Abstract
Aims
The aim of this study was to investigate the causal relationship and evidence of an association between increased adiposity and the risk of incident cardiovascular disease (CVD) events or mortality.
Methods and results
Observational (informing association) and Mendelian randomization (MR) (informing causality) studies were assessed to gather mutually complementary insights and elucidate perplexing epidemiological relationships. Systematic reviews and meta-analyses of observational and MR studies that were published until January 2021 and evaluated the association between obesity-related indices and CVD risk were searched. Twelve systematic reviews with 53 meta-analyses results (including over 501 cohort studies) and 12 MR studies were included in the analysis. A body mass index (BMI) increase was associated with higher risks of coronary heart disease, heart failure, atrial fibrillation, all-cause stroke, haemorrhagic stroke, ischaemic stroke, hypertension, aortic valve stenosis, pulmonary embolism, and venous thrombo-embolism. The MR study results demonstrated a causal effect of obesity on all indices but stroke. The CVD risk increase for every 5 kg/m2 increase in BMI varied from 10% [relative risk (RR) 1.10; 95% confidence interval (CI) 1.01–1.21; certainty of evidence, low] for haemorrhagic stroke to 49% (RR 1.49; 95% CI 1.40–1.60; certainty of evidence, high) for hypertension. The all-cause and CVD-specific mortality risks increased with adiposity in cohorts, but the MR studies demonstrated no causal effect of adiposity on all-cause mortality.
Conclusion
High adiposity is associated with increased CVD risk despite divergent evidence gradients. Adiposity was a causal risk factor for CVD except all-cause mortality and stroke. Half (49%; 26/53) of the associations were supported by high-level evidence. The associations were consistent between sexes and across global regions. This study provides guidance on how to integrate evidence from observational (association) and genetics-driven (causation) studies accumulated to date, to enable a more reliable interpretation of epidemiological relationships.
Keywords: Umbrella review, Meta-analysis, Mendelian randomization study, Cardiovascular disease, Coronary heart disease, Stroke
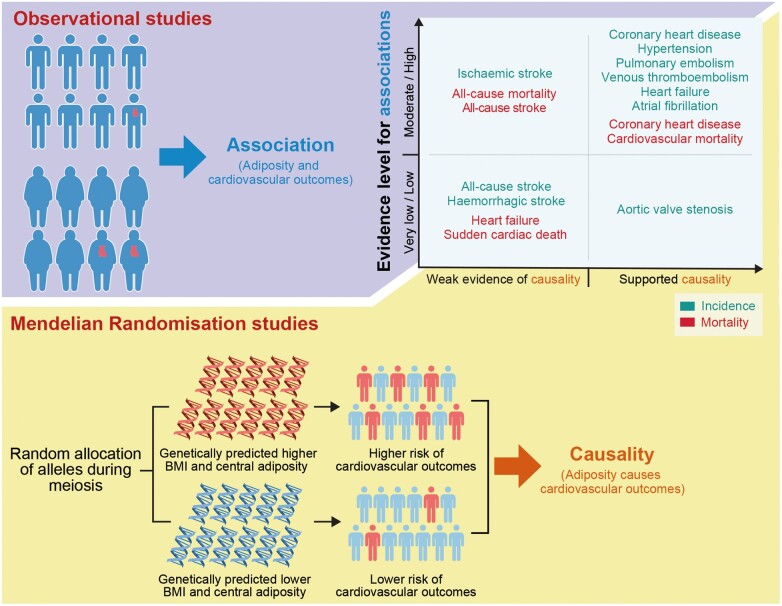
Graphical Abstract
Observational studies (informing associations) and Mendelian randomization studies (informing causality) provided mutually complementary insight and enabled a more reliable interpretation of perplexing epidemiological relationships. This figure was constructed based on the summary of evidence shown in Table 1.
See page 3404 for the editorial comment on this article (doi:10.1093/eurheartj/ehab518)
Introduction
Cardiovascular diseases (CVD) account for over two-thirds of deaths attributable to a high body mass index (BMI),1 and the consequential health outcomes constitute a major proportion of health-related economic burden worldwide.2–6 Despite countermeasures, the outlook is unfavourable, and the incidence of CVD is expected to increase over the next few decades, especially in low- and middle-income countries, as the average BMI increases.7 The association between adiposity and CVD has been extensively studied for decades using Mendelian randomization (MR)8,9 and observational study designs.10,11,12 However, the evidence to date has focused on single clinical associations, leaving evidence on the association between adiposity and multiple cardiovascular outcomes (e.g. stroke, heart failure, atrial fibrillation) unconsolidated.
Umbrella reviews have been increasingly conducted to consolidate the highest level of evidence, namely systematic reviews and meta-analyses, on a given topic.13,14 The most notable difference between a conventional meta-analysis and an umbrella review is that the former uses results from the original study as a fundamental unit for analysis, while the latter uses the results of previous meta-analyses. The umbrella review unites previously published systematic reviews or meta-analyses that usually examine a single clinical association (e.g. adiposity and stroke), systematically merges them to produce multiple clinical endpoints, and provides a bird’s-eye view of a given topic (e.g. adiposity and several CVD).15,16 This umbrella review process involves extensive statistical replication and updating of previous meta-analyses using a uniform analytic model and framework to align and directly compare the relevant information.15 This aspect is a unique strength of the umbrella review in that it enables the consistent and comparative assessment of multiple biases for all relevant outcomes and allows the stratification of findings into distinct evidence levels. Therefore, umbrella reviews help discriminate between mature and immature findings and provide the highest level of evidence to guide decision-making.15
Given that the association between adiposity and CVD outcomes is an epidemiological topic, randomized controlled trials (RCTs) are rarely possible, preventing the elucidation of causal inferences. As an alternative to RCTs, MR studies have been increasingly applied to strengthen causal inferences about associations in observational research.17 The present study is the first to incorporate MR studies into the body of evidence of observational studies and construct an association-to-causality evidence map to aid the more reliable interpretation of epidemiological relationships. This umbrella review accrued a vast amount of relevant evidence on the association between obesity indices and CVD. The results from recently published cohort studies were manually incorporated into existing meta-analyses to update previous results, and over 501 cohorts and 30 million participants were integrated for quantitative syntheses. This work may help contextualize the magnitude of the association and explain the causality of obesity in CVD.
Methods
Literature search and selection criteria
We systematically searched Google Scholar, PubMed, Embase, and the Cochrane Database of Systematic Reviews for systematic reviews and meta-analyses that investigated the association between adiposity indices and cardiovascular health outcomes from inception to 28 January 2021. The adiposity indices of interest included BMI, waist circumference (WC), and waist-to-hip ratio (WHR). We used a predefined search strategy outlined in the Supplementary material online, Appendix for the initial search and replicated it using a search strategy developed by an experienced librarian. We also performed extensive manual searches of the reference lists of the retrieved review articles to identify additional studies. Observational studies were collected to update previous meta-analyses, while MR studies were incorporated to evaluate causality as described in previous umbrella reviews.18,19 We imposed no language restrictions, but all included studies were written in English. The study protocol was published in PROSPERO (CRD42020179469).
Inclusion and exclusion criteria
We included systematic reviews and meta-analyses of prospective cohort studies as well as MR studies that explored the association between obesity indices and cardiovascular outcomes using genetic instruments (GI). We excluded systematic reviews and meta-analyses that evaluated indices other than BMI, WC, and WHR, such as weight loss %, history of bariatric surgery, and adipose tissue volume, as they can increase heterogeneity and hinder a valid synthesis of the results. Studies that included a specific population, such as patients who underwent percutaneous coronary intervention or coronary artery bypass graft or patients who had CVD outcomes of interest [e.g. coronary heart disease (CHD), atrial fibrillation] at baseline were excluded. Studies involving animal or in vitro experiments were excluded.
