Abstract
Background
We conducted a review to compare the sensitivity, specificity, reproducibility, and predictive ability of QuantiFERON-TB Gold Plus (QFT-Plus) with that of QuantiFERON-TB Gold In-Tube (QFT-GIT; QIAGEN, Hilden, Germany) and other latent tuberculosis infection (LTBI) tests.
Methods
We searched MEDLINE, Embase, Web of Science, and the Cochrane Database of Systematic Reviews from January 2013 through May 2020. We included studies comparing QFT-Plus with at least one other LTBI test. We estimated sensitivity from studies of patients with active tuberculosis, and specificity from studies of healthy individuals with low risk of LTBI. Three independent reviewers evaluated eligibility, extracted data, and assessed risk of bias.
Results
Compared with QFT-GIT, the sensitivity of QFT-Plus in patients with active TB was 1.3% higher (95% confidence interval [CI], −0.3% to 2.9%); in 2 studies of patients with very low probability of LTBI, the specificity was 0.9% lower (95% CI, −2.4% to 0.6%). These differences were not statistically significant. The agreement between QFT-Plus and QFT-GIT was high, with a pooled Cohen’s kappa statistic of 0.83 (95% CI, 0.79 to 0.88). The reproducibility of QFT-GIT and QFT-Plus was similarly poor. All participants in the studies to estimate sensitivity were aged ≥15 years, and only 6 were people living with human immunodeficiency virus. We found no studies to assess predictive ability.
Conclusions
QFT-Plus has diagnostic performance that is very similar to that of QFT-GIT. Further studies are needed to assess the sensitivity of QFT-Plus in immunocompromised patients and younger children before concluding if this new version offers advantages.
Keywords: latent tuberculosis infection, interferon-gamma release assay, QuantiFERON-TB Gold Plus, sensitivity, specificity
Based on studies published to date, QuantiFERON-TB Gold Plus (QFT-Plus) and QuantiFERON-TB Gold In-Tube (QFT-GIT) have a very similar diagnostic performance. Further studies are needed to determine if QFT-Plus has a higher sensitivity than QFT-GIT in immunocompromised hosts and young children.
The diagnosis and treatment of individuals with latent tuberculosis infection (LTBI) is an important component of the global strategy to eradicate tuberculosis (TB). Currently, no microbiological test can reliably identify LTBI. Consequently, its diagnosis requires evidence of immune memory against Mycobacterium tuberculosis and the exclusion of active TB. QuantiFERON-TB (QFT) is an interferon-gamma release assay (IGRA), a commercial in vitro test that measures interferon-gamma released by T cells in whole blood in response to exposure to M. tuberculosis-specific antigens [1].
Since there is no reference standard for diagnosing LTBI, studies to evaluate the diagnostic accuracy of LTBI diagnostic tests have used surrogate markers with active TB patients for sensitivity and populations with very low risk of TB exposure for specificity. Because these test parameters are measured in different populations, one cannot analyze the effect of changing diagnostic criteria or thresholds on the sensitivity and specificity (receiver operating characteristic curve analysis). The most certain evidence of the presence of LTBI is the development of active TB among persons with positive LTBI test results who are not treated but followed in longitudinal studies. However, this type of design introduces serious ethical issues.
Previous systematic reviews that evaluated the sensitivity of LTBI diagnostic tests in active TB patients reported a pooled sensitivity of 78%–80% for QuantiFERON-TB Gold test (QFT-G) and 67%–70% for QuantiFERON-TB Gold In-Tube (QFT-GIT), similar to the pooled sensitivity of the tuberculin skin test (TST) (70%–77%) but lower than the sensitivity of the T-SPOT.TB (90%–93%) [2, 3]. The specificity of both QFT tests among individuals with low risk of LTBI was above 96% [2, 3]. However, the sensitivity of both QFTs is reduced in people with immunocompromising conditions such as people living with human immunodeficiency virus (PLHIV) infection [4, 5]. In children, 3 systematic reviews have reported a pooled sensitivity of QFT of 57%–89.6%, similar to that of the T-SPOT.TB (61%–88.5%) and the TST (67%–88.2%) [6–8]. In stratified analyses, the sensitivity of these tests was lower in children aged <5 years [7].
Some studies have found a specific role of the CD8+ T cells in the immune response to M. tuberculosis infection in both adults living with and not living with HIV and young children [9–11]. In an effort to improve the sensitivity of QFT-GIT in young children, people with recent exposure, and people living with HIV, the manufacturer developed QFT-Plus that has 1 additional tube for the induction of cell-mediated immune responses from both CD4+ and CD8+ T cells [12], with a positive test defined as either antigen tube positive. We conducted this review to compare the sensitivity, specificity, and predictive ability of QFT-Plus with QFT-GIT, T-SPOT.TB, and the TST. We also evaluated the agreement of these tests and the within-person reproducibility of QFT-Plus.
METHODS
Protocol and Registration
Analysis methods and inclusion criteria were specified in a protocol registered at PROSPERO: CRD42019146546.
Data Sources and Search Strategy
This review was conducted following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Table 1) [13]. We searched MEDLINE (via Ovid), Embase (via Ovid), Web of Science, and the Cochrane Database of Systematic Reviews for studies published from 1 January 2013 to 9 September 2019, with no language restrictions. The search starting date was based on the fact that the earliest time to enroll patients found in QFT-Plus studies was October 2013. Search keywords were those included in previous QFT-G and QFT-GIT systematic reviews [3], with the addition of the terms “QFT-Plus” and “QuantiFERON Plus” (Supplementary Table 2). We updated the search using the same strategy twice—on 25 November 2019 and on 1 May 2020. We identified additional studies through hand-search of all the issues of the International Journal of Tuberculosis and Lung Disease published from January 2013 to May 2020 and from the reference lists of all included studies as well as from identified reviews of IGRAs published since January 2013.
