Abstract
Nasopharyngeal adenocarcinoma is a rare malignancy that is classified into conventional/surface and salivary-types. Herein we report the case of a 52-year-old male who presented with a right nasopharyngeal mass and right-sided hearing loss. Diagnostic imaging revealed a circumscribed 1.7 cm mass centred in the right antero-lateral aspect of the nasopharynx. A biopsy showed a gland-forming neoplasm that was in continuity with the surface epithelium. The tumor exhibited a nested to micro-papillary architecture, with mild cytologic atypia. Immunohistochemistry demonstrated diffuse staining for CK7, SOX10, and p16; the abluminal layer was highlighted by CK5 and p63, while the luminal cells expressed CD117. The tumor was not amenable to subclassification and was diagnosed as a low-grade nasopharyngeal adenocarcinoma, not otherwise specified (NOS). Subsequent RNA sequencing was performed which identified a novel GOLGB1-BRAF fusion product. Based on its unique morphology and molecular findings, this is presumed to represent a novel subtype of nasopharyngeal adenocarcinoma. In addition to being of diagnostic relevance, this fusion may ultimately represent a potential therapeutic target.
Keywords: BRAF, GOLGB1, nasopharyngeal adenocarcinoma
1. INTRODUCTION
Nasopharyngeal carcinoma (NPC) is the most common malignancy originating in the nasopharynx and is subdivided histologically into keratinizing, nonkeratinizing and basaloid types.1 Epstein–Barr virus infection is recognized as the etiologic agent in the vast majority of NPC cases, while a minority are attributed to high-risk human papillomavirus infection.2 In contrast, primary adenocarcinomas in this site are extremely rare, accounting for only 0.11% to 0.48% of all nasopharyngeal malignancies.3,4 Unlike NPC, the pathogenesis of this heterogeneous group of malignancies remains poorly characterized. Adenocarcinoma of the nasopharynx ostensibly includes nasopharyngeal papillary adenocarcinoma and salivary gland tumors.1,4–12
Herein, we report the case of an adult male who presented with a distinctive nasopharyngeal tumor. Morphologically, it did not correspond to any established subtype of nasopharyngeal adenocarcinoma, and was initially diagnosed as nasopharyngeal adenocarcinoma, not otherwise specified (NOS). Disease-defining fusion events are increasingly recognized amongst tumors of the head and neck. Based on the recent discovery of a diagnostic fusion in another low-grade adenocarcinoma NOS - defining what is now known as “micro-secretory adenocarcinoma”13 - the decision was made to interrogate this tumor by RNA sequencing. Molecular testing subsequently revealed this tumor harbored a novel GOLGB1-BRAF fusion gene.
1.1. Case report
The patient, a 52-year-old male with no significant past medical history, presented to our institution with a right nasopharyngeal mass and concomitant hearing loss. Diagnostic imaging revealed a circumscribed 1.7 cm tumor in the right antero-lateral aspect of the nasopharynx.
The mass was biopsied, showing an invasive gland-forming neoplasm with areas of nested, micropapillary and cribriform architectures. The luminal spaces frequently contained inspissated eosinophilic material. The luminal cells were cuboidal-columnar with eosinophilic cytoplasm. The nuclei were ovoid with mild pleomorphism, prominent nucleoli, and rare mitotic activity (0–1 per 10 HPFs [FD = 0.55 mm]) (Figure 1). Immunohistochemistry was positive for CD117, SOX10, and CK7, with focal immunoreactivity for S100 and nonspecific staining for p16. The abluminal layer was highlighted by CK5 and p63 (Figure 2). The tumor was negative for TTF-1, thyroglobulin, CK20, CDK2, and BRAF. The patient was subsequently taken to the operating room and underwent an endoscopic nasopharyngectomy and en bloc removal of the mass. All intraoperative frozen section margins were negative for malignancy including the deep margin. However, on the final pathologic specimen the tumor focally extended to the deep margin of resection. The patient underwent adjuvant radiotherapy and is currently disease-free 3 years post treatment.
