Abstract
Granuloma annulare (GA) is an inflammatory granulomatous skin disease that can be localized (localized GA) or disseminated (generalized GA), with patch, perforating, and subcutaneous subtypes being less common variants of this benign condition. Recently, new research has emerged that further elucidates GA epidemiology and etiopathogenesis; importantly, new therapeutic options for GA have also been described, although there remains a paucity of randomized controlled studies. In this review, we summarize recent updates on GA epidemiology and etiopathogenesis and offer an updated review of the therapeutic options for GA currently reported in the literature. We hope that the current review galvanizes randomized controlled studies that will in turn help lead to the recommendation of evidence-based treatments for GA.
Key Points
| Granuloma annulare (GA) is a benign skin reaction with an incidence of 0.04% in the US, and has shown to be significantly associated with autoimmune disease, diabetes mellitus, and hyperlipidemia. Likely owing to its relative rarity, little is known about its etiopathogenesis. |
| Recent studies have implicated T-helper 1 (Th1) axis and Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway dysregulation in GA. Certain infectious triggers have also been reported in association with GA, although it is unlikely that they unilaterally induce GA, as GA remains a non-infectious dermatosis. Reports of iatrogenic GA have also been described, although the precise mechanism of these drug eruptions is yet to be elucidated. |
| Management of GA is challenging and is further complicated by the paucity of evidence-based therapies. Based on recent literature, we propose a therapeutic ladder for GA treatment, with topical and intralesional corticosteroids to be the first rung of this ladder. Dapsone, hydroxychloroquine, methotrexate, pentoxifylline, and sulphasalazine may be considered to be the second rung if treatment with topical and intralesional corticosteroids fails. If GA still remains recalcitrant to treatment, then phototherapy and targeted immunomodulators, such as apremilast and tofacitinib, or biologic therapies (adalimumab, dupilumab, etanercept, and infliximab) may be considered. |
Introduction
The first report of granuloma annulare (GA) is credited to Colcott-Fox, who in 1895 described a ‘ringed eruption of the fingers’ in an 11-year-old girl [1]. The term ‘granuloma annulare’ itself was introduced later in 1902 by Radcliffe-Crocker [2]. GA is a condition that remains poorly understood and past attempts to review GA have conceded that the etiopathogenesis eludes facile explanation and that diagnosis and treatment is challenging [3–7]. In 2018, Wang and Khachemoune reviewed literature published up to August 2017 to provide a focused review of therapeutic options for GA. They noted that while localized disease tends to respond well to topical and intralesional corticosteroids, generalized GA remains difficult to manage and refractory to corticosteroid treatment. Furthermore, while they reported phototherapy to be the most well-studied and reliable option for generalized disease, there is otherwise a paucity of evidence-based therapies to establish a paradigm of care for patients with generalized GA [8].
Just in the past few years, a number of observational studies have been published that shed further light on GA. Additional evidence regarding the mechanism underlying GA has emerged and compelling therapeutic strategies have also been described. Nevertheless, there remains a lack of randomized controlled studies that can establish the efficacy of any particular therapeutic option.
In this review, we briefly discuss new evidence that has emerged regarding GA epidemiology, etiology, and pathogenesis, and provide an updated review of the treatment options currently available for GA. We acknowledge the lack of randomized trials to be a concern and as such we critically appraise the current observational studies for bias and provide the level of evidence available for reviewed treatments. Moreover, as cases of effective treatment lend further support to proposed mechanisms of GA pathogenesis, we also attempt to recapitulate GA pathogenesis vis-à-vis treatment.
Methods
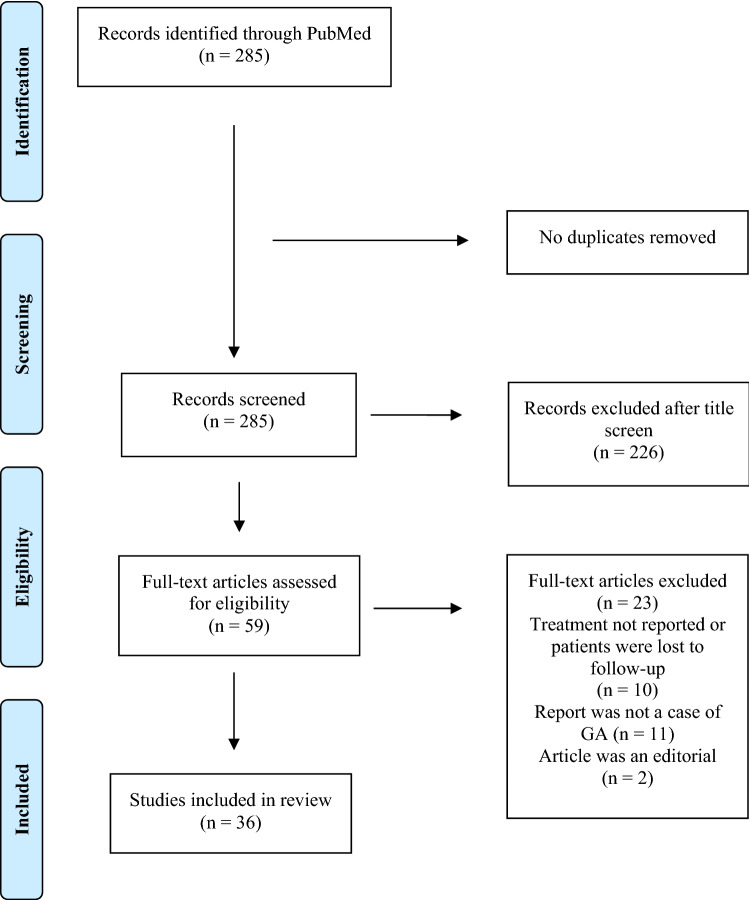
We performed a literature search using PubMed. As the last review of GA treatment by Wang and Khachemoune [8] had captured articles published until August 2017, we included studies published between September 2017 and 20 August 2021. We used the search term ‘granuloma annulare’ to obtain 285 results. Eligible studies were reports that described a response (or lack thereof) to a particular therapy. Case studies and case series were included. We excluded commentaries and editorials as well as studies not published in the English language. A total of 36 studies were included in our review. Our study selection process is illustrated in Fig. 1.
Fig. 1.
Flow diagram outlining the method of study selection. GA granuloma annulare
We assessed the risk of bias in the reviewed studies using the Tool for evaluating the methodological quality of case reports and case series proposed by Murad and colleagues, which evaluates case reports and cases series across four domains: selection, ascertainment, causality, and reporting (Table 1) [9].
Table 1.
Tool for evaluating the methodological quality of case reports and case series, as proposed by Murad and colleagues [9]
| Domains | Leading explanatory questions | Points |
|---|---|---|
| Selection | 1. Does the patient(s) represent(s) the whole experience of the investigator (center) or is the selection method unclear to the extent that other patients with similar presentation may not have been reported? | 1 |
| Ascertainment | 2. Was the exposure adequately ascertained? | 1 |
| 3. Was the outcome adequately ascertained? | 1 | |
| Causality | 4. Were other alternative causes that may explain the observation ruled out? | 1 |
| 5. Was there a challenge/rechallenge phenomenon? | 1 | |
| 6. Was there a dose–response effect? | 1 | |
| 7. Was follow-up long enough for outcomes to occur? | 1 | |
| Reporting | 8. Is the case(s) described with sufficient detail to allow other investigators to replicate the research or to allow practitioners to make inferences related to their own practice? | 1 |
Epidemiology
A 2021 study by Barbieri et al. of 11,608 patients with incident GA and 17,862 patients with prevalent GA helps to establish the epidemiology of GA in the US. The study investigators report the incidence of GA to be 0.04% and note both the incidence and prevalence of disease to be most frequent during the fifth decade of life. Moreover, GA has a predilection for women, with a female-to-male ratio of 3:1. Incidence and prevalence also appears to be more common among Caucasians and individuals with a higher household income and educational attainment. Nevertheless, it is unclear whether this difference is due to genetic and environmental factors or merely due to a lower likelihood of racial minorities and individuals with lower educational attainment seeking medical attention [10]. A recent study of 180 GA patients in the All of Us Research Program, a National Institutes of Health database that includes groups that have been historically underrepresented in research, also reports GA to be more prevalent among White patients compared with non-White patients, suggesting that a genetic predisposition may indeed be likely, although further studies are still needed to establish such an association [11].
The study by Barbieri et al. also provides data on commonly prescribed treatments. Topical and intralesional corticosteroids appear to be the de facto first-line treatments for GA, with 41.5% of GA patients filling a prescription for topical corticosteroids and 9.4% of GA patients receiving injections for intralesional corticosteroids within 6 months of being diagnosed. Tetracycline and hydroxychloroquine (HCQ) were also prescribed as first-line treatments at a frequency of 7.1% and 2.3%, respectively. Interestingly, phototherapy, despite being the most efficacious and evidence-based treatment currently available, was utilized by only 0.5% of patients, suggesting that perhaps logistical and financial constraints may preclude phototherapy from being a viable treatment option [10].
