Abstract
Background
Baloxavir marboxil (baloxavir) is a single-dose, oral antiinfluenza drug with a novel mechanism of action. We compared the incidence of hospitalization in patients treated with baloxavir vs neuraminidase inhibitors.
Methods
In this retrospective, observational, cohort study, we used real-world patient data extracted from a Japanese health insurance claims database. The enrollment period was 1 October 2018 to 17 April 2019. On day 1, eligible patients (N = 339 007) received baloxavir, oseltamivir, zanamivir, or laninamivir. Baseline characteristics were standardized using the inverse probability of treatment weighting method. The primary end point was the incidence of hospitalization (days 2–14). Secondary end points included antibacterial use, secondary pneumonia, and additional antiinfluenza drug use.
Results
Compared with the baloxavir group, the incidence of hospitalization was greater in the oseltamivir group (risk ratio [RR] and 95% confidence interval [CI], 1.41 [1.00–2.00]; risk difference [RD] and 95% CI, 0.06 [.01–.12]) and zanamivir group (RR, 1.85 [1.23–2.78]; RD, 0.11 [.02–.20]). Oseltamivir-treated patients were less likely to require antibacterials than baloxavir-treated patients (RR, 0.87 [.82–.91]). However, oseltamivir-treated patients were more likely to be hospitalized with antibacterials (RR, 1.70 [1.21–2.38]) or antibacterial injection (RR, 1.67 [1.17–2.38]) than baloxavir-treated patients (post hoc analysis). Compared with baloxavir-treated patients, additional antiinfluenza drug use was greater in oseltamivir-, zanamivir-, and laninamivir-treated patients (RR, 1.51 [1.05–2.18], 2.84 [2.04–3.96], and 1.68 [1.35–2.10], respectively).
Conclusions
Baloxavir is an efficacious antiinfluenza treatment that may reduce hospitalization compared with oseltamivir and zanamivir.
Clinical Trials Registration
University hospital Medical Information Network Clinical Trials Registry (UMIN000038159).
Keywords: baloxavir, human influenza, hospitalization, Japan, neuraminidase inhibitor
Baloxavir marboxil is a single-dose oral antiinfluenza drug with a novel mechanism of action. This real-world data study indicates that baloxavir marboxil may reduce hospitalization after influenza outpatient treatment compared with neuraminidase inhibitor treatment.
Influenza is a contagious respiratory illness that ranges from mild to severe. Although most influenza infections resolve without treatment, they can lead to complications and result in hospitalization [1]. In Japan, 4 neuraminidase inhibitors (NAIs; oseltamivir, zanamivir, laninamivir, and peramivir) are approved for the treatment of influenza [1].
Baloxavir marboxil (baloxavir), an antiinfluenza drug with a novel mechanism of action (cap-dependent endonuclease inhibitor), was first approved in Japan [2]. In the trials in adults and adolescents with uncomplicated influenza, 1 day after trial regimen initiation, a single dose of baloxavir significantly reduced the viral load compared with oseltamivir and placebo [3]. In a recent observational study of patients with influenza A, the duration of fever was significantly shorter for baloxavir-treated patients compared with NAI-treated patients [4]. In a phase 3 trial in high-risk influenza patients, baloxavir significantly reduced the incidence of influenza-related complications compared with placebo [5]. Given the ability of baloxavir to significantly reduce the viral load compared with oseltamivir and to reduce the time to alleviation of fever compared with NAI treatment, baloxavir treatment in the real-world setting may provide a superior alternative for alleviating influenza symptoms and thus prevent the onset of complications that lead to hospitalization.
Our aim in this retrospective, observational, cohort study, using data extracted from a health insurance claims database, was to examine the effect of baloxavir treatment in the real-world setting on the incidence of hospitalization, antibacterial use, secondary pneumonia, and additional antiinfluenza drug use compared with NAI treatment in the 2018–2019 influenza season in Japan.
METHODS
Study Design
In this population-based, active comparator, retrospective, cohort study, we used data from the JMDC health insurance claims database (JMDC Inc, Tokyo, Japan). The JMDC database is an epidemiological receipt database that contains inpatient, outpatient, and dispensing receipts received from health insurance associations, allowing for individual patient data to be tracked across multiple facilities. By April 2020, the cumulative dataset consisted of 7.3 million individuals. The data extraction period was 1 April 2018 to 30 April 2019, which included the 2018–2019 influenza season. The study was conducted in accordance with Ethical Guidelines for Medical and Health Research Involving Human Subjects. Informed written consent was not required because the study used deidentified data.
Study Population
The study consisted of 4 influenza outpatient populations: those treated with baloxavir, oseltamivir, zanamivir, or laninamivir. The oseltamivir group was predefined as the primary comparator group. Peramivir was not included as a comparator in this study as it is administered intravenously and would likely be selected under different circumstances from oral and inhalant antiinfluenza drugs.
For the study population, eligible patients were those whose first influenza diagnosis date (day 1, starting date of influenza medical care) was within the enrollment period (1 October 2018 to 17 April 2019), who were continuously registered in the database ≥6 months before day 1, and who received baloxavir, oseltamivir, zanamivir, or laninamivir on day 1. Influenza virus infection was defined by International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) codes J09–J11. Patients were excluded if they were hospitalized in the last 90 days before day 1 or received antibacterials (European Pharmaceutical Market Research Association [EphMRA] Anatomical Therapeutic Chemical [ATC] code J01) on day 1 or in the last 90 days before day 1 or were diagnosed with pneumonia (ICD-10 codes J12–J18) within 3 months before day 1 (including day 1). This was to exclude the possibility that hospitalization, antibacterial administration, or pneumonia was due to a chronic illness (or disease other than influenza) as much as possible. Patients aged <1 year were excluded because baloxavir is not indicated for patients who weigh <10 kg. Patients who died on day 1 were also excluded. To ensure that the outcomes measured in this study were attributable to a single agent, patients who received >1 antiinfluenza drug (baloxavir, oseltamivir, zanamivir, laninamivir, or peramivir) on day 1 were excluded.
