmed proper Cre-mediated excision of Pgr DNA in the AVPV and ARC of KissPRKOs but not in Cre– controls or in tissues known to lack Kiss1 expression (27). Genotypes and occasional occurrence of germline recombination were determined via polymerase chain reaction analysis of tail DNA; germline-recombined (global PRKO) mice were excluded. Female mice were weaned at 3 weeks and housed 2 or 3/cage under a 12–12 light–dark cycle (lights off at 18:00 hours). All experiments were approved by the UCSD IACUC.
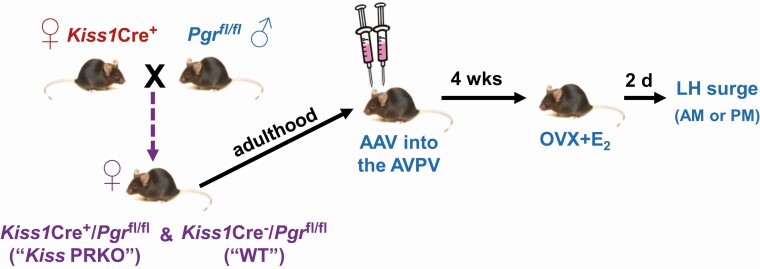
Figure 1.
Experimental design and timeline for virally re-expressing PGR selectively in AVPV kisspeptin neurons of KissPRKO females and then assessing their ability to generate E2-induced LH surges.
AAV Treatment to Induce PR in AVPV Kisspeptin Cells and mCherry Immunohistochemistry Validation
We previously demonstrated that KissPRKO females have impaired ovulatory function and LH surges. Here, we determined if we could rescue the LH surge in KissPRKOs by selectively re-introducing PGR into just AVPV kisspeptin neurons, while all other kisspeptin cells still lack PGR. Adeno-associated viruses (AAVs) were used to deliver a Cre-dependent Pgr construct or a control construct to AVPV kisspeptin neurons (which express Cre in Kiss1Cre+ mice) (Fig. 1). Two- to 3-month-old KissPRKO females (Cre+) and WT controls (Cre–) were anesthetized with isoflurane, and site-specific AVPV-targeted (from bregma: A/P: +0.5 mm; D/V: –5.5 mm, M/L ± 0.3 mm) bilateral injections were made. A 500-nL bolus of either AAV1-CMV-DIO-mPGR-2A-mCherry or AAV2/1-CAG-DIO-mCherry (Vector Biolabs) was infused at a flow rate of 100 nL/min; the former AAV, termed “Pgr,” allows for Pgr and mCherry expression in Cre-expressing cells while the latter AAV, termed “mCh,” was a control AAV with the mCherry reporter but no Pgr construct. After AAV infusion, the needle was left in place for 10 minutes to allow diffusion and prevent backflow. To validate the AAV technique, several nonexperimental mice were perfused and examined for proper targeting via immunohistochemistry (IHC) analysis of mCherry induction in the AVPV region of Cre+ but not WT (Cre–) mice. Because fixed tissue was needed for IHC, these validation mice were separate from the experimental mice evaluated for LH surges and Kiss1+Pgr mRNA coexpression (which required fresh frozen tissue for in situ hybridization). IHC was performed on fixed AVPV-containing brain slices using an mCherry primary antibody (1:5000; Abcam ab167453 (34)) and AlexaFluor 594 donkey antirabbit secondary antibody (1:2000; Jackson 711-585-152 (35)), following previous methods (36). For assessment of LH surges and Pgr mRNA induction after AAV treatment, there were 4 experimental groups: KissPRKOPgr, KissPRKOmCh, WTPgr, and WTmCh. Mice were given 4 weeks to recover prior to LH surge testing and brain collection. Double-label in situ hybridization (ISH) evaluation of Pgr + Kiss1 coexpression was used to confirm successful anatomical targeting of AAV to AVPV Kiss1 neurons; 1 animal with missed targeting was excluded from the study.
