Abstract
Background
Older adults are at increased risk of mortality from influenza infections. We estimated influenza vaccine effectiveness (VE) against mortality following laboratory-confirmed influenza.
Methods
Using a test-negative design study and linked laboratory and health administrative databases in Ontario, Canada, we estimated VE against all-cause mortality following laboratory-confirmed influenza for community-dwelling adults aged >65 years during the 2010–2011 to 2015–2016 influenza seasons.
Results
Among 54 116 older adults tested for influenza across the 6 seasons, 6837 died within 30 days of specimen collection. Thirteen percent (925 individuals) tested positive for influenza, and 50.6% were considered vaccinated for that season. Only 23.2% of influenza test-positive cases had influenza recorded as their underlying cause of death. Before and after multivariable adjustment, we estimated VE against all-cause mortality following laboratory-confirmed influenza to be 20% (95% confidence interval [CI], 8%–30%) and 20% (95% CI, 7%–30%), respectively. This estimate increased to 34% after correcting for influenza vaccination exposure misclassification. We observed significant VE against deaths following influenza confirmation during 2014–2015 (VE = 26% [95% CI, 5%–42%]). We also observed significant VE against deaths following confirmation of influenza A/H1N1 and A/H3N2, and against deaths with COPD as the underlying cause.
Conclusions
These results support the importance of influenza vaccination in older adults, who account for most influenza-associated deaths annually.
Keywords: influenza vaccine, vaccine effectiveness, mortality, older adults
Using the test-negative design and linked databases, influenza vaccine effectiveness against all-cause mortality within 30 days of influenza testing was 20%, increasing to 34% after correcting for exposure misclassification. Therefore, vaccination may prevent deaths following influenza infection in older adults.
Each year, seasonal influenza accounts for an estimated 290 000–650 000 deaths globally, largely among older adults [1, 2]. Influenza-associated mortality varies annually depending on circulating strains and vaccine effectiveness (VE) [3, 4].
Influenza vaccines are 24%–63% effective against influenza infection [4, 5], and 31%–54% effective against influenza-related hospitalizations in older adults [6]. However, there is a dearth of high-quality evidence supporting VE against mortality. Conducting randomized-controlled trials (RCTs) is challenging because very large samples would be required to assess the outcome of death, and RCTs would be unethical because vaccination is recommended in older adults [7, 8]. A Cochrane Review found VE against all-cause mortality to be nearly 50% in older adults [9], but the included observational studies likely suffered from frailty selection bias (ie, frail, undervaccinated individuals are more likely to die, resulting in overestimation of VE) and used nonlaboratory-confirmed outcomes, which may also bias VE [7].
The test-negative design (TND) has emerged as the predominant observational study design for evaluating VE [10]. Because it uses a highly specific outcome (laboratory-confirmed influenza) and it controls for differences in healthcare-seeking behavior between vaccinated and unvaccinated individuals by restricting to individuals tested for influenza, the TND is felt to generate unbiased VE estimates [11]. The objective of this study was to estimate VE against all-cause mortality following laboratory-confirmed influenza among older adults using the TND.
METHODS
Study Population, Setting, and Design
We included community-dwelling adults aged >65 years in the province of Ontario, Canada, who were tested for influenza (as part of clinical care, at the clinician’s discretion) during periods when influenza was actively circulating (using a province-wide threshold level of 5% weekly influenza test positivity, typically between November and May) for the 2010–2011 to 2015–2016 seasons. We restricted to adults aged >65 years to allow for a 1-year lookback period to identify prescriptions that were used to define certain covariates because Ontario Drug Benefit (ODB) coverage starts at age 65 years. We linked data sets containing results of respiratory virus tests performed by a network of public health and academic hospital laboratories to population-based health administrative databases using both deterministic and probabilistic linkage (proportion linked = 97.8%). These data sets were linked using unique encoded identifiers and analyzed at ICES. Details related to these data sets for estimating VE are described elsewhere [12]. All participating laboratories provided ethics approval for this study, and participant informed consent was not required.
Data Sources and Definitions
Laboratory Data
We included results from respiratory specimens tested for influenza by a variety of methods (eg, nucleic acid detection, antigen detection, viral culture). We combined the results from all specimens collected from the same individual on the same day and defined it as a single testing episode. For individuals tested multiple times in the same season, we included their earliest positive testing episode or their earliest testing episode if all were negative.
Mortality Following Laboratory-Confirmed Influenza
Using the death date in Ontario’s Registered Persons Database (RPDB), we restricted the study to those individuals who died from any cause within 30 days of specimen collection, as in other studies [13, 14]. We also considered deaths within 7 days (to minimize uncertainty in the association between influenza vaccination and deaths related to influenza infection) and 90 days (to assess deaths due to underlying chronic conditions that are exacerbated by influenza infection). In addition, we used the Office of the Registrar General–Deaths (ORG-D) database to determine the single underlying cause of death [15]. We grouped them based on common causes of influenza-attributable deaths (Supplementary Table 1) [16]. More details about the mortality data are provided in the Supplementary Methods.
Influenza Vaccination
We used physician and pharmacist billing claims from the Ontario Health Insurance Plan (OHIP) and ODB databases, respectively, to ascertain seasonal influenza vaccination. Individuals who received an influenza vaccine ≥14 days prior to specimen collection (during the same season they were tested) were classified as vaccinated, those who received a vaccine <14 days were excluded from the analysis (because adequate immune responses may take up to 14 days to develop), and all other individuals were considered unvaccinated. During the study period, only trivalent inactivated influenza vaccines were publicly funded for adults ≥65 years in Ontario [12].
Covariates
We identified healthcare encounters associated with each testing episode to determine the setting of specimen collection (intensive care unit [ICU], hospital ward, emergency department, or physician office).