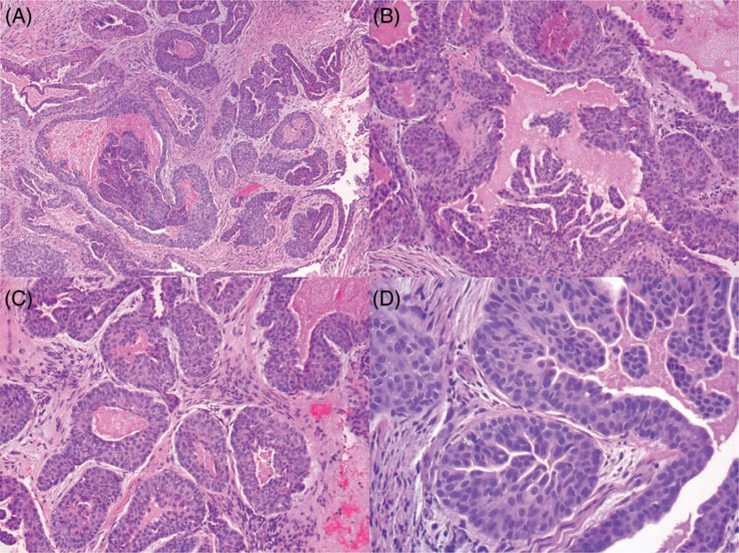
FIGURE 1.
Representative photomicrographs of low-grade nasopharyngeal adenocarcinoma, with GOLGB1-BRAF fusion gene (Hematoxylin and Eosin). A, Low-power magnification showing irregularly shaped invasive glands containing micropapillary luminal projections. B-C, Intermediate magnification highlighting the presence of abundant inspissated luminal material, and diversity of glandular architecture. D, High-power magnification revealing epithelioid cells with abundant eosinophilic cytoplasm; the nuclei are ovoid-round and monomorphic with prominent small nucleoli and inconspicuous mitotic activity
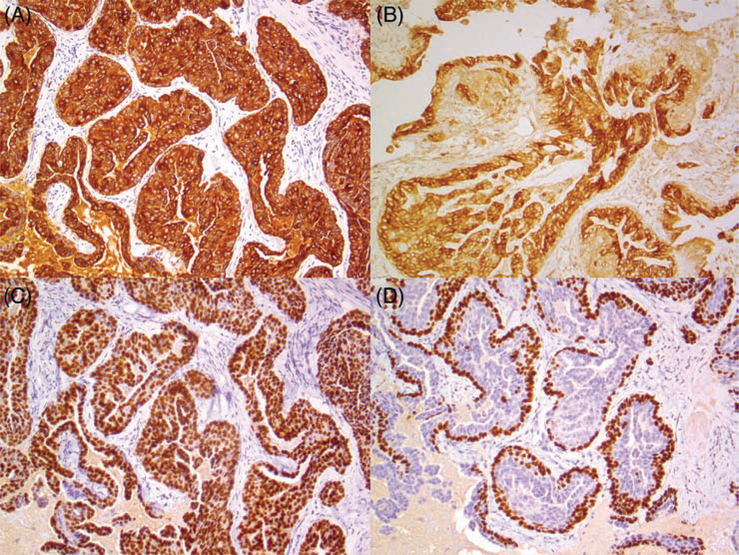
FIGURE 2.
Relevant immunohistochemical stains for low-grade nasopharyngeal adenocarcinoma, with GOLGB1-BRAF fusion gene. There is diffuse cytoplasmic staining for A, CK7, while B, CD117 highlights the luminal layer. There is diffuse nuclear staining with C, SOX10, while D, p63 highlights the abluminal layer
Subsequent testing using the TruSight RNA Fusion Panel (Illumina, San Diego, CA) revealed a novel GOLGB1-BRAF fusion product. The GOLGB1 breakpoint was located at exon 6 of 22 (NCBI Reference Sequence: NM_001256486.1). The BRAF breakpoint was located at exon 9 of 18 (NM_004333.5). While fluorescence in situ hybridization failed to show BRAF rearrangement, independent RT-PCR confirmed the presence of the fusion breakpoint (Figure 3).
