Abstract
Adrenocortical carcinoma (ACC) is a rare tumor, and some histological variants (oncocytic, myxoid, and sarcomatoid ACCs) have been reported in addition to the conventional ACC. Among these subtypes, oncocytic ACC is histologically characterized by the presence of abundant eosinophilic granular cytoplasm in the carcinoma cells owing to the accumulation of mitochondria, which generally yields high 18F-fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET). Herein, we report the case of a 21-year-old woman with oncocytic ACC with low FDG uptake on PET scan. Her circulating levels of androgens were high, and androgen-synthesis enzymes were detected in carcinoma cells. The patient also had hypocholesterolemia. However, glucose transporter 1 (GLUT1) was not detected in the tumor, which was considered to account for the low FDG uptake by the tumor. To the best of our knowledge, this is the first case of low FDG uptake by oncocytic ACC without GLUT1 expression. Additionally, since hypocholesterolemia was reported in 3 previous reports of androgen-producing tumors, a possible correlation between androgenicity in adrenal tumors and the development of hypocholesterolemia could be postulated; however, further investigations are needed for clarification. This case highlights important information regarding the diversity of ACC and its impact on hypocholesterolemia.
Keywords: adrenal incidentaloma, androgen, cholesterol, DHEA-S, ENSAT, SUVmax
Adrenocortical carcinoma (ACC) is a rare malignancy with an annual worldwide incidence of 0.5–2 cases per million population [1]. Apart from the conventional ACC, some histological variants (oncocytic, myxoid, and sarcomatoid ACCs) have been characterized in the latest World Health Organization classification [1, 2]. Oncocytic ACC is histologically characterized by tumor cells with abundant eosinophilic granular cytoplasm, reflecting the accumulation of mitochondria [1–3].
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) has been used to clinically detect various malignant lesions. ACC has been reported to harbor markedly increased FDG uptake, surpassing the liver background [4]. Even in the case of benign adrenocortical tumors, adrenocortical oncocytoma has been reported to be associated with increased FDG uptake owing to the presence of numerous intracellular mitochondria [5] and increased glucose transporter 1 (GLUT1) expression [6]. Herein, we report a rare case of oncocytic ACC with low FDG uptake. To the best of our knowledge, no similar case has been reported previously. GLUT1 expression was very low in the tumor, which could account for the decreased FDG uptake. Additionally, marked hypocholesterolemia was observed in the patient. Only 3 cases of hypocholesterolemia associated with adrenal tumors have been reported in the English literature [7-9], and these concomitant cases will be discussed.
Case Presentation
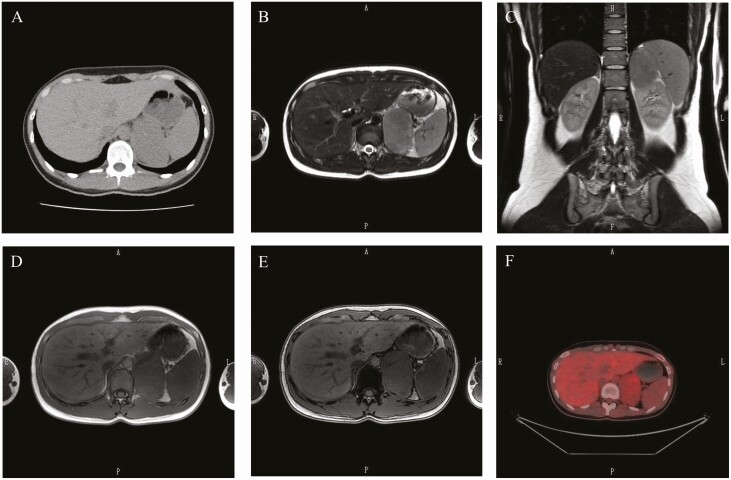
A 21-year-old Japanese woman was referred to our hospital for the characterization of a left adrenal tumor, which was incidentally detected on abdominal computed tomography (CT) after a traffic accident. The oval-shaped tumor measured 7.7 × 4.5 cm2 and had a homogeneous density of 40 Hounsfield units (HU) on a plain CT scan (Fig. 1A).
Figure 1.
(A) Plain computed tomography (CT) scans of the left adrenal area, showing a tumor with clear borders measuring 7.7 × 4.5 cm2. (B–E) Magnetic resonance imaging (MRI). T2-weighted images (B, C) and images in-phase (D) and out-of-phase (E) using chemical shift MRI. (F) Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG). The maximum standardized uptake value (SUVmax) of the left adrenal tumor was 2.8, and the adrenal to liver SUVmax was 0.98.