Outcome Measures
The primary end point was the incidence of hospitalization during days 2−14. Secondary end points included the occurrence of antibacterial (oral and/or injectable) administration (EphMRA-ATC code J01), antibacterial injection (EphMRA-ATC code J01; dosage form, injection drug), pneumonia (ICD-10 codes J12–J18), and additional antiinfluenza drug use (any antiinfluenza drug different from the one received on day 1) during days 2−14. The overall incidence of the secondary end points (prespecified analysis) and the incidence of secondary end points with hospitalization (post hoc analysis) were determined.
Statistical Analyses
The analysis population included all patients who met the eligibility criteria and excluded those who were hospitalized on day 1 (Figure 1). The comparison groups were the baloxavir group (patients who received oral baloxavir on day 1) and each NAI group (patients who received oral oseltamivir, inhaled zanamivir, or inhaled laninamivir on day 1). Comparisons were made between the standardized baloxavir group and the standardized NAI groups (oseltamivir [main comparison group], zanamivir, or laninamivir). Risk ratios (RRs) and risk differences (RDs) were calculated as the incidence of the severity end point in the NAI group divided by, or minus, the incidence in the baloxavir group, respectively. The 95% confidence intervals (CIs) for both the RRs and RDs after standardization of each group in the comparison pair were determined. When the RR and RD 95% CIs did not contain 1 and 0, respectively, differences between groups were considered statistically significant (2-sided significance level, 0.05).
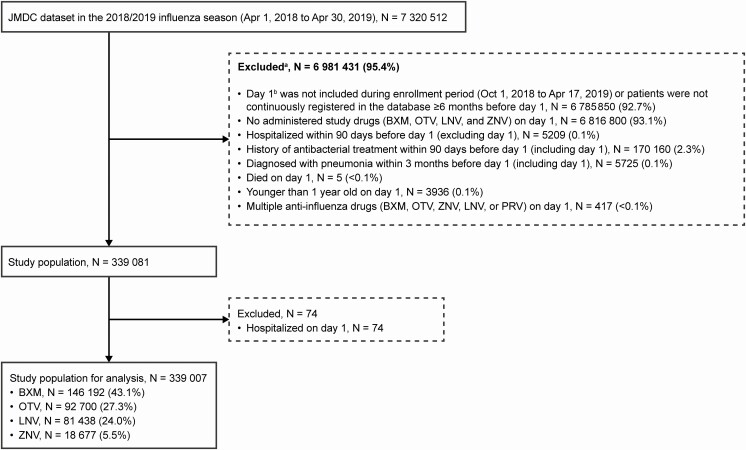
Figure 1.
Flow chart of the study cohort. aPatients could be excluded for ≥1 reason. bDate of earliest diagnosis of influenza virus infection. Abbreviations: BXM, baloxavir marboxil; LNV, laninamivir; OTV, oseltamivir; PRV, peramivir; ZNV, zanamivir.
To standardize the patient baseline demographic and clinical characteristics between the baloxavir group and each NAI group, the propensity score was calculated and the inverse probability of treatment weighting (IPTW) method was applied. To calculate propensity score, a logistic regression analysis was performed using the baloxavir group as the response variable (1 for the baloxavir group, 0 otherwise) and the patient baseline characteristics (covariates) as the explanatory variable. The propensity score calculated as the predicted probability (P) from logistic regression analysis for the baloxavir group for each patient was used as the weight (ie, 1/P for the baloxavir group, 1/(1 – P) for each NAI group).
Covariates (patient baseline characteristics) used to calculate the propensity score were those considered related to influenza severity [6]. Patient age was categorized as following: 1 to <2, ≥2 to <5, ≥5 to <18, ≥18 to <65, and ≥65 years. The age categories were consistent with those most widely used [7]. Comorbidities were diagnosed by ICD-10 code (a receipt corresponding to the ICD-10 code was required within the 6 months prior to the month of day 1 or, if the receipt occurred in the month of day 1, then on a day prior to day 1) or by the administration of disease-related medication (corresponding drug was administered within 180 days before day 1). Covariates included age, gender, influenza virus type (ICD-10 codes J09–J11), and the presence or absence of the following (disease diagnosis was defined by the administration of disease-related medication; corresponding drug was administered within 180 days before day 1 [excluding day 1]): steroid administration (World Health Organization [WHO] ATC code H02), dialysis (defined by the presence/absence of a dialysis-related medical care activity), respiratory coinfection (ICD-10 codes J00–J06, J2) excluding pneumonia, asthma (corresponding drug WHO-ATC code R03), diabetes (corresponding drug WHO-ATC code A10), chronic obstructive lung disease (ICD-10 codes J41–J44), cardiovascular disease (ICD-10 codes I20–I25, Q20–Q28), cerebrovascular disease (ICD-10 codes I60–I69), mental illness including dementia (ICD-10 codes F00–F99), neurological disease (ICD-10 codes G00–G99), anemia (ICD-10 codes D50–D59, D60–D64), immune deficiency (ICD-10 codes D80–D89), liver disease (ICD-10 codes K70–K77), and malignant tumor (ICD-10 codes D00–D09, C00–C97).
The standardized mean difference (SMD) between the comparison groups was calculated for each patient baseline characteristic (by giving 1 if appropriate for the category, 0 for otherwise). Sensitivity analyses of the primary and secondary end points were conducted for the comparison of 2 treatment groups and included patients with an influenza virus diagnostic test on day 1 (data not shown).
Missing values were not replaced and multiplicity was not adjusted for repeated tests. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Demographic and Baseline Clinical Characteristics
During the 2018–2019 influenza season, 7 320 512 individuals were recorded in the JMDC claims database; of these, 339 007 patients met the eligibility criteria for the study analysis population (Figure 1). The most common antiinfluenza drug was baloxavir (N = 146 192), followed by oseltamivir (N = 92 700), laninamivir (N = 81 438), and zanamivir (N = 18 677). Table 1 summarizes the unadjusted patient baseline demographic and clinical characteristics for the study population.
