Abstract
Background
The epidemiology of acute bacterial meningitis has changed substantially since the introduction of conjugate vaccines.
Methods
We analyzed nationwide surveillance data of all cerebrospinal fluid isolates received by the Netherlands Reference Laboratory for Bacterial Meningitis in the Netherlands. We assessed the impact of conjugate vaccines on incidence (defined as episodes per 100 000 population per year) and for different age groups using incidence rate ratios (IRRs), comparing incidence before and after conjugate vaccine introduction.
Results
We analyzed 17 393 episodes, of which 5960 episodes (34%) occurred in preschool children (aged 3 months to 4 years). Overall, bacterial meningitis incidence decreased from 6.37 to 1.58 between 1989–1993 and 2014–2019 (IRR, 0.25 [95% confidence interval {CI}, .23–.26]; P < .001). This decrease was most pronounced in preschool and school-aged children (5–15 years); IRR, 0.10 [95% CI, .09–.12] and 0.08 [95% CI, .06–.10]; both P < .001. The incidence was highest in young infants (<90 days) due to a high incidence of group B Streptococcus and Escherichia coli meningitis (42.48 and 19.49, respectively). Conjugate vaccines effectively reduced the incidence of Haemophilus influenzae type b, Neisseria meningitidis serogroup C, and 10 pneumococcal serotypes (IRRs, .02–.04; P < .001). At the end of the observed period, Streptococcus pneumoniae caused the majority of meningitis cases (829/1616 [51%]), mostly in older adults (aged 45–64 years) and elderly adults (aged ≥65 years; incidence of 1.06 and 1.54, respectively).
Conclusions
Conjugate vaccines reduced the burden of bacterial meningitis, especially in children. The efforts for new measures to prevent bacterial meningitis should be focused on neonates and elderly, as the residual rate of disease is still high in these age groups.
Keywords: bacterial meningitis, conjugate vaccines, surveillance study, epidemiology
Conjugate vaccines have significantly reduced the incidence of bacterial meningitis, especially in children. The impact of vaccination has been limited by concurrent serotype replacement, and disease replacement by non-vaccine-targeted bacteria. The residual incidence remains high in neonates and the elderly.
Bacterial meningitis is associated with high rates of mortality and morbidity [1, 2]. The most common bacteria causing meningitis include Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae [3]. Conjugate vaccines have been developed that target specific serogroups or serotypes of these bacteria that commonly cause bacterial meningitis [3–5]. Over the past 3 decades, these conjugate vaccines have been implemented in routine pediatric immunization programs to lower the burden of disease. In the Netherlands, vaccination against H. influenzae type b (Hib) was initiated in 1993, vaccination against meningococcal serogroup C (MenC) was initiated in 2003, and the first pneumococcal conjugate vaccine (PCV) was implemented in 2006 [6]. The Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM) performs nationwide surveillance and monitors the incidence of bacterial meningitis and sepsis and the effect of vaccination. Our aim was to provide a complete overview of the epidemiology of bacterial meningitis over the past 3 decades in the Netherlands.
METHODS
Patient Identification and Data Collection
Nationwide surveillance data were obtained by the NRLBM. The NRLBM receives approximately 90% of cerebrospinal fluid (CSF) isolates of all patients with bacterial meningitis in the Netherlands (±17 million population) [7, 8]. Limited clinical data, including patient age and sex, are provided in the submission template of each bacterial isolate by medical microbiology laboratories. We included all episodes of patients with a positive CSF culture between 1 July 1988 and 30 June 2019. Episodes with missing patient date of birth were excluded. Episodes with positive blood culture but with negative CSF culture were not included in this study. Meningococcal isolates were serogrouped, and pneumococcal and H. influenzae isolates were serotyped by the NRLBM as previously described [9–11]. Population statistics were obtained from Statistics Netherlands with the use of StatLine [12].
Definitions
We categorized patients into 6 age groups: neonates and young infants (grouped as “infants” [0–89 days]), preschool children (3 months–4 years), school-aged children (5–15 years), young adults (16–44 years), older adults (45–64 years), and elderly adults (≥65 years). Several conjugate vaccines have been implemented in the Netherlands during the observed period: Hib vaccine (October 1993, first vaccination at age 2 months); MenC vaccine (June–November 2002, single vaccination for all children [1–18 years]; September 2002 included in nationwide immunization program for children >14 months) [13], later replaced by a tetravalent meningococcal vaccine additionally covering serogroups A, W, and Y (May 2018); and pneumococcal vaccination against 7 serotypes: 4, 6B, 9V, 14, 18C, 19F, 23F (PCV7; June 2006, at age 2 months) later replaced by a 10-valent vaccine additionally covering serotypes 1, 5, and 7F (PCV10; May 2011) [14]. Serotypes and serogroups were subcategorized according to the conjugate vaccine groups.
Statistical Analysis
Annual incidence rates were calculated as the number of new episodes per 100 000 population per epidemiological year (1 July–30 June, defined as the year on 1 January). We compared mean annual incidences of bacterial meningitis overall, and due to specific pathogens in the first 5 years to the last 6 years of the observed period. To compare incidences of different time periods, we estimated incidence rate ratios (IRRs) using unconditional maximum likelihood estimation (Wald) using the “epitools” package [15]. All statistical tests were 2-sided and were considered statistically significant at a P value of ≤ .05. Analyses were performed using R statistical programming language version 3.6.1.