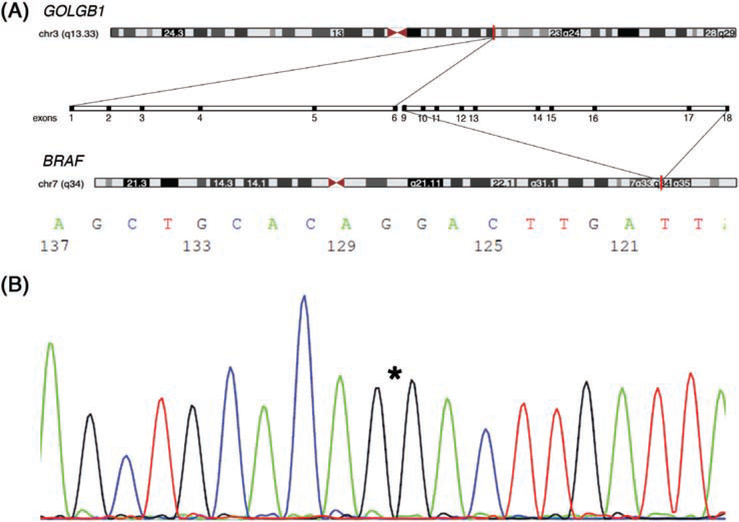
FIGURE 3.
A, Diagrammatic representation of GOLGB1-BRAF fusion gene product; the gene location is highlighted (red) on each chromosome (http://genome.ucsc.edu)26; centrally the expanded view shows the relationship of the exons for each of the gene pairs B, Sanger sequencing independently confirming the results of RNA-Seq; asterisk “*” denotes breakpoints
2. DISCUSSION
The World Health Organization currently subdivides nasopharyngeal adenocarcinomas into nasopharyngeal papillary adenocarcinoma (so-called “common/surface-type”) and salivary gland types.1 The pathogenesis of these rare and heterogeneous neoplasms remains to be fully characterized. We recently identified a novel primary nasopharyngeal adenocarcinoma that was not morphologically or immunophenotypically compatible with this paradigm and, on molecular testing, was found to be characterized by a novel GOLGB1-BRAF fusion product.
Wenig et al (1988) initially reported nine cases of a primary nasopharyngeal papillary adenocarcinoma.12 Histologically, this entity was shown to merge with the overlying normal epithelium, leading the authors to conclude it originated from the surface epithelium. TTF-1 expression was identified in two pediatric cases and based on overlap with papillary thyroid carcinoma (PTC), this entity was designated thyroid-like low-grade nasopharyngeal papillary adenocarcinoma (TL-LGNPPA).14 A relatively consistent description emerged in subsequent reports: these tumors are characterized by a complex arborizing papillary architecture; delicate fibrovascular cores lined by cuboidal-columnar epithelial cells; bland ovoid nuclei with vesicular chromatin; occasional psammoma bodies; immunoreactivity for TTF-1, CK7, CK19, EMA, and vimentin; and, absence of an underlying BRAF mutation.15 TL-LGNPPA can be distinguished from metastatic PTC immunohistochemically by its lack of staining for thyroglobulin and PAX-8, although focal thyroglobulin expression has rarely been reported.16
The gamut of salivary-type adenocarcinomas has been reported in the nasopharynx.4–11 Unsurprisingly, the histopathologic and molecular attributes of nasopharyngeal salivary-type malignancies appears to parallel that of their salivary gland counterparts. Amongst nasopharyngeal adenocarcinomas common- and salivary gland-types appear to have relatively similar proportions. In a series of 48 cases of nasopharyngeal adenocarcinoma 42% were reported to be of salivary-type and 58% were of the traditional type.4 Another series, including 44 cases, reported 64% to be of salivary-type, 29% were the traditional type, and 7% represented metastases.5 Finally, in a series of 67 cases 49% were found to be of salivary-type and 51% of traditional type.8
The tumor in this patient did not resemble a salivary-type neoplasm. Morphologically, it was gland-forming, and lined by plump epithelioid cells with nested and micropapillary architecture. There was continuity with the surface epithelium, raising the possibility of a surface origin; however, in contrast to TL-LGNPPA, this tumor lacked prominent fibrovascular cores, and immunohistochemistry was negative for TTF-1, thereby prompting classification as nasopharyngeal adenocarcinoma not otherwise specified. RNA-Seq subsequently revealed a unique GOLGB1-BRAF fusion product, which was confirmed by RT-PCR, suggesting this represents a novel entity.