Data extraction
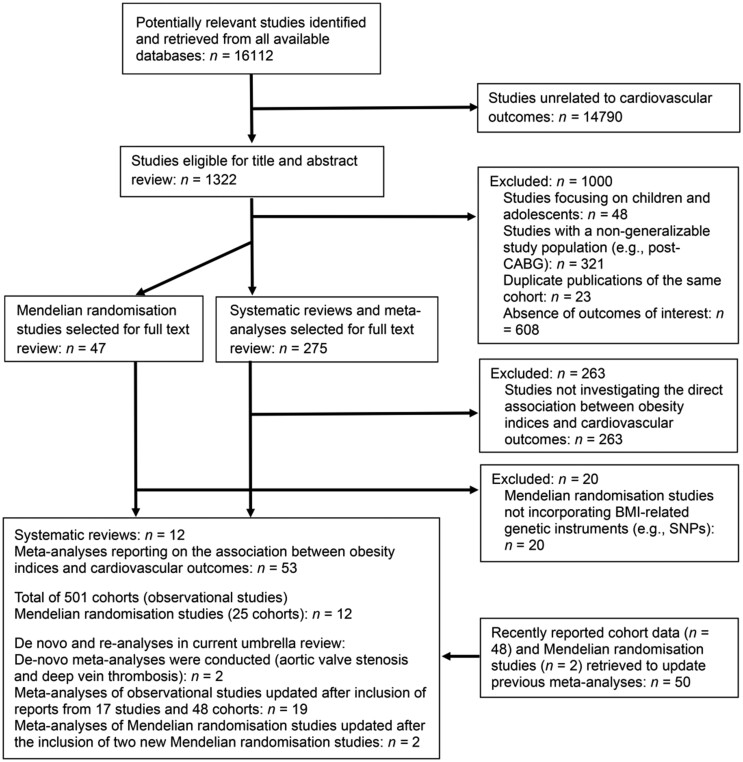
Two researchers (M.S.K. and W.J.K.) independently searched the existing literature and extracted the data. The titles, abstracts, and keywords of each study were reviewed for inclusion, and any ambiguity was resolved through discussion. The study selection process was recorded using the PRISMA flowchart (Figure 1).20
Figure 1.
Flow diagram of the study search and selection process.
The data were collected using a predefined template. The following details were obtained from the included systematic reviews and meta-analyses of observational studies: publication year, number of studies included in the meta-analysis, exposures, comparisons, number of cases and participants, study design, model of effect estimation (random or fixed effects), heterogeneity, and maximally adjusted effect size with 95% confidence interval (CI) for each study (Supplementary material online, Tables S1 and S2). Both categorized (overweight, obese, and severely obese) and continuous (per increase in BMI, WC, and WHR) measures were extracted for qualitative synthesis. From the MR studies, we extracted data on exposure, sample size, instrumental variable method, GI, variance (R2) explained by GI, and maximally adjusted effect estimates with 95% CI (Supplementary material online, Table S3). Mendelian randomization assumptions regarding the reliability of GI (assumption 1) and absence of pleiotropic effects (assumption 2) were evaluated as shown in Supplementary material online, Table S4.21,22
Data analysis
We replicated the meta-analyses and re-analysed the data to uncover the non-explicit details of these meta-analyses necessary to evaluate the relevant biases that were subsequently used to assess the certainty of evidence. The following items were considered to assess bias: heterogeneity among studies using the I2 metric23; the presence of publication bias and small study effect using Egger’s tests (significance threshold, P < 0.10)24; p-curve test detecting p-hacking25–28; and 95% prediction intervals, representing the range within which the effect estimates of future studies will lie with 95% certainty.29–31
We conducted a pairwise meta-analysis using the ‘meta’ package of R (version 3.6.0) software32 to re-analyse and update previous meta-analyses with recently published observational studies. The results are reported in Supplementary material online, Figures S2–S41. We re-analysed and updated previously reported meta-analyses using a generic inverse variance method. For the de novo meta-analysis, the generic inverse variance method was applied to incorporate adjusted results [e.g. adjusted relative risk (RR)] that could not be presented in the dichotomous data (numbers of events and totals). For both re-analysis and de novo meta-analysis, we applied the Hartung–Knapp–Sidik–Jonkman random-effects model since heterogeneity (I2) was generally high and the number of studies was small for multiple outcomes.33,34 We applied a random-effects model because the intergroup heterogeneity was less likely to be introduced by chance. Further details of the methodology and our analytic workflow for pairwise meta-analyses are described elsewhere.35–37 The summary of the effect estimation metrics [odds ratio (OR), RR, and hazard ratio (HR)] presented by each study is shown in Supplementary material online, Table S2.
Subgroup analysis
Pre-specified subgroup analyses were performed to determine whether the results were affected by BMI categories or sex. We conducted a re-analysis by global region at the individual study level to observe global patterns and variations. Since few studies provided individual study data, no bias analysis could have been performed, and regional analyses were not included for evidence classification. We did not conduct subgroup analyses of cohort vs. case-control studies, as all of the included meta-analyses analysed cohort studies. When multiple effect metrics were reported from numerous studies, the effects were pooled regardless of the metrics; however, subgroup analyses for each metric were conducted to evaluate the difference.
Evaluation of the certainty of evidence
We incorporated the MR studies into the body of evidence from the observational studies and constructed an association-to-causality evidence map to enable a more reliable interpretation of the epidemiological relationship. We reviewed any discordance between the observational studies and MR analyses (Table 1). We assessed the certainty of evidence for all reported associations from the observational and MR studies using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework.38
Table 1.
Evidence map of the appraisal of the certainty of evidence (GRADE) of the association between obesity and each cardiovascular outcome
| Main outcomes | Equivalent body fata |
Central adipositya |
Concordance (between observational and MR studies)b | Summary of evidencea | Interpretations and proposals for future study | ||
|---|---|---|---|---|---|---|---|
| BMI category | BMI continuous (per unit increase) | WC | WHR | ||||
| Risk for mortality | |||||||
| All-cause mortality |
|
|
— | — | No (not significant on MR study) | Moderate to high; no causality supported by MR study. Requires cautious interpretation | Results from meta-analyses of observational studies indicate an association; results of MR studies infer causality. Collective evidence suggested that adiposity may not be a causal risk factor for all-cause mortality despite solid associations with moderate to high certainty of evidence. Given that adiposity is a causal risk factor for CVD mortality, it is plausible that CVD mortality acted as a mediator between adiposity and all-cause mortality and complicated a thorough inspection of the actual relationship. Quantitatively synthesized evidence of the association between central adiposity (WC and WHR) and all-cause mortality is lacking, and future studies are required. |
| Heart failure | — | Not significant (very low) | — | — | MR study not reported | Very low | Heart failure-related death may have a very weak link with adiposity. Magnitude of the association and causality remains unsupported. |
| Coronary heart disease | — | High | — | — | Yes | High, with causality supported by MR study | Increased BMI is a causal risk factor for CHD mortality. However, to date, the association between CHD mortality and central adiposity (measured by WC and WHR) was studied at the individual study level, not in systematic reviews and meta-analyses; quantitative synthesis of evidence should be followed with the further accumulation of collective data. |
| Cardiovascular disease | — | High | — | — | Yes | High, with causality supported by MR study | The increase in BMI causes and escalates overall cardiovascular mortality. However, the association between CVD mortality and central adiposity (WC and WHR) is subject to future studies. It requires a further accumulation of collective data (in systematic reviews and meta-analyses). |
| All-cause stroke | — | High | — | — | No (not significant in MR study) | High, without causality supported by MR study. Requires cautious interpretation | MR study suggested no causal effect of adiposity on stroke-specific mortality; the result contradicts those of meta-analyses of observational studies. While adiposity may be associated with stroke death to some degree, more extensive studies may be required to resolve the disparity between the results of observational and MR studies. Prospective investigations on central adiposity (WC and WHR) will provide novel insight into the aetiology of stroke and stroke-specific death. |
| Sudden cardiac death |
|
— | — | — | MR study not reported | Low level of evidence from observational studies; MR study not reported. | There is weak evidence that body fat increases the risk of sudden cardiac death, and the causal relationship remains unknown; future MR studies can elucidate the causality. The association between sudden cardiac death and central adiposity (WC and WHR) will be the subject of future studies. |
| Risk for developing CVD | |||||||
| Coronary heart disease |
|
High | — | — | Yes | Moderate in general, with causality supported by MR study | Collective evidence suggests that the increase in BMI is a causal risk factor for developing CHD. An association between the risk of CHD and central adiposity (WC and WHR) is the subject of a quantitative synthesis. |
| All-cause stroke |
|
Low | — | — |
Yesc (not significant in both observational and MR studies) |
Low, without causality supported by MR study | Adiposity may not cause an all-cause stroke. Magnitude of association and certainty of evidence for the association were weak. An association between the risk of all-cause stroke and central adiposity (WC and WHR) is subject to quantitative synthesis. |
| Haemorrhagic stroke | — | Low | — | — | No (not significant in MR study) | Low, without causality supported by MR study | The MR study suggested no causal effect of adiposity on risk of haemorrhagic stroke; this result contradicts those of reports from the meta-analyses of observational studies. It should be noted that the association was borderline significant on the observational basis, and the directions of the adiposity effect on haemorrhagic stroke were opposite in Europe/North America/Australia vs. Asia, which further weakens the significant associations described in the meta-analysis of observational studies. It would be more reasonable considering that data from observational studies and MR studies are in concord against the association. An association between the risk of haemorrhagic stroke and central adiposity (WC and WHR) will be the subject of future studies. |