Clinical and Histological Presentation and Differential Diagnosis
Clinical Presentation
As the name suggests, GA is a non-infectious granulomatous skin reaction; ‘annulare’ refers to the morphology of the lesions, which, in localized forms of GA, appear as isolated, skin-colored to erythematous circinate papules and plaques. GA can also present in a generalized fashion, with generalized (i.e. disseminated) GA being defined as at least 10 widespread annular plaques [5].
Subcutaneous GA is another variant of GA and is common in the pediatric population. It typically presents as a firm, subcutaneous mass on the lower extremities [12]. Patch type GA is a less common variant and presents as violaceous to erythematous patches that typically involve the bilateral proximal extremities [13]. Perforating GA has also been described and presents as umbilicated papules or pustules with crusting; both localized [14] and generalized [15] forms of perforating GA have been reported. GA appearing on the acral surfaces is rare but has recently been reported on the palms [16, 17] and the soles of the feet [17].
Histological Presentation
GA presents histologically with a focus of necrobiosis surrounded by palisading histiocytes. Additionally, mucin deposition is an important hallmark of GA. Multinucleated giant cells are also a common finding and eosinophils, lymphocytes, and neutrophils infiltrating the dermis may also be observed [18]. Different subtypes of GA have been noted to have certain characteristic features, although the triad of degraded collagen, histiocytic infiltrate, and presence of mucin seem to be common findings across all subtypes of GA.
Differential Diagnosis
In most cases, the diagnosis of GA is straightforward. Albeit rarely, initial clinical impressions can be either ambiguous or misleading, making diagnosis difficult. Notably, GA can resemble other granulomatous skin conditions [19]. The presence of mucin is an important histologic feature that can clinch the diagnosis in the favor of GA, as histological examination of other granulomatous skin diseases such as sarcoidosis and necrobiosis lipoidica does not reveal mucin deposition [5]. Recently, a case of GA appearing on a scar and mimicking sarcoidosis was reported [20]. GA has also been reported to mimic dermatofibroma [21], dermatomyositis [22], papulonecrotic tuberculid [23], psoriasis [24, 25], and tinea cruris [26]. Conversely, borreliosis [27], Churg–Strauss Syndrome [28], Kaposi sarcoma [29], and tuberculoid leprosy [30] have been reported to masquerade as GA. Such case reports underscore the importance of establishing a clinicopathologic correlation before rendering a diagnosis.
Dermoscopic findings may also facilitate diagnosis. Unfocused vessels of variable appearance against a pinkish-reddish background are characteristic but by no means pathognomonic [31, 32]. Recently, ultrasound was reported to be of utility in the diagnosis of subcutaneous GA [33, 34]. As subcutaneous GA is more common among children (who may be more averse to biopsy), ultrasound may represent a novel and useful tool in the diagnosis of this rare GA variant, although further studies are needed to validate the efficacy of ultrasound.
Update on Etiopathogenesis and Disease Associations
Towards a Closer Understanding of Granuloma Annulare (GA) Pathogenesis
Elucidating the pathogenesis of GA has been challenging and our understanding has evolved over the years. Recently, two published studies have advanced our understanding of GA pathogenesis by further uncovering its molecular basis and cytokine signature. Min et al. reported the upregulation of both T-helper (Th) 1 and Th2 pathways in GA lesions compared with healthy skin (of note, previously only the Th1 pathway was implicated in GA pathogenesis). Increased messenger RNA (mRNA) expression of cytokines corresponding to both T-cell axes were also noted: tumor necrosis factor (TNF)-α, interleukin (IL)-1β, interferon (IFN)-γ, and IL-12/23p4 (corresponding to Th1 pathway activation) and IL-4 and IL-31 (corresponding to Th2 pathway activation). The upregulation of IL-4 mRNA expression was particularly pronounced, with a 15,600-fold increase in GA lesional skin versus control skin. Furthermore, Th17 and Th22 axes and the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway were also upregulated [35].
A 2021 study by Wang et al. also found activation of the Th1 and JAK-STAT pathways in GA. In particular, the investigators described the elevation of mRNA of key molecular mediators involved in the JAK-STAT pathway, such as IFN-γ, oncostatin M, and, to a lesser extent, IL-15 and IL-21. Interestingly, Wang et al. described upregulation of ‘M1’ macrophage polarization as well as ‘M2’ macrophage polarization [36]. While contradictory, such a finding may be reconciled by ascribing a biphasic mechanism to GA pathogenesis. First, collagen degradation, mediated by a ‘M1’ macrophage response, is followed by tissue remodeling and mucin deposition mediated by a ‘M2’ macrophage response.
Neither the study by Min et al. nor Wang et al. identified targeting of CD8+ T cells to be of therapeutic potential. While Wang et al. did report an increased abundance of CD8+ T cells [36], the expansion of CD4+ T cells was more pronounced, a finding consistent with previous studies by Modlin et al. [37, 38].
An important inconsistency between the work by Min et al. and Wang et al. is that Wang et al. did not find upregulation of the Th2 pathway that was reported by Min et al. However, plasma levels of IL-4 were elevated in two of the five patients studied by Wang et al. While larger studies evaluating the molecular markers of GA are needed to clarify this discrepancy, the current studies by Min et al. and Wang et al. pave the way for targeted therapies blocking the Th1 and JAK-STAT pathways.
New Evidence on GA Disease Associations
GA and Chronic Comorbidities
GA has been reported to be associated with a host of comorbidities. The link between diabetes mellitus (DM) and GA has been the most discussed and most debated, with smaller cohort studies reporting conflicting findings. A 2021 retrospective cohort study of 51,169 patients with GA is the largest and most rigorous study to date that investigates the link of GA with DM. The study investigators report that 21.1% of patients with GA had DM, while only 13.3% of matched controls had DM. The same study also found GA to be significantly associated with hyperlipidemia, rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE). The study suggests that DM and hyperlipidemia may instigate GA through a common pathway of T-cell dysregulation. Additionally, the association between GA and autoimmune disorders (RA and SLE) lends support to the idea that autoimmunity may be a driver of GA [39].
A recent nested case-control study of 177 GA patients (matched to 708 controls) in the All of Us database also found that GA was significantly associated with autoimmune disease, DM, and hyperlipidemia. The study also found hypothyroidism and ischemic heart disease to be significantly associated with GA [40].
GA and Malignancy
Notably, however, the study of 51,169 GA patients did not find a significant link between hematologic malignant neoplasms and GA [39]. Another study of 5137 GA patients failed to establish a link between GA and solid organ cancers [41]. A 2019 case-control study from Spain of 60 patients matched to 300 controls also did not find an association between GA and malignancy [42].
Nonetheless, the above studies are subject to a number of limitations. Both the 51,169 patient [39] and 5137 patient [41] studies include patients with all subtypes of GA; additionally, the studies include patients of all ages. It is possible then that an association between malignancy and generalized GA in older patients is hidden by cases of localized GA that may not be associated with malignancy. Additionally, the studies do not exclude drug-induced cases of GA, further occulting a possible association between frank GA and malignancy.
The 2019 Spanish study [42], although limited to cases of generalized GA, neither stratifies patients by age nor excludes cases of iatrogenic GA. Considering that malignancies are less frequent occurrences to begin with (compared with DM and hyperlipidemia), the lack of a stringent inclusion and exclusion criteria in studies investigating an association between GA and malignancy leaves this putative link to be still subject to dispute. A study limited to older (i.e. >60 years) patients with frank generalized GA may provide more rigorous results to settle this debate.
GA and Infectious Triggers
Unfortunately, there is a paucity of large-scale studies that investigate the role of infectious agents in triggering GA, although previously both viral and bacterial triggers have been suggested. A 2018 study of 73 biopsies of skin lesions from GA patients discovered Borrelia DNA to be present in 9.6% of biopsies and Chlamydiales DNA to be present in 72.6% of biopsies. However, eight of the patients positive for Chlamydiales DNA were treated with doxycycline for 9 months, but none responded, and only 28.6% of patients positive for Borrelia DNA responded to appropriate antibiotic therapy. Thus, the study authors assert that Borrelia and Chlamydiales do not unilaterally induce GA; rather, they suggest that Borrelia and Chlamydiales are triggers, which, together with other factors, set-up GA pathogenesis [43].
GA in the setting of viral infections has also been noted, with Epstein–Barr virus [44], human immunodeficiency virus [21], and varicella zoster virus (VZV) [45, 46] being recently reported. Two cases of GA triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have also been described [47, 48].
Iatrogenic GA
In January 2021, Shah et al. reviewed granulomatous cutaneous drug eruptions and identified a number of iatrogenic causes of GA: allopurinol, amlodipine, anti-TNFα agents (infliximab, adalimumab, etanercept, and thalidomide), botulinum toxin, dabrafenib, desensitization injections, immune checkpoint inhibitors, intranasal calcitonin, gold, immunizations (hepatitis B and anti-tetanus vaccination), levetiracetam, paroxetine, pegylated IFNα, secukinumab, tocilizumab, topiramate, and vemurafenib [49]. More recently, acetazolamide [50], apremilast [51], ixekizumab [52], mesotherapy [53], phototherapy [54], and measles, mumps, and rubella (MMR) [55], pneumococcal [56], and VZV [57] immunizations have also been implicated in instigating GA. The pathogenesis of drug-induced GA is not easily understood, and the induction of GA by anti-TNFα agents and apremilast in particular is paradoxical, as both TNFα inhibitors and apremilast have also been reported as effective therapies for GA. Instigation of GA by IL-17 inhibitors (ixekizumab and secukinumab) is also intriguing, as Min et al. reported hyperactivation of the Th17 axis in GA. We attempt to explain these paradoxical observations in Sect. 8 of the manuscript. We summarize the recent updates on GA epidemiology and etiopathogenesis in Table 2.