Table 1.
Unadjusted Baseline Demographic and Clinical Characteristics of the Study Population
| Characteristic | Baloxavir N = 146 192 | Oseltamivir N = 92 700 | Laninamivir N = 81 438 | Zanamivir N = 18 677 |
|---|---|---|---|---|
| Age, years | ||||
| 1 to <2 | 104 (0.1) | 3948 (4.3) | 4 (<0.1) | 3 (<0.1) |
| ≥2 to <5 | 1758 (1.2) | 17 789 (19.2) | 213 (0.3) | 53 (0.3) |
| ≥5 to <18 | 45 220 (30.9) | 27 556 (29.7) | 29 907 (36.7) | 12 048 (64.5) |
| ≥18 to <65 | 97 215 (66.5) | 42 426 (45.8) | 50 464 (62.0) | 6513 (34.9) |
| ≥65 | 1895 (1.3) | 981 (1.1) | 850 (1.0) | 60 (0.3) |
| Sex | ||||
| Male | 82 906 (56.7) | 50 628 (54.6) | 44 604 (54.8) | 9522 (51.0) |
| Female | 63 286 (43.3) | 42 072 (45.4) | 36 834 (45.2) | 9155 (49.0) |
| Type of influenza | ||||
| A virus | 106 340 (72.7) | 62 436 (67.4) | 57 119 (70.1) | 12 043 (64.5) |
| B virus | 991 (0.7) | 662 (0.7) | 701 (0.9) | 191 (1.0) |
| A and B virus | 30 (<0.1) | 17 (<0.1) | 19 (<0.1) | 1 (<0.1) |
| Unknown | 38 831 (26.6) | 29 585 (31.9) | 23 599 (29.0) | 6442 (34.5) |
| Steroid use | 472 (0.3) | 270 (0.3) | 302 (0.4) | 47 (0.3) |
| Dialysis use | 17 (<0.1) | 113 (0.1) | 21 (<0.1) | 4 (<0.1) |
| Presence of the following comorbidities: | ||||
| Respiratory coinfectiona | 85 168 (58.3) | 50 204 (54.2) | 46 935 (57.6) | 10 495 (56.2) |
| Asthmab | 16 382 (11.2) | 22 918 (24.7) | 9078 (11.1) | 2947 (15.8) |
| Diabetes mellitusc | 2448 (1.7) | 1206 (1.3) | 1264 (1.6) | 96 (0.5) |
| Chronic obstructive pulmonary disease | 918 (0.6) | 642 (0.7) | 490 (0.6) | 71 (0.4) |
| Cardiovascular disease | 1694 (1.2) | 1064 (1.1) | 917 (1.1) | 121 (0.6) |
| Cerebrovascular disease | 1338 (0.9) | 668 (0.7) | 676 (0.8) | 71 (0.4) |
| Mental disease including dementia | 8509 (5.8) | 5542 (6.0) | 4475 (5.5) | 974 (5.2) |
| Neurological disease | 10 676 (7.3) | 5461 (5.9) | 5695 (7.0) | 919 (4.9) |
| Anemia | 3171 (2.2) | 1982 (2.1) | 2013 (2.5) | 366 (2.0) |
| Immune deficiency | 150 (0.1) | 119 (0.1) | 112 (0.1) | 27 (0.1) |
| Hepatic disease | 4569 (3.1) | 2331 (2.5) | 2508 (3.1) | 281 (1.5) |
| Malignant tumor | 1636 (1.1) | 804 (0.9) | 890 (1.1) | 107 (0.6) |
Data are n (%).
aExcluding pneumonia.
bDiagnosis based on administration of disease-related medication World Health Organization Anatomical Therapeutic Chemical Classification System (WHO-ATC) code R03.
cDiagnosis based on administration of disease-related medication WHO-ATC code A10.
The proportion of patients aged <5 years was higher in the oseltamivir group compared with the other 3 treatment cohorts. For those patients whose influenza type could be determined, most were infected with influenza A. Approximately half of the patients in the study population had a respiratory coinfection, and the proportion of patients with asthma in the oseltamivir group was approximately twice that in the baloxavir group. Baseline demographic and clinical characteristics of patients after standardization using the IPTW method were well balanced across the treatment groups (SMD were all <0.1; Table 2).
Table 2.