Study Selection and Eligibility
We included original full-text reports of studies that compared in a blinded manner the sensitivity, specificity, reproducibility, or predictive ability for TB development of QFT-Plus with QFT-GIT, T-SPOT.TB, and TST. Editorials, narrative reviews, letters, and conference abstracts were excluded. Studies that assessed sensitivity had to have the following characteristics: a cross-sectional or cohort design with 25 or more patients with active TB confirmed by molecular methods, culture, or histopathology, and within 14 days of starting treatment. Studies of clinically diagnosed active TB were included if the definition was prespecified and did not incorporate any LTBI test results. Studies of specificity had a cross-sectional or cohort design of 100 or more participants at a very low risk of LTBI (ie, age <50 years, life-long residents of countries with TB incidence <25/100 000, and no known exposure to patients with pulmonary TB; studies that included healthcare workers were excluded). As in previous reviews [2, 3], we considered that all low-risk persons with positive test results had false-positive results. Studies that assessed agreement reported the results of both tests in more than 90% of the included patients, with an interval between tests of less than 2 weeks. Within-person reproducibility was estimated from studies in which participants were tested more than once. To exclude the possibility of new infections, we excluded studies performed in settings with an annual TB incidence >25/100 000 if the time interval between tests was greater than 6 weeks. We included studies that reported reproducibility only or reported reproducibility as well as sensitivity or specificity. The minimum number of patients for different studies was set to minimize risk of publication bias.
Two investigators (CE. O. and E. O. B.) independently screened the list of titles and abstracts for potential inclusion. Discordances were resolved by consensus or consultation with a third reviewer (M. L. B.).
Data Extraction and Quality Assessment
Two reviewers (CE. O. and E. O. B.) independently extracted data using a standardized form designed for this study and assessed the risk of bias using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool for diagnostic studies [14]. Disagreements were resolved by consensus and discussion with a third reviewer (M. L. B.). Studies were classified at low risk of bias if the risk assessment scored “low” in all domains: patient selection, index test, reference standard, and flow and timing. If any domain was considered to have a “high” risk of bias or if 2 or more domains were classified as “unclear,” the study was rated at “high” risk of bias. If a study was classified as “unclear” in only 1 of the 4 domains, the risk of bias was considered “unclear.” We contacted all corresponding authors of the included articles to obtain additional data and information needed for quality assessment; all but 6 provided the requested information.
Data Synthesis and Statistical Analyses
We calculated sensitivity, specificity, and agreement (with 95% confidence intervals [CIs]) for each study and summarized the results in forest plots. The agreement was estimated using the Cohen’s kappa (κ) statistic [15]. In the primary analysis, we pooled estimates across studies that assessed the same endpoint (ie, sensitivity, specificity, or Cohen’s κ) and used the same comparator (eg, all studies that compared sensitivity of QFT-Plus with QFT-GIT).
Sensitivity and specificity were pooled using a general linear random effects mixed model [16]. Only 2 studies reported both sensitivity and specificity; in these studies, these 2 parameters were also estimated using bivariate random-effects models. We estimated pooled within-study differences in sensitivity and specificity (ie, between QFT-Plus and QFT-GIT and between QFT-Plus and T-SPOT.TB) using data from studies that provided enough information to reconstruct contingency tables for paired diagnostic tests. Then, the difference within each study was pooled with random-effects models using standard inverse-variance weights base (DerSimonian-Laird methods). If the 95% CI of the difference between QFT-Plus and the other LTBI tests did not include zero, the difference was considered to be statistically significant.
To estimate agreement, we pooled the Cohen’s κ using a random-effects model with the DerSimonian-Laird method, as described elsewhere [17]. Finally, we performed subgroup analyses for agreement, pooling the studies according to their risk of bias and to the included population (ie, healthcare workers, persons with LTBI or risk of LTBI, active TB patients, and persons on immunosuppressive therapy). Of the 2 studies that evaluated the reproducibility of QFT-Plus and QFT-GIT, the study design was too heterogeneous, so this outcome was not pooled. Finally, we assessed heterogeneity with I2 tests. Publication bias could not be assessed, as there is no reliable method to assess this for diagnostic studies [18]. All analyses were performed using R (version 3.6.2) with the following packages: meta [19], metafor [20], mada [21].
RESULTS
Study Selection and Description
We identified 4019 studies; 74 were selected for full-text review (Figure 1). Fifty articles were excluded (for detailed reasons, see Supplementary Table 3), leaving 24 studies that met our inclusion criteria [22–45]. Of the 24 included studies, 20 were cross-sectional, 1 was a prospective cohort [45], and 3 combined cross-sectional and prospective designs [31, 37, 44]. Seven studies evaluated sensitivity [22–28], 2 specificity [27, 28], 21 agreement [23–27, 29–44], and 2 reproducibility [31, 45]. No studies were found that assessed predictive ability.
Figure 1.
Flow diagram for search and study selection.
Seven studies conducted in high-income countries compared the sensitivity of QFT-Plus with QFT-GIT (Table 1), while 2 also compared the sensitivity of QFT-Plus with T-SPOT.TB. All 661 active TB patients were aged ≥15 years, of whom only 6 were PLHIV. Of the 2 studies that evaluated specificity, 1 compared QFT-Plus with QFT-GIT and T-SPOT.TB, and the other compared QFT-Plus with QFT-GIT. Both were conducted in Japan, and the median age of the included patients in both studies was 20 years (Supplementary Table 4).
Table 1.