Table 2.
Summary of recent updates on GA epidemiology and etiopathogenesis
| Common comorbidities |
Diabetes; hyperlipidemia [39, 40]; hypothyroidism; ischemic heart disease [40] Association of GA and solid organ [41] and hematological malignancy [39] remains controversial |
| Iatrogenic causes |
For a comprehensive list, refer to the systematic review of granulomatous drug eruptions conducted by Shah et al. in January 2021 [49] More recently, acetazolamide [50], apremilast [51], ixekizumab [52], mesotherapy [53], phototherapy [54], and MMR [55], pneumococcal [56], and VZV [57] immunization have also been reported |
| Infectious triggers |
Bacterial: Borrelia and Chlamydiales [43] Viral: EBV [44], HIV [21], SARS-CoV-2 [47, 48], and VZV [45, 46] |
| Overregulated pathways | Th1 and JAK-STAT [35, 36]. Th2, Th17, and Th22 axes have also been implicated [35] |
EBV Epstein–Barr virus, GA granuloma annulare, HIV human immunodeficiency virus, JAK-STAT Janus kinase-signal transducer and activator of transcription, MMR measles, mumps, rubella, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, Th T-helper, VZV varicella zoster virus
Review of Management Options
Topical and Intralesional Corticosteroids
Topical and intralesional corticosteroids are considered first-line therapies for GA and can induce partial or complete GA regression in some patients, although several cases of GA remain recalcitrant to corticosteroid treatment [8, 10]. Recent single-center retrospective studies of GA treatments further seem to suggest that corticosteroids may have efficacy in the management of GA in some but not all patients. In a retrospective of study of 133 GA patients, 17 of the 55 patients treated with topical triamcinolone and 10 of the 25 patients treated with intralesional triamcinolone experienced improvement [58]. Another retrospective study of 49 generalized GA patients showed similar response rates, with topical corticosteroid treatment leading to stable disease in 46.6% of patients, partial remission in 19% of patients, complete remission in 10.3% of patients, and disease progression in 8.6% of patients. In the same study, of five other patients treated with intralesional triamcinolone, two achieved partial remission and three achieved total remission [59]. A 2021 retrospective study investigated the efficacy of topical corticosteroids alone, intralesional triamcinolone alone, and combination topical and intralesional corticosteroid therapy. All three treatment groups had similar response rates, with partial resolution being seen in 21 (41%) patients treated with topical corticosteroids alone, 7 (50%) patients treated with intralesional triamcinolone alone, and 14 (48%) patients treated with a combination of topical and intralesional corticosteroids. Complete resolution was achieved in 12 (24%) patients treated with topical corticosteroids alone, 2 (14%) patients treated with intralesional triamcinolone alone, and 8 (28%) patients treated with combination topical and intralesional corticosteroids [60]. A recent case series of patients with patch type GA showed a slightly lower response rate to corticosteroids, with 5 of the 20 patients treated with topical corticosteroids showing improvement [13].
Altogether, considering their modest efficacy, relative safety, and low cost, corticosteroids remain viable first-line therapies. However, GA refractory to corticosteroids is common, such that it may become necessary to resort to second-line therapies.
Antimicrobials Revisited
Isolated case reports have shown antimicrobials to be effective in the management of GA, although larger retrospective studies conducted more recently fail to establish their efficacy. One case of generalized GA occurring in the setting of interstitial lung disease was observed to resolve almost completely in response to a 2-month course of doxycycline 100 mg twice daily [61]. A twice-daily regimen of amoxicillin/clavulanic acid 875/125 mg was shown to result in durable control of GA in another patient [62]. However, in a retrospective study of 133 GA patients, of whom eight were treated with minocycline, seven with doxycycline, two with ofloxacin, and two with rifampin, only two of the seven patients treated with doxycycline showed improvement [58]. In another retrospective study of 127 GA patients, only two of the five patients treated with a daily cocktail of rifampin (600 mg), minocycline (100 mg), and ofloxacin (400 mg) responded: one experienced complete resolution and the other experienced partial resolution [60]. As already mentioned, eight GA patients with confirmed positive biopsies for Chlamydiales DNA did not respond to doxycycline, and of the seven patients with confirmed positive biopsies for Borrelia DNA, only two responded to a 9-month course of antibiotic therapy [43].
Larger retrospective studies do suggest some efficacy for dapsone. In a recent retrospective review of 26 generalized GA patients treated with a median daily dose of dapsone 100 mg for a mean duration of 9.8 months, 54% experienced regression of lesions. However, the remissions were not durable in 57% of responders, who experienced lesion relapse. In addition, subclinical myelosuppression was observed in 31% of patients, warranting discontinuation of treatment [63]. Two other case series reported similar response rates to dapsone: three of seven patients treated with dapsone experienced partial remission in the study by Visconti et al. [60], and three of five patients treated with dapsone showed improvement in the study by Rubin and Rosenbach [58]. Given the concern for myelosuppression, dapsone should be used cautiously, especially in immunocompromised at-risk individuals.
Antimalarials Revisited
A 2019 review pooling together the reported cases of antimalarial treatment for GA showed that 25 of 35 patients treated with HCQ, and all of the 12 patients treated with chloroquine, experienced improvement, with an overall response rate of 79.6% [64]. Since the publication of this review, additional reports have emerged that are more mixed. Megna et al. reported complete clearance of disseminated GA in one patient with an 8-week course of HCQ 200 mg twice daily [65], and Xu et al. reported a pediatric patient who experienced complete remission of disseminated GA after 6 months of treatment with HCQ 25 mg once daily [66]. In a retrospective review by Nordmann et al., of the five patients who were treated with HCQ, one patient achieved complete clearance, two achieved partial clearance, and one showed stable disease [59]. However, in the retrospective review conducted by Rubin and Rosenbach, only five of the 12 patients who were treated with HCQ showed improvement [58]. A recent retrospective review of 26 GA patients treated with a median daily dosage of HCQ 400 mg for a mean duration of 10 months showed an even poorer response rate, with only 35% of patients improving with treatment [67]. The poor response reported to HCQ in more recent studies is unfortunate, as HCQ is a cheap and generally well-tolerated drug.
Apremilast
Apremilast is a phosphodiesterase-4 inhibitor that has been licensed for the treatment of psoriasis, psoriatic arthritis, and oral ulcers associated with Behcet’s disease [68]. Several recent case reports recommend further investigation of apremilast in the management of GA. Blum and Altman reported the first two cases of GA treated with apremilast: one patient experienced durable improvement in erythema and induration of lesions in 3 months and the other patient had near complete remission of lesions at 4 months [69]. Bishnoi et al. then reported a case series of four GA patients treated with apremilast, three of whom experienced a reduction in lesion number and one patient who experienced decreased erythema and pruritus; in all four patients, response to apremilast was seen within 6–8 weeks of initiating treatment [70]. Joshi and Tschen reported an additional case where apremilast led to near total resolution of lesions in seven months [71]. Most recently, Hansel et al. reported two patients who also experienced marked improvement of their GA within 8 weeks of treatment [72]. Notably, all patients treated with apremilast for their GA tolerated the medication well. Only two patients in the case series by Bishnoi et al. reported myalgia and mild gastrointestinal symptoms, which were managed with treatment [70].
While the current reported cases are compelling, a paradoxical case of GA induction after starting apremilast therapy for psoriasis has also been reported [51]. Clearly, further randomized studies are needed to evaluate the safety and efficacy of apremilast in the management of GA.
Biologic Therapies Revisited
In 2019, Chen et al. reviewed the role of biologic therapies in the treatment of chronic GA. Based on their pooled analysis, TNFα inhibitors in particular show some promise: 14 of 16 patients treated with adalimumab, one of five patients treated with etanercept, and all three patients treated with infliximab experienced improvement. One patient treated with combination adalimumab and infliximab also experienced improvement [73]. More recently, three separate cases of GA responsive to adalimumab [51], etanercept [74], and infliximab [75] have been added to the literature. The most recent report on infliximab is particularly interesting. The authors also investigated the molecular milieu of the GA lesion microenvironment. They observed expansion of CD183+ cells in GA that was blunted by infliximab treatment [75]. Thus, downregulation of CD183+ cells could explain the role of infliximab in GA. However, this is likely only one piece of the puzzle, as infliximab, along with adalimumab and etanercept, have also been reported to paradoxically induce GA [49]. Knowledge of the association between anti-TNFα agents and GA, then, still remains incomplete and further molecular studies are needed to elucidate this relationship. Altogether, TNFα inhibitors may show efficacy in some GA patients, although patients should be monitored for paradoxical GA exacerbation.