Adjusted Baseline Demographic and Clinical Characteristics of Study Population
| Baloxavir | Oseltamivir | Baloxavir | Laninamivir | Baloxavir | Zanamivir | ||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N = 240 341.4 | N = 238 657.8 | SMD | N = 227 633.9 | N = 227 618.3 | SMD | N = 164 892.3 | N = 163 701.7 | SMD |
| Age, years | |||||||||
| 1 to <2 | 4524.3 (1.9) | 4052.3 (1.7) | 0.01 | 108.0 (<0.1) | 105.5 (<0.1) | <0.01 | 107.0 (0.1) | 119.9 (0.1) | <0.01 |
| ≥2 to <5 | 20 581.4 (8.6) | 19 556.6 (8.2) | 0.01 | 1971.1 (0.9) | 1973.8 (0.9) | <0.01 | 1811.0 (1.1) | 1816.1 (1.1) | <0.01 |
| ≥5 to <18 | 72 693.4 (30.2) | 72 636.0 (30.4) | <0.01 | 75 151.6 (33.0) | 75 181.2 (33.0) | <0.01 | 57 299.9 (34.7) | 57 692.9 (35.2) | 0.01 |
| ≥18 to <65 | 139 660.2 (58.1) | 139 521.4 (58.5) | <0.01 | 147 656.6 (64.9) | 147 605.9 (64.8) | <0.01 | 103 719.4 (62.9) | 102 112.3 (62.4) | 0.01 |
| ≥65 | 2882.2 (1.2) | 2891.5 (1.2) | <0.01 | 2746.6 (1.2) | 2751.9 (1.2) | <0.01 | 1955.0 (1.2) | 1960.4 (1.2) | <0.01 |
| Sex | |||||||||
| Male | 134 638.5 (56.0) | 133 537.5 (56.0) | <0.01 | 127 490.0 (56.0) | 127 395.1 (56.0) | <0.01 | 92 370.5 (56.0) | 88 175.4 (53.9) | 0.04 |
| Female | 105 702.9 (44.0) | 105 120.3 (44.0) | <0.01 | 100 143.9 (44.0) | 100 223.2 (44.0) | <0.01 | 72 521.7 (44.0) | 75 526.3 (46.1) | 0.04 |
| Type of influenza | |||||||||
| A virus | 169 211.8 (70.4) | 168 300.2 (70.5) | <0.01 | 163 433.2 (71.8) | 163 420.6 (71.8) | <0.01 | 118 370.3 (71.8) | 116 356.6 (71.1) | 0.02 |
| B virus | 1683.5 (0.7) | 1657.8 (0.7) | <0.01 | 1693.1 (0.7) | 1686.2 (0.7) | <0.01 | 1182.8 (0.7) | 1203.3 (0.7) | <0.01 |
| A and B virus | 43.2 (<0.1) | 45.2 (<0.1) | <0.01 | 49.4 (<0.1) | 50.0 (<0.1) | <0.01 | 31.0 (<0.1) | 60.7 (<0.1) | 0.01 |
| Unknown | 69 402.8 (28.9) | 68 654.6 (28.8) | <0.01 | 62 458.2 (27.4) | 62 461.5 (27.4) | <0.01 | 45 308.2 (27.5) | 46 081.2 (28.1) | 0.01 |
| Steroid use | 758.1 (0.3) | 748.3 (0.3) | <0.01 | 771.1 (0.3) | 767.5 (0.3) | <0.01 | 517.5 (0.3) | 459.4 (0.3) | <0.01 |
| Dialysis use | 127.8 (0.1) | 129.9 (0.1) | <0.01 | 37.1 (<0.1) | 37.4 (<0.1) | <0.01 | 20.7 (<0.1) | 16.6 (<0.1) | <0.01 |
| Presence of the following comorbidities: | |||||||||
| Respiratory coinfectiona | 134 428.5 (55.9) | 134 519.8 (56.4) | <0.01 | 132 074.8 (58.0) | 131 973.9 (58.0) | <0.01 | 95 659.9 (58.0) | 94 354.7 (57.6) | <0.01 |
| Asthmab | 42 217.2 (17.6) | 39 287.0 (16.5) | 0.03 | 25 473.4 (11.2) | 25 401.5 (11.2) | <0.01 | 19 340.7 (11.7) | 19 599.7 (12.0) | <0.01 |
| Diabetes mellitusc | 3660.1 (1.5) | 3648.3 (1.5) | <0.01 | 3714.1 (1.6) | 3717.2 (1.6) | <0.01 | 2543.5 (1.5) | 2475.8 (1.5) | <0.01 |
| Chronic obstructive pulmonary disease | 1645.8 (0.7) | 1592.2 (0.7) | <0.01 | 1408.1 (0.6) | 1407.1 (0.6) | <0.01 | 989.6 (0.6) | 998.5 (0.6) | <0.01 |
| Cardiovascular disease | 2729.8 (1.1) | 2731.1 (1.1) | <0.01 | 2612.2 (1.1) | 2623.8 (1.2) | <0.01 | 1815.4 (1.1) | 1868.2 (1.1) | <0.01 |
| Cerebrovascular disease | 1997.8 (0.8) | 1995.6 (0.8) | <0.01 | 2017.1 (0.9) | 2023.2 (0.9) | <0.01 | 1409.6 (0.9) | 1446.4 (0.9) | <0.01 |
| Mental disease including dementia | 14 138.7 (5.9) | 14 021.8 (5.9) | <0.01 | 12 997.4 (5.7) | 13 009.7 (5.7) | <0.01 | 9484.1 (5.8) | 9453.6 (5.8) | <0.01 |
| Neurological disease | 16 233.3 (6.8) | 16 174.1 (6.8) | <0.01 | 16 372.8 (7.2) | 16 372.9 (7.2) | <0.01 | 11 597.8 (7.0) | 11 652.8 (7.1) | <0.01 |
| Anemia | 5062.0 (2.1) | 5154.8 (2.2) | <0.01 | 5179.3 (2.3) | 5170.0 (2.3) | <0.01 | 3538.4 (2.1) | 3549.1 (2.2) | <0.01 |
| Immune deficiency | 277.7 (0.1) | 268.6 (0.1) | <0.01 | 261.2 (0.1) | 259.2 (0.1) | <0.01 | 177.8 (0.1) | 198.1 (0.1) | <0.01 |
| Hepatic disease | 6912.4 (2.9) | 6930.9 (2.9) | <0.01 | 7078.3 (3.1) | 7081.3 (3.1) | <0.01 | 4849.3 (2.9) | 4781.4 (2.9) | <0.01 |
| Malignant tumor | 2446.5 (1.0) | 2423.1 (1.0) | <0.01 | 2526.7 (1.1) | 2529.7 (1.1) | <0.01 | 1742.1 (1.1) | 1638.3 (1.0) | <0.01 |
Data are n (%). Adjustment (standardization) was performed according to the inverse probability of treatment weighting method using a propensity score with each item as a covariate. SMD <0.1 indicated the characteristics were balanced between the comparison pairs.
Abbreviation: SMD, standardized mean difference.
aExcluding pneumonia
bDiagnosis based on administration of disease-related medication World Health Organization Anatomical Therapeutic Chemical Classification (WHO-ATC) code R03.
cDiagnosis based on administration of disease-related medication WHO-ATC code A10.