Characteristics of the 7 Cross-Sectional Studies Included in the Pooled Estimates of Sensitivity
| Method of Diagnosis | Extent of Diseases, n/N (%) | Quality Assessment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author, Year (Reference) | Country | Comparator(s) | Participants, n | Patient Selection Method | Agea | Culture, n (%) | Clinical, n (%) | Pulmonary Tuberculosis, n (%) | Acid-Fast Bacilli Smear Positive | Cavitation on Chest X Ray | Human Immunodeficiency Virus Positives, n | Risk of Biasb | Concern Regarding Applicability |
| Fukushima, 2018 [22] |
Japan | QFT-GIT and T-SPOT.TB |
77 | Unclear | 79.9 (16.4) | 77 (100) | 0 (0) | 77 (100) | NR | NR | 0 | High | Highc |
| Hoffmann, 2016 [23] |
Germany | QFT-GIT | 57 | Convenience | NR | 24 (42) | 33 (58) | NR | NR | NR | 2 | High | Low |
| Hong, 2019 [24] |
South Korea | QFT-GIT | 33 | Consecutive | 17 (17, 24) d | 17e (52) | 16e (48) | 33 (100) | 3/33 (9) | 3/33 (9) | 0 | High | Low |
| Horne, 2018 [25] |
United States and Japan | QFT-GIT | 164 | Consecutive | 71 (46, 83)f | 164 (100) | 0 (0) | 145 (88) | NR | NR | 4 | High | Low |
| Petruccioli, 2017 [26] |
Italy | QFT-GIT | 69 | Unclear | 35 (28, 44) | 49 (71) | 20 (29) | 63 (91) | NR | 46/63 (73) | 0g | High | Low |
| Takasaki, 2018 [27] |
Japan | QFT-GIT and T-SPOT.TB |
99 | Unclear | 42 (29, 55)h | 99 (100) | 0 (0) | 97 (98) | 66/99 (67) | NR | 0 | High | Low |
| Yi, 2016 [28] |
Japan | QFT-GIT | 162 | Unclear | 59 (39, 70)i | 162 (100) | 0 (0) | 162 (100) | 138/162 (85) | NR | 0 | High | Low |
Abbreviations: NR, not reported; QFT-GIT, QuantiFERON-TB Gold In-Tube; T-SPOT.TB; .
aMean (standard deviation) or median (interquartile range).
bSee Supplementary Table 7 for detailed explanations of these quality ratings.
cThe results of the QFT-GIT were interpreted using criteria that were different from the manufacturer’s instructions.
dThere were 25 patients aged ≤18 years, the youngest of whom was 15.
eThese numbers differ from those reported in the article. This is the result of contacting the author and reconfirming.
fPatients excluded if aged >70 years in 1 site and >85 years in another site.
gPatients living with human immunodeficiency virus were excluded.
hPatients aged >70 years were excluded.
iPatients aged >80 years were excluded.
Of the 21 studies that assessed agreement between QFT-Plus and other LTBI diagnostic tests, only 1 was conducted in children (Supplementary Table 5). Regarding reproducibility, 2 articles met our inclusion criteria; both compared QFT-Plus with QFT-GIT in adults (Supplementary Table 6).
Assessment of study quality using the QUADAS-2 tool is summarized in Supplementary Figure 1. All studies that assessed sensitivity were considered to have a high risk of bias due to concerns regarding methods of selection of participants (for details, see Supplementary Table 7). Three studies excluded older adults [25, 27, 28], and 5 studies did not include any PLHIV [22, 24, 26–28]. Only 1 study included children, but the minimum age was 15 years in that study [24]. Moreover, 1 study recruited patients nonconsecutively [23], and another had unclear patient selection methods [22]. The latter also used QFT-GIT interpretation standards that differed from those recommended by the manufacturer [22].