The patient underwent a detailed endocrine examination. She was aware of secondary amenorrhea since the age of 20 years; however, she had not paid any particular attention to it. She had no symptoms associated with excess hormone levels in the adrenal cortex or medulla, including hirsutism, and no medical history. Physical examination revealed no significant findings. Clinical parameters were as follows: body height, 162 cm; body weight, 54.0 kg; blood pressure, 110/58 mmHg; and heart rate, 71 beats/minute.
Laboratory data at the time of admission are summarized in Table 1. The complete blood count and blood biochemistry tests were within the normal range, except for extremely low serum total, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol levels. As for the endocrine findings, blood and 24-hour urine catecholamine levels were within the normal range. Plasma aldosterone concentration was high (438 pg/mL); however, plasma renin activity of 2.2 ng/mL/h and aldosterone/renin ratio of 199 were within the normal range; these values may reflect a reduction in salt intake after hospitalization relative to before. Cortisol and adrenocorticotropic hormone (ACTH) levels early in the morning were 10.4 µg/dL and 73.0 pg/mL, respectively. Serum dehydroepiandrosterone (DHEA)-sulfate (DHEA-S), testosterone, and urine 17-ketosteroid levels, especially at the DHEA level, were extremely high, which indicated an excess of adrenal androgens. Because a large adrenal mass was found, the patient underwent an overnight 1 mg dexamethasone suppression test. The cortisol level was 1.7 µg/dL, which ruled out the autonomous secretion of cortisol.
Table 1.
Laboratory findings of the patient
| Peripheral blood | Endocrinological data (plasma or serum) | ||||
|---|---|---|---|---|---|
| WBC | 5370/mm3 | (3300–8600) | Epinephrine | 43 pg/mL | (0–100) |
| RBC | 486 × 104/mm3 | (386–492) | Norepinephrine | 159 pg/mL | (100–450) |
| Hemoglobin | 14.7 g/dL | (11.6–14.8) | Dopamine | 6 pg/mL | (0–20) |
| Hematocrit | 41.7% | (35.1–44.4) | Renin activity | 2.2 ng/mL/h | (0.3–5.4) |
| Platelets | 22.4 × 104/mm3 | (15.8–34.8) | Aldosterone | 438 pg/mL | (29.9–159) |
| ARR | 199 | (<200) | |||
| Biochemical data | ACTH | 73.0 pg/mL | (7.2–63.3) | ||
| Total protein | 6.5 g/dL | (6.6–8.1) | Cortisol | 10.4 μg/dL | (6.2–19.4) |
| Albumin | 4.2 g/dL | (4.1–5.1) | DHEA-S | 4060 μg/dL | (18–391) |
| Total bilirubin | 1.1 mg/dL | (0.4–1.5) | Testosterone | 3.51 ng/mL | (0.11–0.47) |
| AST | 13 U/L | (13–30) | |||
| ALT | 9 U/L | (7–23) | Endocrinological data (urine) | ||
| LDH | 168 U/L | (124–222) | Epinephrine | 4.0 μg/day | (3.4–26.9) |
| ALP | 189 U/L | (106–322) | Norepinephrine | 45.1 μg/day | (48.6–168.4) |
| rGTP | 7 U/L | (9–32) | Metanephrine | 0.09 mg/day | (0.04–0.19) |
| BUN | 10 mg/dL | (8–20) | Normetanephrine | 0.09 mg/day | (0.09–0.33) |
| Creatinine | 0.72 mg/dL | (0.46–0.79) | (17-ketosteroid fraction) | ||
| Natrium | 142 mEq/L | (138–145) | Androsterone | 22.44 mg/day | (0.4–3.0) |
| Potassium | 4.1 mEq/L | (3.6–4.8) | Etiocholanolone | 23.42 mg/day | (0.3–2.5) |
| Chlorine | 105 mEq/L | (101–108) | Dehydroepiandrosterone | 493.86 mg/day | (0.04–2.6) |
| Glucose | 78 mg/dL | (73–109) | 11-ketoandrosterone | 0.70 mg/day | (0.0–0.07) |
| T.chol | 47 mg/dL | (142–220) | 11-ketoetiocholanolone | 1.32 mg/day | (0.03–0.5) |
| Triglyceride | 32 mg/dL | (30–150) | 11-OH androsterone | 8.42 mg/day | (0.22–1.6) |
| HDL-c | 32 mg/dL | (40–103) | 11-OH etiocholanolone | 0.53 mg/day | (0.02–0.65) |
| LDL-c | 9 mg/dL | (65–140) | |||
| sIL-2R | 243 U/mL | (121–613) |
Reference ranges are in parentheses.