We obtained demographic information (age, sex, neighborhood income quintile, and rurality), and information on healthcare utilization prior to testing (number of hospitalizations, physician office visits, prescription medications, and receipt of home care services) from relevant databases. We also determined the presence of comorbidities that increase the risk of influenza complications (ie, anemia, asthma, cancer, chronic obstructive pulmonary disease [COPD], diabetes, dementia, chronic kidney disease, cardiovascular disease, and immunocompromising conditions). Definitions for these comorbidities and the databases used for all covariates are described elsewhere [12].
Statistical Analysis
We compared characteristics and the underlying causes of death between individuals who died within 30 days of testing positive for influenza (cases) with those who died within 30 days of testing negative (controls), and between vaccinated and unvaccinated individuals using χ 2 tests and analysis of variance (ANOVA).
We used multivariable logistic regression to determine the ratio between the odds of vaccination in cases to the odds of vaccination in controls, and calculated VE as 1 – adjusted odds ratio ×100. We selected variables to include in the models a priori based on clinical importance, adjusting for demographic characteristics, previous healthcare use, presence of any comorbidity, month of test, and influenza season (except when stratifying by season). We estimated VE against deaths after laboratory confirmation of any influenza, A/H1N1, A/H3N2, and B by combining the 2010–2011 to 2015–2016 seasons. We also estimated VE against mortality after laboratory-confirmed influenza for each season by stratifying by season of testing. In addition, using all seasons combined, we stratified by receipt of the prior season’s influenza vaccine. We tested for interaction between receipt of seasonal vaccination and stratifying variables to determine whether VE differed between strata. Finally, we also determined cause-specific VE by pooling seasons and subgrouping individuals based on their underlying cause.
In sensitivity analyses, we conducted a quantitative bias analysis using a publicly available macro [17] to correct for influenza vaccination exposure misclassification (because administrative data do not capture vaccinations delivered outside of physician offices and pharmacies), applying sensitivity (69%) and specificity (90%) parameters for influenza vaccination billing codes in the OHIP database for older adults [18]. Furthermore, we described the causes of death and determined VE against deaths occurring in mutually exclusive and cumulative intervals between specimen collection and death (0–7, 8–30, 31–90, 0–90 days) to assess whether the underlying causes and VE varied by time.
All analyses were conducted using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). All tests were 2-sided and used P < .05 as the level of statistical significance.
RESULTS
Of 54 116 community-dwelling older adults tested for influenza across the 6 seasons, 6837 (13%) died within 30 days of specimen collection. Test-positive cases were older, less likely to be male, less likely to have specimens collected in the ICU, less likely to receive influenza vaccine during the season they were tested in, and more likely to have influenza recorded as the underlying cause of death (Table 1). Vaccinated individuals were older, more likely to be male, greater users of some health services (physician office visits, prescriptions) and lower users of others (hospitalizations, home care), more likely to have comorbidities, and more likely to have noninfluenza causes as their underlying cause of death.
Table 1.
Characteristics of Community-Dwelling Adults Aged >65 Years Who Died Within 30 Days of Influenza Testing (n = 6837) During the 2010–2011 to 2015–2016 Seasons in Ontario, Canada
| Characteristic | Influenza-positive (n = 925) | Influenza-negative (n = 5912) | P value | Influenza Vaccinated (n = 3465) | Influenza Unvaccinated (n = 3372) | P valve |
|---|---|---|---|---|---|---|
| Receipt of seasonal influenza vaccine | 424 (45.8%) | 3041 (51.4%) | .002 | 3465 (100.0%) | 0 (0%) | N/A |
| Positive for influenza | 925 (100.0%) | 0 (0%) | N/A | 424 (12.2%) | 501 (14.9%) | .002 |
| Age (y), mean ± SD | 82.58 ± 8.53 | 81.06 ± 8.34 | <.001 | 81.64 ± 8.09 | 80.87 ± 8.66 | <.001 |
| Age group, y | ||||||
| 66–75 | 210 (22.7%) | 1727 (29.2%) | <.001 | 901 (26.0%) | 1036 (30.7%) | <.001 |
| 76–85 | 338 (36.5%) | 2237 (37.8%) | 1370 (39.5%) | 1205 (35.7%) | ||
| ≥86 | 377 (40.8%) | 1948 (32.9%) | 1194 (34.5%) | 1131 (33.5%) | ||
| Male sex | 477 (51.6%) | 3298 (55.8%) | .016 | 1968 (56.8%) | 1807 (53.6%) | .008 |