RESULTS
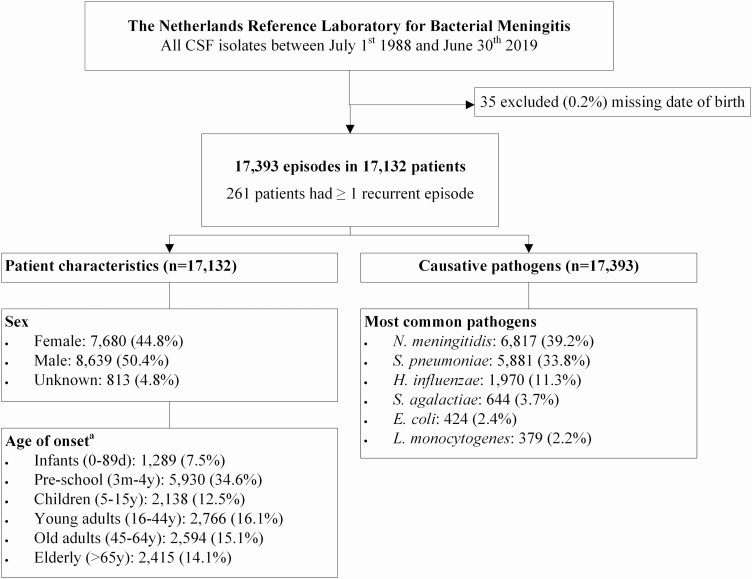
We identified a total of 17 428 episodes of bacterial meningitis. We excluded 35 episodes (0.2%) because of missing patient date of birth. The 17 393 included episodes occurred in 17 132 patients (Figure 1). Two hundred twenty-three patients had 2 episodes, 28 had 3 episodes, 5 had 4 episodes, and 5 had ≥5 episodes. A total of 7796 episodes (45.5%) occurred in females and 8783 (51.3%) in males (sex was unknown in 814 episodes).
Figure 1.
Flowchart baseline characteristics. Abbreviations: CSF, cerebrospinal fluid; E. coli, Escherichia coli; H. influenzae, Haemophilus influenzae; L. monocytogenes, Listeria monocytogenes; N. meningitidis, Neisseria meningitidis; S. agalactiae, Streptococcus agalactiae; S. pneumoniae, Streptococcus pneumoniae. aOnly age of onset of the first episode is described here.
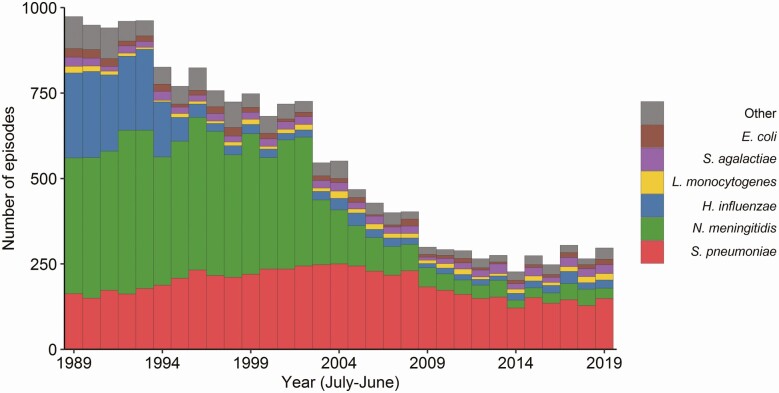
We identified 102 different pathogens (Table 1, Figure 2, and Supplementary Table 1). The median age of patients differed between causative pathogens (Figure 3). Haemophilus influenzae and meningococcal meningitis were predominantly seen in preschool children (1560 of 1970 H. influenzae [79.2%] and 2903 of 6817 meningococcal [48.7%] meningitis episodes), whereas most pneumococcal meningitis cases occurred in older and elderly adults (3200 of 5881 episodes [54.4%]). Five hundred forty-two of 644 Streptococcus agalactiae (84.2%) and 294 of 424 Escherichia coli (69.3%) episodes occurred in infants, together accounting for 63.9% of meningitis cases in this age group. Prior to conjugate vaccine implementation (1989–1993; Figure 4, Table 2, and Supplementary Table 2), N. meningitidis was the most common cause of bacterial meningitis (2.9 episodes per 100 000 population per year), followed by H. influenzae (1.6 per 100 000) and S. pneumoniae (1.1 per 100 000). The incidence of bacterial meningitis was highest in infants (121.5 per 100 000) and preschool children (49.9 per 100 000) in this prevaccination period.
Table 1.
Bacteria Causing Bacterial Meningitis in The Netherlands, July 1988–June 2019
| Pathogen | No. of Episodes (%) | Patient Age, y, Median (IQR) |
|---|---|---|
| Neisseria meningitidis | 6817 (39.2) | 6.3 (1.8–17) |
| Streptococcus pneumoniae | 5881 (33.8) | 50 (4.7–66) |
| Haemophilus influenzae | 1970 (11.3) | 1.7 (0.9–3.7) |
| Streptococcus agalactiae | 644 (3.7) | 15 d (3 d–40 d) |
| Escherichia coli | 424 (2.4) | 27 d (9 d–26 y) |
| Listeria monocytogenes | 379 (2.2) | 67 (54–75) |
A list of all bacteria cultured from the cerebrospinal fluid and received by the National Reference Laboratory for Bacterial Meningitis between July 1988 and June 2019. Data are presented as No. of episodes (%), and the age of patients in whom episodes of the specified pathogen occurred is reported as median (IQR) in years, unless specified otherwise. Only pathogens that are mentioned in the main text are listed here. A full overview is provided in Supplementary Table 1.