Abbreviations: ACTH, adrenocorticotropic hormone; ALP, alkaline phosphatase; ALT, alanine transferase; ARR, aldosterone/renin ratio; AST, aspartate transaminase; BUN, blood urea nitrogen; DHEA-S, dehydroepiandrosterone sulfate; HDL-c, high-density lipoprotein-cholesterol; LDH, lactate dehydrogenase; LDL-c, low-density lipoprotein-cholesterol; RBC, red blood cells; rGTP, γ-glutamyl transferase; sIL-2R, soluble interleukin-2 receptor; T.chol, total cholesterol; WBC, white blood cells.
Magnetic resonance imaging (MRI) revealed a left mass measuring 7.2 × 4.6 cm2 (Fig. 1B-1E). The tumor showed a clear margin and isointense signal on T1-weighted images and iso- to hyperintense on T2-weighted images (Fig. 1B and 1C). On chemical shift MRI of the adrenal glands, the loss of signal intensity was not detected in out-of-phase imaging when compared with that of the spleen (Fig. 1D and 1E). On FDG-PET (Fig. 1F), the maximum standardized uptake value (SUVmax) of the left adrenal tumor was 2.8, and the adrenal to liver SUVmax was 0.98. There were no signals detected anywhere that would make us suspect a malignant tumor.
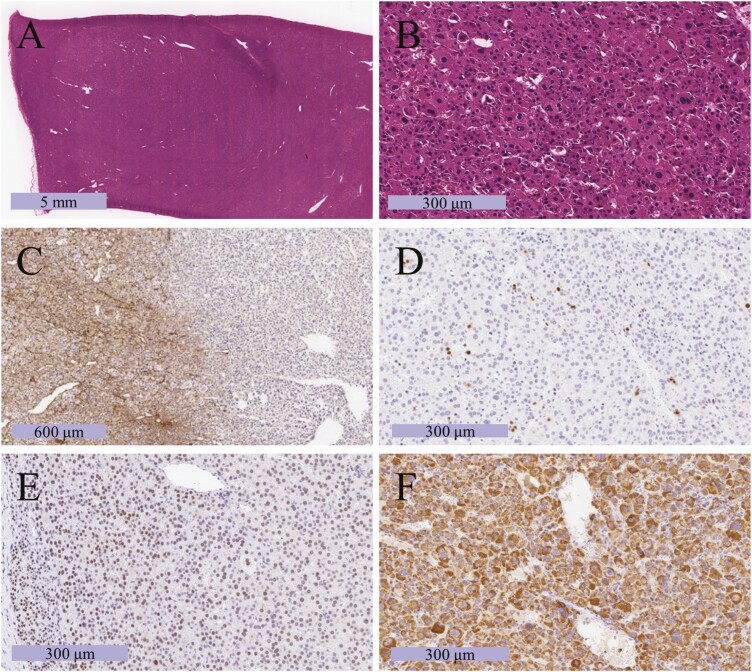
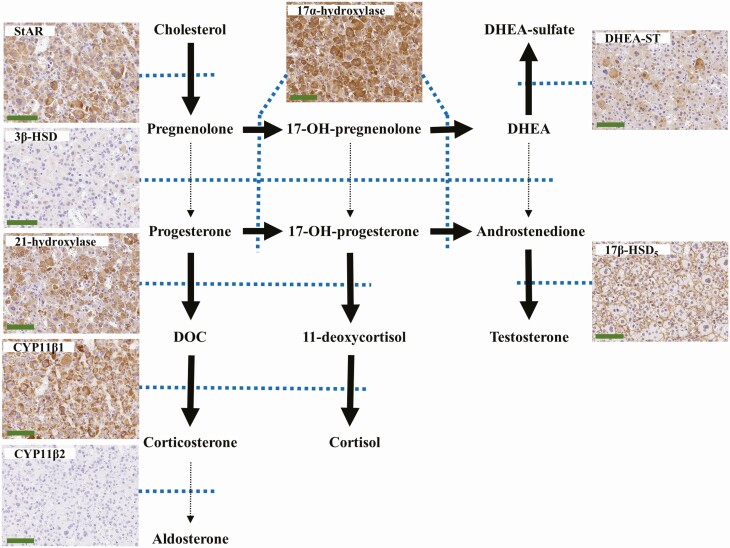
The patient underwent laparoscopic left adrenalectomy. The resected left adrenal gland weighed 130 g, and the tumor measured 82 mm × 50 mm × 50 mm. Representative histological findings are presented in Fig. 2. The tumor cells contained abundant eosinophilic cytoplasm (Fig. 2A). Capsular and sinusoidal invasions were not identified, and the normal adrenal cortex was detected in a compressed fashion near the capsule. Nuclear atypia (Fig. 2B) and a high mitotic index were detected with a diffuse growth pattern (Fig. 2C). The patient’s Weiss score was 4, and the tumor met a major criterion for the Lin-Weiss-Bisceglia system. The Ki-67 labeling index was 6% at hot spots (Fig. 2D). Tumor cells were immunopositive for steroidogenic factor 1 (Fig. 2E), indicating a tumor of the adrenal cortex. Tumor cells were diffusely and intensively immunopositive for mitochondria (Fig. 2F), indicating an oncocytic tumor. To evaluate steroid synthesizability in the resected tumor, we immunohistochemically evaluated the expression of steroidogenic enzymes (Fig. 3). The tumor cells were immunohistochemically positive for steroidogenic acute regulatory proteins (StAR), 21-hydroxylase, 11β-hydroxylase (CYP11β1), 17α-hydroxylase, DHEA-sulfotransferase, and 17β-hydroxysteroid dehydrogenase 5. The tumor was negative for 3β-hydroxysteroid dehydrogenase and 18-hydroxylase (CYP11β2). These results demonstrated that tumor cells produced DHEA and DHEA-S, but not cortisol and aldosterone. The final diagnosis was oncocytic ACC with an androgen-producing ability.