Sensitivity and Specificity of QFT-Plus Compared With QFT-GIT and T-SPOT.TB
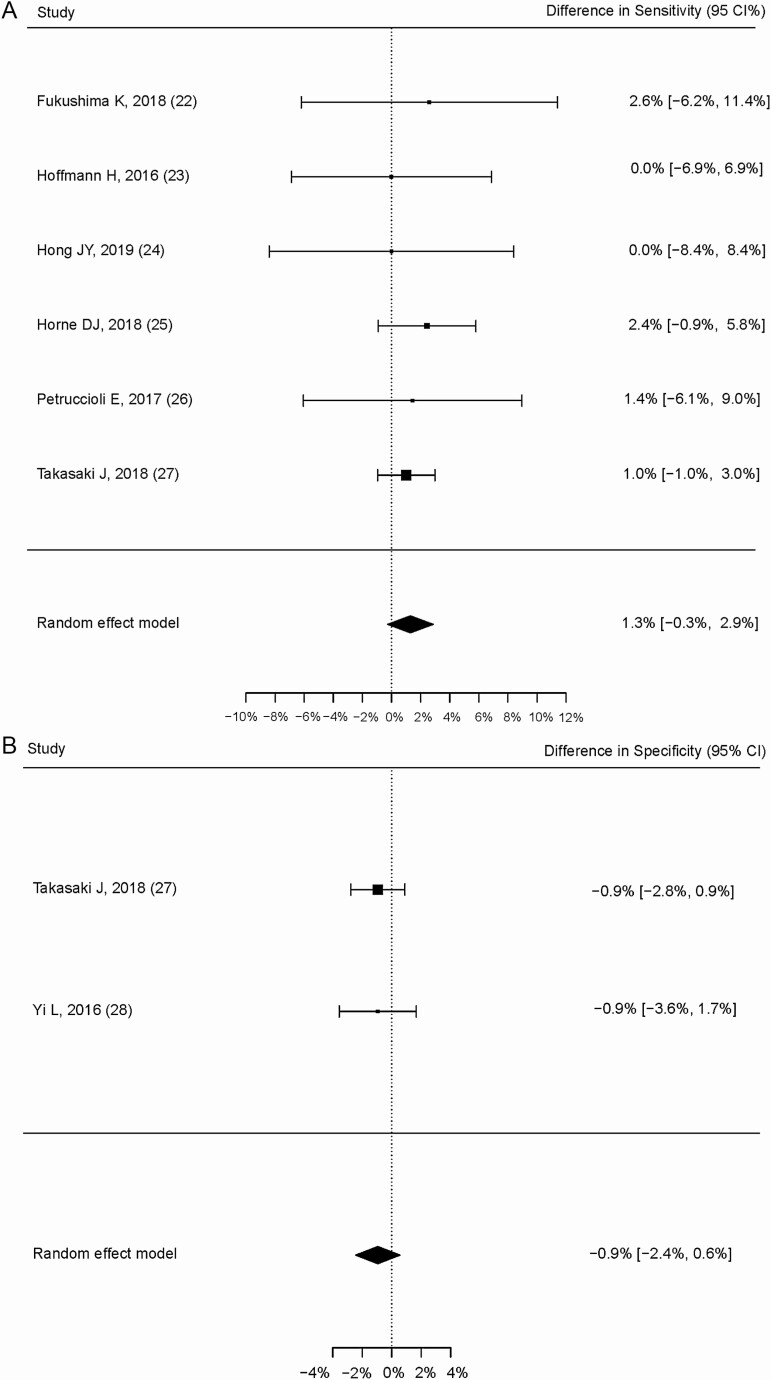
The pooled difference in sensitivity between QFT-Plus and QFT-GIT was 1.3% (95% CI, –.3% to 2.9%; Figure 2A) in 6 studies with 499 participants, where this calculation was possible [22–27]. In 1 study [27], the difference in sensitivity between QFT-Plus and T-SPOT.TB was 2.0% (95% CI, –.7% to 4.8%). The pooled difference in specificity between QFT-Plus and QFT-GIT was –0.9% (95% CI, –2.4% to .6%; Figure 2B). All of the 95% CIs of the differences included zero.
Figure 2.
Pooled within-study differences in sensitivity and specificity. A, Difference in sensitivity between QFT-Plus and QFT-GIT in 6 studies. B, Difference in specificity between QFT-Plus and QFT-GIT. Abbreviations: IFN-γ, interferon gamma; QFT-GIT; QuantiFERON-TB Gold In-Tube; QFT-Plus, QuantiFERON-TB Gold Plus.
As shown in Table 2 and Supplementary Figure 2, pooled estimates of sensitivity were 91.4% (95% CI, 87.5% to 94.2%), 91.4% (95% CI, 88.9% to 93.4%), and 90.2% (95% CI, 61.9% to 98.1%) for QFT-Plus, QFT-GIT, and T-SPOT.TB, respectively. Most of the patients with active TB (89.6%, 592 of 661) had microbiologically confirmed disease, and acid-fast bacilli smear was positive in 207 (69.5%) of 294 patients. When pooled estimates of sensitivity were stratified by the method of diagnosis, the sensitivity of QFT-Plus and QFT-GIT in patients with clinical TB was lower than that of patients with confirmed TB (Table 2).
Table 2.
Pooled Estimates of Sensitivity and Specificity
| Parameter | Test | No. of Studies | Pooled Values in % Estimate (95% Confidence Interval) |
I2 (%) |
|---|---|---|---|---|
| All studies | ||||
| Sensitivity | QFT-Plus | 7 | 91.4 (87.5–94.2) | 50.3 |
| QFT-GIT | 7 | 91.4 (88.9–93.4) | 5.1 | |
| T-SPOT.TB | 2 | 90.2 (61.9–98.1) | 87.0 | |
| Specificity | QFT-Plus | 2 | 97.8 (95.5–98.9) | 0.0 |
| QFT-GIT | 2 | 98.7 (96.7–99.5) | 0.0 | |
| T-SPOT.TBa | 1 | 98.1 (–) | – | |
| Studies that estimated sensitivity and specificity (bivariate estimates) | ||||
| Sensitivity | QFT-Plus | 2 | 95.9 (67.2–99.6) | 70.3b |
| QFT-GIT | 2 | 95.0 (80.2–98.9) | 56.8b | |
| Specificityc | QFT-Plus | 2 | 97.9 (95.5–99.0) | 0.0 |
| QFT-GIT | 2 | 98.8 (96.7–99.6) | 0.0 | |
| Stratified by population | ||||
| Sensitivity, in microbiologically confirmed active TB patients | QFT-Plus | 7 | 92.6 (88.1–91.0) | 55.7 |
| QFT-GIT | 7 | 91.8 (88.5–94.4) | 28 | |
| T-SPOT.TB | 2 | 90.2 (61.9–98.1) | 87.0 | |
| Sensitivity, in clinically diagnosed active TB patients | QFT-Plus | 3 | 85.3 (74.8–91.9) | 0.0 |
| QFT-GIT | 3 | 88.8 (80.9–93.7) | 0.0 |
Abbreviations: QFT-Plus, QuantiFERON-TB Gold Plus; QFT-GIT, QuantiFERON-TB Gold In-Tube; T-SPOT.TB; TB, tuberculosis.
aOnly 1 article reported the specificity of T-SPOT.TB.
bEstimated using univariate model (random-effects–general linear mixed model).
cFalse-positive rate.
