Abstract
Many patients struggle with ongoing symptoms in different domains (physical, mental, cognitive) after hospitalisation for COVID-19, calling out for a multidisciplinary approach. An outpatient multidisciplinary rehabilitation programme, according to a respiratory rehabilitation strategy, was set up for adult patients who were able to attend group sessions during 12 weeks. Results of 22 adult patients with COVID-19, of which 15 had required intensive care, were analysed and some general impressions and challenges of rehabilitation in COVID-19 were reported. Impressive results on physical recovery were determined after 6 weeks and 3 months, with significant improvement of lung function, muscle force and exercise capacity variables. A positive evolution of mental and cognitive burden was present, although less pronounced than the physical recovery. These mental and cognitive consequences seem, next to musculoskeletal and medical complications, the most challenging aspect of rehabilitating patients with COVID-19. These real-world data show feasibility and efficiency of a multidisciplinary respiratory rehabilitation programme after moderate to severe COVID-19 disease.
Keywords: pulmonary rehabilitation, COVID-19
When the Belgian lockdown due to SARS-CoV-2 was implemented in March 2020, we reduced the respiratory rehabilitation programme in our tertiary care hospital to protect our vulnerable patients. Soon, the existing respiratory rehabilitation facilities and staff had to be reorganised to provide a multidisciplinary rehabilitation programme for ambulatory adult patients with COVID-19.
Elderly patients with severe functional or cognitive impairment, or patients who needed inpatient rehabilitation, were considered not eligible for this programme. Other patients willing to take part in outpatient rehabilitation were referred by the treating physician at discharge, on the occasion of a follow-up consultation 6 weeks after discharge, by their general practitioner or after inpatient rehabilitation. These patients underwent clinical assessment, pulmonary function testing, 6-minute walking distance (6MWD) test, hand grip force (HGF), quadriceps force, maximal inspiratory and expiratory pressure, and cardiopulmonary exercise test (CPET). Patients were invited to start the programme if limb muscle force or 6MWD was below 70% of the predicted value, provided symptoms and functional status had deteriorated by COVID-19. Based on our experience with chronic lung diseases, a rehabilitation programme with both endurance and resistance training was set up. Treadmill, cycle ergometer, arm ergometer and stair climbing or step were performed, next to resistance training of lower and upper limbs by leg and chest press. The programme started at 60%–75% of maximal individual performance. Interval training was implemented if the patient was not able to cycle ≥10 min on 80% of his maximal work load during CPET. Progressive overload was obtained by increasing both intensity and duration, based on symptom scores (target Borg dyspnoea and fatigue score 4–6/10). Sessions of 1.5 hours were performed three times a week for 3 months.1 2 The multidisciplinary team was led by a pulmonologist and constituted of physiotherapists, a psychologist, a social worker, a dietitian and an occupational therapist. Patients were evaluated by all team members except the occupational therapist, who was consulted only if indicated. Evaluation was performed after 6 weeks (6MWD and HGF) and 3 months.
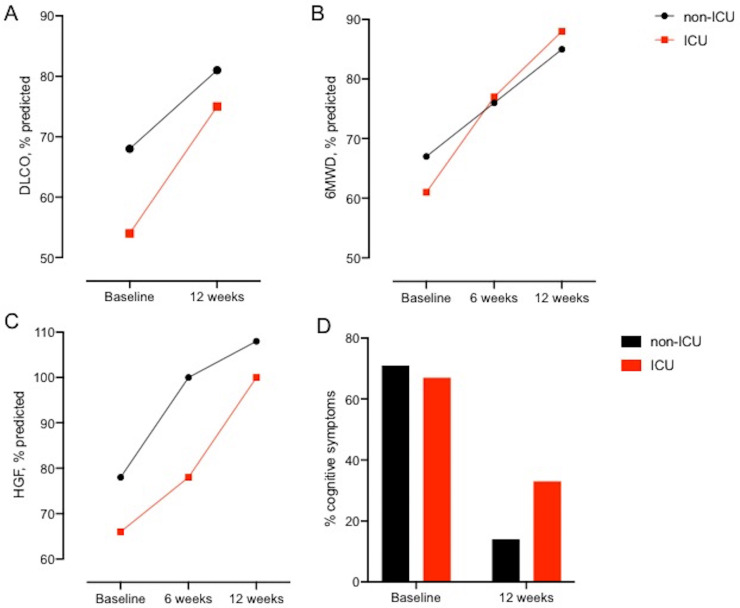