Figure 2.
Photomicrograph of the resected tumor. (A, B) Hematoxylin and eosin staining in low-power (A) and high-power (B) fields. (C) Immunohistochemical labeling for type IV collagen, which can confirm the presence of diffuse proliferation by checking whether the basic chordal structure has been destroyed or not. (D) Immunohistochemical labeling for Ki-67, which can stain cell nuclei during all active phases of the cell cycle and is used as a marker of cell proliferation. The Ki-67 labeling index was 6%. (E) Immunohistochemical labeling for SF-1, which can stain cell nuclei derived from steroidogenic hormone-producing glands such as the adrenal cortex [10]. (F) Immunohistochemical labeling for mitochondria. In the case of the oncocytic tumor, mitochondrial staining can be observed throughout the cytoplasm.
Figure 3.
The steroid synthesis pathway and photomicrographs of immunohistochemical labeling of steroid synthases in the resected tumor. The tumor was positive for steroidogenic acute regulatory protein (StAR), 21-hydroxylase, 11β-hydroxylase (CYP11β1), 17α-hydroxylase, DHEA-sulfotransferase (DHEA-ST), and 17β-hydroxysteroid dehydrogenase (HSD) 5 (17β-HSD5). The tumor was negative for 3β-HSD and 18-hydroxylase (CYP11β2). Blue dotted lines show related enzymes. Bold arrows mean “convertible” because of the existence of related enzymes. Dotted arrows mean “difficult to convert” because of low-level related enzymes. Scale bar: 100 μm. Abbreviations: DHEA: dehydroepiandrosterone, DOC: deoxycorticosterone.
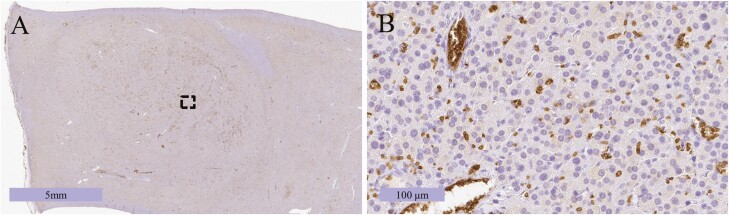
These results led to a new question: why was this tumor negative on FDG-PET? We hypothesized that glucose uptake in this tumor could be suppressed, and immunostaining for GLUT1, which is usually highly expressed in oncocytic ACC, revealed no immunoreactivity in the tumors (Fig. 4).
Figure 4.
(A) Photomicrograph of the resected tumor stained for glucose transporter 1 (GLUT1). (B) Enlarged view of the dotted region in (A). The tumor cells were GLUT1-negative (positive cells were erythrocytes for inner positive control).