Estradiol-induced LH surge
Four weeks after AAV infusion, mice were anesthetized with isoflurane, ovariectomized (OVX), and given subcutaneous Silastic implants (inner diameter: 0.078 inches, outer diameter: 0.125 inches) containing 0.75 µg of 17-β E2 dissolved in sesame oil. This E2 implant is commonly used to induce LH surges and produces elevated serum E2 levels ~16 to 24 pg/mL, resembling mouse proestrus levels (5, 37). This positive feedback paradigm produces an LH surge 2 days later before lights off (5, 13, 14, 37-39). OVX + E2 mice from all 4 treatment groups (KissPRKOPgr, KissPRKOmCh, WTPgr, and WTmCh) were killed 2 days after E2 implantation, either in the morning (09:00-10:00 hours, control time when the LH surge does not occur; n = 4-6/group) or in the afternoon just before lights off (17:30-18:00 hours, time of the expected LH surge; n = 7-10/group) (Fig. 1). Blood was collected at sacrifice and the serum isolated and stored at –20°C. Serum LH was measured by the University of Virginia Ligand Assay Core using a sensitive mouse LH sandwich radioimmunoassay (limit of detectability 0.04 ng/mL) (40, 41). LH surges were defined as >0.70 ng/mL. Brains were collected fresh frozen onto dry ice and stored at –80°C. Brains were sectioned on a cryostat into alternating sets of 20-µm sections onto Superfrost-plus slides and stored at –80°C.
Double-label In Situ Hybridization for Pgr expression in Kiss1 cells
Double-label ISH was used to measure Kiss1 and Pgr coexpression levels in the AVPV. One set of AVPV brain sections was assayed using well-established radio-labeled (33P) Pgr (0.04 pmol/mL) and digoxygenin-labeled (DIG; 1:500) Kiss1 antisense riboprobes, following our standard double-label ISH protocol (14, 27, 42-44). ISH slides were analyzed “blindly” with respect to treatment using microscopy linked to an automated imaging processing system (Dr. Don Clifton, University of Washington) that counts the number of DIG-containing (Kiss1 mRNA) cells under fluorescence microscopy and then quantifies the number of silver grains (Pgr mRNA) overlying each DIG cell under dark-field microscopy. A minimum of 3 AVPV sections (mean: 5.5 sections/animal) and 145 Kiss1 cells (mean: 290 cells/animal) was counted for each animal. Signal-to-background ratios of Pgr expression levels for individual DIG (Kiss1) cells were autocalculated by the program, and a Kiss1 cell considered double-labeled with Pgr if its ratio was >3 (13, 14, 43-46).
Statistical Analyses
All data are expressed as mean ± standard error of the mean. Differences were analyzed by analysis of variance, followed by post hoc comparisons via Fisher’s protected least significant difference test. Statistical significance was set at P < .05.