Results of 22 patients who were enrolled in the programme were analysed, of which 16 completed the 3-month evaluation. Three patients stopped the programme after 6 weeks (two satisfying results, one poor motivation). For three other patients, the programme was interrupted due to interfering medical problems (myasthenia gravis, lumbar discus hernia, severe cognitive dysfunction). Of the 15 patients who were treated in the intensive care unit (ICU), 1 received oxygen by high-flow nasal cannula and 14 were mechanically ventilated of which 1 needed support by extracorporeal membrane oxygenation; moreover, 2 patients were weaned with tracheostomy. The seven other patients stayed on a general low-care COVID-19 unit and received between 0 and 5 L oxygen/min by low-flow nasal cannula or mask. The majority of the patients received hydroxychloroquine (n=19), six patients (all on ICU) were treated with corticosteroids. Baseline characteristics are shown in table 1A, B. All physical variables showed significantly better values after the 3-month programme (table 1C). The 6MWD improved with 86 (53–175) m at 6 weeks and 149 (90–221) m at 3 months. At rehabilitation entry, limb muscle strength was severely impaired, which contrasted with the absence of respiratory muscle weakness. Quadriceps and HGF improved to, respectively, 74% (71–87) and 104% (96–112) of predicted values at 3 months. All patients went from interval to endurance training before week 6, including five patients with supplemental oxygen or ventilatory limitation (maximal exercise ventilation/maximal voluntary ventilation >0.8 during CPET) at the start of the training programme. None of the patients needed specific inspiratory muscle training, although ICU-acquired weakness generally affects also respiratory muscle function3 (table 1C). If severity of COVID-19 disease was considered, physical improvement seems more pronounced in the patients who required intensive care (figure 1A–C), which started of course at a lower level.
Table 1.
Characteristics of patients with COVID-19 at the start and during outpatient rehab programme
| A. Demographics and history | |||
| Number of patients | 22 | ||
| Age, years | 54.5 (47–61) | ||
| Male, n (%) | 15 (68) | ||
| BMI at start rehab, kg/m2 | 28 (25–31) | ||
| History of pulmonary disease, n (%) | 5 (23) | ||
| B. Characteristics of hospitalisation | |||
| ICU stay, n (%) | 15 (68) | ||
| Length of hospital stay, days | 29 (12–39) | ||
| Mechanical ventilation*, days | 14 (11–24) | ||
| MRC sum at ICU discharge*, /60 | 45.5 (41–52) | ||
| Days after hospital discharge, days† | 47 (15–69) | ||
| Prior inpatient rehab, n (%)† | 5 (23) | ||
| C. Physical assessment | |||
| Baseline | 6 weeks | 3 months | |
| Number of patients | 22 | 21 | 16 |
| Oxygen need, n (%) | 2 (9) | 0 (0) | 0 (0) |
| FVC, % pred | 85 (70–97) | 90 (79–105)‡ | |
| FEV1, % pred | 89 (77–98) | 91 (82–103)‡ | |
| TLC, % pred | 86 (74–91) | 95 (76–101)‡ | |
| DLCO, % pred | 56 (50–66) | 75 (60–84)‡ | |
| 6MWD, m | 453 (342–529) | 549 (478–620)§ | 605 (497–655)‡ |
| 6MWD, % pred | 63 (53–73) | 77 (73–85)§ | 88 (76–91)‡ |
| 6MWD, ∆m | NA | 86 (53–175) | 149 (90–221) |
| HGF, % pred | 69 (61–81) | 90 (78–103)§ | 104 (96–112)‡ |
| QF, % pred | 61 (50–70) | 74 (71–87)‡ | |
| MIP, % pred | 89 (74–101) | 107 (83–123)‡ | |
| MEP, % pred | 80 (67–94) | 108 (86–126)‡ | |
| CPET, work load, % pred W | 65 (50–78) | 96 (83–114)‡ | |
| CPET, peak VO2, mL/min/kg | 16 (13–20) | 20 (16–30)‡ | |
| CPET, peak VO2, % pred | 66 (56–73) | 91 (82–108)‡ | |
| D. Mental and cognitive burden | |||
| Baseline | 3 months | ||
| HADS, anxiety score¶ | 5 (0–14) | 6 (0–11) | |
| HADS, anxiety score ≥8¶, n (%) | 5 (26) | 6 (35) | |
| HADS, depression score¶ | 3 (0–11) | 3 (0–13) | |
| HADS, depression score ≥8¶, n (%) | 4 (21) | 2 (12) | |
| Cognitive deficit reported by patient, n (%) | 15 (68) | 6 (27)‡‡ | |
| MoCA** | 25 (20.5–29) | 28 (25–29) | |
| MoCA <26 at discharge**, n (%) | 5 (50) | 2 (20) | |
| Resumed work††, n (%) | 2 (13) | 9 (60)‡‡ | |
Results are presented as number (%) or median (Q1–Q3).