Hospitalization Incidence
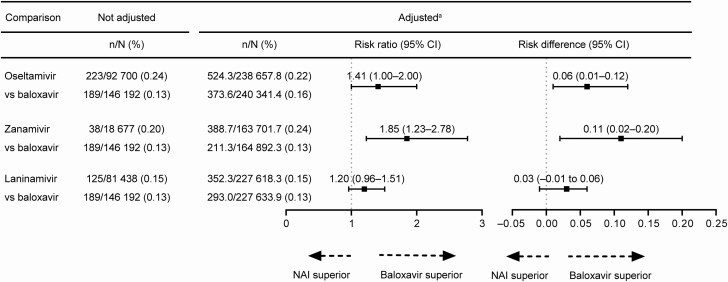
The incidence of hospitalization was greater in the oseltamivir group than in the baloxavir group, with no statistically significant RR of 1.41 (95% CI, 1.00–2.00) and a statistically significant RD of 0.06 (95% CI, .01–.12; Figure 2). The incidence of hospitalization was statistically significantly greater in the zanamivir group than in the baloxavir group, with a significant RR of 1.85 (95% CI, 1.23–2.78) and significant RD of 0.11 (95% CI, .02–.20). No significant difference in the incidence of hospitalization between the laninamivir and baloxavir groups was observed.
Figure 2.
Risk ratio and risk difference of hospitalization. aData were standardized using the inverse probability of treatment weighting method; the risk ratio was calculated using the exposed (baloxavir) group as the denominator and the neuraminidase inhibitor (NAI) group (oseltamivir, zanamivir, or laninamivir) as the numerator. Risk difference was the incidence of the outcome end point in the NAI group minus the incidence in the baloxavir group. Abbreviation: CI, confidence interval.
Antibacterial Administration, Pneumonia, and Additional Antiinfluenza Drug Use
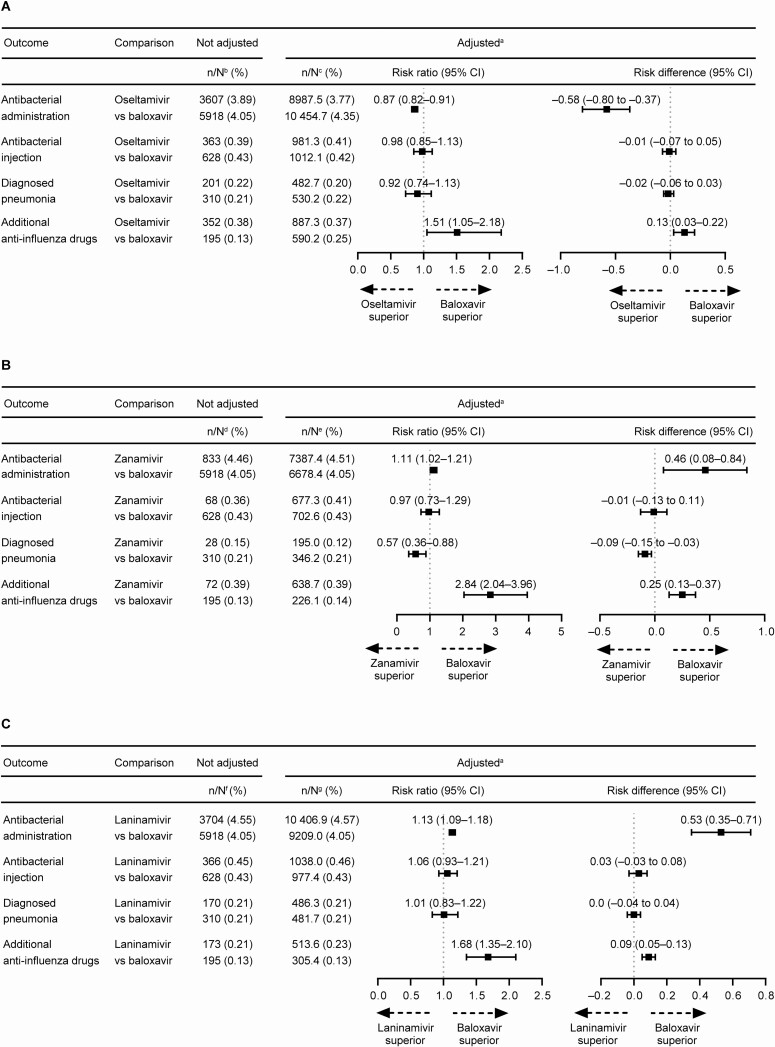
Overall, patients in the oseltamivir group had a reduced risk of requiring antibacterial administration than patients in the baloxavir group (Figure 3A). In contrast, patients in the oseltamivir group had a greater risk of requiring additional antiinfluenza drugs than patients in the baloxavir group (Figure 3A). Patients in the zanamivir group had a reduced risk of pneumonia compared with patients in the baloxavir group (Figure 3B). In the zanamivir and laninamivir groups, patients were more likely to require additional antiinfluenza drugs compared with patients in the baloxavir group (Figure 3B and 3C). Details of additional antiinfluenza drug use and antibacterial administration are listed in Supplementary Tables 1 and 2. Baloxavir and peramivir were most frequently used as additional antiinfluenza drugs, while macrolides and cephalosporins were most frequently used as antibacterial drugs.
Figure 3.
Risk ratio and risk difference of antibacterial administration (oral and/or injectable), injectable antibacterial use, pneumonia, and additional antiinfluenza drug use regardless of hospitalization. (A) Oseltamivir vs baloxavir. (B) Zanamivir vs baloxavir. (C) Laninamivir vs baloxavir. aData were standardized using the inverse probability of treatment weighting method; the risk ratio was calculated using the exposed (baloxavir) group as the denominator and the neuraminidase inhibitor (NAI) group (oseltamivir, zanamivir, or laninamivir) as the numerator. Risk difference was the incidence of the outcome end point in the NAI group minus the incidence in the baloxavir group. bBaloxavir, N = 146 192; oseltamivir, N = 92 700. cBaloxavir, N = 240 341.4; oseltamivir, N = 238 657.8. dBaloxavir, N = 146 192; zanamivir, N = 18 677. eBaloxavir, N = 164 892.3; zanamivir, N = 163 701.7. fBaloxavir, N = 146 192; laninamivir, N = 81 438. gBaloxavir, N = 227 633.9; laninamivir, N = 227 618.3. Abbreviation: CI, confidence interval.