| Rural residence | 81 (8.8%) | 462 (7.8%) | .528 | 257 (7.4%) | 286 (8.5%) | .266 |
| Neighborhood income quintile | ||||||
| 1 (lowest) | 199 (21.5%) | 1311 (22.2%) | .98 | 732 (21.1%) | 778 (23.1%) | .176 |
| 2 | 201 (21.7%) | 1292 (21.9%) | 770 (22.2%) | 723 (21.4%) | ||
| 3 | 174 (18.8%) | 1064 (18.0%) | 642 (18.5%) | 596 (17.7%) | ||
| 4 | 162 (17.5%) | 1059 (17.9%) | 612 (17.7%) | 609 (18.1%) | ||
| 5 (highest) | 185 (20.0%) | 1155 (19.5%) | 696 (20.1%) | 644 (19.1%) | ||
| Missing information | ≤5 (0.4%) | 31 (0.5%) | 13 (0.4%) | 22 (0.7%) | ||
| Season of specimen collection | ||||||
| 2010–2011 | 99 (10.7%) | 760 (12.9%) | <.001 | 409 (11.8%) | 450 (13.3%) | <.001 |
| 2011–2012 | 42 (4.5%) | 407 (6.9%) | 246 (7.1%) | 203 (6.0%) | ||
| 2012–2013 | 184 (19.9%) | 1091 (18.5%) | 577 (16.7%) | 698 (20.7%) | ||
| 2013–2014 | 129 (13.9%) | 1153 (19.5%) | 696 (20.1%) | 586 (17.4%) | ||
| 2014–2015 | 348 (37.6%) | 1439 (24.3%) | 929 (26.8%) | 858 (25.4%) | ||
| 2015–2016 | 123 (13.3%) | 1062 (18.0%) | 608 (17.5%) | 577 (17.1%) | ||
| Month of specimen collection | ||||||
| November | 15 (1.6%) | 190 (3.2%) | <.001 | 65 (1.9%) | 140 (4.2%) | <.001 |
| December | 203 (21.9%) | 856 (14.5%) | 483 (13.9%) | 576 (17.1%) | ||
| January | 328 (35.5%) | 1469 (24.8%) | 883 (25.5%) | 914 (27.1%) | ||
| February | 140 (15.1%) | 1136 (19.2%) | 660 (19.0%) | 616 (18.3%) | ||
| March | 142 (15.4%) | 1109 (18.8%) | 668 (19.3%) | 583 (17.3%) | ||
| April | 83 (9.0%) | 773 (13.1%) | 485 (14.0%) | 371 (11.0%) | ||
| May | 14 (1.5%) | 379 (6.4%) | 221 (6.4%) | 172 (5.1%) | ||
| Setting of specimen collection | ||||||
| Intensive care unit | 279 (30.2%) | 2120 (35.9%) | <.001 | 1258 (36.3%) | 1141 (33.8%) | .094 |
| Hospital ward | 598 (64.6%) | 3616 (61.2%) | 2100 (60.6%) | 2114 (62.7%) | ||
| Emergency department | 38 (4.1%) | 151 (2.6%) | 87 (2.5%) | 102 (3.0%) | ||
| Physician office | 10 (1.1%) | 25 (0.4%) | 20 (0.6%) | 15 (0.4%) | ||
| Hospitalizations in past 3 years, mean ± SD | 1.68 ± 2.11 | 1.81 ± 2.23 | .079 | 1.71 ± 2.08 | 1.88 ± 2.34 | .001 |
| Physician office visits in past year, mean ± SD | 14.00 ± 10.85 | 15.11 ± 11.78 | .007 | 16.39 ± 11.34 | 13.50 ± 11.81 | <.001 |
| Prescriptions in the past year, mean ± SD | 17.02 ± 9.29 | 17.01 ± 9.21 | .991 | 17.75 ± 8.62 | 16.26 ± 9.74 | <.001 |
| Receipt of home care in past year | 574 (62.1%) | 3625 (61.3%) | .668 | 2066 (59.6%) | 2133 (63.3%) | .002 |
| Receipt of prior season’s influenza vaccine | 465 (50.3%) | 3438 (58.2%) | <.001 | 2774 (80.1%) | 1129 (33.5%) | <.001 |
| Medical comorbidities | ||||||
| Anemia | 221 (23.9%) | 1729 (29.2%) | <.001 | 1021 (29.5%) | 929 (27.6%) | .079 |
| Asthma | 260 (28.1%) | 1360 (23.0%) | <.001 | 850 (24.5%) | 770 (22.8%) | .099 |
| Cancer | 315 (34.1%) | 2289 (38.7%) | .007 | 1314 (37.9%) | 1290 (38.3%) | .776 |
| COPD | 505 (54.6%) | 3014 (51.0%) | .041 | 1842 (53.2%) | 1677 (49.7%) | .005 |
| Arrhythmia | 348 (37.6%) | 2065 (34.9%) | .111 | 1272 (36.7%) | 1141 (33.8%) | .013 |
| Ischemic heart disease | 397 (42.9%) | 2375 (40.2%) | .114 | 1428 (41.2%) | 1344 (39.9%) | .254 |
| Congestive heart failure | 480 (51.9%) | 3026 (51.2%) | .689 | 1842 (53.2%) | 1664 (49.3%) | .002 |
| Chronic kidney disease | 274 (29.6%) | 1648 (27.9%) | .272 | 1016 (29.3%) | 906 (26.9%) | .024 |
| Diabetes | 426 (46.1%) | 2480 (41.9%) | .019 | 1546 (44.6%) | 1360 (40.3%) | <.001 |
| Dementia/frailty | 258 (27.9%) | 1331 (22.5%) | <.001 | 757 (21.8%) | 832 (24.7%) | .006 |
| Immunocompromise | 154 (16.6%) | 1084 (18.3%) | .215 | 674 (19.5%) | 564 (16.7%) | .003 |
| History of transient ischemic attack or stroke | 144 (15.6%) | 709 (12.0%) | .002 | 443 (12.8%) | 410 (12.2%) | .434 |
| Any of the above comorbidities | 901 (97.4%) | 5774 (97.7%) | .628 | 3412 (98.5%) | 3263 (96.8%) | <.001 |
| Underlying cause of deatha | ||||||
| Influenza | 215 (23.2%) | 20 (0.3%) | <.001 | 107 (3.1%) | 128 (3.8%) | .002 |
| Pneumonia | 146 (15.8%) | 979 (16.6%) | 605 (17.5%) | 520 (15.4%) | ||
| COPD | 81 (8.8%) | 554 (9.4%) | 356 (10.3%) | 279 (8.3%) | ||
| Acute and other respiratory infections | 45 (4.9%) | 526 (8.9%) | 296 (8.5%) | 275 (8.2%) | ||
| Circulatory system diseases | 153 (16.5%) | 1183 (20.0%) | 686 (19.8%) | 650 (19.3%) | ||
| Cancer | 75 (8.1%) | 1046 (17.7%) | 540 (15.6%) | 581 (17.2%) |
Abbreviations: COPD, chronic obstructive pulmonary disease; N/A, not applicable; SD, standard deviation.
aSum of counts does not equal the total size of the cohort because either the underlying cause of death was not included in one of the selected categories (n = 1782) or individuals were missing underlying cause of death information either because death was not recorded in the Office of the Registrar General-Deaths (ORGD) database or an underlying cause of death was not determined (n = 32).