| Ischaemic stroke | — | High | — | — | No (not significant in MR study) | High, without causality supported by MR study | The MR study suggested no causal effect of adiposity on ischaemic stroke; this result contradicts those of the reports from the meta-analyses of observational studies. While adiposity may be associated with ischaemic stroke to some degree, more extensive studies may be required to resolve the disparity. The findings indicated that ischaemic stroke is more likely to be associated with adiposity than haemorrhagic stroke, which may suggest the underlying mechanism of the adiposity effect on stroke outcomes. Prospective investigations on the association between central adiposity and the risk of ischaemic stroke are necessary. |
| Heart failure |
|
High | High | Moderate | Yes | Heterogeneous for BMI, generally high for central adiposity; causality supported by MR study | Concerning the high level of evidence of the association between continuous BMI and heart failure, the association might indicate the dose–response relationship (inferring causality); however, the weak evidence level of the association between categorical BMI and heart failure might imply that the association is weak or inconclusive. Given the small number of studies investigating the association (≤10 studies), the latter would be more likely. Heterogeneous results across diverse adiposity indices complicate the interpretation and clarification of the association. Further studies should be performed to increase the level of evidence and minimize inconsistency. |
| Atrial fibrillation | Moderate for obese | High | High | Not significant (low) | Yes | Generally high, with causality supported by MR study | Collective evidence suggested that adiposity is a causal risk factor for developing atrial fibrillation. Of note, two central adiposity indices (WC and WHR) provided contradictory results, which should be addressed in future studies. This discrepancy may be partially attributable to the small number of studies; thus, an updated meta-analysis is necessary. |
| Aortic valve stenosis | Low for obese | — | — | — | Yes | Low, with causality supported by MR study | An increased BMI was associated with an increased risk of developing AVS. However, the magnitude of the association indicated by the meta-analysis of observational studies should be interpreted with caution due to the small sample sizes and cohorts. A prospective updated meta-analysis incorporating a larger number of studies is warranted. Moreover, evidence of the association between central adiposity (WC and WHR) and AVS is lacking, implying that this area requires future investigation. |
| Hypertension | — | High | High | High | Yes | High, with causality supported by MR study | The collective evidence suggested that adiposity is a causal risk factor for developing hypertension. The findings of equivalent body fat (BMI) and central adiposity (WC and WHR) consistently support this association. Both BMI and central adiposity may be reliable indicators for measuring risk and understanding the aetiology of hypertension. |
| Pulmonary embolism | High for obese | — | — | — | Yes | High, with causality supported by MR study | Adiposity might be a causal risk factor for developing PE. An association between the risk of PE and central adiposity (WC and WHR) will be the subject of future studies. |
| Venous thrombo-embolism | Moderate for obese | — | — | — | Yes | Moderate, with causality supported by MR study | Adiposity is likely a causal risk factor for developing VTE. For both PE and VTE, collective evidence of the effect of increased central adiposity (WC and WHR) is lacking. |
GRADE (Grading of Recommendations, Assessment, Development, and Evaluation): High: The evidence provides a very good indication of the likely effect; the likelihood that the effect will be substantially different is low. Moderate: The evidence provides a good indication of the likely effect; the likelihood that the effect will be substantially different is moderate. Low: The evidence provides some indication of the likely effect; however, the likelihood that it will be substantially different (a large enough difference that it might have an effect on a decision) is high. Very low: The evidence does not provide a reliable indication of the likely effect; the likelihood that the effect will be substantially different (a large enough difference that it might have an effect on a decision) is very high.
AVS, aortic valve stenosis; BMI, body mass index; CHD, coronary heart disease; CVD, cardiovascular disease (including CHD and stroke); MR, Mendelian randomization; PE, pulmonary embolism; VTE, venous thrombo-embolism; WHR, waist-to-hip ratio; WC, waist circumference.
High, moderate, low, and very low include outcomes with statistical significance.
Results from meta-analyses of observational studies indicate an association, while results of MR studies suggest causality.
The association between BMI and the risk of stroke in overweight populations reported in observational studies and the risk of stroke reported in Mendelian randomization studies are consistently not significant..
The GRADE framework accounts for study limitations, risk of bias, imprecision, indirectness, inconsistency, publication bias, large magnitude of effect, and dose-response associations. For the study design in the GRADE framework, we assigned the high level for MR studies given that RCTs are rarely possible for non-interventional epidemiological topics and MR studies are usually deemed as an alternative to RCTs for such topics17,21; we assigned a moderate level for large prospective cohort studies and a low level for retrospective studies, since numerous studies suggested a differentiation of evidence levels between prospective and retrospective cohort studies.39–41 The small study effects were judged by the GRADE’s imprecision and publication bias evaluation.42 The risk of bias evaluation was tailored for observational and MR studies because the study designs involved different biases. Observational studies are more susceptible to confounding, reverse causation, and selection biases, while MR studies are more commonly affected by weak instrument bias and pleiotropic effects.21,43 The risk of bias was evaluated using the Newcastle-Ottawa scale (NOS)44 for observational studies and a modified NOS for MR studies. The evaluation of all other components of GRADE was adhered to the guideline.45 The 53 previously reported associations were classified according to the GRADE framework and are presented as evidence maps (Graphical abstract and Table 1). Further methodological details are provided in the Methods section of the Supplementary material online.
Results
Literature review
Of the 16 112 studies identified in the reviewed databases, 1322 were eligible for title and abstract review. After the exclusion of 1000 studies that met our pre-specified exclusion criteria, 275 systematic reviews with meta-analyses and 47 MR studies were subjected to full-text review. The full-text review led to the exclusion of 283 further studies; thus, 12 systematic reviews and 53 meta-analyses of over 501 non-overlapping cohort studies and 12 MR studies (25 cohorts) were included in the final analyses. The PECO (population, exposure, comparison, and outcome) of the included studies were as follows: meta-analyses investigated the impact of increased adiposity (E) vs. normal condition (C) on the risk of CVD outcomes (O) in the general population (P). The search and selection processes are presented in Figure 1. The inclusion/exclusion criteria and adjustment profiles of the included meta-analyses are summarized in Supplementary material online, Table S9.
Meta-analyses of observational studies
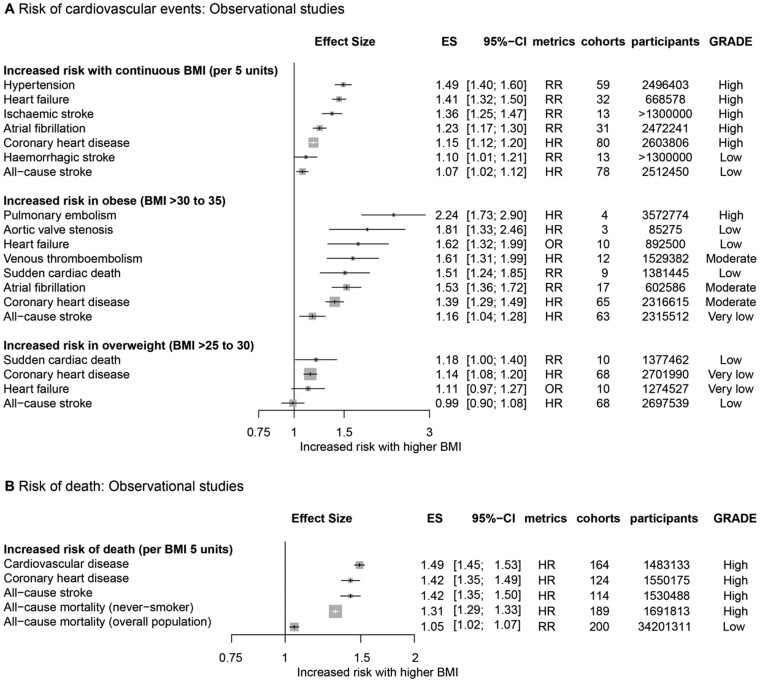
All but 6 of the 53 associations were statistically significant according to the random-effect model results (Supplementary material online, Table S7). The increase in the risk of developing CVD for every 5 kg/m2 increase in BMI varied from 10% (RR, 1.10; 95% CI, 1.01–1.21; certainty of evidence, low) for haemorrhagic stroke to 49% (RR, 1.49; 95% CI, 1.40–1.60; certainty of evidence, high) for hypertension (Figure 2A). The risk of cardiovascular events was increased in the overweight population (BMI > 25–30 kg/m2) vs. the reference group with normal BMI values (HR, 1.14; 95% CI, 1.08–1.20; certainty of evidence, very low for CHD, and RR, 1.18; 95% CI, 1.00–1.40; certainty of evidence, low for sudden cardiac death) (Figure 2A). The risk of developing CVD was increased in the obese population (BMI > 30–35 kg/m2) compared with the normal group (HR, 1.16; 95% CI, 1.04–1.28; certainty of evidence, very low for all-cause stroke to HR, 2.24; 95% CI, 1.73–2.90; certainty of evidence, high for pulmonary embolism) (Figure 2A). The risk of mortality for every 5 kg/m2 (BMI) increment was escalated to divergent extents (RR, 1.05; 95% CI, 1.02–1.07; certainty of evidence, low for all-cause mortality to HR, 1.49; 95% CI, 1.45–1.53; certainty of evidence, high for CVD mortality) (Figure 2B).