Results
mCherry Localization in the AVPV of Cre+ mice and Pgr Upregulation in Kiss PRKO Females
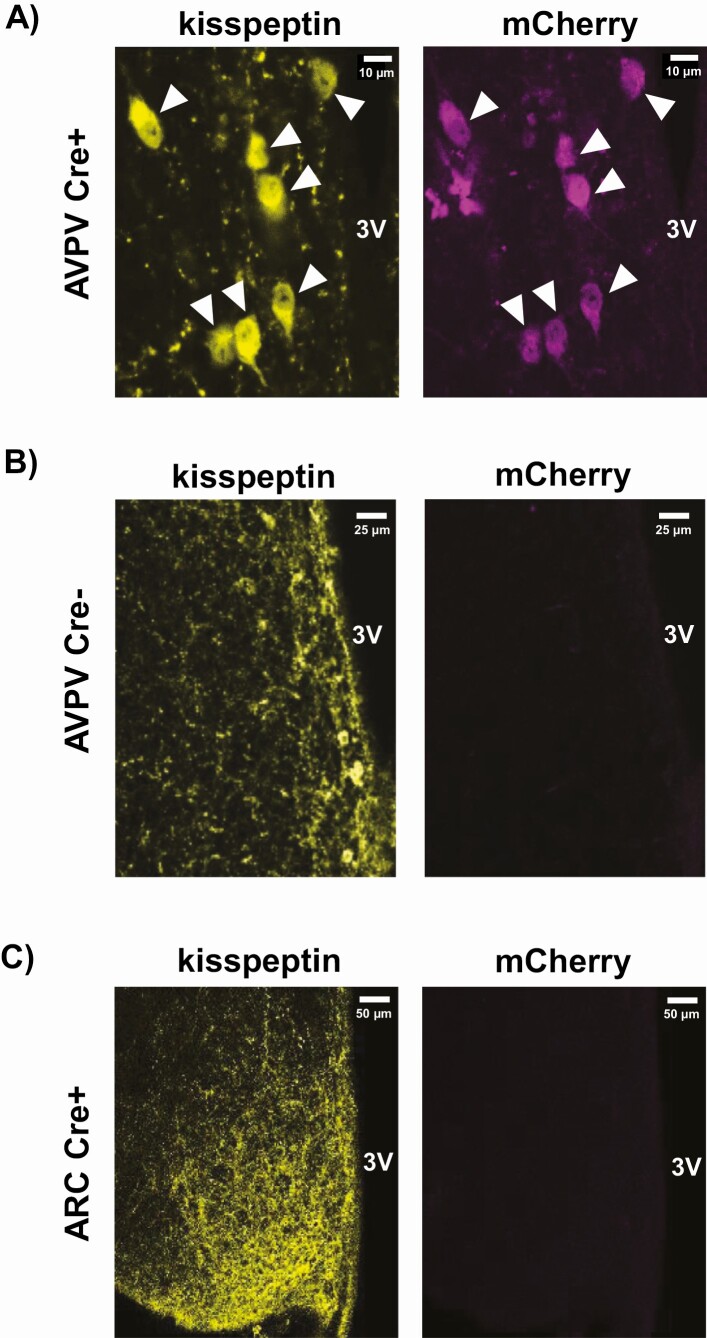
Pgr or mCh AAV was infused into the AVPV of adult Cre+ (KissPRKO) females and Cre– (WT) controls (Fig. 1). We first confirmed proper localization and targeting of the AAV injection, using IHC to identify mCherry expression in the AVPV of Cre+ mice but not Cre– controls (Fig. 2A and 2B). We also confirmed no spread of mCherry expression in the ARC (Fig. 2C).
Figure 2.
Double-label IHC verification of proper mCherry expression in AVPV kisspeptin cells of (A) Cre+ mice (KissPRKO) but not (B) Cre- mice (WT controls) after targeted AAV injection into the AVPV. Kisspeptin staining is yellow; mCherry is pseudo-colored magenta. White arrowheads in (A) denote kisspeptin neurons. Importantly, there was no spread of AVPV-infused AAV to the ARC region, indicated by (C) complete absence of mCherry in kisspeptin cells in the ARC of Cre+ animals. Scale bars are shown in white for each image.