For the 6 seasons combined, VE against all-cause mortality following laboratory-confirmed influenza before and after adjustment was 20% (95% CI, 8%–30%) and 20% (95% CI, 7%–30%), respectively (Table 2). This increased to 34% (95% CI, 20%–46%) after correcting for exposure misclassification. Adjusted VE was 48% (95% CI, 15%–68%), 30% (95% CI, 11%–45%), and 26% (95% CI, –2%, 47%) against deaths following infection with A/H1N1, A/H3N2, and B, respectively. Adjusted VE against unsubtyped influenza A was lower at –8% (95% CI, –35%, 14%). Among season-specific estimates, VE was only significant for 2014–2015 (VE = 26%; 95% CI, 5%–42%). We did not find significant VE in either stratum based on prior season vaccination, nor did they differ (test for interaction, P = .21). We found significant VE against deaths with COPD coded as the underlying cause (VE = 49%; 95% CI, 16%–69%).
Table 2.
Influenza Vaccine Effectiveness Estimates for Community-Dwelling Adults Aged >65 Years Against All-Cause Mortality Following Laboratory-Confirmed Influenza During the 2010–2011 to 2015–2016 Influenza Seasons in Ontario, Canada (n = 6837)
| Analysis | Test-positive cases, No. Vaccinated/Total | Test-negative controls, No. Vaccinated/Total | Unadjusted VE% (95% CI) | Adjusted VE%a (95% CI) | Misclassification Corrected Adjusted VE% (95% CI) |
|---|---|---|---|---|---|
| Any influenza | 424 / 925 | 3041 / 5912 | 20 (8, 30) | 20 (7, 30) | 34 (20, 46) |
| Influenza A | 343 / 759 | 3041 / 5912 | 22 (9, 33) | 18 (3, 30) | 35 (21, 48) |
| A/H1N1b | 27 / 79 | 2273 / 4473 | 50 (20, 69) | 48 (15, 68) | 66 (32, 79) |
| A/H3N2 | 134 / 327 | 3041 / 5912 | 34 (18, 48) | 30 (11, 45) | 52 (34, 64) |
| A/unsubtyped | 182 / 353 | 3041 / 5912 | 0 (–25, 19) | –8 (–35, 14) | –5 (–44, 22) |
| Influenza B | 81 / 166 | 3041 / 5912 | 10 (–22, 34) | 26 (–2, 47) | 27 (–14, 52) |
| By season | |||||
| 2010–2011 | 42 / 99 | 367 / 760 | 21 (–20, 48) | 14 (–36, 46) | 29 (–28, 61) |
| 2011–2012 | 24 / 42 | 222 / 407 | –11 (–111, 42) | –12 (–128, 45) | –42 (–359, 49) |
| 2012–2013 | 79 / 184 | 498 / 1091 | 10 (–23, 35) | –3 (–44, 27) | 10 (–39, 41) |
| 2013–2014 | 62 / 129 | 634 / 1153 | 24 (–9, 47) | 32 (0, 53) | 46 (10, 68) |
| 2014–2015 | 161 / 348 | 768 / 1439 | 25 (5, 41) | 26 (5, 42) | 43 (22, 59) |
| 2015–2016 | 56 / 123 | 552 / 1062 | 23 (–12, 47) | 20 (–19, 46) | 36 (–9, 61) |
| By prior season vaccination | |||||
| Yes | 330 / 465 | 2444 / 3438 | 1 (–23, 20) | –2 (–27, 18) | N/A |
| No | 94 / 460 | 597 / 2474 | 19 (–3, 37) | 19 (–5, 37) | 31 (4, 51) |
| Underlying cause of deathc | |||||
| Influenza | 97 / 215 | 10 / 20 | 18 (–106, 67) | 18 (–146, 73) | 31 (–128, 81)d |
| Pneumonia | 75 / 146 | 530 / 979 | 11 (–27, 37) | 12 (–26, 39) | 22 (–28, 53) |
| COPD | 35 / 81 | 321 / 554 | 45 (12, 66) | 49 (16, 69) | 71 (47, 85)d |
| Acute and other respiratory infections | 22 / 45 | 274 / 526 | 12 (–62, 52) | 12 (–70, 54) | 22 (–77, 65)d |
| Circulatory system diseases | 74 / 153 | 612 / 1183 | 13 (–22, 38) | 20 (–14, 44) | 27 (–17, 55) |
| Cancer | 33 / 75 | 507 / 1046 | 16 (–34, 48) | 7 (–54, 44) | 23 (–53, 61) |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; VE, vaccine effectiveness.
aModels were adjusted for age, sex, rurality, neighborhood income quintile, receipt of homecare, number of hospitalizations in past 3 years, number of physician office visits in past year, number of prescription drugs in past year, presence of any comorbidity, calendar time, and influenza season (expect when stratifying by influenza season).
b1439 test-negative controls from influenza season 2014–2015 were removed from the analysis because there were no cases positive for influenza A/H1N1 in that season.
cSum of counts does not equal the total size of the cohort because either the underlying cause of death was not included in one of the selected categories (n = 1782) or individuals were missing underlying cause of death information either because death was not recorded in Office of the Registrar General–Deaths (ORG-D) or underlying cause of death was not determined (n = 32).
d Misclassification corrected unadjusted VE estimates were reported because models for misclassification corrected adjusted VE estimates did not converge.