The postoperative course of the patient was unremarkable. Amenorrhea, which was the only symptom of androgen excess, improved after surgery. Postoperative steroid treatment was not required. Mitotane was not administered because of the low-grade nature of the ACC. Postoperative blood cortisol and ACTH levels early in the morning were 6.7 µg/dL and 27.7 pg/mL, respectively. Plasma aldosterone concentration of 73.6 pg/mL, plasma renin activity of 0.8 ng/mL/h, and aldosterone/renin ratio of 92 were within the normal ranges. Serum DHEA-S level regressed to 293 μg/dL and testosterone level to 0.35 ng/mL. Serum total cholesterol, triglyceride, HDL-cholesterol, and LDL-cholesterol levels were 142 mg/dL, 44 mg/dL, 51 mg/dL, and 82 mg/dL, respectively, which were within the normal ranges. At 1 year postoperatively, there were no signs of ACC recurrence.
Discussion
A large left adrenal tumor measuring >7 cm was incidentally detected in our patient. The tumor showed a density of approximately 40 HU on a CT scan. Our case was of a functional tumor, but even if it was a nonfunctional tumor, consideration of surgery would have been appropriate. In some papers, tumors measureing >4 cm [11, 12] should be considered for surgery; in others, tumors measureing >4.6 cm or with an attenuation of >20 HU on CT scan [13], should be considered for surgery, because of the possibility of malignancy. On chemical shift MRI of the adrenal glands, the loss of signal intensity was not detected on out-of-phase imaging when compared with that of the spleen, suggesting the possibility of malignancy rather than adenoma [14]. The FDG-PET scan was negative in our case; however, adrenalectomy was performed because the tumor was functional, and imaging findings other than the FDG-PET scan were suspicious for carcinoma.
The pathological diagnosis after surgery was oncocytic ACC. Weiss’s criteria are considered the gold standard criteria for diagnosing ACC, with a combined score of ≥3 considered as malignancy [1, 2]. In our patient, the following 4 criteria were met: high nuclear grade, <25% of clear cells, diffuse architecture, and high mitotic index. Because the first 3 of Weiss’s criteria are characteristic of oncocytic neoplasms, Weiss’s criteria could result in the overdiagnosis during the pathological diagnosis of oncocytic ACC; hence, it may be better to use the Lin-Weiss-Bisceglia system [1, 2, 15]. In our patient, 1 major Lin-Weiss-Bisceglia criterion, a high mitotic index, was met, indicating malignancy. However, the differentiation between benign and malignant adrenal oncocytic tumors in pathological diagnosis remains controversial.
FDG-PET is useful for the differential diagnosis of benign and malignant lesions. Several studies on adrenocortical neoplasms demonstrated that the cutoff values of SUVmax for adrenal lesions ranged from 2.5 to 5.2, and the cutoff value of adrenal to liver SUVmax ratio ranged from 1.53 to 1.8 [16-19]. However, it is also true that the patients included in those studies were diagnosed with metastatic adrenal carcinoma. Owing to the rarity of ACC, little evidence exists for the cutoff values. Groussin et al reported that in a study of 22 ACC cases and 43 adrenocortical adenoma cases, using a cutoff value of >1.45 for the adrenal to liver SUVmax ratio could differentiate between ACC and adrenocortical adenoma [4]. In the same study, an SUVmax cutoff value of >3.4 for adrenal lesions was also proposed [4]. Tessonnier et al reported that 37 patients with ACC harbored median uptake values of SUVmax = 11 (range, 3-56) on FDG-PET scans [20]. In our patient, the SUVmax for the left adrenal tumor was 2.8, and the adrenal to liver SUVmax was 0.98. Therefore, this tumor was considered benign on FDG-PET scans, contrary to the malignant findings in the pathological diagnosis.
One major question in our case was the extremely low FDG uptake despite the presence of oncocytic ACC. Most aggressive malignant tumors, such as ACC, are known to utilize aerobic glycolysis to derive a substantial amount of energy [21]. Therefore, a large amount of glucose is usually taken up by malignant cells. Labeled deoxy-glucose, which is a glucose analog used in FDG-PET, enters the cell through specific transmembrane carrier proteins, especially GLUT1 [22]. Even in the case of benign tumors, oncocytic tumors such as those of the parotid gland, renal cells, adrenocortical cells, and thyroid Hurthle cells have been reported to be associated with increased FDG uptake [6, 21, 23, 24] owing to the presence of numerous intracellular mitochondria [5] and increased GLUT1 expression [6]. FDG uptake was expected to be high in our case considering the oncocytic nature of the lesion. Therefore, to further explore the pathogenesis of low FDG uptake in this oncocytic tumor, we hypothesized that tumoral glucose uptake could be suppressed, and proceeded to investigate GLUT1 expression. No immunoreactivity of GLUT1 was detected, which was one of the reasons for FDG-PET negativity. The reason for the decreased expression of GLUT1 in this tumor is unknown; however, it has been reported that GLUT1 expression is not always observed in conventional ACCs that are not oncocytic [25, 26]. Since no similar cases have been reported in the literature, additional reports of oncocytic ACC cases are needed to clarify this phenomenon.