Recently, Song reported that dupilumab, an IL-4 and IL-13 inhibitor, could successfully control GA [76], and also reported that tildrakizumab, an IL-23 inhibitor, was actually ineffective in the management of GA in another patient [77].
Methotrexate
Two retrospective studies suggest methotrexate to be a promising second-line therapy for GA. In a study of 15 patients treated with a median weekly dose of methotrexate 10 mg for a mean duration of 11 months, 60% of patients responded to treatment [78]. Another study of 11 patients reported a similar response rate: 64% of patients witnessed improvement, of whom 43% achieved complete clearance and 57% achieved partial clearance. While the majority of patients tolerated methotrexate well, two patients experienced gastrointestinal adverse effects and hair loss that warranted treatment cessation [79]. Additionally, one patient in the retrospective review by Rubin and Rosenbach was treated with methotrexate, and responded to treatment [58]. Given its immunomodulatory function and low cost, methotrexate may be considered a second-line therapy for GA, although continuous monitoring for adverse effects (e.g. myelosuppression) is crucial.
Pentoxifylline Revisited
Pentoxifylline is a methylxanthine derivative that has anti-inflammatory properties, although its mechanism of immunomodulation is not fully understood [80]. Recent studies suggest that pentoxifylline may be a viable therapy for the management of GA in some patients. In a three-patient case series, all patients treated with pentoxifylline witnessed complete clearance of their GA lesions; however, two patients experienced GA flare-ups: one was managed by transiently increasing pentoxifylline dosage and the other was managed with intralesional corticosteroids [81]. In the 127-patient retrospective study conducted by Visconti et al., of the 27 patients treated with pentoxifylline 400 mg thrice daily, 56% experienced partial remissions and 15% experienced complete resolution [60]. Indeed, while the response rate to pentoxifylline was high, not all patients experienced improvement and it is likely that pentoxifylline may not be efficacious in all patients. Both patients treated with pentoxifylline in the retrospective review by Rubin and Rosenbach experienced lesion persistence [58]. Nevertheless, pentoxifylline, like HCQ, is a cheap and relatively well tolerated small molecule that may merit further study in randomized controlled trials.
Phototherapy Revisited
In their 2018 review, Wang and Khachemoune identified phototherapy to be the most well-studied and efficacious management option for GA [8]. Recent studies lend further support to the efficacy of phototherapy, yet not all patients treated with phototherapy respond robustly and some fail to respond altogether.
Of all the phototherapy modalities, photodynamic (PD) therapy seems to be supported by the most evidence [8]. A 2020 retrospective review of 13 GA patients treated with a mean of three PD therapy sessions adds to this evidence base. In all patients, methyl aminolevulinate or aminolevulinic acid was applied for 3 h, illuminated with LED 635 nm with a fluence of 37 J/cm2. Seven patients experienced complete regression of their GA and four patients experienced partial improvement. PD treatment was well tolerated in all patients, with only two experiencing slight hyperpigmentation [82].
Ultraviolet A1 (UVA1) also appears to be an effective phototherapy modality. In a retrospective review of nine patch GA patients, Aichelburg et al. reported four patients to be treated with UVA1 phototherapy, of whom two experienced complete remission while the other two experienced partial remissions [83]. In another case series of five GA patients treated with UVA1, two experienced complete clearance, two experienced partial clearance, and one patient did not respond to treatment [84]. In the retrospective study by Nordmann et al., of the 20 patients receiving UVA1 therapy, three experienced full remission, six achieved partial remission, seven had stable disease, and one experienced disease progression [59].
Narrowband UVB (NB-UVB) also appears promising. In the retrospective review by Aichelburg et al., of the two individuals treated with NB-UVB, one achieved complete regression of GA while the other achieved partial regression [83]. Another case of patch GA showing excellent response to NB-UVB has been reported [13]. Furthermore, two recent isolated case reports demonstrate the efficacy of NB-UVB in the management of generalized GA [85, 86]. However, in the study by Visconti et al., a low response rate to NB-UVB therapy was observed, with only one of the four patients treated experiencing partial resolution [60]. A similarly low response rate was observed by Nordmann et al.: of the eight patients treated with NB-UVB, none achieved full remission, three showed partial clearance, four had stable disease, and one patient had disease progression [59].
Aichelburg et al. reported oral psoralen plus UVA (PUVA) to be effective in all three patients who were treated [83]. Moreover, of the 11 patients treated with PUVA in the study by Nordmann et al., three patients experienced complete remission, four experienced partial remission, three had stable disease, and one experienced disease progression [59].
In the study by Rubin and Rosenbach, four of the eight patients treated with phototherapy responded. Unfortunately, the investigators did not specify the phototherapy subtype used [58].
Altogether, phototherapy continues to appear as an effective option for some patients. Considering its relative safety profile and moderate efficacy, phototherapy is a reasonable second-line treatment for GA. However, it may be worth noting that phototherapy is expensive and may not be an affordable option for all patients. Additionally, phototherapy use has declined in recent years in favor of targeted therapies [87, 88]. Thus, despite its evidence base, phototherapy may not be as practical as oral therapies.
Tofacitinib
Tofacitinib is a JAK inhibitor that is quickly gaining popularity in dermatology for a wide range of applications, and has shown some off-label efficacy in inflammatory conditions such as alopecia areata, atopic dermatitis, psoriasis, and vitiligo [89]. In a case series of five GA patients treated with oral tofacitinib, three patients experienced complete involution of lesions while two others experienced marked improvement [36]. An additional case of GA responding to oral tofacitinib was recently reported by Damsky et al. [90]. The report by Damsky et al. is particularly interesting because the investigators also characterize the molecular changes induced by tofacitinib treatment. Of note, they report a reduction in not only the cytokines involved in the JAK-STAT pathway but also a reduction in non-JAK-STAT pathway-dependent cytokines, such as TNFα. The investigators therefore propose that JAK-STAT pathway dysregulation may be the proximal pathogenic event that incites the immune dysregulation that is observed in GA [90].
Two patients with near total resolution of their GA in response to topical tofacitinib have also been described, suggesting that the topical formulation of the drug may also be efficacious [91, 92]. It is worth noting though that JAK-STAT inhibitors are expensive and may not be covered by insurance for off-label applications. Thus, JAK-STAT inhibitors may need to be reserved as treatments of last resort.
Other Therapies
In addition to the therapies discussed above, a diverse assortment of other therapies for GA have been reported, highlighting the lack of an established paradigm of care for GA. Sulphasalazine has shown a modicum of efficacy in the management of GA. A retrospective study of 13 GA patients treated with sulphasalazine found that 11 patients responded to treatment. Sulphasalazine was generally well tolerated, although three patients discontinued therapy due to adverse effects (elevated liver function tests, gastrointestinal distress, neutropenia, and xerostomia) [93].
Potassium iodide (KI) is another treatment that has been suggested for GA, although recent studies yield disappointing results. An 11-patient retrospective study found that KI led to improvement in only four patients [94]. Furthermore, in the retrospective study by Nordmann et al., of the 12 patients treated with KI, none experienced complete resolution, three experienced partial resolution, two had stable disease, and two had GA progression [59].
The larger retrospective studies previously cited also report small numbers of individual patients treated with a myriad of other therapies. In the 133-patient retrospective study by Rubin and Rosenbach, the following additional therapies were reported: local radiation (with only one patient being treated, who did show improvement), niacinamide (with neither of the two patients treated showing improvement), oral isotretinoin (with one of the two patients treated showing improvement), oral corticosteroid (with three of the six patients treated showing improvement), tacrolimus cream (with one of the five patients treated showing improvement), and zileuton (with none of the three patients treated showing improvement) [58]. In the 127-patient retrospective study by Visconti et al., three patients were treated with isotretinoin 20 mg twice daily, of whom only one showed partial improvement; one patient was also treated with cyclosporine 100 mg daily but did not show improvement [60].
In 2018, a report of radial pulse therapy (a form of extracorporeal shock wave therapy) was found to improve GA in one patient [95]. While novel, radial pulse therapy is impractical for disseminated GA and no further reports have emerged that validate its efficacy in localized GA.
Control of common comorbidities (diabetes and hyperlipidemia) may also be an indirect therapy for GA. Indeed, both diabetes and hyperlipidemia have been associated with T-cell dysregulation [96, 97] and control of these comorbidities may dampen overactive T-cell reactions that are central to GA pathogenesis. In 2020, Patrun and Hadžavdić reported a diabetic patient with disseminated GA who experienced complete resolution of lesions after starting insulin treatment [98]. Another GA patient with hyperglycemia and hyperlipidemia was reported to experience partial resolution of GA after 2 months of treatment with atorvastatin, fenofibrate, glimepiride, and metformin [99]. These case studies suggest that management of comorbid diabetes and hyperlipidemia may, in and of itself, be a therapeutic strategy to address GA. We summarize the recently reported treatments for GA in Table 3 and indicate the level of evidence for each therapeutic strategy.
Table 3.