Abbreviation: IQR, interquartile range.
Figure 2.
Number of episodes of bacterial meningitis. Histogram showing the number of isolates received per pathogen per epidemiological year (1 July–30 June) from July 1988 to June 2019.
Figure 3.
Age distribution per pathogen. Histograms showing the proportion of cases occurring in at a specific age for Streptococcus agalactiae (A), Escherichia coli (B), Haemophilus influenzae (C), Neisseria meningitidis (D), Streptococcus pneumoniae (E), and Listeria monocytogenes (F).
Figure 4.
Incidence of bacterial meningitis and the impact of vaccination. A, Incidence of bacterial meningitis due to Haemophilus influenzae (blue), Neisseria meningitidis (green), and Streptococcus pneumoniae (red) between June 1988 and July 2019. Lines represent the number of new episodes per 100 000 population per year. The black vertical lines represent the timing of implementation of each vaccine. The dotted lines represent the incidence of the serogroups or (sero)types targeted by the implemented vaccines, type b for H. influenzae (dotted blue line), serogroup C for N. meningitidis (dotted green line), the 10 serotypes targeted by 10-valent pneumococcal conjugate vaccine (PCV) (dotted-dashed red line) and, below, the 7 serotypes targeted by 7-valent PCV (dotted red line) for S. pneumoniae. B–G, Incidence of bacterial meningitis due to H. influenzae (blue), N. meningitidis (green), and S. pneumoniae (red) per epidemiological year (symbols) with the fitted loess regression and the corresponding 95% confidence intervals for infants (aged <90 days; B), preschool children (aged 3 months–4 years; C), school-aged children (aged 5–15 years; D), young adults (aged 16–44 years; E), older adults (aged 45–64 years; F), and elderly adults (aged ≥65 years; G). Abbreviations: Hib, Haemophilus influenzae vaccine against serotype b; MenACWY, vaccine against serogroups A, C, W, and Y meningococci; MenC, vaccine against serogroup C meningococci; PCV7, 7-valent pneumococcal vaccine (serotypes 4, 6B, 9V, 14, 18C, 19F, 23F); PCV10, 10-valent pneumococcal vaccine (additional serotypes 1, 5, 7F).
Table 2.
Bacterial Meningitis in The Netherlands, July 1988–June 2019
| 1989–1993 (Baseline) | 1994–1998 | 1999–2003 | 2004–2008 | 2009–2013 | 2014–2019 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Group and Pathogen | No. of Cases | Incidence | No. of Cases | Incidence | No. of Cases | Incidence | No. of Cases | Incidence | No. of Cases | Incidence | No. of Cases | Incidence | IRR (95% CI) Baseline vs 2014–2019 |
| All pathogens | |||||||||||||
| All ages | 4786 | 6.37 | 3901 | 5.03 | 3420 | 4.28 | 2250 | 2.76 | 1420 | 1.71 | 1616 | 1.58 | 0.25 (.23–.26) |
| Neonates (0–89 d) | 293 | 121.53 | 286 | 118.65 | 223 | 87.88 | 178 | 75.19 | 130 | 57.44 | 198 | 77.17 | 0.63 (.53–.76) |
| Preschool-aged (3 mo-4 y) | 2236 | 49.91 | 1431 | 30.76 | 1160 | 24.45 | 609 | 12.97 | 269 | 6.14 | 255 | 5.09 | 0.10 (.09–.12) |
| School-aged (5–15 y) | 719 | 7.23 | 658 | 6.41 | 475 | 4.40 | 178 | 1.62 | 71 | 0.65 | 71 | 0.56 | 0.08 (.06–.10) |
| Adults (16–44 y) | 800 | 2.23 | 681 | 1.94 | 609 | 1.77 | 338 | 1.01 | 200 | 0.62 | 218 | 0.57 | 0.26 (.22–0.30) |
| Older adults (45–64 y) | 381 | 2.57 | 426 | 2.52 | 525 | 2.80 | 476 | 2.31 | 403 | 1.81 | 440 | 1.60 | 0.62 (.54–.71) |
| Elderly (≥65 y) | 357 | 3.70 | 419 | 4.07 | 428 | 3.93 | 471 | 4.04 | 347 | 2.64 | 434 | 2.32 | 0.63 (.55–.72) |
| Haemophilus influenzae | 1178 | 1.57 | 320 | 0.41 | 117 | 0.15 | 139 | 0.17 | 75 | 0.09 | 141 | 0.14 | 0.09 (.07–.10) |
| Type b | 1133 | 1.51 | 261 | 0.34 | 37 | 0.05 | 60 | 0.07 | 28 | 0.03 | 46 | 0.04 | 0.03 (.02–.04) |
| Neisseria meningitidis | 2157 | 2.87 | 2003 | 2.59 | 1681 | 2.10 | 535 | 0.66 | 234 | 0.28 | 207 | 0.20 | 0.07 (.06–.08) |
| Serogroup C | 419 | 0.56 | 220 | 0.28 | 386 | 0.48 | 30 | 0.04 | 9 | 0.01 | 8 | 0.01 | 0.01 (.01–.03) |
| Streptococcus pneumoniae | 826 | 1.10 | 1055 | 1.36 | 1182 | 1.48 | 1170 | 1.43 | 819 | 0.98 | 829 | 0.81 | 0.74 (.67–.81) |
| PCV7 serotypesa | 371 | 0.49 | 491 | 0.63 | 581 | 0.73 | 544 | 0.67 | 115 | 0.14 | 37 | 0.04 | 0.07 (.05–.10) |
| PCV10 – 7 serotypesb | 87 | 0.12 | 96 | 0.12 | 104 | 0.13 | 152 | 0.19 | 128 | 0.15 | 35 | 0.03 | 0.30 (.20–.44) |
| Listeria monocytogenes | 73 | 0.10 | 68 | 0.09 | 72 | 0.09 | 51 | 0.06 | 35 | 0.04 | 48 | 0.05 | 0.48 (.34–.69) |
| Streptococcus agalactiae | 102 | 0.14 | 102 | 0.13 | 110 | 0.14 | 108 | 0.13 | 90 | 0.11 | 132 | 0.13 | 0.95 (.73–1.23) |
| Escherichia coli | 103 | 0.14 | 89 | 0.11 | 65 | 0.08 | 56 | 0.07 | 43 | 0.05 | 68 | 0.07 | 0.48 (.36–.66) |
Overview of bacterial meningitis episodes in the Netherlands between July 1988 and June 2019 per 5-year period (6 years for last observation period), subcategorized per age group and the 6 most common pathogens, with the vaccine targeted serotype/serogroup if applicable.