Oncocytic ACC produced androgens in our patient. Because of the rarity of oncocytic ACC, there have been only a few reports; however, approximately one-third of oncocytic ACCs have been reported to produce androgens [27, 28]. To the best of our knowledge, ours is the first report of a detailed analysis of the expression of steroidogenic enzymes in functional oncocytic ACC. The results revealed that the status of the enzymes in the tumor tissue and corresponding circulating hormone levels were consistent, which also confirmed the usefulness of immunohistochemical evaluation of steroidogenic enzymes in exploring the features of neoplastic steroidogenesis in functioning adrenocortical tumors. Additionally, serum androgen levels decreased after surgery, and amenorrhea clinically improved. Recently, Harada et al reported a similar case of oncocytic ACC; however, it was nonfunctional [29], and neither PET results nor GLUT1 immunostaining were reported.
ACC is a rare tumor; thus, treatment decisions are difficult. However, the European Network for the Study of Adrenal Tumors (ENSAT) and the European Society of Endocrinology have published guidelines on the management of adrenocortical carcinoma in adults [30]. Before surgery, our case was evaluated as ACC amenable to complete resection (stage II according to the ENSAT staging system). Complete resection was performed, and the Ki-67 labeling index was <10%; therefore, the risk of recurrence was determined to be low/moderate. The guidelines above, however, did not necessarily mention that adjuvant mitotane therapy was always necessary but emphasized that the requirement of adjuvant mitotane therapy should be discussed on an individual basis. Our patient was not administered mitotane postoperatively because of the oncocytic nature of ACC, which was reported to have a better clinical outcome than conventional ACC [27, 28, 31]. Additionally, our case was GLUT1 negative. The higher expression of GLUT1 was associated with a worse prognosis in ACC; particularly, high GLUT1 expression in ACC indicated increased glucose uptake, which correlates with aggressive behavior [25, 26]. As expected, no recurrence was detected in our patient at this juncture, 1 year after the surgery. Long-term imaging and biochemical (eg, blood DHEA-S level) follow-up are warranted since the outcome and clinical behavior of GLUT1-negative oncocytic ACC remain uncertain.
In our patient, marked hypocholesterolemia was observed. To date, 3 similar cases have been reported in the English literature [7-9]. The clinical features and diagnoses of the 4 patients, including ours, are summarized in Table 2. In all these reports, the cases involved women, and serum androgen levels were high, similar to our study. However, the tumors were reported as benign adenomas, which is different from our case of the malignant tumor. The pathogenesis of hypocholesterolemia in patients with adrenal tumors has been reported to be subsequent to increased LDL receptor activity and unrestricted uptake of LDL by the adrenal tumor [9], but not the effect of increased serum levels of androgens on LDL receptors [9]. Because all 4 cases, including our case, showed high androgen levels, and because there have been no reports of hypocholesterolemia in patients with adrenal tumors other than androgen-producing tumors, we assume that androgen-producing tumors themselves could play a role in the development of hypocholesterolemia; however, further investigation is required for clarification.
Table 2.
Summary of case reports on hypocholesterolemia associated with androgen-producing adrenal tumors
| Author, year (ref) | Age (year) | Gender | Symptom | Excess hormone | T.chol (pre-operation) | T.chol (post-operation) | Diagnosis, affected side, tumor size |
|---|---|---|---|---|---|---|---|
| Leichter & Daughada, 1974 [8] | 48 | Female | Hirsutism, amenorrhea | Androgen | 95 mg/dL | 185 mg/dL | Benign adenoma, right, 20 cm |
| Nakagawa et al., 1995 [9] | 16 | Female | Hirsutism, amenorrhea | Androgen, cortisol | 23 mg/dL* | > 115mg/dL* | Benign adenoma, right, 10 cm |
| Benvenga, 1995 [7] | 20 | Female | Hirsutism, oligomenorrhea | Androgen, cortisol | 30 mg/dL* | 127 mg/dL* | Nonmalignant adrenal tumor, right, 15 cm |
| Present case | 21 | Female | Amenorrhea | Androgen | 47 mg/dL | 142 mg/dL | Oncocytic ACC, left, 8.2 cm |
Abbreviations: ACC, Adrenocortical carcinoma; T.chol, total cholesterol; ref, reference.