Review of recently reported treatments for GA, and their corresponding level of evidence
| Treatment | Level of evidence | References |
|---|---|---|
| Antimicrobials | ||
| Amoxicillin/clavulanic acid | D | [62] |
| Dapsone | C | [58, 60, 63] |
| Doxycycline | D | [43, 58] |
| Minocycline + ofloxacin + rifampin | D | [58] |
| Apremilast | D | [69–72] |
| Biologic agents | ||
| Adalimumab | D | [51] |
| Etanercept | D | [74] |
| Infliximab | D | [75] |
| Dupilumab | D | [76] |
| Control of comorbidities | ||
| Diabetes control | D | [98] |
| Hyperlipidemia control | D | [99] |
| Corticosteroids | ||
| Intralesional corticosteroids | C | [58–60] |
| Intralesional + topical corticosteroids | C | [60] |
| Oral corticosteroid | D | [58] |
| Topical corticosteroids | C | [58–60] |
| Hydroxychloroquine | C | [58, 59, 65–67] |
| Local radiation | D | [58] |
| Methotrexate | C | [58, 78, 79] |
| Oral isotretinoin | D | [58] |
| Pentoxifylline | C | [60] |
| Phototherapy | ||
| NB-UVB | C | [13, 59, 60, 83, 85, 86] |
| Photodynamic therapy | B | [82] |
| PUVA | C | [59, 83] |
| UVA1 | C | [59, 83, 84] |
| Potassium iodide | D | [59] |
| Radial Pulse therapy | D | [95] |
| Sulphasalazine | C | [93] |
| Tacrolimus | D | [58] |
| Tofacitinib | D | [36, 90–92] |
B lesser quality randomized controlled trial or prospective study, C case-control study or retrospective study, D case series or case reports, GA granuloma annulare, NB-UVB narrow-band ultraviolet B, PUVA psoralen plus ultraviolet A, UVA1 ultraviolet A1
Does Treatment Recapitulate Pathogenesis?
Recent studies of successful and unsuccessful treatments for GA, although limited by size and their observational nature, nevertheless allow us to an opportunity to contemplate GA pathogenesis vis-à-vis treatment. The moderate efficacy of corticosteroids [58, 59, 81] and other anti-inflammatory agents, such as dapsone [63], methotrexate [79], pentoxifylline [81], and sulphasalazine [93], is consistent with the long-established inflammatory nature of GA. However, not all cases of GA are responsive to global anti-inflammatory therapy, suggesting that the inflammation seen in GA exists along a spectrum: milder and moderate GA can be controlled by topical and intralesional corticosteroids, yet severe cases remain recalcitrant to non-targeted immunosuppression.
The relative inefficacy of antimicrobials supports the putative non-infectious etiology of GA [43, 58]. The response to antimicrobials that is seen in some GA patients is most likely due to the anti-inflammatory rather than bactericidal activity of antimicrobials. The moderate response seen to dapsone [63] and doxycycline [43, 58] supports this hypothesis, as both dapsone and doxycycline also have strong anti-inflammatory action.
The excellent response of GA to anti-TNFα therapy seen in several patients [51, 73–75] supports recent studies that implicate overactivation of the Th1 pathway in GA [35, 36]. Recent cases demonstrating response of GA to apremilast [69–72] further indite Th1 axis dysregulation in GA, as apremilast also downregulates TNFα expression. The response of GA to dupilumab, as reported by Song et al. [76] suggests that the Th2 pathway may also be hyperactive in GA, supporting the study by Min et al., which found a marked increase in IL-4 mRNA expression in GA lesional skin compared with non-lesional skin [35]. However, as the study by Wang et al. did not show elevated IL-4 mRNA expression, such an association is tenuous.
The regression of GA in response to oral [36, 90] and topical [91, 92] tofacitinib is consistent with the studies by Min et al. [35] and Wang et al. [36], both of which identify JAK-STAT pathway dysregulation in GA. Furthermore, as previously discussed, JAK-STAT pathway upregulation may be the initial event driving GA pathogenesis [90]; thus, JAK inhibitors may represent targeted upstream therapies for GA.
A recent case of GA unresponsive to tildrakizumab [77], an IL-23 inhibitor, is somewhat inconsistent with the study by Min et al., which demonstrated Th17 axis upregulation in GA [35]. Nonetheless, the inefficacy of Th17 blockade may not be entirely incongruent with the study by Min et al. Indeed, while Min et al. observed Th17 pathway upregulation, they also reported Th1 and Th2 pathway hyperactivation [35]. We speculate that perhaps the Th1 and Th2 pathways are the major axes that incite GA, whereas the Th17 pathway, although upregulated, is not the major culprit in GA pathogenesis. Thus, perhaps a Th17 blockade alone is insufficient to induce GA regression. Perhaps, a randomized study involving biologics targeting the Th1, Th2, and Th17 axes may not only help establish an evidence-based biologic therapy for GA but may also help in the ascertainment of GA pathogenesis
GA induced by IL-17 and TNFα inhibitors [49] is more difficult to explain and defies our current understanding of GA pathogenesis; however, we can attempt an explanation by considering the relationship between the Th1 and Th17 arms of the immune system. Studies of autoimmunity in mice have shown that ablating either axis causes an upregulation of the other [100], leading us to speculate that in some patients, Th1 blockade causes pronounced upregulation of the Th17 axis, and vice versa.
Perhaps upregulation of the other axis is overcompensating, inducing GA. Nevertheless, this explanation is inadequate to explain the report of apremilast-induced GA [51], as apremilast suppresses both IL-17 and TNFα production. Clearly, GA pathogenesis is not amenable to simple explanation and further studies are needed to better understand the immunology underpinning GA.
Risk-of-Bias Assessment
Among the 36 studies we reviewed using the Tool for evaluating the methodological quality of case reports and case series [9], five studies were graded a score of 3, six studies a score of 4, 21 studies a score of 5, and four studies a score of 6, out of a best possible score of 8 points.
Review Limitations
We acknowledge our review to be subject to important limitations. Notably, the quality of studies we reviewed limits the strength of our findings: of the 36 studies reviewed, none had a score of 7 or 8, and 89% had scores ≤ 5/8. More specifically, only one of the studies reporting response to GA treatment adequately ruled out the possibility of spontaneous GA remission. Indeed, spontaneous resolution of GA has been reported in almost 2% of GA patients [58]; thus, it is possible that some of the reports we reviewed represent instances of spontaneous GA remission rather than bona fide responses to treatment. Moreover, only two studies reported a dose–response effect to treatment and only one described a challenge/rechallenge phenomenon. Altogether then, the reviewed studies were weakest in the ‘causality’ domain defined by Murad and colleagues [9], suggesting that the presented therapeutic options are supported largely by correlation but not causation. Additionally, while the majority (81%) of the studies we reviewed employed a satisfactory inclusion criterion (i.e. biopsy-proven GA), some studies did not specify the precise inclusion criteria while others included GA patients diagnosed only by clinical examination. As previously discussed, histopathological analysis is crucial for establishing a definitive GA diagnosis, since GA can mimic (and be mimicked by) a host of other conditions. Thus, some of the cases included in our review may not be GA but rather other dermatologic entities masquerading as GA.
Our review is also limited by our methodology of study selection. As we only included published studies, publication bias represents a significant limitation of our review. As a consequence, ostensibly efficacious treatments may merely be a reflection of the overrepresentation of positive outcomes in the literature rather than their true therapeutic potential. While the larger retrospective studies we include in this review partially offset this bias, we also include a number of case reports and case series of 10 or fewer patients that limit our conclusions. Selection bias is an additional limitation of our review, as we excluded studies not published in English.
Furthermore, while we strived to report dosage and treatment duration whenever possible, some studies did not report this information, rendering comparison of studies difficult. Finally, the lack of a ubiquitously utilized scale to measure GA severity resulted in heterogeneity in the manner in which response rates to treatment were reported (e.g. differing definitions of ‘partial’ and ‘complete’ resolution across studies), precluding a direct side-by-side comparison of each treatment option. Although a GA Investigator Global Assessment (IGA) was designed by Min and Lebwohl in 2016 [101], none of the studies we reviewed employed this scale in evaluating the response of GA to treatment.
Conclusions
In the past couple of years, new literature has been published on the topic of GA and we have gained a better understanding of GA epidemiology; recent studies have also helped further elucidate its pathogenesis by identifying Th1, JAK-STAT, and perhaps also Th2 pathway dysregulation in GA lesional skin. The long-debated association between GA and DM now seems to be established, while the link between GA and malignancy remains controversial. New bacterial and viral triggers have also been reported, although it remains unlikely that they unilaterally induce GA. A number of reports of iatrogenic GA have also been recently added to the literature, although the pathogenesis of these drug eruptions eludes facile explanation, as there is some overlap between the drugs that induce GA and the drugs that treat it.
In regard to therapy, several case reports, case series, and observational studies have emerged that present compelling response to certain treatments, although the significance of their findings remains limited. Large retrospective studies seem to support the efficacy of topical and intralesional corticosteroids in some but not all patients. For GA refractory to corticosteroids, other therapies may need to be utilized. Phototherapy remains the most well-studied option, but recent reports emphasize that not all patients undergoing phototherapy respond. Furthermore, financial and logistical considerations remain barriers to accessing this treatment modality. Dapsone, HCQ, methotrexate, pentoxifylline, and sulphasalazine are cheaper, orally available therapies that have been reported to be of some efficacy in larger retrospective studies and may also be considered viable second-line therapies for GA. Targeted immunomodulators, such as apremilast and tofacitinib, and biologic therapies (such as TNFα inhibitors and dupilumab) may also be indicated off-label in GA, although the evidence base for these therapies is limited to isolated case reports and small case series. Management of common comorbidities (e.g. diabetes and hyperlipidemia) may also be adjunctive.