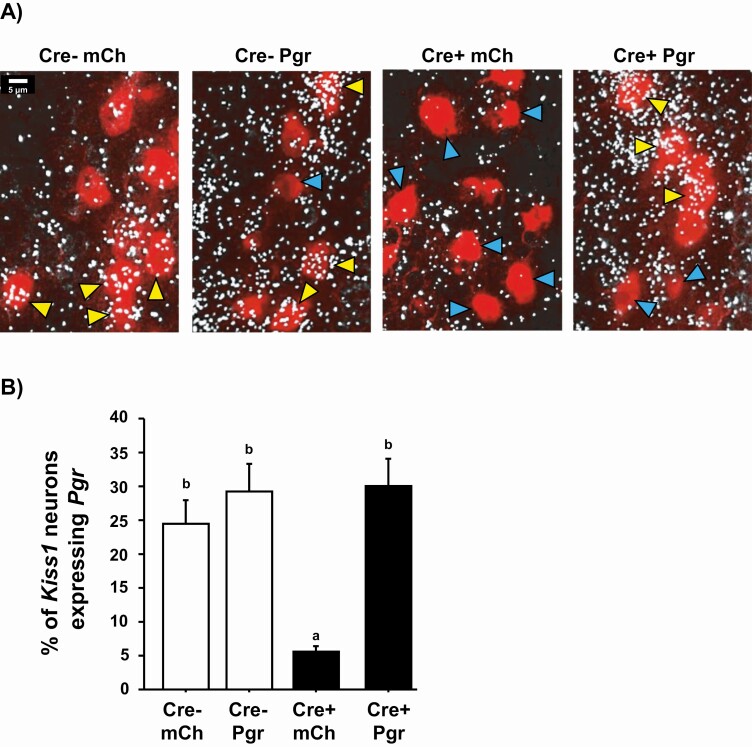
Next, to verify that our Pgr AAV treatment upregulated Pgr levels in Kiss1 cells of Cre+ mice, Pgr + Kiss1 coexpression levels in the AVPV were determined with double ISH. Pgr was coexpressed with Kiss1 at similar levels between WTPgr and WTmCh (Fig. 3), as expected for Cre– controls. As we previously reported, KissPRKOs normally have much less Prg coexpression than WTs, evidenced by significantly lower % Pgr + Kiss1 coexpression in KissPRKOmCh vs either WTPgr and WTmCh controls (P < .05 for each; Fig. 3). Confirming the AAV-mediated upregulation of Pgr, KissPRKOPgr had significantly increased % Prg + Kiss1 coexpression vs KissPRKOmCh (P < .05; Fig. 3), with similar % coexpression as the WT control groups (Fig. 3). Mean levels of Pgr mRNA in Kiss1 neurons were also similar between KissPRKOPgr and WTPgr controls (13.5 ± 1.5 vs 12.0 ± 1.8 grains/cell, P = .53). Thus, the experimental paradigm effectively reintroduced Pgr expression in the AVPV of KissPRKOs that normally lack PR in kisspeptin neurons.
Figure 3.
Very low coexpression of Pgr in AVPV Kiss1 neurons of KissPRKOmCh females and upregulation of Pgr coexpression in KissPRKOs after “Pgr AAV” treatment, as assessed with double-label ISH. (A) Representative microscope images of Pgr (white silver grains) and Kiss1 (red fluorescence) coexpression in AVPV neurons of OVX + E2 females 4 weeks after treatment with either Pgr AAV or control mCh AAV. Yellow arrowheads denote example Kiss1 cells with Pgr coexpression; blue arrowheads denote examples of nondouble-labeled Kiss1 cells. (B) Mean % of Kiss1+Pgr coexpression in the AVPV of all 4 afternoon groups (n = 7-10/group). Different letters above the bars signify significantly different (P < .05) from each other.