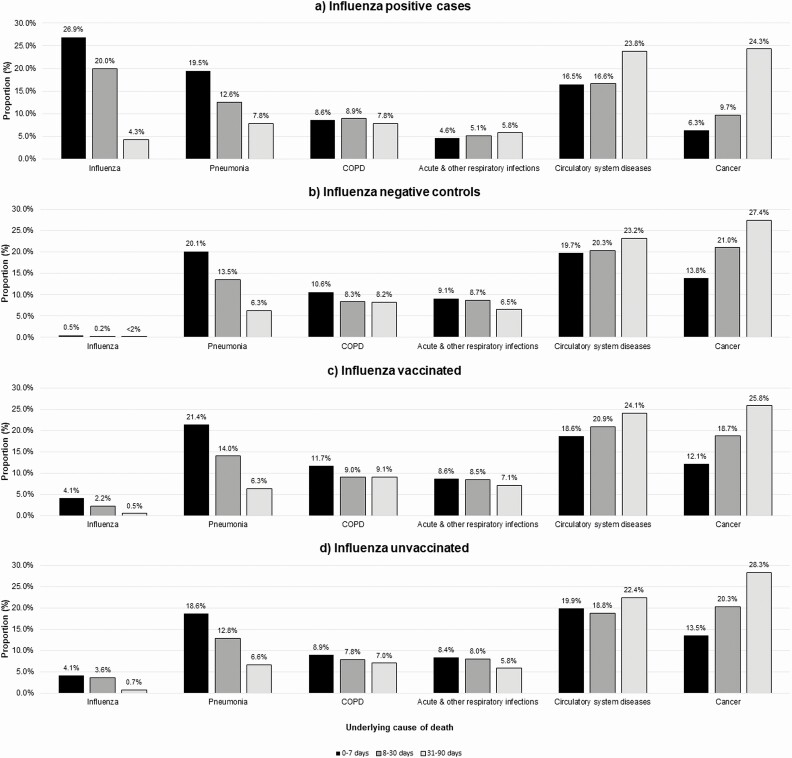
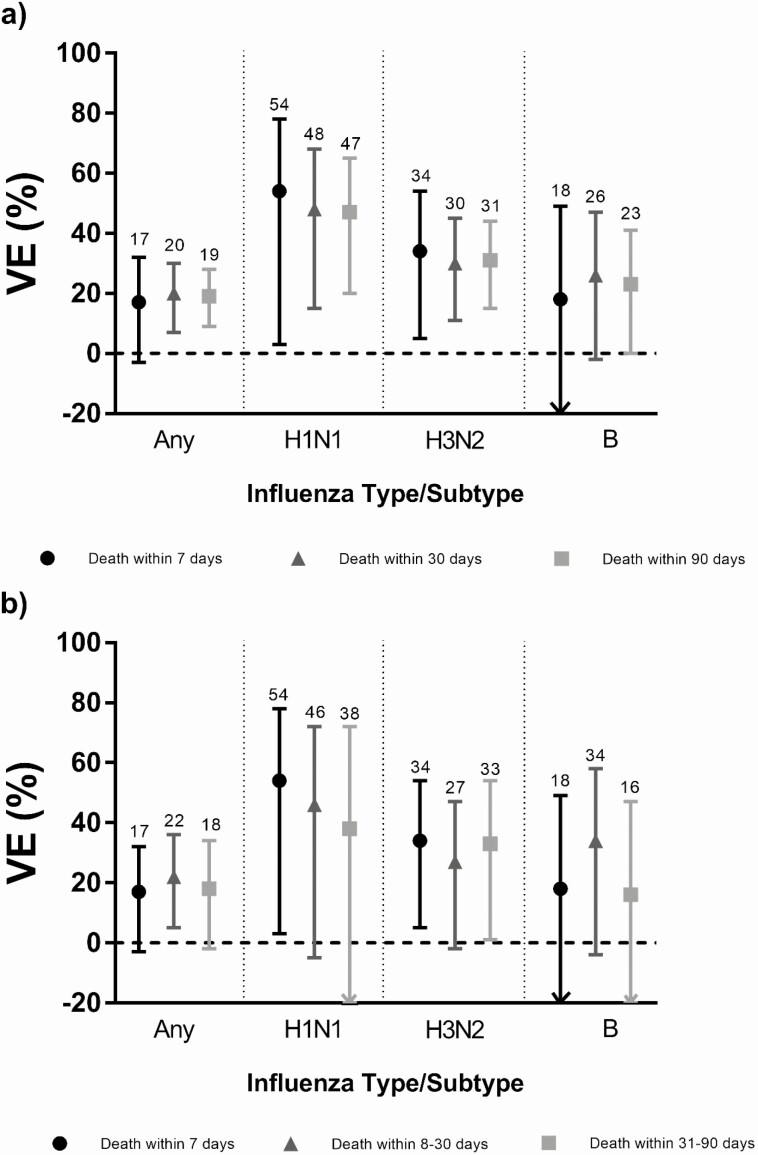
Among cases, the proportions with influenza or pneumonia as the underlying cause of death decreased with increasing time since specimen collection, whereas the proportions increased for some non-influenza causes. Among controls, the temporal patterns in the proportions of noninfluenza causes were similar to those of cases other than for COPD and respiratory infections. The proportions and temporal patterns were similar between vaccinated and unvaccinated individuals (Figure 1). VE estimates were comparable when considering death within 7, 90, 8–30, and 31–90 days after specimen collection (Figure 2, Supplementary Tables 2a and 2b). Using these alternate periods, the point estimates were consistently higher among individuals who did not receive the prior season’s vaccine. VE was significant against deaths within 90 days with circulatory system diseases as the underlying cause (VE = 24%; 95% CI, 0%–42%) (Supplementary Table 3).
Figure 1.