Considering the findings of our review, we propose a therapeutic ladder for the management of GA, with the first rung being topical and intralesional corticosteroids. If patients do not respond to corticosteroids, therapy can be escalated to the second rung, i.e. dapsone, HCQ, methotrexate, pentoxifylline, and sulphasalazine. If GA still remains recalcitrant to treatment, then phototherapy and targeted immunomodulators, such as apremilast and tofacitinib, or biologic therapies (adalimumab, dupilumab, etanercept, and infliximab) may be considered. Finally, it is worth emphasizing that GA (commonly the localized variant but only rarely the disseminated variant) is a self-limited disease and can resolve of its own accord. Thus, in patients who do not desire treatment of lesions for cosmetic reasons, simple reassurance regarding the benign nature of GA may suffice.
Now more than a century has passed since Dr. Colcott-Fox described a ‘ringed eruption of the fingers’ [1], yet the precise etiopathogenesis and treatment of GA remains defiantly elusive. Current literature on GA treatment is limited to observational studies; there remains a paucity of randomized controlled trials, and a gold standard for GA treatment is yet to be identified. Further studies on some of the therapies we have reviewed may be warranted so that evidence-based therapies for GA can be established.
Declarations
Funding
Not applicable.
Conflict of Interest
Tejas P. Joshi and Madeleine Duvic declare no conflicts of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Availability of Data and Material
Articles used in this review are available in the public domain.
Code Availability
Not applicable.
Author Contributions
TPJ conceived the idea for this review, performed the literature search, and wrote the first draft of the manuscript. MD provided expert guidance and critically revised the work. Both authors read and approved the final submitted version.
References
- 1.Colcott-Fox T. Ringed eruptions of the fingers. Br J Dermatol. 1895;7:91–95. [Google Scholar]
- 2.Little EG. Granuloma Annulare. Br J Dermatol. 1908;20:317–335. [Google Scholar]
- 3.Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467–479. doi: 10.1016/j.jaad.2015.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457–465. doi: 10.1016/j.jaad.2015.03.054. [DOI] [PubMed] [Google Scholar]
- 5.Thornsberry LA, English JC., III Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279–290. doi: 10.1007/s40257-013-0029-5. [DOI] [PubMed] [Google Scholar]
- 6.Lukács J, Schliemann S, Elsner P. Treatment of generalized granuloma annulare—a systematic review. J Eur Acad Dermatol Venereol. 2015;29:1467–1480. doi: 10.1111/jdv.12976. [DOI] [PubMed] [Google Scholar]
- 7.Keimig EL. Granuloma Annulare. Dermatol Clin. 2015;33:315–329. doi: 10.1016/j.det.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Khachemoune A. Granuloma annulare: a focused review of therapeutic options. Am J Clin Dermatol. 2018;19:333–344. doi: 10.1007/s40257-017-0334-5. [DOI] [PubMed] [Google Scholar]
- 9.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbieri JS, Rodriguez O, Rosenbach M, Margolis D. Incidence and prevalence of granuloma annulare in the United States. JAMA Dermatol. 2021;157:824–830. doi: 10.1001/jamadermatol.2021.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leasure AC, Damsky W, Cohen JM. Prevalence of granuloma annulare in the United States: a cross-sectional study in the All of Us Research Program. Int J Dermatol. In press. 10.1111/ijd.15832 [DOI] [PMC free article] [PubMed]
- 12.Endo Y, Sekiguchi A, Motegi S, Ishikawa O. Subcutaneous granuloma annulare on the heel: a case report and review of the Japanese published work. J Dermatol. 2020;47:677–679. doi: 10.1111/1346-8138.15352. [DOI] [PubMed] [Google Scholar]
- 13.Khanna U, North JP. Patch-type granuloma annulare: an institution-based study of 23 cases. J Cutan Pathol. 2020;47:785–793. doi: 10.1111/cup.13707. [DOI] [PubMed] [Google Scholar]
- 14.Pap N, Bradamante M, Ljubojević HS. Localized perforating granuloma annulare. Acta Dermatovenerol Croat. 2019;27:33–36. [PubMed] [Google Scholar]
- 15.Salzmann M, Rendon A, Toberer F, Hassel JC. Generalized perforating granuloma annulare: a case report. J Dtsch Dermatol Ges. 2021;19:585–587. doi: 10.1111/ddg.14442. [DOI] [PubMed] [Google Scholar]
- 16.Muse M, Prohaska J, Shah M, White W, Appel J. Granuloma annulare on the palms. Dermatol Online J. 2021;27:13030/qt0m50398n. [PubMed]
- 17.Rai T. Papular granuloma annulare of palms and soles. Indian Dermatol Online J. 2017;8:511–513. doi: 10.4103/idoj.IDOJ_338_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee D, Kaur M, Punia RPS, Bhalla M, Handa U. Evaluating the unusual histological aspects of granuloma annulare: a study of 30 cases. Indian Dermatol Online J. 2018;9:409–413. doi: 10.4103/idoj.IDOJ_75_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawryluk EB, Izikson L, English JC. Non-infectious granulomatous diseases of the skin and their associated systemic diseases: an evidence-based update to important clinical questions. Am J Clin Dermatol. 2010;11:171–181. doi: 10.2165/11530080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Shibayama A, Sugita K, Narukawa K, Fujiwara Y, Goto H, Shiomi T, et al. Granuloma annulare can occur on a scar, mimicking sarcoidosis. Clin Exp Dermatol. 2017;42:920–921. doi: 10.1111/ced.13210. [DOI] [PubMed] [Google Scholar]
- 21.Akay BN, Atak MF, Kirmizi A, Farabi B. Granuloma annulare mimicking eruptive dermatofibroma in an HIV-positive male: a challenge with distinct dermatoscopic findings. Dermatol Ther. 2020;33:e13375. doi: 10.1111/dth.13375. [DOI] [PubMed] [Google Scholar]
- 22.Maoz K, Greenberger S, Maly A, Merims S, Tirosh I, Barzilai A, et al. Subcutaneous granuloma annulare mimicking dermatomyositis. Pediatr Dermatol. 2020;37:687–689. doi: 10.1111/pde.14167. [DOI] [PubMed] [Google Scholar]
- 23.Chae MH, Shin JY, Lee JY, Yoon TY. Perforating granuloma annulare mimicking papulonecrotic tuberculid. Ann Dermatol. 2018;30:716–720. doi: 10.5021/ad.2018.30.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob JS, Krenek G, Tschen J. Perforating granuloma annulare mimicking psoriasis. Cureus. 2020;12:e9983. doi: 10.7759/cureus.9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witkoff BM, Ivanov NN, Trotter SC. Perforating granuloma annulare appearing as a psoriasiform lesion. Case Rep Dermatol. 2019;11:233–238. doi: 10.1159/000501875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orleans RA, Magro CM, Varghese GI. A case of granuloma annulare mimicking tinea cruris. Dermatol Online J. 2018;24:13030/qt3kz6h4kj. [PubMed]
- 27.Saggini A, Cerroni L. Cutaneous borreliosis: an insidious mimicker of patch-type granuloma annulare. J Cutan Pathol. 2020;47:876–878. doi: 10.1111/cup.13760. [DOI] [PubMed] [Google Scholar]
- 28.Zhu H, Huang M, Huang W. Anti-neutrophil cytoplasmic antibodies-negative Churg-Strauss syndrome presenting as granuloma annulare-like lesions: an unusual cutaneous presentation and a diagnostic pitfall. Indian J Dermatol Venereol Leprol. 2021;87:259–262. doi: 10.25259/IJDVL_797_19. [DOI] [PubMed] [Google Scholar]
- 29.Aghazadeh N, Bridges AG, Camilleri MJ, Peters MS, Comfere NI. Kaposi sarcoma misdiagnosed as granuloma annulare: a case of mistaken identity. J Cutan Pathol. 2021;48:318–321. doi: 10.1111/cup.13815. [DOI] [PubMed] [Google Scholar]
- 30.Zhu TH, Kamangar F, Silverstein M, Fung MA. Borderline tuberculoid leprosy masquerading as granuloma annulare: a clinical and histological pitfall. Am J Dermatopathol. 2017;39:296–299. doi: 10.1097/DAD.0000000000000698. [DOI] [PubMed] [Google Scholar]
- 31.Errichetti E, Lallas A, Apalla Z, Di Stefani A, Stinco G. Dermoscopy of granuloma annulare: a clinical and histological correlation study. Dermatology. 2017;233:74–79. doi: 10.1159/000454857. [DOI] [PubMed] [Google Scholar]
- 32.Errichetti E, Stinco G. Dermatoscopy of granulomatous disorders. Dermatol Clin. 2018;36:369–375. doi: 10.1016/j.det.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Vázquez-Osorio I, Quevedo A, Rodríguez-Vidal A, Rodríguez-Díaz E. Usefulness of ultrasonography in the diagnosis of subcutaneous granuloma annulare. Pediatr Dermatol. 2018;35:e200–e201. doi: 10.1111/pde.13470. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez Bandera AI, Stewart N, Beato MJ, de Lucas LR. Comment on “Usefulness of ultrasonography in the diagnosis of subcutaneous granuloma annulare”. Pediatr Dermatol. 2018;35:875–876. doi: 10.1111/pde.13615. [DOI] [PubMed] [Google Scholar]