Positive Feedback Induction of the LH Surge Is Rescued in KissPRKOPgr Females
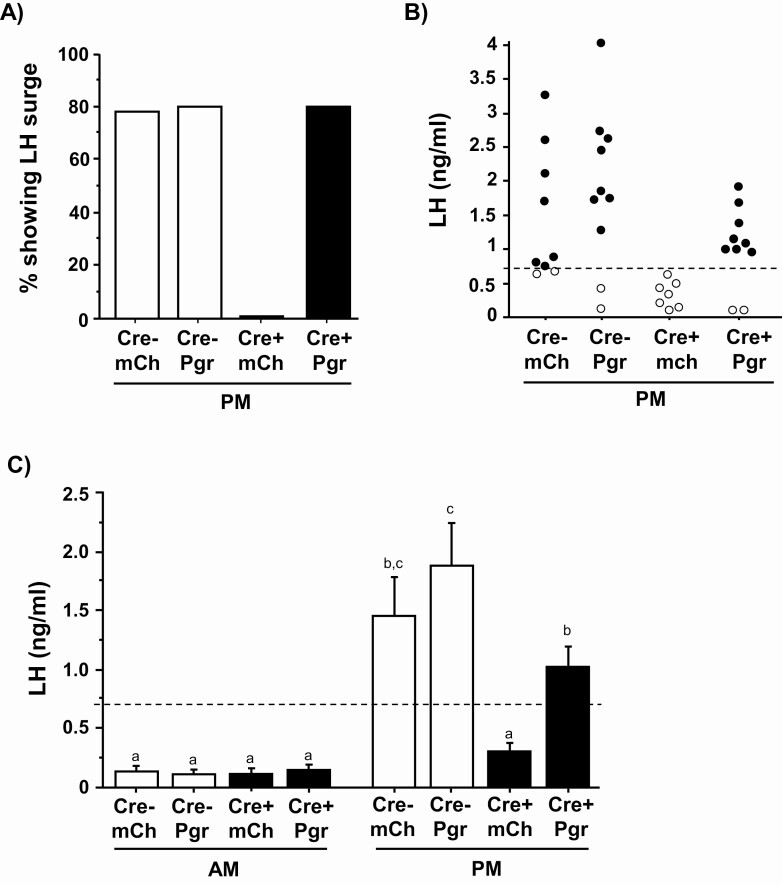
We hypothesized that the previously observed loss of LH surges in KissPRKOs (27) reflects an impairment in the AVPV kisspeptin system caused by absent PGR signaling in this specific neural population. We tested whether selective reintroduction of PGR into AVPV kisspeptin neurons, while leaving PGR absent in all other kisspeptin cells, could rescue the LH surge in OVX + E2 females. All 4 groups exhibited low LH in the morning, as expected for this nonsurge time, with no group differences (Fig. 4C). In the afternoon, the overall occurrence of an LH surge was greatly increased in KissPRKOPgr compared with KissPRKOmCh females and was not different between KissPRKOPgr and WTPgr and WTmCh control groups (Fig. 4A). Indeed, virtually all afternoon KissPRKOPgr females demonstrated a surge, whereas no afternoon KissPRKOmCh females surged (Fig. 4B).
Figure 4.
Selective reintroduction of PGR into AVPV kisspeptin neurons rescues the LH surge in OVX + E2 females. (A) The occurrence of LH surges in each group, represented by the % of females demonstrating a surge after treatment with a validated E2 positive feedback regimen known to elicit an LH surge in the afternoon but not morning time-point (n = 7-10/afternoon group and n = 4-6/morning group). The LH surge occurrence was very low in KissPRKOmCh females and increased to WT levels in KissPRKOPgr females. (B) Individual dot plot of afternoon LH values showing the high proportion of animals achieving an LH surge in both WTs and KissPRKOPgr (Cre+ Pgr) females but not KissPRKOmCh (Cre+ mCh) females. Dashed horizontal line designates LH surge threshold (>0.70 ng/mL). (C) Mean serum LH levels in OVX + E2 females at the morning and afternoon time-periods demonstrating elevated surge-levels of LH in afternoon KissPRKOPgr but not afternoon KissPRKOmCh females (n = 7-10/afternoon group and n = 4-6/morning group). Different letters above the bars signify significantly different (P < .05) from each other.
In the afternoon, mean LH was significantly elevated in both WT control groups vs morning values, whereas mean LH of afternoon KissPRKOmCh females was lower (P < .05 vs each afternoon WT group; Fig. 4C) and did not differ significantly from morning groups, similar to our previous report (27). By contrast, afternoon KissPRKOPgr females exhibited elevated mean LH vs all morning groups and vs afternoon KissPRKOmCh females (P < .05 for each; Fig. 4C). The mean LH of afternoon KissPRKOPgr females was not significantly different than afternoon WTmCh controls but was lower than WTPgr (Fig. 4C), due primarily to 1 very high WTPgr value (Fig. 4B; not significantly different if that value excluded). Collectively, these results demonstrate that reintroduction of Pgr selectively in AVPV kisspeptin cells rescues the circadian-timed LH surge.