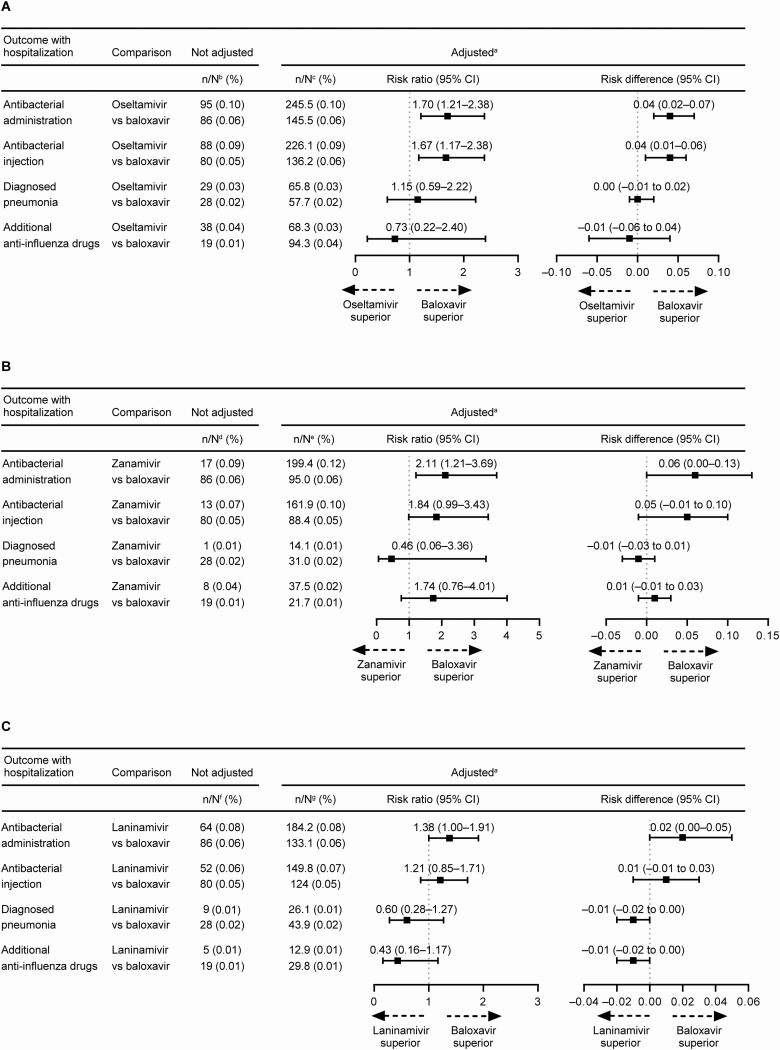
As post hoc analysis, the incidence of secondary end points with hospitalization were assessed. Patients treated with oseltamivir had a significantly greater risk of requiring hospitalization with antibacterial administration and hospitalization with antibacterial injection compared with baloxavir-treated patients (Figure 4A). Patients treated with zanamivir had a significantly greater risk of requiring hospitalization with antibacterial administration compared with baloxavir-treated patients (Figure 4B). Patients in the laninamivir group were 1.38 times more likely to require hospitalization with antibacterial administration than the baloxavir group, although this risk was not significantly different (95% CI, 1.00–1.91; Figure 4C).
Figure 4.
Risk ratio and risk difference of antibacterial administration (oral and/or injectable), injectable antibacterial use, pneumonia, and additional antiinfluenza drug use with hospitalization. (A) Oseltamivir vs baloxavir. (B) Zanamivir vs baloxavir. (C) Laninamivir vs baloxavir. aData were standardized using the inverse probability of treatment weighting method; the risk ratio was calculated using the exposed (baloxavir) group as the denominator and the neuraminidase inhibitor (NAI) group (oseltamivir, zanamivir, or laninamivir) as the numerator. Risk difference was the incidence of the outcome end point in the NAI group minus the incidence in the baloxavir group. bBaloxavir, N = 146 192; oseltamivir, N = 92 700. cBaloxavir, N = 240 341.4; oseltamivir, N = 238 657.8. dBaloxavir, N = 146 192; zanamivir, N = 18 677. eBaloxavir, N = 164 892.3; zanamivir, N = 163 701.7. fBaloxavir, N = 146 192; laninamivir, N = 81 438. gBaloxavir, N = 227 633.9; laninamivir, N = 227 618.3. Abbreviation: CI, confidence interval.
DISCUSSION
To our knowledge, this is the first study to use population-based, real-world patient data to investigate the incidence of hospitalization after outpatient influenza treatment with baloxavir compared with NAIs. The incidence of hospitalization was lower in baloxavir-treated patients than in oseltamivir-treated patients with no statistically significant RR and a statistically significant RD while that in baloxavir-treated patients was lower compared with zanamivir-treated patients with statistically significant RR/RD. These results suggest that baloxavir reduces the risk of hospitalization compared with oseltamivir or zanamivir.
In this study, the administration of antibacterial treatment (both oral and injectable) upon hospitalization was significantly reduced for baloxavir-treated patients compared with oseltamivir-treated patients. However, the incidence of antibacterial administration regardless of hospitalization was significantly higher with baloxavir than with oseltamivir. One possible explanation for this discrepancy is that the outpatient administration of antibacterial drugs may have contributed to the reduced incidence of hospitalization in the baloxavir group. However, for all cohorts, the incidence of hospitalization was increased in those patients who received outpatient antibacterials (Supplementary Table 3). This is not surprising as the requirement for antibacterials indicates that a patient may be experiencing a secondary infection, which can lead to complications that require hospitalization. These results suggest that the lower hospitalization incidence for baloxavir-treated patients compared with oseltamivir-treated patients was not due to the higher antibacterial administration rate. Studies have shown that patients aged ≥16 years hospitalized with influenza have a high and persistent viral load [8, 9]. Although the study results from patients aged ≥16 years may not always be applicable to all age groups, the lower incidence of hospitalization observed for the baloxavir group in this study compared with the oseltamivir group may be attributable to baloxavir’s ability to reduce the viral load more rapidly than oseltamivir [3]. This is also consistent with our observation that the incidence of additional administration of antiinfluenza drugs was significantly lower for the baloxavir group compared with the oseltamivir group (Figure 3A). The reason why the baloxavir group had a higher incidence of antibacterial administration is not known and is a limitation. As possible reasons, since diphasic fever is often observed in young children with influenza [10, 11], oral antibacterials, which might be prescribed for inadequate reasons in Japan [12], might have been more frequently prescribed for baloxavir-treated outpatients due to its single-dose administration and concerns about development of secondary infections such as bacterial infection or baloxavir-insensitive strains in pediatric patients [13].