*Values that had been reported in millimole per liter were converted to milligram per deciliter by dividing by 0.0259.
In conclusion, we report the case of a patient with an oncocytic ACC with low 18F-FDG uptake. Particularly, the ACC masquerades as a benign lesion on PET/CT scans, and immunohistochemical analysis indicated low GLUT1 expression in the tumor. Since there are no similar cases reported in the literature, additional case reports are needed to prove this phenomenon. Moreover, the presence of hypocholesterolemia with adrenal masses has been reported in 4 cases, including ours, all of which involved androgen-producing tumors. Therefore, a possible correlation between androgenicity in adrenal tumors and the development of hypocholesterolemia could be proposed; however, further investigation is warranted.
Glossary
Abbreviations
- ACC
adrenocortical carcinoma
- ACTH
adrenocorticotropic hormone
- CT
computed tomography
- DHEA
dehydroepiandrosterone
- DHEA-S
dehydroepiandrosterone sulfate
- ENSAT
European Network for the Study of Adrenal Tumors
- FDG
18F-fluorodeoxyglucose
- GLUT1
glucose transporter 1
- HDL
high-density lipoprotein
- HU
Hounsfield unit
- LDL
low-density lipoprotein
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- SUV
standardized uptake value
Additional Information
Disclosures: The authors have no conflicts of interest to report. The authors declare that they have no competing interests.
Data Availability
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Consent for Publication:
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- 1. Torti JF, Correa R. Adrenal Cancer. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available at: https://www.ncbi.nlm.nih.gov/pubmed/31536189. Accessed July 17, 2021. [Google Scholar]
- 2. Lam AK. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr Pathol. 2017;28(3):213-227. [DOI] [PubMed] [Google Scholar]
- 3. Botsios D, Blouhos K, Vasiliadis K, Asimaki A, Tsalis K, Betsis D. Adrenocortical oncocytoma – a rare tumor of undefined malignant potential: report of a case. Surg Today. 2007;37(7):612-617. [DOI] [PubMed] [Google Scholar]
- 4. Groussin L, Bonardel G, Silvéra S, et al. 18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients. J Clin Endocrinol Metab. 2009;94(5):1713-1722. [DOI] [PubMed] [Google Scholar]
- 5. Kim DJ, Chung JJ, Ryu YH, Hong SW, Yu JS, Kim JH. Adrenocortical oncocytoma displaying intense activity on 18F-FDG-PET: a case report and a literature review. Ann Nucl Med. 2008;22(9):821-824. [DOI] [PubMed] [Google Scholar]
- 6. Sato N, Nakamura Y, Takanami K, et al. Case report: adrenal oncocytoma associated with markedly increased FDG uptake and immunohistochemically positive for GLUT1. Endocr Pathol. 2014;25(4):410-415. [DOI] [PubMed] [Google Scholar]
- 7. Benvenga S. Another case of hypocholesterolemia associated with virilizing adrenal adenoma. J Clin Endocrinol Metab. 1995;80(11):3391-3392. [DOI] [PubMed] [Google Scholar]
- 8. Leichter SB, Daughaday WH. Massive steroid excretion and hypocholesterolemia with an adrenal adenoma. Report of a case. Ann Intern Med. 1974;81(5):638-640. [DOI] [PubMed] [Google Scholar]
- 9. Nakagawa T, Ueyama Y, Nozaki S, et al. Marked hypocholesterolemia in a case with adrenal adenoma–enhanced catabolism of low density lipoprotein (LDL) via the LDL receptors of tumor cells. J Clin Endocrinol Metab. 1995;80(1):92-96. [DOI] [PubMed] [Google Scholar]
- 10. Sbiera S, Schmull S, Assie G, et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J Clin Endocrinol Metab. 2010;95(10):E161-E171. [DOI] [PubMed] [Google Scholar]
- 11. Menegaux F, Chéreau N, Peix JL, et al. Management of adrenal incidentaloma. J Visc Surg. 2014;151(5):355-364. [DOI] [PubMed] [Google Scholar]
- 12. Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab. 2010;95(9):4106-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kahramangil B, Kose E, Remer EM, . et al. A modern assessment of cancer risk in adrenal incidentalomas: analysis of 2219 patients. Ann Surg. Published online June 11, 2020. doi: 10.1097/SLA.0000000000004048. [DOI] [PubMed] [Google Scholar]