Discussion
Ovulation is dependent on sex steroid–induced positive feedback induction of GnRH/LH surges. Ample evidence implicates the AVPV kisspeptin system in mediating the positive feedback effects of E2 on the LH surge (reviewed in (3, 9). Along with E2, P4 is also critical for the LH surge, and we and another group recently reported that P4 action specifically in kisspeptin cells is necessary for females to generate proper LH surges (27, 29). However, neither in vivo study determined which exact kisspeptin population mediates this necessary action of PGR. AVPV kisspeptin neurons coexpress PGR (27, 28), suggesting they are key targets for P4 action. Supporting this, mHypoA51 cells (representing AVPV kisspeptin cells) respond to P4 by increasing Kiss1 expression and in vitro kisspeptin release (24, 25). To formally test whether AVPV kisspeptin cells are critical for positive feedback induction of the LH surge, we reintroduced Pgr expression selectively into AVPV kisspeptin neurons of KissPRKO females using targeted AAV technology. We demonstrate that such upregulation of PGR in kisspeptin neurons specifically within the AVPV is sufficient to rescue the E2-induced LH surge in KissPRKO females, suggesting these neurons are targets of the endogenous P4 that promotes the surge.
Female KissPRKO mice were previously shown to display impacted fertility, including fewer litters and a notable reduction in litter size (27). The reduced fecundity was traced to diminished ovulatory events in KissPRKO females, reflected by fewer corpora lutea and diminished or absent LH surges in response to elevated E2 (positive feedback) (27, 29). These sequelae mirrored rats in which hypothalamic P4 synthesis was blocked (19). Moreover, we previously demonstrated that P4 stimulated the release of intracellular calcium in mHypoA51 cells, mirroring our prior in vivo results that KissPRKO AVPV kisspeptin neurons were not properly activated by E2, correlating with impaired LH surges. Here, we both verified and extended that finding to show that the deficit is likely in kisspeptin neurons specifically in the AVPV, as selective reintroduction of PGR in just AVPV kisspeptin neurons was sufficient to rescue both the occurrence and magnitude of LH surges. These findings support prior evidence linking the AVPV kisspeptin population to the circadian-timed E2-induced LH surge and also supports recent findings that P4 can act in vitro in AVPV mHypoA51 kisspeptin cells to activate MAPK and Src kinases and induce kisspeptin release (24). Importantly, our present data also indicate that PGR is not required in other kisspeptin populations, such as the ARC, medial amygdala, or bed nucleus of the stria terminalis, for proper LH surges, though we cannot rule out if PGR action in those populations is sufficient for surge induction.
Two KissPRKOPgr females did not show an LH surge, but this was not due to insufficient Pgr induction, as Pgr + Kiss1 coexpression was not different between those 2 nonsurging mice and the other 8 surging KissPRKOPgr mice or surging WT controls (data not shown). Rather, in typical mouse LH surge studies, a small percentage (10-20%) of normal animals sometimes do not surge (for reasons unknown) and the 2 nonsurging KissPRKOPgr animals likely reflect that normal outcome, similar to the 2 nonsurging WTPgr (Fig. 4B).
Our findings demonstrate that signaling of endogenous P4 directly in AVPV kisspeptin cells is essential for the positive feedback induction of LH surges. These findings further highlight the critical importance of P4 signaling, along with E2 signaling, in positive feedback induction of LH surge. We have proposed that the source of P4 in this LH surge process is neural rather than ovarian, derived in astrocytes of the AVPV region, under the influence of elevated E2 (18-22). Indeed, because all our mice were OVX, any requisite P4 for the surge was nonovarian in origin, further underlining the role of neuroP in E2 triggered LH surges and ovulation.