- 35.Min MS, Wu J, He H, Sanz-Cabanillas JL, Del Duca E, Zhang N, et al. Granuloma annulare skin profile shows activation of T-helper cell type 1, T-helper cell type 2, and Janus kinase pathways. J Am Acad Dermatol. 2020;83:63–70. doi: 10.1016/j.jaad.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 36.Wang A, Rahman N-T, McGeary MK, Murphy M, McHenry A, Peterson D, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795–1809. doi: 10.1016/j.jaci.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Modlin RL, Horwitz DA, Jordan RR, Gebhard JF, Taylor CR, Rea TH. Immunopathologic demonstration of T lymphocyte subpopulations and interleukin 2 in granuloma annulare. Pediatr Dermatol. 1984;2:26–32. doi: 10.1111/j.1525-1470.1984.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 38.Modlin RL, Vaccaro SA, Gottlieb B, Gebhard JF, Linden CE, Forni M, et al. Granuloma annulare. Identification of cells in the cutaneous infiltrate by immunoperoxidase techniques. Arch Pathol Lab Med. 1984;108:379–382. [PubMed] [Google Scholar]
- 39.Barbieri JS, Rosenbach M, Rodriguez O, Margolis DJ. Association of granuloma annulare with Type 2 Diabetes, hyperlipidemia, autoimmune disorders, and hematologic malignant neoplasms. JAMA Dermatol. 2021;157:817–823. doi: 10.1001/jamadermatol.2021.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leasure AC, Damsky W, Cohen JM. Comorbidities associated with granuloma annulare: a case-control study in the All of Us research program. J Am Acad Dermatol. 2021 doi: 10.1016/j.jaad.2021.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbieri JS, Rosenbach M, Rodriguez O, Margolis DJ. Granuloma annulare is not associated with solid-organ malignancies: a cohort study. J Am Acad Dermatol. 2021 doi: 10.1016/j.jaad.2021.04.098. [DOI] [PubMed] [Google Scholar]
- 42.Gabaldón VH, Haro-González-Vico V. Lack of an association between generalized granuloma annulare and malignancy: a case-control study. J Am Acad Dermatol. 2019;80:1799–1800. doi: 10.1016/j.jaad.2019.02.056. [DOI] [PubMed] [Google Scholar]
- 43.Tolkki L, Hokynar K, Meri S, Panelius J, Puolakkainen M, Ranki A. Granuloma Annulare and Morphea: Correlation with Borrelia burgdorferi Infections and Chlamydia-related Bacteria. Acta Derm Venereol. 2018;98:355–360. doi: 10.2340/00015555-2831. [DOI] [PubMed] [Google Scholar]
- 44.Guglielmo A, Virdi A, Misciali C, Gabrielli L, Veronesi G, Corsini I, et al. Generalized granuloma annulare-like eruption secondary to acute Epstein-Barr virus infection. Int J Dermatol. 2021;60:e110–e112. doi: 10.1111/ijd.15274. [DOI] [PubMed] [Google Scholar]
- 45.Mann E, Maruthi R, Friedland MH, Chung HJ, McGee JS. Case of post-herpetic, isotopic granuloma annulare (GA), followed by generalized GA. J Dermatol. 2019;46:e476–e477. doi: 10.1111/1346-8138.15088. [DOI] [PubMed] [Google Scholar]
- 46.Al Ali A, Alkhodair R, Thuraisingam T, Gerstein W, Watters K. Multiple granuloma annulare lesions presenting simultaneously with herpes zoster infection: Wolf’s isotopic response. JAAD Case Rep. 2018;4:631–632. doi: 10.1016/j.jdcr.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.García-Gil MF, Monte Serrano J, García García M, Matovelle Ochoa C, Ara-Martín M. Granuloma annulare triggered by SARS-CoV-2 infection. The first reported case. J Dermatol. 2021;48:e1–2. doi: 10.1111/1346-8138.15594. [DOI] [PubMed] [Google Scholar]
- 48.Monte-Serrano J, García-Gil MF, García-García M, Casas-Flecha I, Matovelle-Ochoa C, Ara-Martín M. Granuloma annulare triggered by SARS-CoV-2 infection: immunohistochemical staining. Dermatol Ther. 2021;34:e14897. doi: 10.1111/dth.14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah N, Shah M, Drucker AM, Shear NH, Ziv M, Dodiuk-Gad RP. Granulomatous cutaneous drug eruptions: a systematic review. Am J Clin Dermatol. 2021;22:39–53. doi: 10.1007/s40257-020-00566-4. [DOI] [PubMed] [Google Scholar]
- 50.Guimaraes MJ, Gomes J, Caldas R, Almeida F, Brito C. Subcutaneous granuloma annulare induced by acetazolamide. Pediatr Dermatol. 2020;37:1181–1182. doi: 10.1111/pde.14347. [DOI] [PubMed] [Google Scholar]
- 51.Fässler M, Schlapbach C. Granuloma annulare arising under systemic psoriasis therapy successfully treated with adalimumab. JAAD Case Rep. 2020;6:832–834. doi: 10.1016/j.jdcr.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gray AR, Davies OMT, White K, Ortega-Loayza AG. Granuloma annulare following initiation of interleukin-17a antagonist. Clin Exp Dermatol. 2021;46:924–926. doi: 10.1111/ced.14579. [DOI] [PubMed] [Google Scholar]
- 53.Robati RM, Bahmanjahromi A, Bidari-Zerehpoosh F. Periorbital granuloma annulare following mesotherapy. Dermatol Ther. 2020;33:e14326. doi: 10.1111/dth.14326. [DOI] [PubMed] [Google Scholar]
- 54.Fonda-Pascual P, de Gálvez MV, Aguilera J, Herrera-Ceballos E. Photoinduced granuloma annulare confirmed by experimental exposure to UVA light. Actas Dermosifiliogr. 2021;112:190–192. doi: 10.1016/j.ad.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Samaran Q, Clark E, Secco L-P, Poujade L, Schwob E, Bessis D, et al. Granulomatous dermatitis following measles, mumps, and rubella vaccination. Pediatr Dermatol. 2021 doi: 10.1111/pde.14687. [DOI] [PubMed] [Google Scholar]
- 56.García-Gil MF, Álvarez-Salafranca M, Martínez García A, Ara-Martín M. Generalized granuloma annulare after pneumococcal vaccination. An Bras Dermatol. 2021;96:59–63. doi: 10.1016/j.abd.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nichani P, Micieli JA. Granuloma annulare, scalp necrosis, and ischemic optic neuropathy from giant cell arteritis after varicella-zoster virus vaccination. J Neuroophthalmol. 2021;41:e145–e148. doi: 10.1097/WNO.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 58.Rubin CB, Rosenbach M. Granuloma annulare: a retrospective series of 133 patients. Cutis. 2019;103:102–106. [PubMed] [Google Scholar]
- 59.Nordmann TM, Kim J-R, Dummer R, Anzengruber F. A monocentric, retrospective analysis of 61 patients with generalized granuloma annulare. Dermatology. 2020;236:369–374. doi: 10.1159/000507247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Visconti MJ, Ashack KA, Ashack RJ. Granuloma annulare: strengthening potential associations and pentoxifylline as a therapeutic option. J Dermatol Treat. 2021;32:381–382. doi: 10.1080/09546634.2019.1662366. [DOI] [PubMed] [Google Scholar]
- 61.Eid E, Zein-El-Dine S, Kurban M, Abbas O. Generalized granuloma annulare associated with interstitial lung disease: Good response to doxycycline. Dermatol Ther. 2021;34:e14864. doi: 10.1111/dth.14864. [DOI] [PubMed] [Google Scholar]
- 62.Chandan N, Boen M, Lake EP, Aronson I. Successful treatment of two individual cases of generalized granuloma annulare with amoxicillin/clavulanic acid and a combination of doxycycline and pentoxifylline. Dermatol Online J. 2018;24:13030/qt9161p8z0. [PubMed]
- 63.Hrin ML, Bashyam AM, Feldman SR, Huang WW. Oral dapsone for the treatment of generalized granuloma annulare: a retrospective case series. J Am Acad Dermatol. 2021 doi: 10.1016/j.jaad.2021.03.045. [DOI] [PubMed] [Google Scholar]
- 64.Mostafa N, Phan K, Smith SD. Antimalarial therapy for granuloma annulare: a systematic review. J Dermatolog Treat. 2020 doi: 10.1080/09546634.2020.1801973. [DOI] [PubMed] [Google Scholar]
- 65.Megna M, Sidikov A, Ruggiero A, Fabbrocini G, Zaslavsky D, Nasyrov R, et al. A case of generalized granuloma annulare successfully treated by hydroxychloroquine. Dermatol Ther. 2020;33:e13894. doi: 10.1111/dth.13894. [DOI] [PubMed] [Google Scholar]
- 66.Xu Q, Gu Y, Li Y, Ling B, Yu H, Yao Z. Concurrence of generalized perforating and subcutaneous granuloma annulare in a 4-year-old boy with latent tuberculosis infection successfully treated with low-dose hydroxychloroquine. J Dermatol. 2020;47:e71–e72. doi: 10.1111/1346-8138.15152. [DOI] [PubMed] [Google Scholar]
- 67.Hrin ML, Feldman SR, Huang WW. Hydroxychloroquine for generalized granuloma annulare: 35% response rate in a retrospective case series of 26 patients. J Am Acad Dermatol. 2021 doi: 10.1016/j.jaad.2021.06.867. [DOI] [PubMed] [Google Scholar]
- 68.Otezla® (apremilast) tablets, for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf. Accessed 17 Jul 2021.