Figure 2.
Collective results of observational studies. (A) Increased risk of cardiovascular events with elevated continuous and categorical body mass index. (B) Increased risk of death with elevated continuous body mass index. All results are based on random-effect models. The cohort and participant columns display the number of independent cohorts and the total number of participants incorporated in the meta-analysis for the outcome. The certainty of evidence underlying each association between body mass index and cardiovascular outcomes was evaluated using the GRADE framework. BMI, body mass index; ES, effect size; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HR, hazard ratio; OR, odds ratio; RR, risk ratio or relative risk.
Mendelian randomization studies
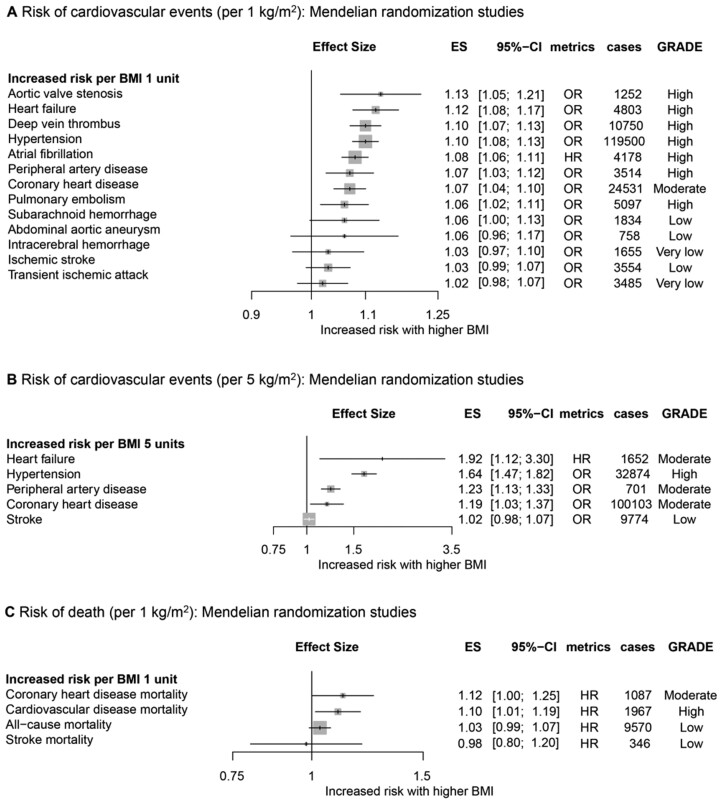
A total of 12 MR analyses (25 cohorts) were identified and classified into 22 outcomes (Supplementary material online, Table S3). The proportion of variance (R2) explained by GI was 1.6–1.82%. Thirteen of the 22 outcomes were supported by a statistical power greater than 80%. All but transient ischaemic attacks (TIAs) and CHD (per 1 kg/m2) met the MR assumptions. Every 1 kg/m2 increment in BMI was associated with an increased risk of pulmonary embolism, CHD, peripheral artery disease, atrial fibrillation, hypertension, deep vein thrombosis, heart failure, and aortic valve stenosis (Figure 3A); every 5 kg/m2 increment in BMI was associated with an increased risk of CHD, peripheral artery disease, hypertension, and heart failure (Figure 3B); and every 1 kg/m2 increment in BMI was associated with an increased risk of death from CVD and CHD (Figure 3C).
Figure 3.
Collective results of Mendelian randomization studies. (A) Increased risk of cardiovascular events per 1 kg/m2 increase in body mass index. (B) Increased risk of cardiovascular events per 5 kg/m2 increase in body mass index. (C) Increased risk of death per 1 kg/m2 increase in body mass index. All results are based on random-effects models. The cohort and cases columns display the number of independent cohorts and the number of cases incorporated in the meta-analysis for the outcome. BMI, body mass index; ES, effect size; HR, hazard ratio; OR, odds ratio; RR, risk ratio or relative risk.
Subgroup analyses
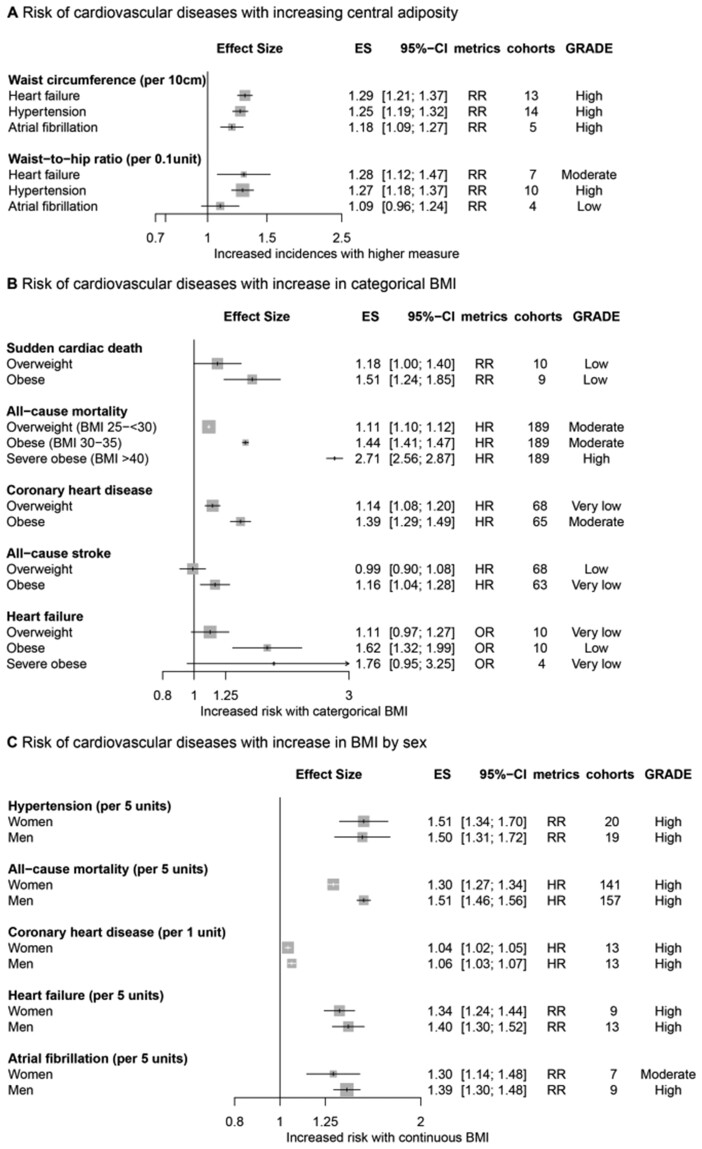
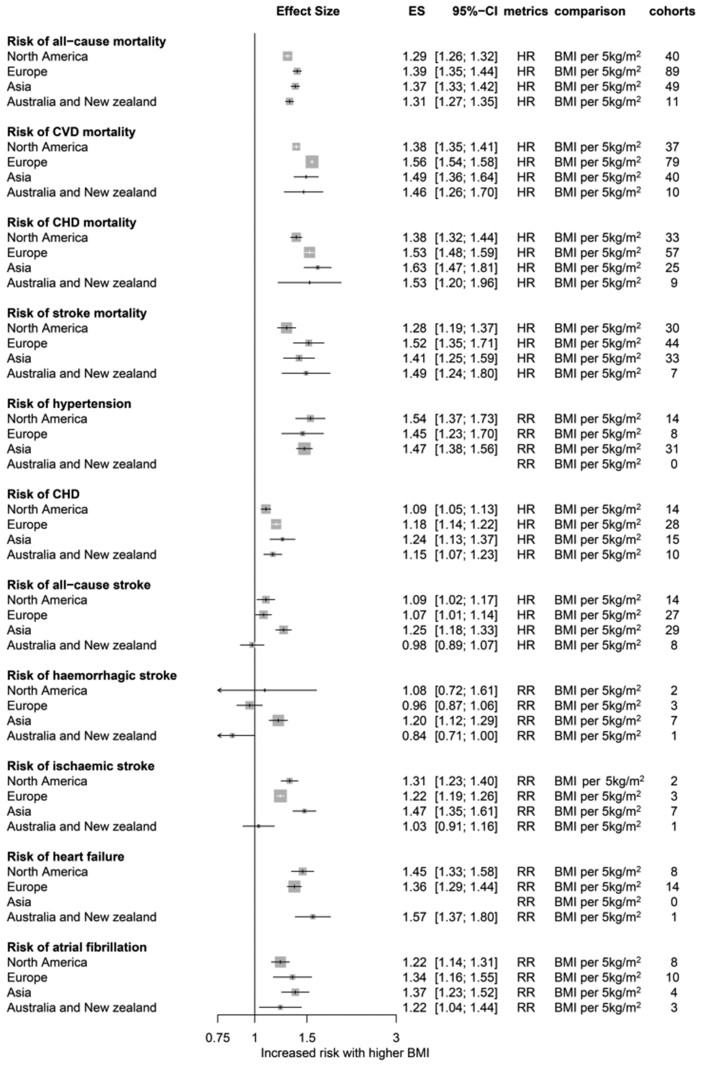
The associations of CVD outcomes with other adiposity measures, including WC and WHR, were consistent with those of BMI (Figure 4A). The risk of CVD outcomes showed a dose-dependent increase with a stepwise increase in BMI categories (Figure 4B). Obese men had a higher risk of CVD than obese women (Figure 4C), but the difference was not statistically significant except for all-cause mortality. According to the regional analysis (Figure 5), European and Asian populations were prone to greater cardiovascular mortality per 5 kg/m2 increase in BMI than North American populations. While the overall patterns were consistent for diverse cardiovascular phenotypes, the associations were heterogeneous for stroke among regions.