Proportion with underlying causes of death for varying intervals from specimen collection to death among community-dwelling older adults during the 2010–2011 to 2015–2016 influenza seasons in Ontario, Canada. Sum of the proportions for each interval per exposure group does not equal 100%. Of the 10 150 individuals who died within 90 days of specimen collection, 2698 individuals had an underlying cause of death that was not included in one of the selected categories, and 51 individuals do not have an underlying cause of death, because either their death was not registered in the Office of the Registrar General–Death database or their underlying cause of death was not determined. Abbreviation: COPD, chronic obstructive pulmonary disease.
Figure 2.
Influenza vaccine effectiveness for community-dwelling adults aged >65 years against all-cause mortality following laboratory-confirmed influenza for cumulative intervals (a) and mutually exclusive intervals (b) from specimen collection to death for any influenza and by type/subtype. Abbreviation: VE, vaccine effectiveness.
DISCUSSION
We observed that receipt of seasonal influenza vaccine was associated with an overall 20% reduced risk of all-cause mortality following laboratory-confirmed influenza, which increased to 34% after correcting for exposure misclassification. We found significant VE under the following circumstances: against deaths after A/H1N1 and A/H3N2 infection; during the 2014–2015 season; and for deaths coded as being due to COPD. Also, we found consistent results when using different intervals from specimen collection to death.
Other studies that used the TND to determine VE against mortality following laboratory-confirmed influenza in older adults had higher VE estimates than ours, but differences were likely due to the control group used. In assessing the effectiveness of repeated vaccination during the 2013–2014 and 2014–2015 seasons in Spain, Casado et al found that receipt of influenza vaccine during the current season and at least one prior season prevented fatal influenza infections (VE = 70%; 95% CI, 34%–87%) [13]. However, instead of using test-negative patients as controls, they used patients hospitalized for causes other than influenza and acute respiratory illness. Nichols et al examined 30-day mortality among older adults hospitalized for influenza-related reasons during the 2011–2012 to 2013–2014 influenza seasons in Canada, and estimated VE to be 75% (95% CI, 44%–88%) [14]. However, they did not restrict controls to those who died. This likely resulted in frailty selection bias because frail individuals are overrepresented in the unvaccinated test-positive group, which biases VE away from the null [7]. Nation et al determined that VE during the 2010–2017 seasons in Australia among adults ≥65 years was 17% (95% CI, –28%, 46%). However, they measured in-hospital deaths and used test-negative survivors as their control group [19]. Finally, Suzuki et al estimated VE against influenza pneumonia death among older Japanese adults from 2012 to 2014 to be 71% (95% CI, –63%, 95%) [20]. This study is the most comparable to ours in terms of control selection; however, it used a more specific outcome.
Despite the variability in VE across study seasons, by pooling data from all seasons, we estimated the overall effect of vaccination against all-cause mortality and observed significant VE against deaths after A/H1N1 and A/H3N2 infection. Influenza B cases were consistently detected in later months (March–May). Thus, waning immunity might explain the apparent diminished protection against severe influenza B infections [21].
Interestingly, we found significant VE only during the 2014–2015 season. This season was known to have markedly low VE because the predominant circulating A/H3N2 virus was antigenically drifted from the vaccine strain [22]. This suggests that influenza vaccines might be more effective in preventing more severe infections than less severe infections. Two studies observed higher VE against more serious outcomes compared to less serious outcomes. One found significant VE against A/H3N2-related hospitalizations (VE = 43%; 95% CI, 5%–66%) in adults aged ≥18 years during the 2014–2015 season when VE was low in ambulatory settings [23]. Another study of adults aged ≥18 years during the 2012–2015 influenza seasons found VE point estimates to be consistently higher for patients in the ICU than those on general wards [24]. The authors surmised that if vaccination conferred greater protection against more severe outcomes, influenza positivity should be lower for vaccinated ICU patients than vaccinated ward patients. However, the proportions were similar. Thus, the difference in VE was due to higher influenza positivity in unvaccinated ICU patients compared to unvaccinated ward patients [24]. In our study, influenza positivity among vaccinated subjects was lower in those who died within 30 days (12%) compared to those who survived greater than 30 days (19%), and the VE among the latter group was 22% (95% CI, 18%–25%; data not shown). Thus, although mortality can be used to assess whether vaccination is more effective in preventing serious outcomes than less serious outcomes, we did not find a meaningful difference in VE between individuals who died versus those who survived.
We did not find significant VE against mortality based on receipt of prior season’s vaccination for most intervals likely due to limited sample sizes, except against deaths within 90 days among individuals who did not receive the prior season’s vaccination. Therefore, we could not verify the findings from Casado et al [13]. However, as previously mentioned, that study used a control group that biased their VE estimate away from the null.
Using death certificate data, we found that 23% of influenza-positive individuals had influenza recorded as their cause of death, which was the most frequent cause for deaths within 30 days of testing. Cancer and circulatory system diseases were the most common causes for deaths >30 days after testing, which suggests that deaths can occur later due to exacerbations of comorbidities precipitated by influenza infection and listed instead as the underlying cause [25]. Other studies have also examined causes of death among those with laboratory-confirmed influenza but were limited by small numbers. One study found that among 32 individuals who were aged ≥65 years with laboratory-confirmed influenza hospitalization and died within 30 days of testing, 28% had influenza registered as the underlying cause [26]. Another study found that 25% of deaths within 84 days of laboratory-confirmed influenza among all ages (n = 40) had influenza listed as the underlying cause [27]. To our knowledge, our study is the largest to examine laboratory-confirmed influenza and cause of death among older adults.