Abbreviations: CI, confidence interval; IRR, incidence rate ratio; PCV7, 7-valent pneumococcal conjugate vaccine; PCV10, 10-valent pneumococcal conjugate vaccine.
aPCV7 serotypes: Number of cases/incidence of pneumococcal meningitis caused by serotypes within PCV7 (serotype 4, 6B, 9V, 14, 18C, 19F, and 23F).
bPCV10 – 7 serotypes: Number of cases/incidence of pneumococcal meningitis caused by serotypes additionally covered by PCV10 (serotypes 1, 5, and 7F).
The incidence of bacterial meningitis due to any pathogen decreased from 6.37 in 1989–1993 to 1.58 episodes per 100 000 population per year in 2014–2019 (IRR, 0.25 [95% confidence interval {CI}, .23–.26]; P < .001). This decrease was most pronounced in preschool and school-aged children (IRR, 0.10 [95% CI, .09–.12] and 0.08 [95% CI, .06–.10], respectively; both P < .001). The incidence of bacterial meningitis remained highest in infants, mainly due to a high incidence of S. agalactiae (42.48 per 100 000 infants) and E. coli meningitis (19.49 per 100 000 infants). Pneumococcal meningitis is currently most common, with a mean annual incidence of 0.81 episodes per 100 000 population per year in 2014–2019.
The incidence of H. influenzae meningitis declined from 1.57 per 100 000 population in 1989–1993 to 0.14 per 100 000 in 2014–2019 (IRR, 0.09 [95% CI, .07–.10]; Figure 4 and Table 2). Hib accounted for 1133 of 1175 (96.4%) of H. influenzae meningitis cases before Hib vaccination in 1993. Prior to vaccination, the proportion of H. influenzae meningitis cases due to type b was significantly lower in adults (≥16 years; 18 of 45 cases [40.0%]) compared to children (<16 years; 1115/1130 cases [98.7%]) (P < .001). The incidence of Hib declined from 1.44 per 100 000 population per year prior to vaccination (1991–1993) to 0.04 afterward (2000–2002; IRR, 0.02; P < .001; Supplementary Table 3). The absolute decline in Hib incidence was largest in the preschool children, in whom incidence decreased from 22.94 to 0.46 episodes per 100 000 per year. The relative reduction in Hib incidence was similar for the nonvaccinated age groups (IRR, 0.02 [95% CI, .02–.04]). Incidence of non–type b typeable H. influenzae has increased since Hib vaccination (0.002 in 1989–1993 to 0.014 episodes per 100 000 population per year in 2014–2019; IRR, 5.14 [95% CI, 1.17–22.61]; P = .01), mainly due to type f (11 of 14 non–type b typeable H. influenzae cases [79%] in 2014–2019). Also, we observed an increase in nontypeable H. influenzae meningitis (0.05 in 1989–1993 to 0.08 episodes per 100 000 population per year in 2014–2019 (IRR, 1.49 [95% CI, 1.02–2.17]; P = .04), which was most pronounced in older adults (IRR, 2.23 [95% CI, .98–5.10]; P = .05) and elderly adults (IRR, 2.27 [95% CI, .86–6.00]; P = .08).
The incidence of N. meningitidis meningitis decreased from 2.87 per 100 000 population in 1989–1993 to 0.20 per 100 000 population in 2014–2019 (IRR, 0.07 [95% CI, .06–.08]; Figure 4 and Table 2). Twenty-two percent of this reduction in meningococcal meningitis was attributable to a decline in MenC, which in its peak from July 2001 and June 2002 caused 153 of 377 meningococcal meningitis episodes (41%). A rapid decrease in MenC meningitis followed vaccination in June 2002, from 0.62 to 0.01 between 2000–2002 and 2009–2011 (IRR, 0.02 [95% CI, .01–.04]; P < .001; Supplementary Table 4). This decline was similar in all age categories. Meningitis due to serogroup B, a non-vaccine-targeted serogroup, declined from 2.23 to 0.16 per 100 000 population per year between 1989–1993 and 2014–2019 (IRR, 0.07 [95% CI, .06–.08]; P < .001). This relative decline due to serogroup B was most pronounced in school-aged children, with an IRR of 0.03 (95% CI, .02–.05). Overall, the mean annual incidence of meningococcal meningitis in school-aged children dropped from 5.95 per 100 000 children in 1989–1993 to 0.19 per 100 000 in 2014–2019 (Supplementary Table 2). From 2015 onward, there was an increase in the number of serogroup W meningitis episodes from 0 in 2015 to 9 in 2018. Thirteen of 25 of the serogroup W meningococcal meningitis cases identified between 2015 and 2019 occurred in preschool and school-aged children.