Figure 4.
Subgroup analyses of risk of cardiovascular diseases for central adiposity (A), categorical body mass index (B), and sex (C). All results are based on random-effects models. The cohort and participant columns display the number of independent cohorts and the total number of participants incorporated in the meta-analysis for the outcome. The certainty of evidence was evaluated using the GRADE framework. BMI, body mass index; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RR, risk ratio or relative risk.
Figure 5.
Risks of cardiovascular incidences and mortalities were re-analysed according to regions. BMI, body mass index; CVD, cardiovascular disease; CHD, coronary heart disease; ES, effect size; HR, hazard ratio; OR, odds ratio; RR, risk ratio or relative risk.
Level of evidence
The certainty of evidence derived from the observational and MR studies was evaluated using the GRADE framework (Supplementary material online, Tables S5 and S6). Of the 53 meta-analyses that investigated the effect of obesity on CVD-related outcomes, 26 associations (49%) were supported by high evidence certainty (GRADE) as described in the evidence map (Supplementary material online,Table S7). The MR study results were more likely to be susceptible to the small study effects than the observational study results; along with the absolute smaller sample sizes of the MR studies, according to the GRADE evaluations, 50% (11/22) of all outcomes derived from the MR studies were imprecise (Supplementary material online, TableS6) vs. only 20% (11/53) of all outcomes derived from the observational studies (Supplementary material online, Table S5). To avoid reverse causation bias, concordance between the observational and MR analyses results in the direction and/or the statistical significance of associations was reviewed and summarized in Table 1. The quality of the included meta-analyses evaluated using the AMSTAR2 tool was generally moderate (Supplementary material online, Table S8).
Discussion
This umbrella review provides a comprehensive overview of the existing evidence on the association between obesity and CVD by stratifying the association between obesity and each CVD outcome into distinct evidence levels. Twelve systematic reviews with 53 meta-analyses comprising over 501 cohorts and 30 million participants were subjected to a quantitative synthesis and quality assessment. As observational studies can suggest an association but are unable to make claims about causation, MR studies were included to determine causality. Therefore, we provide results from observational and MR studies in parallel to contextualize both the magnitude of association and the causality. The novel findings and insights provided by this umbrella review are summarized in Table 1. This work is a landmark study in that it provides guidance on how to integrate evidence from observational and genetics-driven studies accumulated to date to enable a more reliable interpretation of epidemiological relationships.
We also re-analysed all published data to uncover the non-explicit details, particularly the heterogeneous impact of adiposity among various global regions. To the best of our knowledge, this is the first region-specific quantitative synthesis of all cardiovascular outcomes relative to adiposity. The overall patterns were consistent across regions for diverse cardiovascular phenotypes; however, there were some exceptions; for example, European and Asian populations showed greater increases in cardiovascular death risk per 5 kg/m2 increase in BMI than North American populations. In addition, the associations of BMI with stroke were heterogeneous among regions, particularly for haemorrhagic stroke risk.
An increase in BMI was associated with a higher risk of developing all specific CVD; risks of CHD, heart failure, atrial fibrillation, all-cause stroke, haemorrhagic stroke, ischaemic stroke, hypertension, aortic valve stenosis, pulmonary embolism, and venous thrombo-embolism increased with BMI in observational studies (informing association), consistent with MR study results (informing causality) with the exception of stroke (Figures 2 and 3). In our subgroup analyses, the risk of developing CVD showed a proportional and dose-dependent increase with a step-up in BMI categories, and obese men were more prone to unfavourable CVD outcomes than obese women (Figure 4), although the difference was not statistically significant. The associations between CVD outcomes and other adiposity measures, including WC and WHR, were consistent with those for BMI.
Of note, all-cause mortality significantly increased with higher BMI in observational analyses, although this association was not significant in the MR analyses (Table 1). Such discordance may be explained by the intrinsic limitations of observational studies in managing living confounders. Although a significant association between obesity and all-cause mortality rate was observed in more than 200 collective cohorts with adjustments for age, sex, and smoking,46 this association should be interpreted cautiously as the association may involve residual confounding factors such as diverse comorbidities such as diabetes mellitus and dyslipidaemia; Aune et al.46 reported significant associations between adiposity and all-cause mortality in crude analysis, but not in sensitivity analyses that adjusted for such potential intermediate traits (Supplementary material online, Table S9). It is plausible that the observational results of all-cause mortality may have been overestimated by comorbidities and other intermediate or surrogate causes for death in cohorts that are not necessarily driven by cardiovascular impairment.
Several points should be considered for the proper interpretation of the MR results. Mendelian randomization studies rely on certain assumptions,21,22 of which that regarding horizontal pleiotropy is known to be the most challenging to address. The horizontal pleiotropic effect represents the effects of GI (e.g. variants) on multiple biological pathways, which confounds interpretation of the MR results.17,21 We checked for the assumptions for each MR study and confirmed that no horizontal pleiotropy was suspected with all but TIA and CHD (per 1 kg/m2 increase) (Supplementary material online, Table S4). However, this does not necessarily weaken our analyses; although an MR result for TIA was reported in this review, it was not used in the interpretation (Table 1) since TIA was not investigated in observational studies; while CHD (per 1 kg/m2 increase) was at risk of pleiotropy, CHD (per 5 kg/m2 increase) still met the assumptions, and because both CHD MR results similarly supported positive causation of adiposity on CHD, it is less likely to alter the interpretations.
For the MR analysis, Wade et al.47 used the polygenic risk score (PRS), comprising 77 single nucleotide polymorphisms associated with BMI as reported in the Genetic Investigation of Anthropometric Traits (GIANT) consortium, as the GI, which explained 1.82% of the variance (equivalent to at least >60 in F-statistics). The proportion of variance (R2) explained by the GI was deemed acceptable, as it was >10 in F-statistics,21,48–50 and the explanatory power of PRS for obesity was deemed reliable.51 While there are some limitations to the MR approaches incorporated in our study, such as potential pleiotropic effect (e.g. TIA) and limited statistical power for certain CVD phenotypes (Supplementary material online, Table S3),22,52 it is likely that any potential biases are less marked than those of observational studies47 because the assumptions for MR were generally met.21 The triangulation of different methodologies is essential for inferring definite conclusions with proper causal inference,47 and the findings from the MR studies may add to the current body of evidence implicating obesity as a risk factor for cardiovascular health outcomes.