We observed significant VE against deaths caused by COPD. We have previously shown that vaccination is associated with reduced influenza-associated hospitalizations among older adults with COPD [28], and the current study reinforces the benefits of vaccination for this population. Furthermore, VE nearly reached significance against deaths within 90 days caused by circulatory system diseases (including cardiovascular events). Influenza infection is known to initiate cardiovascular events that can lead to death [29, 30], and our findings support the vaccine’s effectiveness in preventing serious cardiovascular outcomes [31]. We did not find significant VE against deaths with influenza as the underlying cause, possibly due to influenza being less frequently designated as the underlying cause among individuals with comorbidities [26].
By linking laboratory and health administrative data, we created a large cohort to study VE against a rare outcome, overcoming sample size challenges faced by previous studies. However, our study has several limitations. First, the TND assumes that healthcare-seeking behavior between cases and controls is similar, but this can depend on disease severity as those with severe influenza-related illness might have died before seeking medical care [32]. This would underestimate the vaccine’s true impact against mortality. Second, we may not have completely eliminated frailty selection bias because our control group may have been less frail and more likely to have been vaccinated than cases [7]. Although the proportions with any comorbidity were similar between cases and controls, cases had a higher proportion specifically with dementia/frailty compared to controls (28% vs 23%, P < .001). Furthermore, although vaccinated individuals had a higher proportion with any comorbidity, the proportion with dementia/frailty was lower among vaccinated than unvaccinated individuals (22% vs 25%, P = .006). Consequently, because individuals with dementia/frailty are overrepresented among unvaccinated cases, we might be overestimating VE against mortality but not likely to the extent of other studies that used suboptimal control groups. In a post hoc analysis, we controlled for dementia/frailty separately from all other comorbidities and found that VE changed minimally (VE = 19%; 95% CI, 6%–30%), which suggests that dementia/frailty is not a confounder, which is consistent with a study by Talbot et al [33]. Third, specimens were collected as part of routine clinical care and testing procedures varied by institution. However, we have validated the use of these specimens for estimating VE [12], particularly in inpatients who comprise the majority of our mortality cohort. Fourth, we used specimen collection date rather than illness onset date (due to data availability), which would bias our estimates toward the null because of the inclusion of false-negative controls. However, the VE estimates are similar between groups with mutually exclusive intervals from specimen collection to death. Using illness onset date would lengthen that interval and reassign individuals to the next interval. Fifth, the VE estimate against unsubtyped influenza A was outside of the range between the estimates for A/H1N1 and A/H3N2. However, we have reported elsewhere that individuals positive for subtyped influenza A were representative of all those positive for influenza A in our cohort [34]. Sixth, our main VE estimate increased notably after adjusting for exposure misclassification, underscoring the variability in VE estimates when using Ontario’s administrative databases to ascertain vaccination status, with underreporting due to vaccination also occurring in alternative settings. However, imperfect specificity biases VE estimates more than imperfect sensitivity [35, 36] and our estimates (unadjusted and adjusted for exposure misclassification) represent a minimum range of VE against this rare outcome. Finally, despite controlling for potential confounders, residual confounding might have affected our results.
CONCLUSION
We observed 20%–34% VE against all-cause mortality following laboratory-confirmed influenza among community-dwelling older adults across 6 influenza seasons in Ontario, Canada. Influenza vaccination was associated with reduced risk of influenza-associated deaths even during a season when the vaccine was mismatched to the circulating strain. These findings reiterate the importance of influenza vaccination in older adults, who account for most influenza-related deaths.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank IMS Brogan Inc. for use of their Drug Information Database.
Disclaimers. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI) and by Cancer Care Ontario (CCO). However, the analyses, conclusions, opinions, and statement expressed herein are those of the authors, and not necessarily those of CIHI or CCO. No endorsement by ICES, PHO, MOHLTC, CIHI, or CCO is intended or should be inferred. Parts of this report are based on Ontario’s Office of the Registrar General (ORG) information on deaths, the original source of which is Service Ontario. The views expressed therein are those of the author and do not necessarily reflect those of ORG or the Ministry of Government Services.
Financial support. This work was supported by an operating grant from the Canadian Institutes of Health Research (PJT 159516). J. C. K. is supported by a Clinician Scientist Award from the University of Toronto Department of Family and Community Medicine. This study was also supported by Public Health Ontario (PHO) and ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The study sponsors did not participate in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Potential conflicts of interest. A. J. M. has received grants and personal fees from Sanofi Pasteur (for investigator initiated research, paid to institution, and honoraria from advisory boards), grants and personal fees from Merck (for investigator initiated research and honoraria from advisory boards), personal fees from GlaxoSmithKine for honoraria from advisory boards, and grants from Seqirus (contracts for research paid to institution), unrelated to this study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Preliminary results of this study were presented at the Options X for the Control of Influenza Conference in Singapore on 29 August 2019.
References
- 1.Iuliano AD, Roguski KM, Chang HH, et al. ; Global Seasonal Influenza-associated Mortality Collaborator Network . Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391:1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Public Health Agency of Canada. Canadian immunization guide chapter on influenza and statement on seasonal influenza vaccine for 2018–2019.2018. Available at: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2018–2019.html. Accessed 2 September 2020.