As seen in Table 2 and Supplementary Figure 3, pooled estimates of specificity were 97.8% (95% CI, 95.5% to 98.9%), 98.7% (95% CI, 96.7% to 99.5%), and 98.1% in QFT-Plus, QFT-GIT, and T-SPOT.TB, respectively. Given that only 1 article reported the specificity of T-SPOT.TB, no 95% CI was provided. Two studies evaluated both sensitivity and specificity. In the bivariate analysis, the sensitivity of QFT-Plus was 95.9% (95% CI, 67.2 to 99.6; area under the curve [AUC], 0.937); the sensitivity of QFT-GIT was 95.0% (95% CI, 80.2 to 98.9; AUC, 0.940); and the specificity in QFT-Plus and QFT-GIT was 97.9% (95% CI, 95.5% to 99.0%) and 98.8% (95% CI, 96.7% to 99.6%), respectively.
Agreement of QFT-Plus With QFT-GIT, T-SPOT.TB, and TST
Agreement between QFT-Plus and QFT-GIT in all included studies was almost perfect, with an estimated pooled Cohen’s κ statistic of 0.83 (95% CI, .79 to .88; Table 3 and Supplementary Figure 4). QFT-Plus and T-SPOT.TB had a substantial agreement, with an estimated pooled κ statistic of 0.78 (95% CI, .63 to.93). In the only article that reported agreement between QFT-Plus and TST, the κ value was 0.46. The agreement between QFT-Plus and the other IGRAs was significant or almost perfect in most groups when stratified on a population basis.
Table 3.
Pooled Estimates of Agreement Between Tests Results
| Parameter | Tests Compared | No. of Studies | Pooled Kappa Values Estimate (95% Confidence Interval) |
I2 (%) |
|---|---|---|---|---|
| All studies | ||||
| QFT-Plus vs QFT-GIT | 19 | .83 (.79–.88) | 74.1 | |
| QFT-Plus vs T-SPOT.TB | 5 | .78 (.63–.93) | 95.1 | |
| QFT-Plus vs tuberculin skin test | 1 | .46 (.38–.54) | –a | |
| Stratified by quality | ||||
| Low risk of bias | QFT-Plus vs QFT-GIT | 14 | .83 (.77–.89) | 77.1 |
| QFT-Plus vs T-SPOT.TB | 3 | .79 (.49–1.00) | 81.9 | |
| High or unclear risk of bias | QFT-Plus vs QFT-GIT | 5 | .85 (.82–.88) | 2.4 |
| QFT-Plus vs T-SPOT.TB | 2 | .75 (.68–.81) | 33.8 | |
| Stratified by population | ||||
| Healthcare workers | QFT-Plus vs QFT-GIT | 5 | .75 (.60–.89) | 86.9 |
| Persons with LTBI or risk of LTBI | QFT-Plus vs QFT-GIT | 9 | .84 (.79–.88) | 15.1 |
| Active tuberculosisb | QFT-Plus vs QFT-GIT | 7 | .79 (.63–.95) | 72.3 |
| Persons on immunosuppressive therapy | QFT-Plus vs QFT-GIT | 2 | .86 (.80–.92) | 0.0 |
| Persons on immunosuppressive therapy | QFT-Plus vs T-SPOT.TB | 2 | 0.64 (.37–.91) | 35.5 |
Abbreviations: LTBI, latent tuberculosis infection; QFT-Plus, QuantiFERON-TB Gold Plus; QFT-GIT, QuantiFERON-TB Gold In-Tube; T-SPOT.TB; TB, tuberculosis.
aOnly 1 article reported the agreement of QFT-Plus and tuberculin skin test.
bOne of the 8 studies that reported active tuberculosis patients was excluded from the analysis because the results of the 2 tests were perfectly agreed and the data did not converge.
Reproducibility of QFT-Plus Compared With QFT-GIT
Knierer et al analyzed reproducibility by repeating QFT-Plus and QFT-GIT once weekly for 4 weeks [45] (Supplementary Table 6). In this study, 4 of 93 negative QFT-Plus tests converted from negative to positive (4.3%; 95% CI, 1.4% to 11.3%), and 2 of 29 positive tests reverted to negative (6.9%; 95% CI, 1.2% to 24.2%). Of 91 with negative QFT-GIT, 2 converted (2.2%; 95% CI, 0.4% to 8.5%), and 1 of 31 with positive QFT-GIT reverted (3.2%; 95% CI, .2% to 18.5%). Chien et al tested QFT-Plus and QFT-GIT 3 times over 4 weeks [31]. Of 74 patients who were initially positive with QFT-Plus, 16 reverted in the second test (21.6%), and 5 of the 16 with reversion converted back to positive on the third test (31.3%). Among 66 patients with positive QFT-GIT in the initial test, 15 (22.7%) reverted to negative in the second test, 5 of those 15 patients (33.3%) converted back to positive in the third test.
Indeterminate Results of QFT-Plus Compared With QFT-GIT or T-SPOT.TB
As seen in Table 4, the pooled estimate of rates of indeterminate QFT-Plus was 1.5% (95% CI, .5% to 4.9%) in the studies that evaluated sensitivity, it was 0% (95% CI, .05% to 2.3%) in the studies that evaluated specificity, and it was 0.3% (95% CI, .07% to .94%) in the studies that evaluated agreement. These results were similar to the rates of indeterminate tests with QFT-GIT and were lower than the proportion of the results that were invalid and/or borderline in T-SPOT.TB.
Table 4.