The risk of the incidence of all CVDs except stroke was significantly increased with obesity in both observational and MR analyses (Figures 2 and 3). A large number of mediators released by the adipose tissue may play a key role in the link between obesity and CVD. Adipose tissues release bioactive mediators that influence alterations in lipids, coagulation, fibrinolysis, and inflammation, leading to endothelial dysfunction and atherosclerosis.53 Atherosclerosis is the principal origin of CVD54,55; it synergistically interacts with hypertension, and both factors aggravate one another.54,56 It is notable that hypertension was the most vulnerable entity affected by BMI in our analysis; the increase in the risk of developing CVD for every 5 kg/m2 increase in BMI ranged from 10% (RR, 1.10; 95% CI, 1.01–1.21) for haemorrhagic stroke to 49% (RR, 1.49; 95% CI, 1.40–1.60) for hypertension. Other CVDs may be the consequence of atherosclerosis and hypertension, as these entities possibly represent a pathophysiological basis and are thus major risk factors for CVD.55,57–60
Several MR studies have reported that BMI has no causal effect on stroke,61–63 and stroke was the least affected entity in our analysis. This result corroborates that of a recent study conducted by Khera et al.51 in which stroke occurred less frequently than most other CVDs, such as hypertension and venous thrombo-embolism, in high BMI PRS carriers (10th percentile); this observation probably indicates that the genetic drivers for obesity have a weak causal effect on the development of stroke. The discordance in the results of observational and MR analyses for stroke (Table 1) in our umbrella review may suggest that stroke pathophysiology involves a complex mechanism in which obesity is only a minor part.64 Other explanations for the discordance include the possible heterogeneous interactive effects of different adiposity measures (e.g. BMI and fat mass index) and stroke subtypes; for example, a positive association of fat mass index with ischaemic stroke, but not haemorrhagic stroke, was reported by an MR study.65
Obesity is a multifactorial disease that results from interactions between genetics and lifestyle.66,67 The heritability for obesity is known to be around 40%,66,68 while the remainder can be explained by lifestyle factors, which suggests that obesity is a modifiable risk factor.69 In this context, the causal effect of obesity on nearly all specific cardiovascular outcomes suggested in this study provides an enthusiastic prospect in which lifestyle modification to reduce adiposity can result in the overall reduction of cardiovascular health problems and substantial health-economic burden.70 This study supports the assertion that reducing adiposity through interventional approaches such as bariatric surgery,71 promotion of educational equality,72 lifestyle modifications including healthy diets,73–78 and increasing physical activities77 may better improve one’s well-being,79 even more so than previously expected, by affecting multiple vascular health outcomes. Our results also support the diet and lifestyle recommendations proposed by the American Heart Association80 and European Society of Cardiology81 and further specify the benefits. Future studies should aim to provide empirical evidence of the effect of lifestyle modifications targeted at reducing adiposity on cardiovascular benefits.
Limitations
This study has several limitations. First, umbrella reviews have intrinsic limitations in that they focus only on existing meta-analyses; therefore, important phenotypes that were not assessed at the meta-analysis level may be overlooked. To minimize the disregard of clinically relevant cardiovascular phenotypes, we independently conducted de novo meta-analyses for certain CVDs (e.g. aortic valve stenosis) that have not been meta-analysed despite a sufficient number of published original studies. Second, when meta-analyses are outdated, they may provide incomplete conclusions with less power, which may directly affect the analyses of subsequent umbrella reviews. As a countermeasure, we updated 19 meta-analyses of observational studies by incorporating recent reports from 35 cohorts to reflect up-to-date conclusions. Third, most genetic studies conducted to date have been conducted of European ancestry82; the MR studies included in this review used genome-wide association study summary statistics from European-ancestry cohorts, such as the UK Biobank, METASTROKE, DIAGRAM, GIANT, and GLGC consortia. This European bias should be resolved in future genetic studies by focusing on diverse ancestries. Fourth, as with previous umbrella reviews of obesity, we did not analyse the effect of underweight on CVD outcomes.83,84 Although a lower BMI is known to affect CVD outcomes and the association constitutes a J-shaped curve, this analysis was beyond the scope of this investigation because our research question mainly lies in whether and to what extent adiposity causally affects various CVD outcomes. Fifth, some analyses may be susceptible to type I error by repeating statistical significance tests of updated meta-analyses and involving small studies.85,86 The small studies may draw underpowered conclusions and subsequently feature an increased likelihood of type II error as well.85 To consider such small study effects, we examined the composite of sample size, width of confidence interval, and statistical power to judge the ‘imprecision’ of the GRADE framework and downgraded the evidence level for the detection of such deficiencies. And finally, although we conducted subgroup analyses for potential modifiers such as sex and region, there are likely residual effect modifiers. Furthermore, correlations among outcomes may influence pooled effect sizes. Such residual effect modifiers and correlations among outcomes are subject to future exploration with meta-regressions and multivariate meta-analyses.87,88
Conclusions
Although obesity as a risk factor for various cardiovascular outcomes has been extensively studied for decades, only 26 of the 53 (49%) associations reported here were supported by high-level evidence. While other associations could be genuine, various degrees of uncertainty remain. The results of this study corroborated the causative effect of obesity on nine of 16 CVD-related outcomes, and the remaining four mortality outcomes and three risk factors for incident stroke (all-cause, ischaemic, and haemorrhagic stroke) are at risk of potential reverse causation bias and require further clarification.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors thank Sooyung Park, an experienced librarian at Samsung Medical Center, for assistance with the search strategy and searching for replication.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (no. 2019R1A2C4070496). M.S.K. and HH.W. had full access to all data in the study. HH.W. made the final decision to submit for publication.
Conflict of interest: None declared.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1.GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang G, Zheng Z-J, Heath G, Macera C, Pratt M, Buchner D.. Economic burden of cardiovascular disease associated with excess body weight in US adults. Am J Prev Med 2002;23:1–6. [DOI] [PubMed] [Google Scholar]
- 3.Cappuccio FP, Miller MA.. Cardiovascular disease and hypertension in sub-Saharan Africa: burden, risk and interventions. Intern Emerg Med 2016;11:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emamgholipour S, Sari AA, Pakdaman M, Geravandi S.. Economic burden of cardiovascular disease in the southwest of Iran. Int Cardiovasc Res J 2018;12:6–12. [Google Scholar]
- 5.Gaziano TA.Economic burden and the cost-effectiveness of treatment of cardiovascular diseases in Africa. Heart 2008;94:140–144. [DOI] [PubMed] [Google Scholar]
- 6.Luengo-Fernandez R, Leal J, Gray A, Petersen S, Rayner M.. Cost of cardiovascular diseases in the United Kingdom. Heart 2006;92:1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand SS, Yusuf S.. Stemming the global tsunami of cardiovascular disease. Lancet 2011;377:529–532. [DOI] [PubMed] [Google Scholar]
- 8.TikkanenE, , GustafssonS, , KnowlesJW, , PerezM, , BurgessS, , Ingelsson E.. Body composition and atrial fibrillation: a Mendelian randomization study. Eur Heart J2019;40:1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LauridsenBK, , StenderS, , KristensenTS, , KofoedKF, , KøberL, , NordestgaardBG, , Tybjærg-Hansen A.. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur Heart J2018;39:385–393. [DOI] [PubMed] [Google Scholar]