Given the anatomical proximity to the nasal cavity, the differential diagnosis of a nasopharyngeal adenocarcinoma perhaps includes a nasal cavity origin. The World Health Organization divides primary sinonasal adenocarcinomas into intestinal and nonintestinal types.1 The morphology and immunophenotype in our patient is incompatible with intestinal-type adenocarcinoma. Nonintestinal-type adenocarcinomas, which are less common than their intestinal counterparts, and further subdivided into low-grade and high-grade types, likewise do not appear to show significant morphologic or immunophenotypic overlap with this tumor. However, it is worth noting that subsets of these tumors have variably been reported to harbor BRAF V600E point mutations,17 or fusions involving ETV6.18,19
BRAF is a proto-oncogene that encodes a serine/threonine kinase involved in the MAPK signaling pathway.20 It is known to be mutated in a variety of cancers including melanoma, thyroid, colorectal, lung, prostate, and ovarian cancers, amongst others.20 Gene fusions involving BRAF are less common, but have been reported in subsets of carcinoma, glioma, melanoma, and soft tissue neoplasms.21 GOLGB1 encodes golgin subfamily B member 1, a ubiquitously expressed coiled coil protein associated with the membrane of the golgi complex.22,23 Interestingly, this protein has been shown to be a regulator of palatogenesis in mammals, with loss-of-function mutations causing cleft palate in murine models.23 Gene fusions involving GOLGB1 have been reported in the context of myeloproliferative neoplasms.24,25 To our knowledge the GOLGB1-BRAF fusion gene has not previously been reported in the literature, and further study is necessary to determine whether it is unique to the adenocarcinoma described herein.
In summary, we report a primary low-grade nasopharyngeal adenocarcinoma characterized by a novel GOLGB1-BRAF fusion gene. This tumor was morphologically distinctive, with invasive glands lined by plump epithelioid cells assuming a micropapillary-cribriform pattern, and inspissated luminal secretions. Primary nasopharyngeal adenocarcinomas are rare, and the incidence of this subtype remains to be characterized; nevertheless, in addition to TL-LGNPPA, this is presumed to represent a subset of so-called traditional “surface-type” tumors, rather than one of salivary gland derivation. Further studies are necessary to characterize the spectrum of clinical, pathologic, and molecular attributes of this tumor; and, for now, we have tentatively labelled this a “low-grade nasopharyngeal adenocarcinoma with BRAF-rearrangement”. The identification of this fusion is relevant for diagnostic purposes, and it is conceivable that there may be a potential role for targeted kinase inhibitors in some situations.
Acknowledgments
Funding information
Panov 2 Research Fund
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Ethics Statement
This study was performed following institutional REB approval.
Informed Consent
Informed consent was obtained from the patient reported in this study.
DATA AVAILABILITY STATEMENT
N/A
REFERENCES
- 1.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO Classification of Head and Neck Tumours. 4th ed. Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 2.Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu T, Li ZM, Gu MF, et al. Primary nasopharyngeal adenocarcinoma: a review. Asia Pac J Clin Oncol. 2012;8(2):123–131. [DOI] [PubMed] [Google Scholar]
- 4.Guo ZM, Liu WW, He JH. A retrospective cohort study of nasopharyngeal adenocarcinoma: a rare histological type of nasopharyngeal cancer. Clin Otolaryngol. 2009;34(4):322–327. [DOI] [PubMed] [Google Scholar]
- 5.Pineda-Daboin K, Neto A, Ochoa-Perez V, Luna MA. Nasopharyngeal adenocarcinomas: a clinicopathologic study of 44 cases including immunohistochemical features of 18 papillary phenotypes. Ann Diagn Pathol. 2006;10(4):215–221. [DOI] [PubMed] [Google Scholar]
- 6.Kuo T, Tsang NM. Salivary gland type nasopharyngeal carcinoma: a histologic, immunohistochemical, and Epstein-Barr virus study of 15 cases including a psammomatous mucoepidermoid carcinoma. Am J Surg Pathol. 2001;25(1):80–86. [DOI] [PubMed] [Google Scholar]