Pooled Estimates of Indeterminate Results
| Parameter | Test | No. of Studies | n/N | Estimate (95% Confidence Interval) (%) | I2 (%) |
|---|---|---|---|---|---|
| Studies that evaluated sensitivity | QFT-Plus | 7 | 15/661 | 1.5 (.5–4.9) | 64.2 |
| QFT-GIT | 7 | 17/661 | 1.9 (.6–5.7) | 70.3 | |
| T-SPOT.TB | 2 | 8/176 | 4.3 (1.7–10.6) | 34.4 | |
| Studies that evaluated specificity | QFT-Plusa | 2 | 0/318 | 0.0 (.05–2.3) | 0.0 |
| QFT-GITa | 2 | 0/318 | 0.0 (.05–2.3) | 0.0 | |
| T-SPOT.TB | 1 | 2/106 | 1.9 (–) | – | |
| Studies that evaluated agreement | QFT-Plus | 19b | 51/5878 | 0.3 (.07–.94) | 90.7 |
| QFT-GIT | 19b | 47/5878 | 0.4 (.13–1.16) | 89.6 | |
| T-SPOT.TB | 5c | 59/897 | 4.6 (3.6–5.7) | 0.0 |
Abbreviations: QFT-Plus, QuantiFERON-TB Gold Plus; QFT-GIT, QuantiFERON-TB Gold In-Tube; T-SPOT.TB; TB, tuberculosis.
aThese studies pooled using random-effects model, by inverse method, applying a 0.5 continuity correction for empty cells. All other estimates from general linear mixed method.
bIncludes 5 studies that reported sensitivity.
cIncludes 1 study that reported sensitivity.
DISCUSSION
We compared the diagnostic accuracy, agreement, and reproducibility of QFT-Plus, the latest version of the QuantiFERON assay, with QFT-GIT and other tests for LTBI. Compared with QFT-GIT, QFT-Plus had a clinically and statistically nonsignificant increase in sensitivity, with a loss of specificity of similar magnitude. The agreement between the 2 tests was very high, confirming that there is little difference in the performance of the 2 tests. The sensitivity and specificity of QFT-Plus and T-SPOT.TB were similar, although this is based on limited information. Only 2 studies assessed the reproducibility of QFT-Plus; these 2 studies reported high rates of spontaneous conversion and reversion, similar to QFT-GIT.
Hence, we found no evidence that QFT-Plus has superior sensitivity or specificity for the diagnosis of LTBI compared with QFT-GIT. One prior review suggested that QFT-Plus may have higher sensitivity than QFT-GIT [46]. In this review, the pooled sensitivity of QFT-Plus was compared with the pooled sensitivity of QFT-GIT from a different set of studies included in an earlier systematic review [47]. In our study, we estimated the sensitivity of the 2 tests only in populations that underwent both tests simultaneously. Interestingly, the sensitivity of both tests was substantially higher than that of QFT-GIT in all previous reviews [2, 3, 47], which may reflect differences in the populations. We speculate that the estimates of the sensitivity of QFT-Plus and QFT-GIT in our review might be higher as a consequence of the exclusion of younger children, older persons, and PLHIV, and possibly selecting persons with less severe TB disease than in prior studies. Our within-study estimates of differences in sensitivity and specificity between QFT-Plus and the other LTBI tests should have been much less affected by these potential biases.
Given the nearly identical diagnostic performance and little change in test procedures, the only potential advantage of QFT-Plus compared with QFT-GIT would be a lower cost. Regarding the material costs, we collected our experience at 3 sites in Supplementary Table 8. Although the material price per test of QFT-Plus is the same as for QFT-GIT, the overall cost for QFT-Plus per test increased, mostly as a consequence of fewer tests now being performed per enzyme-linked immunosorbent assay plate, with a resultant increase in technician time and materials costs.
Our study has limitations. All studies that estimated sensitivity were considered to have a high risk of bias because of concerns regarding patient selection methods. Second, there were very few studies that compared QFT-Plus and T-SPOT.TB or TST. Third, we could not identify studies of the predictive ability of QFT-Plus. Given the excellent agreement of QFT-Plus with QFT-GIT, we can infer that these outcomes would likely be similar to those already established in previous studies that used QFT-GIT. Fourth, as very few PLHIV and children were included, we cannot assess whether QFT-Plus has superior sensitivity in these high-risk population groups. In 2 studies that were not included in this study because they did not compare QFT-Plus to any other LTBI test, the sensitivity of QFT-Plus in active TB patients living with HIV infection was 68.7%–85% [48, 49]. In 3 other studies excluded because they did not have a comparator test or were published after our search period, the sensitivity of QFT-Plus in children with active TB was 82.9%–84.2% [50–52].
The development of active TB disease following testing in untreated persons is currently considered the optimal reference standard, but we could not find any longitudinal studies that measured incident TB disease following QFT-Plus testing. Hence, we estimated sensitivity and specificity from cross-sectional studies in which these test characteristics were assessed in separate populations, with sensitivity assessed in persons with active TB disease, and specificity in populations considered to have very low likelihood of prior exposure. This approach has several limitations. Optimal cut-points cannot be defined as these require results of sensitivity and specificity from the same population. Sensitivity may be underestimated because persons with active TB have impaired cell-mediated immunity, which will affect immune-based tests, such as the IGRA. Specificity may be underestimated, as TB infection still can occur even in populations considered at low risk of TB exposure.