- 10.Romero-CorralA, , SomersVK, , Sierra-JohnsonJ, , KorenfeldY, , BoarinS, , KorinekJ, , JensenMD, , ParatiG, , Lopez-Jimenez F.. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J2010;31:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ChenG-C, , ArthurR, , IyengarNM, , KamenskyV, , XueX, , Wassertheil-SmollerS, , AllisonMA, , ShadyabAH, , WildRA, , SunY, , BanackHR, , ChaiJC, , Wactawski-WendeJ, , MansonJE, , StefanickML, , DannenbergAJ, , RohanTE, , Qi Q.. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur Heart J2019;40:2849–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IliodromitiS, , Celis-MoralesCA, , LyallDM, , AndersonJ, , GraySR, , MackayDF, , NelsonSM, , WelshP, , PellJP, , GillJMR, , Sattar N.. The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: a cohort study of 296 535 adults of white European descent. Eur Heart J 2018;39:1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papatheodorou S.Umbrella reviews: what they are and why we need them. Eur J Epidemiol 2019;34:543–546. [DOI] [PubMed] [Google Scholar]
- 14.Biondi-Zoccai G.Umbrella reviews. In: Evidence Synthesis with Overviews of Reviews and Meta-Epidemiologic Studies. Cham, Switzerland: Springer International; 2016;3–391. [Google Scholar]
- 15.Fusar-Poli P, Radua J.. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health 2018;21:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P.. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. JBI Evidence Implement 2015;13:132–140. [DOI] [PubMed] [Google Scholar]
- 17.Pingault J-B, O'Reilly PF, Schoeler T, Ploubidis GB, Rijsdijk F, Dudbridge F.. Using genetic data to strengthen causal inference in observational research. Nat Rev Genet 2018;19:566–580. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Meng X, Timofeeva M, Tzoulaki I, Tsilidis KK, Ioannidis JP, Campbell H, Theodoratou E.. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ 2017;357:j2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Köhler CA, Evangelou E, Stubbs B, Solmi M, Veronese N, Belbasis L, Bortolato B, Melo MCA, Coelho CA, Fernandes BS, Olfson M, Ioannidis JPA, Carvalho AF.. Mapping risk factors for depression across the lifespan: an umbrella review of evidence from meta-analyses and Mendelian randomization studies. J Psychiatr Res 2018;103:189–207. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–341. [DOI] [PubMed] [Google Scholar]
- 21.Davies NM, Holmes MV, Smith GD.. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018;362:k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emdin CA, Khera AV, Kathiresan S.. Mendelian randomization. JAMA 2017;318:1925–1926. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J, Thompson SG.. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Smith GD.. Bias in location and selection of studies. BMJ 1998;316:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Aert RCM, Wicherts JM, van Assen M.. Publication bias examined in meta-analyses from psychology and medicine: a meta-meta-analysis. PLoS One 2019;14:e0215052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Assen MA, van Aert RC, Wicherts JM.. Meta-analysis using effect size distributions of only statistically significant studies. Psychol Methods 2015;20:293–309. [DOI] [PubMed] [Google Scholar]
- 27.Mertens G, Engelhard IM.. A systematic review and meta-analysis of the evidence for unaware fear conditioning. Neurosci Biobehav Rev 2020;108:254–268. [DOI] [PubMed] [Google Scholar]
- 28.Simonsohn U, Nelson LD, Simmons JP.. p-Curve and effect size: correcting for publication bias using only significant results. Perspect Psychol Sci 2014;9:666–681. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP.Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol 2008;37:1158–1160. [DOI] [PubMed] [Google Scholar]
- 30.Riley RD, Higgins JP, Deeks JJ.. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 31.IntHout J, Ioannidis JP, Rovers MM, Goeman JJ.. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;6:e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu C, Niu Y, Wu J, Gu H, Zhang C.. Software and package applicating for network meta-analysis: a usage-based comparative study. J Evid Based Med 2018;11:176–183. [DOI] [PubMed] [Google Scholar]
- 33.IntHout J, Ioannidis JP, Borm GF.. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014;14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sidik K, Jonkman JN.. Simple heterogeneity variance estimation for meta‐analysis. J R Stat Soc Ser C (Appl Stat) 2005;54:367–384. [Google Scholar]
- 35.Kim A, Kim MS, Park YJ, Choi WS, Park HK, Paick SH, Choo MS, Kim HG.. Clinical outcome of single-incision slings, excluding TVT-Secur, vs standard slings in the surgical management of stress incontinence: an updated systematic review and meta-analysis. BJU Int 2019;123:566–584. [DOI] [PubMed] [Google Scholar]
- 36.Kim A, Kim MS, Park YJ, Choi WS, Park HK, Paick SH, Kim HG.. Retropubic versus transobturator mid urethral slings in patients at high risk for recurrent stress incontinence: a systematic review and meta-analysis. J Urol 2019;202:132–142. [DOI] [PubMed] [Google Scholar]
- 37.Kim MS, An MH, Kim WJ, Hwang TH.. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. PLoS Med 2020;17:e1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, Kessels AG, Guyatt GH; GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
- 39.Shekelle PG, Maglione MA, Luoto J, Johnsen B, Perry TR.. AHRQ methods for effective health care. In:Global Health Evidence Evaluation Framework. Rockville, MD: Agency for Healthcare Research and Quality (US; ) 2013;3–24. [PubMed] [Google Scholar]
- 40.Ho PM, Peterson PN, Masoudi FA.. Evaluating the evidence: is there a rigid hierarchy? Circulation 2008;118:1675–1684. [DOI] [PubMed] [Google Scholar]
- 41.Katz DL, Karlsen MC, Chung M, Shams-White MM, Green LW, Fielding J, Saito A, Willett W.. Hierarchies of evidence applied to lifestyle Medicine (HEALM): introduction of a strength-of-evidence approach based on a methodological systematic review. BMC Med Res Methodol 2019;19:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Coello PA, Guyatt GH, Yepes-Nuñez JJ, Akl EA, Hazlewood G, Pardo-Hernandez H, Etxeandia-Ikobaltzeta I, Qaseem A, Williams JW, Tugwell P, Flottorp S, Chang Y, Zhang Y, Mustafa RA, Rojas MX, Xie F, Schünemann HJ.. GRADE guidelines: 20. Assessing the certainty of evidence in the importance of outcomes or values and preferences–inconsistency, imprecision, and other domains. J Clin Epidemiol 2019;111:83–93. [DOI] [PubMed] [Google Scholar]
- 43.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G.. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008;27:1133–1163. [DOI] [PubMed] [Google Scholar]
- 44.Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute 2011. p1–12. [Google Scholar]
- 45.Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schunemann H, Guyatt G.. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015;350:h870. [DOI] [PubMed] [Google Scholar]
- 46.Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, Romundstad P, Vatten LJ.. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016;353:i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wade KH, Carslake D, Sattar N, Davey Smith G, Timpson NJ.. BMI and mortality in UK Biobank: revised estimates using Mendelian randomization. Obesity 2018;26:1796–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haycock PC, Burgess S, Wade KH, Bowden J, Relton C, Davey Smith G.. Best (but oft-forgotten) practices: the design, analysis, and interpretation of Mendelian randomization studies. Am J Clin Nutr 2016;103:965–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pierce BL, Burgess S.. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol 2013;178:1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burgess S, Thompson SG.. Bias in causal estimates from Mendelian randomization studies with weak instruments. Stat Med 2011;30:1312–1323. [DOI] [PubMed] [Google Scholar]
- 51.Khera AV, Chaffin M, Wade KH, Zahid S, Brancale J, Xia R, Distefano M, Senol-Cosar O, Haas ME, Bick A, Aragam KG, Lander ES, Smith GD, Mason-Suares H, Fornage M, Lebo M, Timpson NJ, Kaplan LM, Kathiresan S.. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell 2019;177:587–596.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith GD, Ebrahim S.. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol 2004;33:30–42. [DOI] [PubMed] [Google Scholar]
- 53.Van Gaal LF, Mertens IL, Christophe E.. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–880. [DOI] [PubMed] [Google Scholar]
- 54.Cachofeiro V, Miana M, Heras N, Martín-Fernandez B, Ballesteros S, Balfagon G, Lahera V.. Inflammation: a link between hypertension and atherosclerosis. Curr Hypertens Rev 2009;5:40–48. [Google Scholar]
- 55.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Virani SS, Williams KA, Yeboah J, Ziaeian B.. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 2019;74:e177–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulz E, Gori T, Münzel T.. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res 2011;34:665–673. [DOI] [PubMed] [Google Scholar]