- 14. Blake MA, Cronin CG, Boland GW. Adrenal imaging. AJR Am J Roentgenol. 2010;194(6):1450-1460. [DOI] [PubMed] [Google Scholar]
- 15. Papotti M, Libè R, Duregon E, Volante M, Bertherat J, Tissier F. The Weiss score and beyond–histopathology for adrenocortical carcinoma. Horm Cancer. 2011;2(6):333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ciftci E, Turgut B, Cakmakcilar A, Erturk SA. Diagnostic importance of 18F-FDG PET/CT parameters and total lesion glycolysis in differentiating between benign and malignant adrenal lesions. Nucl Med Commun. 2017;38(9):788-794. [DOI] [PubMed] [Google Scholar]
- 17. Kunikowska J, Matyskiel R, Toutounchi S, Grabowska-Derlatka L, Koperski L, Królicki L. What parameters from 18F-FDG PET/CT are useful in evaluation of adrenal lesions? Eur J Nucl Med Mol Imaging. 2014;41(12):2273-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okada M, Shimono T, Komeya Y, et al. Adrenal masses: the value of additional fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) in differentiating between benign and malignant lesions. Ann Nucl Med. 2009;23(4):349-354. [DOI] [PubMed] [Google Scholar]
- 19. Vos EL, Grewal RK, Russo AE, et al. Predicting malignancy in patients with adrenal tumors using 18 F-FDG-PET/CT SUVmax. J Surg Oncol. 2020;122(8):1821-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tessonnier L, Ansquer C, Bournaud C, et al. (18)F-FDG uptake at initial staging of the adrenocortical cancers: a diagnostic tool but not of prognostic value. World J Surg. 2013;37(1): 107-112. [DOI] [PubMed] [Google Scholar]
- 21. Hofman MS, Hicks RJ. How We Read Oncologic FDG PET/CT. Cancer Imaging. 2016;16(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rubello D, Rufini V, Casara D, Calcagni ML, Samanes Gajate AM, Shapiro B. Clinical role of positron emission tomography (PET) in endocrine tumours. Panminerva Med. 2002;44(3):185-196. [PubMed] [Google Scholar]
- 23. Coppola M, Romeo V, Verde F, et al. Integrated imaging of adrenal oncocytoma: a case of diagnostic challenge. Quant Imaging Med Surg. 2019;9(11):1896-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiesner W, Engel H, von Schulthess GK, Krestin GP, Bicik I. FDG PET-negative liver metastases of a malignant melanoma and FDG PET-positive Hurthle cell tumor of the thyroid. Eur Radiol. 1999;9(5):975-978. [DOI] [PubMed] [Google Scholar]
- 25. Fenske W, Völker HU, Adam P, et al. Glucose transporter GLUT1 expression is an stage-independent predictor of clinical outcome in adrenocortical carcinoma. Endocr Relat Cancer. 2009;16(3):919-928. [DOI] [PubMed] [Google Scholar]
- 26. Pinheiro C, Granja S, Longatto-Filho A, et al. Metabolic reprogramming: a new relevant pathway in adult adrenocortical tumors. Oncotarget. 2015;6(42):44403-44421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mills JK, Khalil M, Pasieka J, Kong S, Xu Y, Harvey A. Oncocytic subtypes of adrenal cortical carcinoma: Aggressive in appearance yet more indolent in behavior? Surgery. 2019;166(4):524-533. [DOI] [PubMed] [Google Scholar]
- 28. Wong DD, Spagnolo DV, Bisceglia M, Havlat M, McCallum D, Platten MA. Oncocytic adrenocortical neoplasms–a clinicopathologic study of 13 new cases emphasizing the importance of their recognition. Hum Pathol. 2011;42(4):489-499. [DOI] [PubMed] [Google Scholar]
- 29. Harada K, Yasuda M, Nakano Y, et al. A rare case of oncocytic adrenocortical carcinoma clinically presented as an incidentaloma. Endocr J. 2020;67(8):883-888. [DOI] [PubMed] [Google Scholar]
- 30. Fassnacht M, Dekkers OM, Else T, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1-G46. [DOI] [PubMed] [Google Scholar]
- 31. Renaudin K, Smati S, Wargny M, et al. ; for Comete-Cancer Network . Clinicopathological description of 43 oncocytic adrenocortical tumors: importance of Ki-67 in histoprognostic evaluation. Mod Pathol. 2018;31(11):1708-1716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.