*In ICU patients only.
†Five patients were discharged from the hospital to an inpatient rehabilitation facility where they stayed between 5 and 80 days before they started this outpatient programme.
‡Difference between baseline and 3 months significant with p<0.01 using Wilcoxon matched-pairs signed-rank test.
§Difference between baseline and 6 weeks significant with p<0.001.
¶n=19.
**n=10 (all ICU).
††Fifteen patients were working before illness, some resumed work part-time.
‡‡Difference between baseline and 3 months significant with p<0.05 using Fisher’s exact test.
BMI, body mass index; CPET, cardiopulmonary exercise test; DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HADS, Hospital Anxiety and Depression Scale; HGF, hand grip force; ICU, intensive care unit; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; MoCA, Montreal Cognitive Assessment; MRC, Medical Research Council; 6MWD, 6-minute walking distance; NA, not available; QF, quadriceps force; TLC, total lung capacity; VO2, oxygen consumption.
Figure 1.
Difference in improvement between patients who stayed in the intensive care unit (ICU) and not. These graphs show the difference between patients who stayed in the ICU (n=15) and not (n=7). (A) Improvement in diffusing capacity for carbon monoxide (DLCO) between baseline and 12 weeks after rehabilitation. (B) Improvement in % predicted of 6-minute walking distance (6MWD) between baseline, 6 weeks and 12 weeks after start of rehabilitation. (C) Improvement in % predicted of hand grip force (HGF) between baseline, 6 weeks and 12 weeks after start of rehabilitation. (D) Percentage of patients who reported cognitive deficits at baseline and 12 weeks after start of rehabilitation.
Although these significant improvements on functional outcomes highlight the potential of a multidisciplinary respiratory rehabilitation programme, a number of COVID-19-specific challenges were encountered. First, many patients struggled with anxiety (26%), depressed mood (21%) and cognitive dysfunction (68%) (table 1D). Given the extraordinary circumstances during admission (ie, isolation from family, caregivers in personal protection equipment, uncertainty about prognosis) and possibly because of direct and indirect viral effects on the central nervous system, we anticipated a higher prevalence of post-intensive care syndrome (PICS) in COVID-19 survivors compared with other post-critical care patients.4 In 27% of patients, referral was made for psychological or cognitive therapy. Exercise training, especially with companions, has a positive influence on mental health and cognitive symptoms.5 Nevertheless, at 3 months, anxiety assessed by Hospital Anxiety and Depression Scale was present in even more patients than at baseline, possibly influenced by ongoing pandemic and the impending second wave. The fact that 40% of patients who were working before COVID-19 had not resumed work after 3 months, despite the large physical improvement, may be attributed to these mental and cognitive consequences. Family members should also be actively involved in the rehabilitation process. Fourteen per cent of family members needed psychological support to cope with the trauma of a critical ill family member. As the cognitive deficits and mental health issues were also common in the non-ICU patients with COVID-19, other factors than disease severity are likely affecting the burden of symptoms (figure 1D). Second, we encountered more musculoskeletal issues compared with our experiences in the chronic lung disease group. Shoulder complaints due to immobilisation and positioning are a known, but not fully understood problem, post-ICU.6 Frozen shoulders and rotator cuff complaints occurred in six patients and clinically diagnosed foot drop was present in two patients. As these problems require specific medical attention, advice may need to be sought. Twenty-seven per cent of our patients were referred for specific individual physiotherapy. Furthermore, we experienced that these patients needed medical follow-up. Four patients (18%) were diagnosed with diabetes during COVID-19 hospitalisation, of which three could stop antidiabetic treatment during the rehabilitation programme. Although acute kidney injury was present in five patients (23%) during hospitalisation, only one patient had residual renal impairment at the start of rehabilitation. We are aware of cardiac and hepatobiliary complications after COVID-19, but we did not encounter these in our group.
Our first impressions concur with an expert consensus-based statement of the European Respiratory Society and American Thoracic Society advocating for multidisciplinary screening and interventions in patients with COVID-19 after hospital discharge.7 Although results of previous, mostly homebased, studies of rehabilitation after ICU stay are disappointing,8 we do believe that a supervised multidisciplinary rehabilitation can make a difference in the recovery of patients with COVID-19 for whom ambulatory multidisciplinary programmes are scarce and underfinanced.9 Other authors could also show the merit of telehealth-based interventions in COVID-19 survivors.10 Further studies should explore the full potential of different programmes in the recovery of COVID-19 and non-COVID-19 ICU survivors.