The incidence of pneumococcal meningitis increased from 1.10 to 1.48 episodes per 100 000 per year between 1989–1993 and 2004–2006 (IRR, 1.34 [95% CI, 1.22–1.48]; P < .001; Figure 4, Table 2, and Supplementary Table 5). This increase was most evident shortly following the reduction of Hib, and there was significant negative correlation between the incidence of pneumococcal meningitis and H. influenzae meningitis (r = –0.94; P < .001). Before the implementation of PCVs, 2177 of 3785 pneumococcal meningitis cases (57.5%) were due to serotypes included in PCV10. This proportion was significantly higher in children (aged <16 years; 974/1379 [70.6%]) compared to adults (aged ≥16 years; 1203/2406 [50.0%]; P < .001). Pneumococcal meningitis due to serotypes targeted by PCV7 vaccination diminished from 0.76 to 0.03 episodes per 100 000 per year between 2004–2006 and 2018–2019 (IRR, 0.03 [95% CI, .02–.07]; P < .001), followed by a reduction of the 3 serotypes additionally targeted by PCV10 from 0.17 to 0.01 per 100 000 between 2010–2011 and 2018–2019 (IRR, 0.07 [95% CI, .02–.18]; P < .001; Supplementary Table 5). Since the introduction of vaccination, there has been an increase in non-PCV serotypes from 0.56 to 0.77 episodes per 100 000 per year between 2004–2006 and 2018–2019 (IRR, 1.36 [95% CI, 1.15–1.61]; P < .001). The incidence of meningitis due to pneumococcal serotype 19A increased from 0.04 to 0.08 (IRR, 1.92 [95% CI, 1.07–3.41]; P = .03). The overall decline in pneumococcal meningitis over the past 3 decades was most pronounced in preschool and school-aged children (IRR, 0.39 [95% CI, .31–.49] and 0.32 [95% CI, .21–.49], respectively, both P < .001). The change in incidence in pneumococcal meningitis in older adults and elderly was limited (IRR, 1.01 [95% CI, 0.83–1.23], P = .900 and 0.79 [95% CI, 0.66–0.95], P =. 014; respectively).
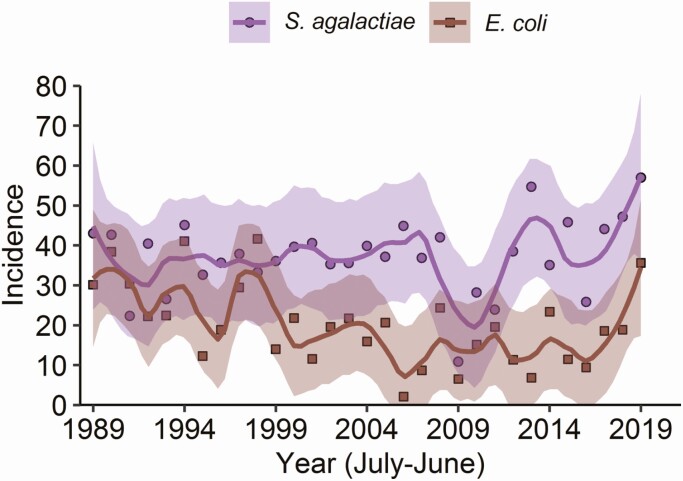
Among the causative pathogens where no vaccination has been implemented, S. agalactiae, E. coli, and Listeria monocytogenes were the most common pathogens. The overall incidence of L. monocytogenes meningitis decreased from 0.10 per 100 000 population in 1989–1993 to 0.05 per 100 000 population in 2014–2019 (IRR, 0.48 [95% CI, .34–.69]; Supplementary Table 2). This was most pronounced in older and elderly adults (IRR, 0.28 [95% CI, .14–.59], P < .001 and 0.46 [95% CI, 0.23–0.91], P = .03, respectively). S. agalactiae and E. coli meningitis predominantly occur in infants (Figure 5). The incidence of S. agalactiae did not change significantly in this age group over time (from 34.84 in 1989–1993 to 42.48 per 100 000 infants per year in 2014–2019; IRR, 1.22 [95% CI, .92–1.62]), while there was a decrease in the incidence of E. coli meningitis (from 28.62 in 1989–1993 to 19.49 per 100 000 infants per year in 2014–2019 (IRR, 0.68 [95% CI, .47–.98]; P = .04; Supplementary Table 2).
Figure 5.
Incidence of group B Streptococcus and Escherichia coli meningitis in neonates. Incidence of bacterial meningitis due to Streptococcus agalactiae (purple) and E. coli (brown) in infants (aged <90 days) between June 1988 and July 2019. Lines represent the number of new episodes per 100 000 population per year.