- 7.Schramm VL, Imola MJ. Management of nasopharyngeal salivary gland malignancy. Laryngoscope. 2001;111(9):1533–1544. [DOI] [PubMed] [Google Scholar]
- 8.Liu TR, Chen FJ, Qian CN, et al. Primary salivary gland type carcinoma of the nasopharynx: therapeutic outcomes and prognostic factors. Head Neck. 2010;32(4):435–444. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Park SE, Bae HG, et al. Epithelial-myoepithelial carcinoma of the nasopharynx: a case report and review of the literature. Oncol Lett. 2015;10(2):927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin J, He XY. Basal cell adenocarcinoma of the nasopharyngeal minor salivary glands: a case report and review of the literature. BMC Cancer. 2018;18(1):878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Fu Y, Wang H, et al. Myoepithelial carcinoma of the nasopharynx: rare case report with clinicopathologic and immunohistochemical features review of literature. Head Neck. 2018;40(6): E62–E67. [DOI] [PubMed] [Google Scholar]
- 12.Wenig BM, Hyams VJ, Heffner DK. Nasopharyngeal papillary adenocarcinoma. A clinicopathologic study of a low-grade carcinoma. Am J Surg Pathol. 1988;12(12):946–953. [PubMed] [Google Scholar]
- 13.Bishop JA, Weinreb I, Swanson D, et al. Microsecretory adenocarcinoma: a novel salivary gland tumor characterized by a recurrent MEF2C-SS18 fusion. Am J Surg Pathol. 2019;43(8):1023–1032. [DOI] [PubMed] [Google Scholar]
- 14.Carrizo F, Luna MA. Thyroid transcription factor-1 expression in thyroid-like nasopharyngeal papillary adenocarcinoma: report of 2 cases. Ann Diagn Pathol. 2005;9(4):189–192. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Wei J, Yao X, Wang C. Clinicopathological features of low-grade thyroid-like nasopharyngeal papillary adenocarcinoma. Cancer Res Treat. 2017;49(1):213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozer S, Kayahan B, Cabbarzade C, Bugdayci M, Kosemehmetoglu K, Yucel OT. Thyroid-like papillary adenocarcinoma of the nasopharynx with focal thyroglobulin expression. Pathology. 2013;45(6):622–624. [DOI] [PubMed] [Google Scholar]
- 17.Franchi A, Innocenti DR, Palomba A, et al. Low prevalence of K-RAS, EGF-R and BRAF mutations in sinonasal adenocarcinomas. Implications for anti-EGFR treatments. Pathol Oncol Res. 2014;20(3): 571–579. [DOI] [PubMed] [Google Scholar]
- 18.Andreasen S, Skálová A, Agaimy A, et al. ETV6 gene rearrangements characterize a morphologically distinct subset of sinonasal low-grade non-intestinal-type adenocarcinoma: a novel translocation-associated carcinoma restricted to the sinonasal tract. Am J Surg Pathol. 2017;41 (11):1552–1560. [DOI] [PubMed] [Google Scholar]
- 19.Andreasen S, Kiss K, Melchior LC, Laco J. The ETV6-RET gene fusion is found in ETV6-rearranged low-grade sinonasal adenocarcinoma without NTRK3 involvement. Am J Surg Pathol. 2018;42(7):985–988. [DOI] [PubMed] [Google Scholar]
- 20.Hussain MR, Baig M, Mohamoud HS, et al. BRAF gene: from human cancers to developmental syndromes. Saudi J Biol Sci. 2015;22(4): 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross JS, Wang K, Chmielecki J, et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int J Cancer. 2016;138(4):881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linstedt AD, Hauri HP. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol Biol Cell. 1993;4(7):679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan Y, Zhang N, Liu H, Xu J, Jiang R. Golgb1 regulates protein glycosylation and is crucial for mammalian palate development. Development. 2016;143(13):2344–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troadec E, Dobbelstein S, Bertrand P, et al. A novel t(3;13)(q13;q12) translocation fusing FLT3 with GOLGB1: toward myeloid/lymphoid neoplasms with eosinophilia and rearrangement of FLT3? Leukemia. 2017;31(2):514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naumann N, Schwaab J, Metzgeroth G, et al. Fusion of PDGFRB to MPRIP, CPSF6, and GOLGB1 in three patients with eosinophilia-associated myeloproliferative neoplasms. Genes Chromosomes Cancer. 2015;54(12):762–770. [DOI] [PubMed] [Google Scholar]
- 26.Kent WJ, Sugnet CW, Furey TS, et al. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]