In conclusion, we found minimal differences in sensitivity and specificity between QFT-Plus and QFT-GIT and excellent agreement between these 2 tests. Our findings can reassure clinicians that use of QFT-Plus in place of QFT-GIT should not result in any clinically significant change in diagnostic performance for LTBI in nonimmunocompromised adults. However, there is insufficient data to determine whether the sensitivity of QFT-Plus is superior to that of QFT-GIT among immunocompromised persons and young children, a stated objective of the manufacturer. Studies that compare QFT-Plus with other LTBI tests in these populations are needed, as well as longitudinal studies to evaluate the predictive ability of this new test.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Protocol registered at PROSPERO: CRD42019146546
Notes
Financial support. No funding sources directly supported this study. M. L. B. has salary support from the Canadian Institutes of Health Research (foundation grant FDN – 143350).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention . Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 2010; 59:1–25. [PubMed] [Google Scholar]
- 2.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–54. [DOI] [PubMed] [Google Scholar]
- 3.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santin M, Muñoz L, Rigau D. Interferon-γ release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PLoS One 2012; 7:e32482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cattamanchi A, Smith R, Steingart KR, et al. . Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr 2011; 56:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sollai S, Galli L, de Martino M, Chiappini E. Systematic review and meta-analysis on the utility of interferon-gamma release assays for the diagnosis of Mycobacterium tuberculosis infection in children: a 2013 update. BMC Infect Dis 2014; 14:S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandalakas AM, Detjen AK, Hesseling AC, Benedetti A, Menzies D. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis 2011; 15:1018–32. [DOI] [PubMed] [Google Scholar]
- 8.Laurenti P, Raponi M, de Waure C, Marino M, Ricciardi W, Damiani G. Performance of interferon-γ release assays in the diagnosis of confirmed active tuberculosis in immunocompetent children: a new systematic review and meta-analysis. BMC Infect Dis 2016; 16:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiacchio T, Petruccioli E, Vanini V, et al. . Polyfunctional T-cells and effector memory phenotype are associated with active TB in HIV-infected patients. J Infect 2014; 69:533–45. [DOI] [PubMed] [Google Scholar]
- 10.Lancioni C, Nyendak M, Kiguli S, et al. ; Tuberculosis Research Unit . CD8+ T cells provide an immunologic signature of tuberculosis in young children. Am J Respir Crit Care Med 2012; 185:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozot V, Vigano S, Mazza-Stalder J, et al. . Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol 2013; 43:1568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.QIAGEN. QuantiFERON®-TB Gold Plus (QFT®-Plus) package insert. Available at: https://www.quantiferon.com/us/wp-content/uploads/sites/13/2019/07/L1095849-R05-QFT-Plus-ELISA-IFU-USCA.pdf. Accessed 20 February 2020.
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiting PF, Rutjes AW, Westwood ME, et al. ; QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155:529–36. [DOI] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–74. [PubMed] [Google Scholar]
- 16.Hamza TH, van Houwelingen HC, Stijnen T. The binomial distribution of meta-analysis was preferred to model within-study variability. J Clin Epidemiol 2008; 61:41–51. [DOI] [PubMed] [Google Scholar]
- 17.Sun S. Meta-analysis of Cohen’s kappa. Health Serv Outcomes Res Methodol 2011; 11:145–63. [Google Scholar]
- 18.Macaskill PGC, Deeks JJ, Harbord RM, Takwoingi Y.In: Deeks JJ, BP, Gatsonis C, eds. Cochrane handbook for systematic reviews of diagnostic test accuracy. Oxford, United Kingdom: The Cochrane Collaboration, 2010:46–7. Available at: http://srdta.cochrane.org/. Accessed 20 February 2020. [Google Scholar]
- 19.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019; 22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viechtbauer W. Conducting meta-analyses in R with the metafor package. 2010. J Stat Softw 2010; 36:48. Available at: https://www.jstatsoft.org/article/view/v036i03. Accessed 8 June 2020. [Google Scholar]
- 21.Doebler P. Meta-analysis of diagnostic accuracy. 2019. Available at: https://cran.r-project.org/web/packages/mada/mada.pdf. Accessed 8 June 2020.
- 22.Fukushima K, Kubo T, Kaneko Y, et al. . Comparison study of sensitivity of QuantiFERON® TB Gold Plus with existing IGRAs in the patients with active pulmonary tuberculosis. Kekkaku. 2018; 93:517–23. [Google Scholar]
- 23.Hoffmann H, Avsar K, Gores R, Mavi SC, Hofmann-Thiel S. Equal sensitivity of the new generation QuantiFERON-TB Gold plus in direct comparison with the previous test version QuantiFERON-TB Gold IT. Clin Microbiol Infect 2016; 22:701–3. [DOI] [PubMed] [Google Scholar]
- 24.Hong JY, Park SY, Kim A, Cho SN, Hur YG. Comparison of QFT-Plus and QFT-GIT tests for diagnosis of M. tuberculosis infection in immunocompetent Korean subjects. J Thorac Dis 2019; 11:5210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horne DJ, Jones BE, Kamada A, et al. . Multicenter study of QuantiFERON-TB Gold Plus in patients with active tuberculosis. Int J Tuberc Lung Dis 2018; 22:617–21. [DOI] [PubMed] [Google Scholar]
- 26.Petruccioli E, Vanini V, Chiacchio T, et al. . Analytical evaluation of QuantiFERON- Plus and QuantiFERON- Gold In-tube assays in subjects with or without tuberculosis. Tuberculosis (Edinb) 2017; 106:38–43. [DOI] [PubMed] [Google Scholar]
- 27.Takasaki J, Manabe T, Morino E, et al. . Sensitivity and specificity of QuantiFERON-TB Gold Plus compared with QuantiFERON-TB Gold In-Tube and T-SPOT.TB on active tuberculosis in Japan. J Infect Chemother 2018; 24:188–92. [DOI] [PubMed] [Google Scholar]