Acknowledgments
The authors thank Ruby Parra, Angela Wong, Tina Keshishian, Melinda Mittelman-Smith, and Shannon Stephens for technical and experimental support.
Financial Support: This research was supported by National Institutes of Health (NIH) grants R01 HD090161, R01 HD100580, and R01 HD042635. M.A.M. was supported by NIH F32 HD097965. Additional support provided by National Institute of Child Health and Human Development (NICHD) grants P50 HD012303 (U.C. San Diego) and R24 HD1020614 (University of Virginia Ligand Core).
Glossary
Abbreviations
- AAV
adeno-associated virus
- ARC
arcuate nucleus
- AVPV
anteroventral periventricular
- DIG
digoxygenin
- E2
estradiol
- GnRH
gonadotropin-releasing hormone
- ISH
in situ hybridization
- KissPRKOs
transgenic mice lacking
- PGR
exclusively in kisspeptin cells
- KO
knockout
- LH
luteinizing hormone
- OVX
ovariectomized
- PGR
progesterone receptor
Additional Information
Disclosure Statement: The authors have nothing to disclose.
Data Availability
Some or all data generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1.Freeman ME. Neuroendocrine control of the ovarian cycle in the rat. In: Neill JD ed. Physiology of Reproduction. 3rd ed. Elsevier; 2006:2283-2326. [Google Scholar]
- 2.Wintermantel TM, Campbell RE, Porteous R, et al. . Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52(2):271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol. 2012;24(1):131-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28(35):8691-8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88(6):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottsch ML, Cunningham MJ, Smith JT, et al. . A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004;145(9):4073-4077. [DOI] [PubMed] [Google Scholar]
- 8.Kauffman AS, Gottsch ML, Roa J, et al. . Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774-1783. [DOI] [PubMed] [Google Scholar]
- 9.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57(2):277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adachi S, Yamada S, Takatsu Y, et al. . Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53(2):367-378. [DOI] [PubMed] [Google Scholar]
- 11.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686-3692. [DOI] [PubMed] [Google Scholar]
- 12.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poling MC, Luo EY, Kauffman AS. Sex differences in steroid receptor coexpression and circadian-timed activation of kisspeptin and RFRP-3 neurons may contribute to the sexually dimorphic basis of the LH surge. Endocrinology. 2017;158(10):3565-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homma T, Sakakibara M, Yamada S, et al. . Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod. 2009;81(6):1216-1225. [DOI] [PubMed] [Google Scholar]
- 16.Chappell PE, Lydon JP, Conneely OM, O’Malley BW, Levine JE. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology. 1997;138(10):4147-4152. [DOI] [PubMed] [Google Scholar]
- 17.Chappell PE, Schneider JS, Kim P, et al. . Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology. 1999;140(8):3653-3658. [DOI] [PubMed] [Google Scholar]
- 18.Micevych P, Sinchak K. The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Front Endocrinol (Lausanne). 2011;2:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008;290(1-2):44-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78(1):29-35. [DOI] [PubMed] [Google Scholar]
- 21.Sinchak K, Mohr MA, Micevych PE. Hypothalamic astrocyte development and physiology for neuroprogesterone induction of the luteinizing hormone surge. Front Endocrinol (Lausanne). 2020;11:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohr MA, Wong AM, Tomm RJ, Soma KK, Micevych PE. Pubertal development of estradiol-induced hypothalamic progesterone synthesis. Horm Behav. 2019;111:110-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittelman-Smith MA, Rudolph LM, Mohr MA, Micevych PE. Rodent models of non-classical progesterone action regulating ovulation. Front Endocrinol (Lausanne). 2017;8:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittelman-Smith MA, Wong AM, Micevych PE. Estrogen and progesterone integration in an in vitro model of RP3V kisspeptin neurons. Neuroendocrinology. 