Finally, developing a COVID-19 rehabilitation—alongside the existing and ongoing respiratory rehabilitation programmes—has been challenging for the team in terms of facilities, infrastructure, staffing and infection control measures. PICS and musculoskeletal problems posed additional challenge as they are out of the comfort zone of respiratory physicians, requiring expansion of the team. Nevertheless, both team members and patients do experience this multidisciplinary COVID-19 rehabilitation as very rewarding.
We recognise that the sample size is small and that our observations may not be generalised to the entire population with COVID-19. In particular, the benefits of rehabilitation for patients who suffered from mild COVID-19 still need to be identified. The absence of a control group makes comparison with natural recovery impossible. Nevertheless, these real-world data are worthwhile to show advantages and challenges of rehabilitation after moderate to severe COVID-19. In conclusion and in agreement with other reports, a multidisciplinary respiratory rehabilitation programme is feasible and taps into the needs of selected patients with COVID-19,11 12 who seem to rapidly improve on admission to the programme.
Acknowledgments
Thanks to the respiratory rehabilitation team of UZ Leuven for all the efforts they did for the COVID-19 programme: Kim Decaluwé, Bart Vrijsen, Heleen Demeyer, Veronica Barbier, Iris Coosemans, Lode Claes, Nele Vandenbergh, Astrid Blondeel, Sofie Breuls, Marieke Wuyts, Dirk Delva, Linda Stans, Rina Droogmans, Jasmien Van Den Bergh and Roos Odeyn.
Footnotes
Contributors: SE organised the rehabilitation programme, collected data, interpreted the results and drafted the manuscript. AH organised the rehabilitation programme and collected data. DL, HB, GH and NL contributed to interpretation and critically revised the manuscript. TT, RG and WJ supervised the rehabilitation programme, contributed to the interpretation of the results and critically revised the manuscript. All authors have read and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Collection of patient data was approved by the institution’s ethics committee (s64081).
References
- 1.Spruit MA, Singh SJ, Garvey C, et al. An official American thoracic Society/European respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13–64. 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 2.Burtin C, Saey D, Saglam M, et al. Effectiveness of exercise training in patients with COPD: the role of muscle fatigue. Eur Respir J 2012;40:338–44. 10.1183/09031936.00111811 [DOI] [PubMed] [Google Scholar]
- 3.Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care 2015;19:274. 10.1186/s13054-015-0993-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med 2012;40:502–9. 10.1097/CCM.0b013e318232da75 [DOI] [PubMed] [Google Scholar]
- 5.Hopkins RO, Suchyta MR, Farrer TJ, et al. Improving post-intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med 2012;186:1220–8. 10.1164/rccm.201206-1022CP [DOI] [PubMed] [Google Scholar]
- 6.Gustafson OD, Rowland MJ, Watkinson PJ, et al. Shoulder impairment following critical illness: a prospective cohort study. Crit Care Med 2018;46:1769–74. 10.1097/CCM.0000000000003347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spruit MA, Holland AE, Singh SJ, et al. COVID-19: interim guidance on rehabilitation in the hospital and Post-Hospital phase from a European respiratory Society and American thoracic Society-coordinated international Task force. Eur Respir J 2020. 10.1183/13993003.02197-2020. [Epub ahead of print: 13 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly B, Salisbury L, O'Neill B. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness. Cochrane Database Syst Rev 2015;2015:CD008632. 10.1002/14651858.CD008632.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polastri M, Nava S, Clini E, et al. COVID-19 and pulmonary rehabilitation: preparing for phase three. Eur Respir J 2020;55. 10.1183/13993003.01822-2020. [Epub ahead of print: 25 Jun 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hameed F, Palatulan E, Jaywant A, et al. Outcomes of a COVID-19 recovery program for patients hospitalized with SARS-CoV-2 infection in New York City: a prospective cohort study. Pm R 2021;13:609–17. 10.1002/pmrj.12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitacca M, Carone M, Clini EM, et al. Joint statement on the role of respiratory rehabilitation in the COVID-19 crisis: the Italian position paper. Respiration 2020;99:493–9. 10.1159/000508399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermann M, Pekacka-Egli A-M, Witassek F, et al. Feasibility and efficacy of cardiopulmonary rehabilitation after COVID-19. Am J Phys Med Rehabil 2020;99:865–9. 10.1097/PHM.0000000000001549 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.