DISCUSSION
Over the past 3 decades, the incidence of H. influenzae meningitis declined from 1.57 to 0.14 per 100 000 population in the Netherlands. Prior to Hib vaccination, the first ever conjugate vaccine implemented in humans, 25% of all cases in our cohort were caused by H. influenzae. Introduction of Hib vaccination reduced the incidence of Hib meningitis from 1.44 to 0.04 episodes per 100 000 population per year. This impact of 97% is consistent with several other studies worldwide [16]. Type b caused 96% of H. influenzae meningitis episodes. Ever since vaccination, H. influenzae is an uncommon cause of bacterial meningitis, accounting for only 8.8% of cases in 2014–2019. The majority of the H. influenzae meningitis cases are due to nontypeable strains [17], and are mainly identified in predisposed patients and patients of extreme age [18]. In adults with bacterial meningitis, H. influenzae is often seen in patients with CSF leakage [19].
The incidence of N. meningitidis meningitis decreased from 2.87 per 100 000 population in 1989–1993 to 0.20 per 100 000 population in 2014–2019. MenC vaccination, which was implemented in 2002 following the outbreak during 1999–2001, diminished MenC meningitis, which at that time represented 27.4% of N. meningitidis meningitis cases [8]. The largest decrease of meningococcal meningitis was due to the reduction of serogroup B, while no conjugate vaccine targeting serogroup B has been introduced. This decline can best be regarded to as a natural fluctuation. Natural fluctuations, characterized by alternating periods of high incidence followed by periods of low incidence, are common in N. meningitidis disease [8, 20–22]. Factors driving epidemics are not clearly understood [23, 24]. The peaks with high incidence are caused by meningococcal genotypes expressing a certain set of antigens [8, 25, 26]. These genotypes disappear when herd immunity has been developed, which provides opportunity to new genotypes with a different set of expressed antigens [25, 26].
S. pneumoniae has become the most common pathogen to cause bacterial meningitis in the Netherlands. Interventions to prevent pneumococcal meningitis have not been as effective as the interventions implemented in H. influenzae meningitis. Also, the decline in pneumococcal meningitis was relatively small when compared to the (partially natural) decline seen in meningococcal meningitis. The implementation of PCV7 and PCV10 has effectively reduced the rate of pneumococcal meningitis due to vaccine serotypes. However, the overall impact of these conjugate vaccines for pneumococcal meningitis was limited. The proportion of pneumococcal meningitis cases caused by vaccine serotypes prior to vaccination was relatively small, especially in adults in whom only half of pneumococcal meningitis cases were due to vaccine serotypes. In addition, there was evidence of serotype replacement following the eradication of PCV serotypes, with a subsequent increase of 35% in pneumococcal meningitis caused by nonvaccine serotypes. Though we also identified an increase in non-vaccine-targeted H. influenzae serotypes, this has had a small impact as the proportion of cases caused by non–type b capsulated and nontypeable H. influenzae strains was low.
Both H. influenzae and S. pneumoniae are colonizers of the human nasopharynx. We observed an increase in incidence of pneumococcal meningitis following the eradication of H. influenzae meningitis. We hypothesize this may be related to natural competition in colonization, similar to that of the mechanisms behind serotype replacement [27, 28]. Increased pneumococcal carriage in preschool children may have served as a reservoir for increased adult pneumococcal disease, while nasopharyngeal carriage of Hib in preschool children was not clearly associated with invasive disease in adults (a limited proportion of H. influenzae meningitis in adults was due to type b). Almost all nonvaccinated healthy adults have protective immunoglobulin G antibody levels against Hib capsular polysaccharide, probably from nasopharyngeal Hib carriage in childhood [29].
Due to herd protection, conjugate vaccines have also led to a decline in the incidence of meningitis in the nonvaccinated population, including in older adults and elderly. MenC meningitis has almost completely been eradicated in adults and elderly following the immunization of children. Herd protection occurred promptly following the implementation of MenC vaccination. The catch-up campaign may have facilitated the eradication of MenC from the target population and may thereby have accelerated herd protection [30]. The impact of herd protection was limited in H. influenzae meningitis, likely because children are a less evident reservoir for H. influenzae disease in adults (as mentioned before). PCV7 and PCV10 conjugate vaccines in children have effectively eradicated vaccine types in elderly adults. However, the burden of bacterial meningitis remains high in adults and the elderly due to the high rate of pneumococcal meningitis, combined with the relatively low proportion of PCV10 serotypes causing meningitis and the rapid pneumococcal serotype replacement in these age groups. In elderly, additional vaccination with polysaccharide conjugate vaccine targeting 23 serotypes will be recommended from fall 2020 onward in the Netherlands [31].
Overall, the reduction in incidence of bacterial meningitis was most substantial in preschool children, from 49.9 to 5.1 per 100 000 population, and in school-aged children, from 7.23 to 0.56 per 100 000 population. Rates of disease in infants and older adults and the elderly are, however, still high. The most common pathogens in neonates are S. agalactiae and E. coli. Intrapartum antibiotic prophylaxis for women in labor with increased risk for perinatal S. agalactiae infection was introduced in the Netherlands in 1998 [32]. This strategy has likely reduced the incidence of invasive S. agalactiae infection in the first days of life, mainly presenting as sepsis without meningitis. It has, however, had no impact on S. agalactiae infections after the first week of life, where meningitis is more common [33, 34]. The finding that low levels of maternal antibodies to capsular polysaccharide antigens were associated with neonatal S. agalactiae infection, possibly related to inadequate transplacental transmission of maternal antibodies to the unborn child, has sparked interest in maternal immunization [35]. Several conjugate vaccines are under development to further limit neonatal S. agalactiae disease, but have not been implemented yet [36, 37].