- 57.Palla M, Saber H, Konda S, Briasoulis A.. Masked hypertension and cardiovascular outcomes: an updated systematic review and meta-analysis. Integr Blood Press Control 2018;11:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thompson AM, Hu T, Eshelbrenner CL, Reynolds K, He J, Bazzano LA.. Antihypertensive treatment and secondary prevention of cardiovascular disease events among persons without hypertension: a meta-analysis. JAMA 2011;305:913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li W, Zhao J, Song L, Chen S, Liu X, Wu S.. Combined effects of carotid plaques and hypertension on the risk of cardiovascular disease and all-cause mortality. Clin Cardiol 2020;43:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez CJ, Swett K, Agarwal SK, Folsom AR, Fox ER, Loehr LR, Ni H, Rosamond WD, Chang PP.. Systolic blood pressure levels among adults with hypertension and incident cardiovascular events: the atherosclerosis risk in communities study. JAMA Intern Med 2014;174:1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holmes MV, Lange LA, Palmer T, Lanktree MB, North KE, Almoguera B, Buxbaum S, Chandrupatla HR, Elbers CC, Guo Y, Hoogeveen RC, Li J, Li YR, Swerdlow DI, Cushman M, Price TS, Curtis SP, Fornage M, Hakonarson H, Patel SR, Redline S, Siscovick DS, Tsai MY, Wilson JG, van der Schouw YT, FitzGerald GA, Hingorani AD, Casas JP, de Bakker PIW, Rich SS, Schadt EE, Asselbergs FW, Reiner AP, Keating BJ.. Causal effects of body mass index on cardiometabolic traits and events: a Mendelian randomization analysis. Am J Hum Genet 2014;94:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lyall DM, Celis-Morales C, Ward J, Iliodromiti S, Anderson JJ, Gill JMR, Smith DJ, Ntuk UE, Mackay DF, Holmes MV, Sattar N, Pell JP.. Association of body mass index with cardiometabolic disease in the UK Biobank: a Mendelian randomization study. JAMA Cardiol 2017;2:882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fall T, Hägg S, Mägi R, Ploner A, Fischer K, Horikoshi M, Sarin A-P, Thorleifsson G, Ladenvall C, Kals M, Kuningas M, Draisma HHM, Ried JS, van Zuydam NR, Huikari V, Mangino M, Sonestedt E, Benyamin B, Nelson CP, Rivera NV, Kristiansson K, Shen H-Y, Havulinna AS, Dehghan A, Donnelly LA, Kaakinen M, Nuotio M-L, Robertson N, de Bruijn RFAG, Ikram MA, Amin N, Balmforth AJ, Braund PS, Doney ASF, Döring A, Elliott P, Esko T, Franco OH, Gretarsdottir S, Hartikainen A-L, Heikkilä K, Herzig K-H, Holm H, Hottenga JJ, Hyppönen E, Illig T, Isaacs A, Isomaa B, Karssen LC, Kettunen J, Koenig W, Kuulasmaa K, Laatikainen T, Laitinen J, Lindgren C, Lyssenko V, Läärä E, Rayner NW, Männistö S, Pouta A, Rathmann W, Rivadeneira F, Ruokonen A, Savolainen MJ, Sijbrands EJG, Small KS, Smit JH, Steinthorsdottir V, Syvänen A-C, Taanila A, Tobin MD, Uitterlinden AG, Willems SM, Willemsen G, Witteman J, Perola M, Evans A, Ferrières J, Virtamo J, Kee F, Tregouet D-A, Arveiler D, Amouyel P, Ferrario MM, Brambilla P, Hall AS, Heath AC, Madden PAF, Martin NG, Montgomery GW, Whitfield JB, Jula A, Knekt P, Oostra B, van Duijn CM, Penninx BWJH, Davey Smith G, Kaprio J, Samani NJ, Gieger C, Peters A, Wichmann H-E, Boomsma DI, de Geus EJC, Tuomi T, Power C, Hammond CJ, Spector TD, Lind L, Orho-Melander M, Palmer CNA, Morris AD, Groop L, Järvelin M-R, Salomaa V, Vartiainen E, Hofman A, Ripatti S, Metspalu A, Thorsteinsdottir U, Stefansson K, Pedersen NL, McCarthy MI, Ingelsson E, Prokopenko I; for the European Network for Genetic and Genomic Epidemiology (ENGAGE) consortium. The role of adiposity in cardiometabolic traits: a Mendelian randomization analysis. PLoS Med 2013;10:e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boden-Albala B, Sacco RL.. Lifestyle factors and stroke risk: exercise, alcohol, diet, obesity, smoking, drug use, and stress. Curr Atheroscler Rep 2000;2:160–166. [DOI] [PubMed] [Google Scholar]
- 65.Larsson SC, Bäck M, Rees JM, Mason AM, Burgess S.. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J 2020;41:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Vliet-Ostaptchouk JV, Snieder H, Lagou V.. Gene-lifestyle interactions in obesity. Curr Nutr Rep 2012;1:184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jääskeläinen T, Paananen J, Lindström J, Eriksson JG, Tuomilehto J, Uusitupa M; Finnish Diabetes Prevention Study Group; Genetic predisposition to obesity and lifestyle factors–the combined analyses of twenty-six known BMI- and fourteen known waist:hip ratio (WHR)-associated variants in the Finnish Diabetes Prevention Study. Br J Nutr 2013;110:1856–1865. [DOI] [PubMed] [Google Scholar]
- 68.Day FR, Loos RJ.. Developments in obesity genetics in the era of genome-wide association studies. J Nutrigenet Nutrigenomics 2011;4:222–238. [DOI] [PubMed] [Google Scholar]
- 69.Eckel RH, Krauss RM.. American Heart Association call to action: obesity as a major risk factor for coronary heart disease. AHA Nutrition Committee. Circulation 1998;97:2099–2100. [DOI] [PubMed] [Google Scholar]
- 70.Ghanta RK, LaPar DJ, Zhang Q, Devarkonda V, Isbell JM, Yarboro LT, Kern JA, Kron IL, Speir AM, Fonner CE, Ailawadi G.. Obesity increases risk-adjusted morbidity, mortality, and cost following cardiac surgery. J Am Heart Assoc 2017;6:e003831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou X, Yu J, Li L, Gloy VL, Nordmann A, Tiboni M, Li Y, Sun X.. Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients: systematic review and meta-analysis. Obes Surg 2016;26:2590–2601. [DOI] [PubMed] [Google Scholar]
- 72.Carter AR, Gill D, Davies NM, Taylor AE, Tillmann T, Vaucher J, Wootton RE, Munafò MR, Hemani G, Malik R, Seshadri S, Woo D, Burgess S, Davey Smith G, Holmes MV, Tzoulaki I, Howe LD, Dehghan A.. Understanding the consequences of education inequality on cardiovascular disease: Mendelian randomisation study. BMJ 2019;365:l1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ge L, Sadeghirad B, Ball GDC, da Costa BR, Hitchcock CL, Svendrovski A, Kiflen R, Quadri K, Kwon HY, Karamouzian M, Adams-Webber T, Ahmed W, Damanhoury S, Zeraatkar D, Nikolakopoulou A, Tsuyuki RT, Tian J, Yang K, Guyatt GH, Johnston BC.. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ 2020;369:m696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ho FK, Gray SR, Welsh P, Petermann-Rocha F, Foster H, Waddell H, Anderson J, Lyall D, Sattar N, Gill JMR, Mathers JC, Pell JP, Celis-Morales C.. Associations of fat and carbohydrate intake with cardiovascular disease and mortality: prospective cohort study of UK Biobank participants. BMJ 2020;368:m688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drouin-Chartier J-P, Chen S, Li Y, Schwab AL, Stampfer MJ, Sacks FM, Rosner B, Willett WC, Hu FB, Bhupathiraju SN.. Egg consumption and risk of cardiovascular disease: three large prospective US cohort studies, systematic review, and updated meta-analysis. BMJ 2020;368:m513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Z-H, Zhong W-F, Liu S, Kraus VB, Zhang Y-J, Gao X, Lv Y-B, Shen D, Zhang X-R, Zhang P-D, Huang Q-M, Chen Q, Wu X-B, Shi X-M, Wang D, Mao C.. Associations of habitual fish oil supplementation with cardiovascular outcomes and all cause mortality: evidence from a large population based cohort study. BMJ 2020;368:m456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB.. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun Y, Liu B, Snetselaar LG, Robinson JG, Wallace RB, Peterson LL, Bao W.. Association of fried food consumption with all cause, cardiovascular, and cancer mortality: prospective cohort study. BMJ 2019;364:k5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y, Schoufour J, Wang DD, Dhana K, Pan A, Liu X, Song M, Liu G, Shin HJ, Sun Q, Al-Shaar L, Wang M, Rimm EB, Hertzmark E, Stampfer MJ, Willett WC, Franco OH, Hu FB.. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ 2020;368:l6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J.. Summary of American Heart Association diet and lifestyle recommendations revision 2006. Arterioscler Thromb Vasc Biol 2006;26:2186–2191. [DOI] [PubMed] [Google Scholar]
- 81.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M-T, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen M-L, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sirugo G, Williams SM, Tishkoff SA.. The missing diversity in human genetic studies. Cell 2019;177:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Martin-Hirsch P, Tsilidis KK.. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ 2017;356:j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Mitra A, Terzidou V, Bennett P, Martin-Hirsch P, Tsilidis KK.. Obesity and gynaecological and obstetric conditions: umbrella review of the literature. BMJ 2017;359:j4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mascha EJ.Alpha, beta, meta: guidelines for assessing power and type I error in meta-analyses. Anesth Analg 2015;121:1430–1433. [DOI] [PubMed] [Google Scholar]
- 86.Thomas J, Askie LM, Berlin JA, Elliott JH, Ghersi D, Simmonds M, Takwoingi Y, Tierney JF, Higgins JP.. Prospective approaches to accumulating evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane; 2019. p547–568. [Google Scholar]
- 87.Riley RD, Jackson D, Salanti G, Burke DL, Price M, Kirkham J, White IR.. Multivariate and network meta-analysis of multiple outcomes and multiple treatments: rationale, concepts, and examples. BMJ 2017;358:j3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Riley RD, Moons KG, Snell KI, Ensor J, Hooft L, Altman DG, Hayden J, Collins GS, Debray TP.. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 2019;364:k4597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.