Baloxavir compared favorably with both inhaled NAIs (zanamivir and laninamivir), although direct comparisons are limited because of possible confounding due to the different routes of administration. In comparison with the zanamivir group, baloxavir-treated outpatients had a reduced incidence of hospitalization and hospitalization that required antibacterials (oral/injectable). However, the baloxavir group had an increased risk of pneumonia compared with the zanamivir group. Physicians may have been less likely to prescribe zanamivir, which requires 10 inhalations over 5 days, to patients with compromised lung function; such patients are also more likely to develop pneumonia. Although we adjusted for underlying patient baseline characteristics, including chronic respiratory diseases, the condition of some patients with compromised lung function may not have been captured in the database. Therefore, we have not adjusted for this condition in those patients, and it was possible that those patients were less likely to be prescribed zanamivir. Nevertheless, we are unable to determine the reason for the difference in pneumonia incidence. Both zanamivir- and laninamivir-treated patients in this study were more likely to require additional antiinfluenza drugs than baloxavir-treated patients. The additional antiinfluenza treatments may have been prescribed if the attending physician had concerns that the inhaled formulation was not properly inhaled, for example, in children [14, 15], or if a patient’s symptoms had not improved [16]. Analysis of data from a double-blind, randomized, clinical trial indicated that nonpersistence with influenza medication was greater for inhaled medication compared with oral medication (hazard ratio, 1.23; P = .043) [17]. In Japan, a survey of adherence to oseltamivir therapy revealed that 21% of patients discontinued oseltamivir, with patients revealing that a shorter treatment plan would be preferred [18]. In the current study, treatment adherence was not examined, which is a limitation of the study. However, baloxavir treatment consists of a single oral dose, and therefore concerns of nonadherence to this antiinfluenza therapy, in particular in response to adverse side effects, are not relevant.
Extracting data from the health insurance claims database allowed us to compare the incidence of hospitalization associated with multiple antiinfluenza drugs in real-world patient data with a sample size >330 000. However, we acknowledge that oseltamivir, given its oral route of administration, was the only true active comparator for baloxavir. This study was further strengthened by using the IPTW method as we were able to adjust for the differences in treatment choice between patients based on demographics, influenza type, and the presence of other factors related to influenza severity.
Several limitations arose from using data from the health insurance claims database. The proportion of elderly patients (aged ≥65 years) in the database is low (approximately 1% [19]) because retired individuals no longer belong to an employer-based health insurance association; therefore, the database is not representative of the aging population (28% are aged ≥65 years [20]). In addition, the number of patients aged <5 years treated with baloxavir, laninamivir, or zanamivir is also limited compared with those treated with oseltamivir. Therefore, our study results may not be applicable to this population. Receipt information in the database does not include information on the period from the onset of influenza to the start of treatment, temperature data, and findings on influenza symptoms, so severity of influenza symptoms at the start of treatment is unknown. The accuracy and the severity of the diagnosis of the covariates used to calculate the propensity score are unknown. Similarly, the accuracy and the severity of the diagnosis of pneumonia, a secondary outcome measure, were also unknown. As this was an observational study, other unmeasured confounding factors may have existed. Also, although significant differences can be identified in observational studies of large datasets, it is very difficult to verify causal relationships. Additional studies to determine why hospitalization incidence was lower in the baloxavir group are required. Finally, the observational period for this study covered the 2018–2019 influenza season when both influenza A/H1N1 and A/H3N2 were the major influenza types; therefore, data for influenza B were limited.
In conclusion, the results of this study using real-world influenza patient data indicate that a single oral dose of baloxavir may reduce hospitalization compared with NAI treatment and provides an alternative antiinfluenza treatment option.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. All authors participated in the interpretation of study results and in the drafting, critical revision, and approval of the final version of the manuscript. T. K., H. Miyauchi, K. Honda, M. F., H. W., and Y. K. were involved in the study design and data analyses. M. F., Y. A., and H. W. conducted the statistical analysis.
Acknowledgments. The authors thank all study participants. Shionogi & Co, Ltd was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Financial support. This work was supported by Shionogi & Co, Ltd, manufacturer/licensee of baloxavir marboxil. Medical writing assistance was provided by Rebecca Lew, PhD, CMPP, and Prudence Stanford, PhD, of ProScribe—Envision Pharma Group and was funded by Shionogi & Co, Ltd. ProScribe’s services complied with international guidelines for Good Publication Practice.