Our study has several limitations. Over the past 3 decades, changes in clinical practices in performing a lumbar puncture and the rate of negative CSF cultures due to more timely administration of antibiotics may have affected the number of isolates received. Also, there may have been variation in the submission rate of isolates from medical microbiology laboratories to the NRLBM. With an estimated 10% of patients having a contraindication to undergo a lumbar puncture, a false-negative culture rate of 25% [38], and 90% send-in rate by microbiology laboratories, actual incidences are likely more than 50% higher. We have previously shown that the number of isolates received by the NRLBM is similar to the number of notifications received by the Dutch National Institute for Public Health and the Environment system [8]. A recent study showed that of all cases reported to the NRLBM and the notification system, 93% were reported to the NRLBM and 94% to the notification system [39]. We did not have clinical data from described episodes, which precluded us from excluding patients with nosocomial meningitis. Also, our results are derived from patients in the Netherlands only and thus do not reflect findings in other countries.
In conclusion, the epidemiology of bacterial meningitis in the Netherlands has changed over the past 3 decades. The impact of conjugate vaccines has varied per pathogen and per age group because of the different proportions covered by the vaccine. In addition, replacement of disease, either due to serotypes not included in vaccines or the increase of other pathogens due to changes in nasopharyngeal carriage, has partly limited conjugate vaccine impact. The efforts for preventive measures for bacterial meningitis warrant a shift from preschool children toward neonates and the elderly given the residual high rate of disease in these age groups.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the research analysts of the Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM) for the collection of samples and their work on the analyses of the bacterial isolates.
Financial support. This work was supported by the Netherlands Organisation for Health Research and Development (ZonMw; NWO-Vidi-Grant [grant number 917.17.308 to M. C. B.]); NWO-Vici-Grant (grant number 918.19.627 to D. v. d. B); Academic Medical Center (AMC PhD Scholarship to D. L. H. K.); AMC Innovative Impulse Grant (to M. W. B. and D.v.d.B.); Steun Emma Foundation (grant to M. W. B. and D.v.d.B.); and Pfizer (investigator-initiated research grant number WI173197 to A. v. d. E.). The NRLBM is partially financed by the National Institute for Public Health and the Environment, Bilthoven. This work was partly supported by a grant from the Meningitis Research Foundation (project number 1502.0, Group B Streptococcal Genome Library) awarded to A. v. d. E.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.van de Beek D, Brouwer MC, Thwaites GE, Tunkel AR. Advances in treatment of bacterial meningitis. Lancet 2012; 380:1693–702. [DOI] [PubMed] [Google Scholar]
- 2.van de Beek D, Brouwer MC, Hasbun R, Koedel U, Whitney CG, Wijdicks E. Community-acquired bacterial meningitis. Nat Rev Dis Primers 2016; 2:16074. [DOI] [PubMed] [Google Scholar]
- 3.McIntyre PB, O’Brien KL, Greenwood B, van de Beek D. Effect of vaccines on bacterial meningitis worldwide. Lancet 2012; 380:1703–11. [DOI] [PubMed] [Google Scholar]
- 4.Schuchat A, Robinson K, Wenger JD, et al. . Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med 1997; 337:970–6. [DOI] [PubMed] [Google Scholar]
- 5.Thigpen MC, Whitney CG, Messonnier NE, et al. . Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–25. [DOI] [PubMed] [Google Scholar]
- 6.Bijlsma MW, Brouwer MC, Kasanmoentalib ES, et al. . Community-acquired bacterial meningitis in adults in the Netherlands, 2006-14: a prospective cohort study. Lancet Infect Dis 2016; 16:339–47. [DOI] [PubMed] [Google Scholar]
- 7.Netherlands Reference Laboratory for Bacterial Meningitis (AMC/RIVM). Bacterial meningitis in the Netherlands: annual report 2016. Amsterdam, the Netherlands: University of Amsterdam, 2017. [Google Scholar]
- 8.Bijlsma MW, Bekker V, Brouwer MC, Spanjaard L, van de Beek D, van der Ende A. Epidemiology of invasive meningococcal disease in the Netherlands, 1960–2012: an analysis of national surveillance data. Lancet Infect Dis 2014; 14:805–12. [DOI] [PubMed] [Google Scholar]
- 9.Monge S, Hahné SJ, de Melker HE, Sanders EA, van der Ende A, Knol MJ. Effectiveness of the DTPa-HBV-IPV/Hib vaccine against invasive Haemophilus influenzae type b disease in the Netherlands (2003-16): a case-control study. Lancet Infect Dis 2018; 18:749–57. [DOI] [PubMed] [Google Scholar]
- 10.Knol MJ, Hahné SJM, Lucidarme J, et al. . Temporal associations between national outbreaks of meningococcal serogroup W and C disease in the Netherlands and England: an observational cohort study. Lancet Public Health 2017; 2:e473–82. [DOI] [PubMed] [Google Scholar]
- 11.Wagenvoort GH, Sanders EA, Vlaminckx BJ, et al. . Invasive pneumococcal disease: clinical outcomes and patient characteristics 2–6 years after introduction of 7-valent pneumococcal conjugate vaccine compared to the pre-vaccine period, the Netherlands. Vaccine 2016; 34:1077–85. [DOI] [PubMed] [Google Scholar]
- 12.Statistics Netherlands. StatLine. Available at: https://www.cbs.nl. Accessed 1 April 2020.