- 69.Blum S, Altman D. Treatment of generalized granuloma annulare with apremilast: a report of 2 cases. JAAD Case Rep. 2019;5:976–978. doi: 10.1016/j.jdcr.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bishnoi A, Raj D, Vinay K, Dogra S. Refractory Generalized Granuloma Annulare Treated With Oral Apremilast. JAMA dermatology (Chicago, IL). United States: American Medical Association; 2019;155:1318. [DOI] [PubMed]
- 71.Joshi TP, Tschen J. Apremilast in the management of disseminated granuloma annulare. Cureus. 2021;13:e14918. doi: 10.7759/cureus.14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hansel K, Biondi F, Bianchi L, Tramontana M, Marietti R, Stingeni L. Generalized granuloma annulare successfully treated with apremilast: report of two cases and literature review. Clin Exp Dermatol. 2021 doi: 10.1111/ced.14806. [DOI] [PubMed] [Google Scholar]
- 73.Chen A, Truong AK, Worswick S. The role of biologics in the treatment of chronic granuloma annulare. Int J Dermatol. 2019;58:622–626. doi: 10.1111/ijd.14350. [DOI] [PubMed] [Google Scholar]
- 74.Antoñanzas J, Rodríguez-Garijo N, Tomás-Velázquez A, Estenaga A, Andrés-Ramos I, España AA. Treatment of recalcitrant reactive granulomatous dermatitis: Granuloma annulare subtype with etanercept. Dermatol Ther. 2020;33:e14081. doi: 10.1111/dth.14081. [DOI] [PubMed] [Google Scholar]
- 75.Bürgler C, Vinay K, Häfliger S, Klötgen H-W, Yawalkar N. Infliximab reduces activated myeloid dendritic cells, different macrophage subsets and CXCR3-positive cells in granuloma annulare. J Dermatol. 2019;46:808–811. doi: 10.1111/1346-8138.14981. [DOI] [PubMed] [Google Scholar]
- 76.Song EJ, Bezecny J, Farrer S. Recalcitrant generalized granuloma annulare treated successfully with dupilumab. JAAD Case Rep. 2021;7:1–2. doi: 10.1016/j.jdcr.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song EJ. Tildrakizumab ineffective in generalized granuloma annulare. JAAD Case Rep. 2021;7:3–4. doi: 10.1016/j.jdcr.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hrin ML, Bowers NL, Feldman SR, Huang WW. Methotrexate for generalized granuloma annulare: A 60% response rate in a retrospective case series of 15 patients. J Am Acad Dermatol. 2021 doi: 10.1016/j.jaad.2021.07.037. [DOI] [PubMed] [Google Scholar]
- 79.Naka F, Strober BE. Methotrexate treatment of generalized granuloma annulare: a retrospective case series. J Dermatolog Treat. 2018;29:720–724. doi: 10.1080/09546634.2018.1447075. [DOI] [PubMed] [Google Scholar]
- 80.Hassan I, Dorjay K, Anwar P. Pentoxifylline and its applications in dermatology. Indian Dermatol Online J. 2014;5:510–516. doi: 10.4103/2229-5178.142528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong GN, Wee E, Tam M, Kelly R. Pentoxifylline as a treatment for granuloma annulare. Australas J Dermatol. 2019;60:328–331. doi: 10.1111/ajd.13074. [DOI] [PubMed] [Google Scholar]
- 82.García-Malinis AJ, Gracia-Cazaña T, Planas Linares D, Agón-Banzo PJ, Gilaberte Y. Granuloma annulare: report of 13 patients treated with photodynamic therapy. J Eur Acad Dermatol Venereol. 2021;35:e211–e214. doi: 10.1111/jdv.16935. [DOI] [PubMed] [Google Scholar]
- 83.Aichelburg MC, Pinkowicz A, Schuster C, Volc-Platzer B, Tanew A. Patch granuloma annulare: clinicopathological characteristics and response to phototherapy. Br J Dermatol. 2019;181:198–199. doi: 10.1111/bjd.17606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pitney T, Pitney MJ. A retrospective review of UVA1 treatment: an Australian experience from a single centre. Australas J Dermatol. 2020;61:318–323. doi: 10.1111/ajd.13321. [DOI] [PubMed] [Google Scholar]
- 85.Dawson A, Glassman SJ. Generalized granuloma annulare responsive to narrowband UVB. Cutis. 2019;103:E14–E15. [PubMed] [Google Scholar]
- 86.Muylaert BPB, Almada R, de Vasconcelos RCF. Granuloma annulare treated with narrowband UVB phototherapy. An Bras Dermatol. 2017;92:82–84. doi: 10.1590/abd1806-4841.20174994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aubin F, Chaignot C, Gallais-Serezal I. Société Française de Photodermatologie. Decline in the use of phototherapy in France from 2010 to 2019. Br J Dermatol. 2021 doi: 10.1111/bjd.20384. [DOI] [PubMed] [Google Scholar]
- 88.Eadie E, Gallacher M, Gorczynski A, Smith L, Dawe RS. Photonet, the Managed Clinical Network for Ultraviolet (UV) Phototherapy in Scotland. Response to Decline in use of phototherapy in France from 2010 to 2019. Br J Dermatol. 2021 doi: 10.1111/bjd.20586. [DOI] [PubMed] [Google Scholar]
- 89.Maloney NJ, Zhao J, Tegtmeyer K, Lee EY, Cheng K. Off-label studies on apremilast in dermatology: a review. J Dermatol Treat. 2020;31:131–140. doi: 10.1080/09546634.2019.1589641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Damsky W, Thakral D, McGeary MK, Leventhal J, Galan A, King B. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612–621. doi: 10.1016/j.jaad.2019.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Durgin JS, Shields BE, Rosenbach M. Generalized granuloma annulare: a widespread response to limited application of compounded 2% topical tofacitinib. JAAD Case Rep. 2020;6:1113–1115. doi: 10.1016/j.jdcr.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Damsky W, King BA. Treatment of granuloma annulare with tofacitinib 2% ointment. JAAD Case Rep. 2020;6:69–71. doi: 10.1016/j.jdcr.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang YW, Lehrer MD, Mangold AR, Yiannias JA, Nelson SA, Pittelkow MR. Treatment of granuloma annulare and related granulomatous diseases with sulphasalazine: a series of 16 cases. J Eur Acad Dermatol Venereol. 2021;35:211–215. doi: 10.1111/jdv.16356. [DOI] [PubMed] [Google Scholar]
- 94.Anzengruber F, Mergenthaler C, Murer C, Dummer R. Potassium Iodide for cutaneous inflammatory disorders: a monocentric. Retrospective Study. Dermatology. 2019;235:137–143. doi: 10.1159/000494614. [DOI] [PubMed] [Google Scholar]
- 95.Mickel M, Kunstfeld R, Crevenna R. Granuloma annulare and radial pulse therapy: preliminary findings. J Clin Aesthet Dermatol. 2018;11:32–34. [PMC free article] [PubMed] [Google Scholar]
- 96.Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis G-A, Vogiatzi G, Papaioannou S, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14:50–59. doi: 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mailer RKW, Gisterå A, Polyzos KA, Ketelhuth DFJ, Hansson GK. Hypercholesterolemia enhances T cell receptor signaling and increases the regulatory T cell population. Sci Rep. 2017;7:15655. doi: 10.1038/s41598-017-15546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patrun S, Ljubojević HS. Unusual case of granuloma annulare associated with diabetes mellitus. Acta Dermatovenerol Croat. 2020;28:45–46. [PubMed] [Google Scholar]
- 99.Virath R, Mehta S, Balai M, Meena M, Gupta LK. Eruptive Xanthoma and Granuloma Annulare in Association with Metabolic Disorder. Indian J Dermatol. 2021;66:199–201. doi: 10.4103/ijd.IJD_421_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Min MS, Lebwohl M. Treatment of recalcitrant granuloma annulare (GA) with adalimumab: a single-center, observational study. J Am Acad Dermatol. 2016;74:127–133. doi: 10.1016/j.jaad.2015.09.015. [DOI] [PubMed] [Google Scholar]