- 3.Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of hospitalization attributable to influenza and RSV in the US during 1997–2009, by age and risk status. BMC Public Health 2017; 17:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–51. [DOI] [PubMed] [Google Scholar]
- 5.Darvishian M, van den Heuvel ER, Bissielo A, et al. Effectiveness of seasonal influenza vaccination in community-dwelling elderly people: an individual participant data meta-analysis of test-negative design case-control studies. Lancet Respir Med 2017; 5:200–11. [DOI] [PubMed] [Google Scholar]
- 6.Rondy M, El Omeiri N, Thompson MG, Levêque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75:381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 2007; 7:658–66. [DOI] [PubMed] [Google Scholar]
- 8.Verhees RAF, Dondorp W, Thijs C, Dinant GJ, Knottnerus JA. Influenza vaccination in the elderly: is a trial on mortality ethically acceptable? Vaccine 2018; 36:2991–7. [DOI] [PubMed] [Google Scholar]
- 9.Demicheli V, Jefferson T, Di Pietrantonj C, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2018; 2:CD004876. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan SG, Feng S, Cowling BJ. Potential of the test-negative design for measuring influenza vaccine effectiveness: a systematic review. Expert Rev Vaccines 2014; 13:1571–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. [DOI] [PubMed] [Google Scholar]
- 12.Kwong JC, Buchan SA, Chung H, et al. Can routinely collected laboratory and health administrative data be used to assess influenza vaccine effectiveness? Assessing the validity of the Flu and Other Respiratory Viruses Research (FOREVER) Cohort. Vaccine 2019; 37:4392–400. [DOI] [PubMed] [Google Scholar]
- 13.Casado I, Domínguez Á, Toledo D, et al. ; Project PI12/02079 Working Group . Repeated influenza vaccination for preventing severe and fatal influenza infection in older adults: a multicentre case-control study. CMAJ 2018; 190:E3–E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols MK, Andrew MK, Hatchette TF, et al. Influenza vaccine effectiveness to prevent influenza-related hospitalizations and serious outcomes in Canadian adults over the 2011/12 through 2013/14 influenza seasons: a pooled analysis from the Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS Network). Vaccine 2018; 36:2166–75. [DOI] [PubMed] [Google Scholar]
- 15.Chiu M, Lebenbaum M, Lam K, et al. Describing the linkages of the immigration, refugees and citizenship Canada permanent resident data and vital statistics death registry to Ontario’s administrative health database. BMC Med Inform Decis Mak 2016; 16:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schanzer DL, Tam TWS, Langley JM, Winchester BT. Influenza-attributable deaths, Canada 1990–1999. Epidemiol Infect 2007; 135:1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox MP, Lash TL, Greenland S. A method to automate probabilistic sensitivity analyses of misclassified binary variables. Int J Epidemiol 2005; 34:1370–6. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz KL, Jembere N, Campitelli MA, Buchan SA, Chung H, Kwong JC. Using physician billing claims from the Ontario Health Insurance Plan to determine individual influenza vaccination status: an updated validation study. CMAJ Open 2016; 4:E463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nation ML, Moss R, Spittal MJ, Kotsimbos T, Kelly PM, Cheng AC. Influenza vaccine effectiveness against influenza-related mortality in Australian hospitalized patients: a propensity score analysis. Clin Infect Dis 2021; 72:99–107. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki M, Katsurada N, Le MN, et al. Effectiveness of inactivated influenza vaccine against laboratory-confirmed influenza pneumonia among adults aged ≥65 years in Japan. Vaccine 2018; 36:2960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kissling E, Nunes B, Robertson C, et al. I-MOVE multicentre case-control study 2010/11 to 2014/15: is there within-season waning of influenza type/subtype vaccine effectiveness with increasing time since vaccination? Eurosurveillance 2016; 21:30201. [DOI] [PubMed] [Google Scholar]
- 22.Skowronski DM, Chambers C, Sabaiduc S, et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrie JG, Ohmit SE, Cheng CK, et al. Influenza vaccine effectiveness against antigenically drifted influenza higher than expected in hospitalized adults: 2014–2015. Clin Infect Dis 2016; 63:1017–25. [DOI] [PubMed] [Google Scholar]
- 24.Thompson MG, Pierse N, Sue Huang Q, et al. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine 2018; 36:5916–25. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Frequently asked questions about estimated flu burden | CDC. Available at: https://www.cdc.gov/flu/about/burden/faq.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fflu%2Fabout%2Fdisease%2Fus_flu-related_deaths.htm#deaths. Accessed 2 September 2020.
- 26.Casado I, Martínez-Baz I, Floristán Y, Chamorro J, Ezpeleta C, Castilla J; Network for Influenza Surveillance in Hospitals of Navarra . Cause of death in hospitalized patients with laboratory-confirmed influenza. An Sist Sanit Navar 2015; 38:263–8. [DOI] [PubMed] [Google Scholar]
- 27.Muscatello DJ, Amin J, MacIntyre CR, et al. Inaccurate ascertainment of morbidity and mortality due to influenza in administrative databases: a population-based record linkage study. PLoS One 2014; 9:e98446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gershon AS, Chung H, Porter J, et al. Influenza vaccine effectiveness in preventing hospitalizations in older patients with chronic obstructive pulmonary disease. J Infect Dis 2020; 221:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018; 378:345–53. [DOI] [PubMed] [Google Scholar]
- 30.Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51:1701794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Udell JA, Farkouh ME, Solomon SD, Vardeny O. Does influenza vaccination influence cardiovascular complications? Expert Rev Cardiovasc Ther 2015; 13:593–6. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol 2016; 184:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talbot HK, Nian H, Chen Q, Zhu Y, Edwards KM, Griffin MR. Evaluating the case-positive, control test-negative study design for influenza vaccine effectiveness for the frailty bias. Vaccine 2016; 34:1806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwong JC, Chung H, Jung JK, et al. The impact of repeated vaccination using 10-year vaccination history on protection against influenza in older adults: a test-negative design study across the 2010/11 to 2015/16 influenza seasons in Ontario, Canada. Eurosurveillance 2020; 25:1900245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson ML. Use of self-reported vaccination status can bias vaccine effectiveness estimates from test-negative studies. Vaccine X 2019; 1:100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Smedt T, Merrall E, Macina D, Perez-Vilar S, Andrews N, Bollaerts K. Bias due to differential and non-differential disease- and exposure misclassification in studies of vaccine effectiveness. PLoS One 2018; 13:e0199180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.