2018;106(2):101-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mittelman-Smith MA, Wong AM, Kathiresan AS, Micevych PE. Classical and membrane-initiated estrogen signaling in an in vitro model of anterior hypothalamic kisspeptin neurons. Endocrinology. 2015;156(6):2162-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Yang L, Lin N, Pan X, Zhu Y, Chen X. Aging-related changes in RP3V kisspeptin neurons predate the reduced activation of GnRH neurons during the early reproductive decline in female mice. Neurobiol Aging. 2014;35(3):655-668. [DOI] [PubMed] [Google Scholar]
- 27.Stephens SB, Tolson KP, Rouse ML Jr, et al. . Absent progesterone signaling in kisspeptin neurons disrupts the LH Surge and impairs fertility in female mice. Endocrinology. 2015;156(9):3091-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephens SBZ, Kauffman AS. Estrogen regulation of the molecular phenotype and active translatome of AVPV kisspeptin neurons. Endocrinology. 2021; 162(9): bqab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gal A, Lin PC, Cacioppo JA, et al. . Loss of fertility in the absence of progesterone receptor expression in kisspeptin neurons of female mice. PLoS One. 2016;11(7):e0159534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011;152(5):2020-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Giorgio NP, Semaan SJ, Kim J, et al. . Impaired GABAB receptor signaling dramatically up-regulates Kiss1 expression selectively in nonhypothalamic brain regions of adult but not prepubertal mice. Endocrinology. 2014;155(3):1033-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephens SB, Chahal N, Munaganuru N, Parra RA, Kauffman AS. Estrogen stimulation of kiss1 expression in the medial amygdala involves estrogen receptor-α but not estrogen receptor-β. Endocrinology. 2016;157(10):4021-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephens SBZ, Di Giorgio NP, Liaw RB, et al. . Estradiol-dependent and -independent stimulation of kiss1 expression in the amygdala, BNST, and lateral septum of mice. Endocrinology. 2018;159(9):3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RRID:AB_2571870. https://scicrunch.org/resolver/AB_2571870 [Google Scholar]
- 35.RRID:AB_2340621. https://scicrunch.org/resolver/AB_2340621 [Google Scholar]
- 36.Falcy BA, Mohr MA, Micevych PE. Immunohistochemical amplification of mCherry fusion protein is necessary for proper visualization. Methodsx. 2020;7(20):100946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci U S A. 2005;102(43):15682-15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephens SBZ, Rouse ML, Tolson KP, et al. . Effects of selective deletion of tyrosine hydroxylase from kisspeptin cells on puberty and reproduction in male and female mice. eNeuro 2017;4(3): ENEURO.0150-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88(6):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.RRID:AB_2665514. https://scicrunch.org/resolver/AB_2665514 [Google Scholar]
- 41.RRID:AB_2665513. http://antibodyregistry.org/AB_2665513 [Google Scholar]
- 42.Yang JA, Hughes JK, Parra RA, Volk KM, Kauffman AS. Stress rapidly suppresses in vivo LH pulses and increases activation of RFRP-3 neurons in male mice. J Endocrinol. 2018;239(3):339-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151(12):5807-5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semaan SJ, Kauffman AS. Daily successive changes in reproductive gene expression and neuronal activation in the brains of pubertal female mice. Mol Cell Endocrinol. 2015;401:84-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo E, Stephens SB, Chaing S, Munaganuru N, Kauffman AS, Breen KM. Corticosterone blocks ovarian cyclicity and the LH surge via decreased kisspeptin neuron activation in female mice. Endocrinology. 2016;157(3):1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esparza LA, Schafer D, Ho BS, Thackray VG, Kauffman AS. Hyperactive LH pulses and elevated kisspeptin and NKB gene expression in the arcuate nucleus of a PCOS mouse model. Endocrinology. 2020;161(4):bqaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.