- 28.Yi L, Sasaki Y, Nagai H, et al. . Evaluation of QuantiFERON-TB Gold Plus for detection of Mycobacterium tuberculosis infection in Japan. Sci Rep 2016; 6:30617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal S, Nguyen DT, Lew JD, Graviss EA. Performance and variability of QuantiFERON Gold Plus assay associated with phlebotomy type. PLoS One 2018; 13:e0207892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barcellini L, Borroni E, Brown J, et al. . First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur Respir J 2016; 48:1411–9. [DOI] [PubMed] [Google Scholar]
- 31.Chien JY, Chiang HT, Lu MC, et al. . QuantiFERON-TB Gold Plus is a more sensitive screening tool than QuantiFERON-TB Gold In-Tube for latent tuberculosis infection among older adults in long-term care facilities. J Clin Microbiol 2018; 56:e00427–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallegos Morales EN, Knierer J, Schablon A, Nienhaus A, Kersten JF. Prevalence of latent tuberculosis infection among foreign students in Lübeck, Germany tested with QuantiFERON-TB Gold In-Tube and QuantiFERON-TB Gold Plus. J Occup Med Toxicol 2017; 12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Igari H, Ishikawa S, Nakazawa T, et al. . Lymphocyte subset analysis in QuantiFERON-TB Gold Plus and T-Spot.TB for latent tuberculosis infection in rheumatoid arthritis. J Infect Chemother 2018; 24:83–7. [DOI] [PubMed] [Google Scholar]
- 34.Igari H, Akutsu N, Ishikawa S, et al. . Positivity rate of interferon-gamma release assays for estimating the prevalence of latent tuberculosis infection in renal transplant recipients in Japan. J Infect Chemother 2019; 25:537–42. [DOI] [PubMed] [Google Scholar]
- 35.Kay AW, DiNardo AR, Dlamini Q, et al. . Evaluation of the QuantiFERON-Tuberculosis Gold Plus assay in children with tuberculosis disease or following household exposure to tuberculosis. Am J Trop Med Hyg 2019; 100:540–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SH, Jo KW, Shim TS. QuantiFERON-TB Gold PLUS versus QuantiFERON- TB Gold In-Tube test for diagnosing tuberculosis infection. Korean J Intern Med 2020; 35:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon HW, Gaur RL, Tien SS, Spangler M, Pai M, Banaei N. Evaluation of QuantiFERON-TB Gold-Plus in health care workers in a low-incidence setting. J Clin Microbiol 2017; 55:1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pieterman ED, Liqui Lung FG, Verbon A, et al. . A multicentre verification study of the QuantiFERON-TB Gold Plus assay. Tuberculosis 2018; 108:136–42. [DOI] [PubMed] [Google Scholar]
- 39.Ryu MR, Park MS, Cho EH, et al. . Comparative evaluation of QuantiFERON-TB Gold In-Tube and QuantiFERON-TB Gold Plus in diagnosis of latent tuberculosis infection in immunocompromised patients. J Clin Microbiol 2018; 56:e00438–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel SAR, Cavanaugh M, Ku JH, Kawamura LM, Winthrop KL. Specificity of QuantiFERON-TB Plus, a new-generation interferon gamma release assay. J Clin Microbiol 2018; 56:e00629–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theel ES, Hilgart H, Breen-Lyles M, et al. . Comparison of the QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold In-Tube interferon gamma release assays in patients at risk for tuberculosis and in health care workers. J Clin Microbiol 2018; 56:e00614–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuyuzaki M, Igari H, Okada N, Suzuki K. Variation in interferon-γ production between QFT-Plus and QFT-GIT assays in TB contact investigation. Respir Investig 2019; 57:561–5. [DOI] [PubMed] [Google Scholar]
- 43.Venkatappa TK, Punnoose R, Katz DJ, et al. . Comparing QuantiFERON-TB Gold Plus with other tests to diagnose Mycobacterium tuberculosis infection. J Clin Microbiol 2019; 57:e00985–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Xin H, Wang D, et al. . Serial testing of Mycobacterium tuberculosis infection in Chinese village doctors by QuantiFERON-TB Gold Plus, QuantiFERON-TB Gold in-Tube and T-SPOT.TB. J Infect 2019; 78:305–10. [DOI] [PubMed] [Google Scholar]
- 45.Knierer J, Gallegos Morales EN, Schablon A, Nienhaus A, Kersten JF. QFT-Plus: a plus in variability? —Evaluation of new generation IGRA in serial testing of students with a migration background in Germany. J Occup Med Toxicol 2017; 12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sotgiu G, Saderi L, Petruccioli E, et al. . QuantiFERON TB Gold Plus for the diagnosis of tuberculosis: a systematic review and meta-analysis. J Infect 2019; 79:444–53. [DOI] [PubMed] [Google Scholar]
- 47.Sester M, Sotgiu G, Lange C, et al. . Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J 2011; 37:100–11. [DOI] [PubMed] [Google Scholar]
- 48.Petruccioli E, Chiacchio T, Navarra A, et al. . Effect of HIV-infection on QuantiFERON-plus accuracy in patients with active tuberculosis and latent infection. J Infect 2020; 80:536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Telisinghe L, Amofa-Sekyi M, Maluzi K, et al. . The sensitivity of the QuantiFERON(®)-TB Gold Plus assay in Zambian adults with active tuberculosis. Int J Tuberc Lung Dis 2017; 21:690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen DT, Phan H, Trinh T, et al. . Sensitivity and characteristics associated with positive QuantiFERON-TB Gold-Plus assay in children with confirmed tuberculosis. PLoS One 2019; 14:e0213304-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buonsenso D, Delogu G, Perricone C, et al. . Accuracy of QuantiFERON-TB Gold Plus test for diagnosis of Mycobacterium tuberculosis infection in children. J Clin Microbiol 2020; 58:e00272–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soler-Garcia A, Gamell A, Santiago B, et al. . Diagnostic accuracy of QuantiFERON-TB Gold Plus assays in children and adolescents with tuberculosis disease. J Pediatr 2020; 223:212–5.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.