- 13.de Greeff SC, de Melker HE, Spanjaard L, van den Hof S, Dankert J. The first effect of the national vaccination campaign against meningococcal-C disease: a rapid and sharp decrease in the number of patients [in Dutch]. Ned Tijdschr Geneeskd 2003; 147:1132–5. [PubMed] [Google Scholar]
- 14.National Institute for Public Health and the Environment. The national immunisation programme in the Netherlands—surveillance and developments in 2018–2019. Report 2019-0193. Bilthoven, the Netherlands: RIVM, 2019. [Google Scholar]
- 15.Aragon TJ. Epitools: epidemiology tools. R package version 0.5–10.1.2020. Available at: https://CRAN.R-project.org/package=epitools. Accessed 20 June 2020.
- 16.Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010; 23:467–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouwer MC, van de Beek D, Heckenberg SG, Spanjaard L, de Gans J. Community-acquired Haemophilus influenzae meningitis in adults. Clin Microbiol Infect 2007; 13:439–42. [DOI] [PubMed] [Google Scholar]
- 18.Campos J, Hernando M, Román F, et al. , Group of Invasive Haemophilus Infections of the Autonomous Community of Madrid, Spain . Analysis of invasive Haemophilus influenzae infections after extensive vaccination against H. influenzae type b. J Clin Microbiol 2004; 42:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ter Horst L, Brouwer MC, van der Ende A, van de Beek D. Community-acquired bacterial meningitis in adults with cerebrospinal fluid leakage. Clin Infect Dis 2020; 70:2256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore PS. Meningococcal meningitis in sub-Saharan Africa: a model for the epidemic process. Clin Infect Dis 1992; 14:515–25. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez K, Lingani C, Aderinola OM, et al. . Meningococcal meningitis outbreaks in the African meningitis belt after meningococcal serogroup a conjugate vaccine introduction, 2011–2017. J Infect Dis 2019; 220:S225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenwood B. Manson lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg 1999; 93:341–53. [DOI] [PubMed] [Google Scholar]
- 23.Borrow R, Alarcón P, Carlos J, et al. , Global Meningococcal Initiative . The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines 2017; 16:313–28. [DOI] [PubMed] [Google Scholar]
- 24.Halperin SA, Bettinger JA, Greenwood B, et al. . The changing and dynamic epidemiology of meningococcal disease. Vaccine 2012; 30(Suppl 2):B26–36. [DOI] [PubMed] [Google Scholar]
- 25.Bambini S, Piet J, Muzzi A, et al. . An analysis of the sequence variability of meningococcal fHbp, NadA and NHBA over a 50-year period in the Netherlands. PLoS One 2013; 8:e65043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kremer PHC, Lees JA, Ferwerda B, et al. . Diversification in immunogenicity genes caused by selective pressures in invasive meningococci. Microb Genom 2020; 6:mgen000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block SL, Hedrick J, Harrison CJ, et al. . Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J 2004; 23:829–33. [DOI] [PubMed] [Google Scholar]
- 28.Spijkerman J, Prevaes SM, van Gils EJ, et al. . Long-term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PLoS One 2012; 7:e39730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nix EB, Hawdon N, Gravelle S, et al. . Risk of invasive Haemophilus influenzae type b (Hib) disease in adults with secondary immunodeficiency in the post-Hib vaccine era. Clin Vaccine Immunol 2012; 19:766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flasche S, Ojal J, Le Polain de Waroux O, et al. . Assessing the efficiency of catch-up campaigns for the introduction of pneumococcal conjugate vaccine: a modelling study based on data from PCV10 introduction in Kilifi, Kenya. BMC Med 2017; 15:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institute for Public Health and the Environment. Guideline of national coordination of infectious disease control, part of the Netherlands National Institute for Public Health and the Environment—pneumococcal vaccine. Bilthoven, the Netherlands: RIVM, 2020. [Google Scholar]
- 32.Netherlands Association for Obstetrics and Gynecology. Preventie van perinatale groep-B-streptokokkenziekte.Utrecht, the Netherlands: NVOG, 1998. [Google Scholar]
- 33.Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine 2013; 31(Suppl 4):D7–12. [DOI] [PubMed] [Google Scholar]
- 34.Ohlsson A, Shah VS. Intrapartum antibiotics for known maternal group B streptococcal colonization. Cochrane Database Syst Rev 2014; 1:CD007467. [DOI] [PubMed] [Google Scholar]
- 35.Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med 1976; 294:753–6. [DOI] [PubMed] [Google Scholar]
- 36.Nuccitelli A, Rinaudo CD, Maione D. Group B Streptococcus vaccine: state of the art. Ther Adv Vaccines 2015; 3:76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vornhagen J, Adams Waldorf KM, Rajagopal L. Perinatal group B streptococcal infections: virulence factors, immunity, and prevention strategies. Trends Microbiol 2017; 25:919–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khatib U, van de Beek D, Lees JA, Brouwer MC. Adults with suspected central nervous system infection: a prospective study of diagnostic accuracy. J Infect 2017; 74:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandwagt DAH, van der Ende A, Ruijs WLM, de Melker HE, Knol MJ. Evaluation of the surveillance system for invasive meningococcal disease (IMD) in the Netherlands, 2004–2016. BMC Infect Dis 2019; 19:860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.