Potential conflicts of interest. E. O., M. F., Y. A., H. W., Y. K., and K. Hara are employees of Shionogi & Co, Ltd. M. F., Y. A., Y. K., and K. Hara report company stock in Shionogi & Co. T. K., S. I., H. Miyauchi, and K. Honda are employees of Shionogi Pharmacovigilance Center Co, Ltd, and report company stock in Shionogi & Co. H. Mukae has received honoraria for lecturing and research grants from Shionogi & Co, Ltd and Chugai Pharmaceutical Co, Ltd, and grants and honoraria for lecturing from Daiichi Sankyo Co, Ltd. H. Mukae also reports personal fees from AbbVie GK, Asahi Kasei Pharma Corporation, Astellas Pharma, AstraZeneca K.K., Bristol-Myers Squibb, Eli Lilly Japan K.K., FUJIFILM Toyama Chemical Co, Ltd, Gilead Sciences Inc, Kyorin Pharmaceutical Co, Ltd, Meiji Seika Pharma Co, Ltd, Insmed Incorporated, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, MSD Co, Ltd, Nihon Pharmaceutical Co, Ltd, Nippon Boehringer Ingelheim Co, Ltd, Novartis Pharma K.K, Pfizer Inc, Sumitomo Dainippon Pharma Co, Ltd, Taiho Pharmaceutical Co, Ltd, Taisho Pharma Co, Ltd, Teijin Home Healthcare Ltd, and Toa Shinyaku Co, Ltd and grants from Asahi Kasei Pharma Corporation, Astellas Pharma Inc, FUJIFILM Toyama Chemical Co, Ltd, Kyorin Pharmaceutical Co, Ltd, Meiji Seika Pharma Co, Ltd, Pfizer Inc, Taiho Pharmaceutical Co, Ltd, Taisho Pharma Co, Ltd, Teijin Pharma Ltd, Toa Shinyaku Co, Ltd, and Torii Pharmaceutical Co, Ltd outside the submitted work. T. M. reports grants from Pfizer, MSD, Astellas, Toyama Chemical, Meiji Seika Pharma, Shionogi, Asahi Kasei Pharma, Daiichi Sankyo, and Kyorin; consulting fees from Pfizer, Toyama Chemical, and Asahi Kasei Pharma; and lecture honoraria from Pfizer, MSD, Astellas, Meiji Seika Pharma, Shionogi, Sumitomo Dainippon Pharma, Taisho Toyama Pharmaceutical, and Torii Pharmaceutical outside the submitted work. T. T. reports personal fees from MSD Co Ltd, Pfizer Inc, and Sumitomo Dainippon Pharma Co, Ltd and grants from MSD Co Ltd and Sumitomo Dainippon Pharma Co, Ltd outside the submitted work. The remaining author: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Zaraket H, Saito R. Japanese surveillance systems and treatment for influenza. Curr Treat Options Infect Dis 2016; 8:311–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heo YA. Baloxavir: first global approval. Drugs 2018; 78:693–7. [DOI] [PubMed] [Google Scholar]
- 3.Hayden FG, Sugaya N, Hirotsu N, et al. ; Baloxavir Marboxil Investigators Group . Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379:913–23. [DOI] [PubMed] [Google Scholar]
- 4.Yoshii N, Tochino Y, Fujioka M, et al. . The comparison of the efficacy of baloxavir and neuraminidase inhibitors for patients with influenza A in clinical practice. Intern Med 2020; 59:1509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ison MG, Portsmouth S, Yoshida Y, et al. . Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis 2020; 20:1204–14. [DOI] [PubMed] [Google Scholar]
- 6.Ono S, Ono Y, Matsui H, Yasunaga H. Factors associated with hospitalization for seasonal influenza in a Japanese nonelderly cohort. BMC Public Health 2016; 16:922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mertz D, Kim TH, Johnstone J, et al. . Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ 2013; 347:f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee N, Chan PK, Hui DS, et al. . Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009; 200:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee N, Chan PK, Rainer TH, Hui D, Choi KW, Cockram CS. Influenza virus load in hospitalised patients. Hong Kong Med J 2013; 19 Suppl 4:15–8. [PubMed] [Google Scholar]
- 10.Ishiguro N, Koseki N, Kaiho M, et al. . Clinical effectiveness of four neuraminidase inhibitors (oseltamivir, zanamivir, laninamivir, and peramivir) for children with influenza A and B in the 2014-2015 to 2016-2017 influenza seasons in Japan. J Infect Chemother 2018; 24:449–57. [DOI] [PubMed] [Google Scholar]
- 11.Koseki N, Kaiho M, Kikuta H, et al. . Comparison of the clinical effectiveness of zanamivir and laninamivir octanoate for children with influenza A(H3N2) and B in the 2011-2012 season. Influenza Other Respir Viruses 2014; 8:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Y, Fujitomo Y, Soeda H, et al. . A nationwide questionnaire survey of clinic doctors on antimicrobial stewardship in Japan. J Infect Chemother 2020; 26:149–56. [DOI] [PubMed] [Google Scholar]
- 13.Hirotsu N, Sakaguchi H, Sato C, et al. . Baloxavir marboxil in Japanese pediatric patients with influenza: safety and clinical and virologic outcomes. Clin Infect Dis 2020; 71:971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eiland LS, Eiland EH. Zanamivir for the prevention of influenza in adults and children age 5 years and older. Ther Clin Risk Manag 2007; 3:461–5. [PMC free article] [PubMed] [Google Scholar]
- 15.Murasaka T, Ikemura K, Enokiya T, et al. . Impact of the number of repeated inhalations and patient characteristics on the residual amount of inhaled laninamivir octanoate hydrate dry powder in pediatric patients with influenza. J Pharm Health Care Sci 2017; 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seki M, Sakai-Tagawa Y, Yasuhara A, Watanabe Y. Adult influenza A (H3N2) with reduced susceptibility to baloxavir or peramivir cured after switching anti-influenza agents. IDCases 2019; 18:e00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flicoteaux R, Protopopescu C, Tibi A, et al. . Factors associated with non-persistence to oral and inhaled antiviral therapies for seasonal influenza: a secondary analysis of a double-blind, multicentre, randomised clinical trial. BMJ Open 2017; 7:e014546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ushijima K, Higuchi S, Fujimura A. Survey of compliance with oseltamivir phosphate therapy in Japan. Am J Ther 2009; 16:8–10. [DOI] [PubMed] [Google Scholar]
- 19.Saokaew S, Sugimoto T, Kamae I, Pratoomsoot C, Chaiyakunapruk N. Healthcare databases in Thailand and Japan: potential sources for health technology assessment research. PLoS One 2015; 10:e0141993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statistics Bureau, Ministry of Internal Affairs and Communications. Statistical handbook of Japan 2019. Chapter 2: population. Available at: https://www.stat.go.jp/english/data/handbook/pdf/2019all.pdf#page=23. Accessed 9 July 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.