Abstract
Purpose
A number of studies performed in the operating room evaluated the hemodynamic effects of the fluid challenge (FC), solely considering the effect before and after the infusion. Few studies have investigated the pharmacodynamic effect of the FC on hemodynamic flow and pressure variables. We designed this trial aiming at describing the pharmacodynamic profile of two different FC infusion times, of a fixed dose of 4 ml kg−1.
Methods
Forty-nine elective neurosurgical patients received two consecutive FCs of 4 ml kg−1 of crystalloids in 10 (FC10) or 20 (FC20) minutes, in a random order. Fluid responsiveness was defined as stroke volume index increase ≥ 10%. We assessed the net area under the curve (AUC), the maximal percentage difference from baseline (dmax), time when the dmax was observed (tmax), change from baseline at 1-min (d1) and 5-min (d5) after FC end.
Results
After FC10 and FC20, 25 (51%) and 14 (29%) of 49 patients were classified as fluid responders (p = 0.001). With the exception of the AUCs of SAP and MAP, the AUCs of all the considered hemodynamic variables were comparable. The dmax and the tmax were overall comparable. In both groups, the hemodynamic effects on flow variables were dissipated within 5 min after FC end.
Conclusions
The infusion time of FC administration affects fluid responsiveness, being higher for FC10 as compared to FC20. The effect on flow variables of either FCs fades 5 min after the end of infusion.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10877-021-00756-3.
Keywords: Fluid challenge, Pharmacodynamic, Neurosurgery, Fluids, Hemodynamics, Fluid responsiveness
Introduction
The appropriate fluid management in the perioperative period is an important, and still partially unclear, chapter of clinical practice for anesthesiologists [1–4]. Increasing evidence suggests that intraoperative fluid therapy should be tailored to individual patient’s physiology to target fluid administration to specific stroke volume (SV) responses, or its surrogates [5–7]. For this reason, small repeated and fast boluses challenging the cardiovascular system should be preferred to continuous and prolonged infusions [8–10].
The fluid challenge (FC) is defined as a small amount of fluid given in a short period of time to assess whether the preload reserve of the patient can increase SV with further administration of fluids [11]. A number of studies performed in the operating room evaluated the hemodynamic effects of FC solely considering the effect before and after the infusion [12]. Recently, Aya demonstrated that at least 4 ml kg−1 should be infused to effectively challenge the preload, additionally showing that the hemodynamic effect of the FC is dissipated within 10 min [13].
The approach of Aya et al. [13] considers the FC as a drug evoking a systemic response on flow (i.e. SV) and pressure variables [i.e. systolic arterial pressure (SAP)]. Accordingly, the pharmacodynamic effect is evaluated by considering the magnitude (i.e. the maximal changes from baseline obtained for a specific variable), the global effect [i.e. considering the area under the curve (AUC) obtained by plotting the changes overtime] and the persistence of the hemodynamic response after the end of FC administration. The infusion time of FC administration, which ranges in the literature between 5 and 30 min [12], may influence the magnitude of SV response and, in turn, the number of patients defined as fluid responders [5]. Since several intraoperative pathways of hemodynamic optimization are based on the response to repeated FCs [6, 10, 14–18], a prolonged infusion time may potentially affect fluid responsiveness and, in turn, wrongly drive intraoperative fluid management and eventually affect postoperative outcomes.
Since FC is a test embedding at least three variables (i.e., the amount of fluid; the time needed to complete the administration, and the SV change threshold used to define a positive response), the role of one single component on the final outcome can be addressed only by keeping the others fixed. Therefore, we designed this multicenter, in-patient randomized trial performed on elective patients scheduled for supine neurosurgery, hypothesizing that a FC of 4 ml kg−1 infused over 10 min would be associated with a higher rate of fluid responders, as compared to a FC infused over 20 min. Furthermore, we described the pharmacodynamic profiles of the two different FC infusion times.
Materials and methods
Patients
This prospective multicenter randomized trial was carried out in the operating rooms of three Italian tertiary hospitals: the Humanitas Research Hospital (Rozzano, Milano), the University hospital “Maggiore della Carità” (Novara), and the San Bortolo Hospital (Vicenza). The protocol was designed in accordance with the principles outlined in the Declaration of Helsinki; the study was approved by all the local institutional ethics committees [Ethical Committee of the Coordinator Center—Humanitas Research Hospital, Rozzano (Milano; Italy); Protocol Number 92/19; 19 February 2019], and prospectively registered (NCT03810118). Informed consent was obtained from all the participants.
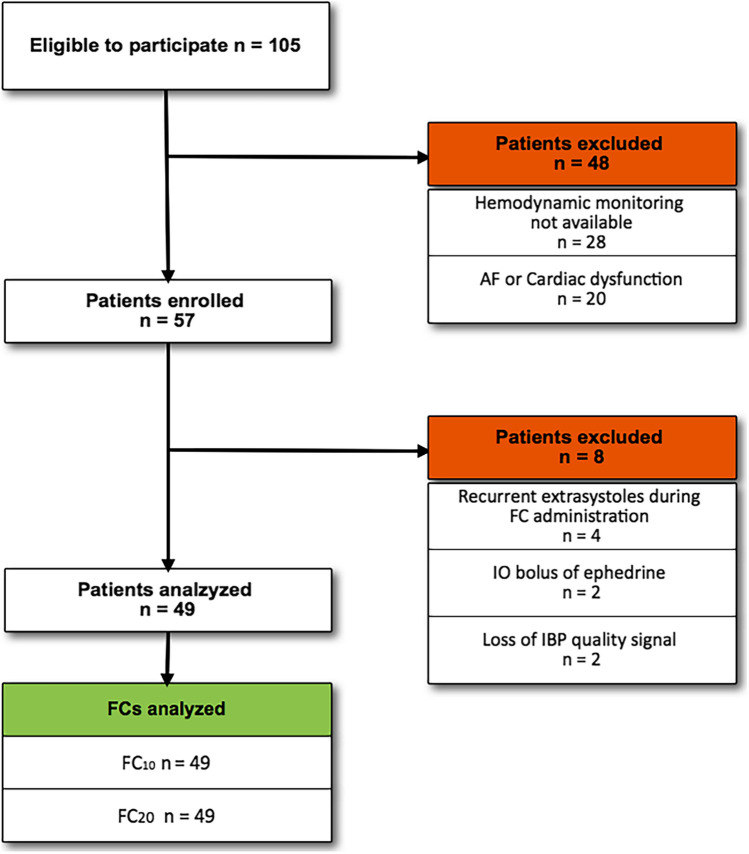
We enrolled adult patients with a body weight < 100 kg (for technical limitations regarding FC infusion, see Supplemental Table 1 in the Supplementary Information), scheduled for elective supine neurosurgery and requiring a FC. The decision to administer a FC was at the discretion of the attending physician. The preoperative exclusion criteria were (1) any recurrent cardiac arrhythmia; (2) reduced left (ejection fraction < 30%) or right (systolic peak velocity of tricuspid annular motion < 0.17 m/s) ventricular systolic function. Once enrolled, the patient can be additionally excluded due to the occurrence of one of the following intraoperative conditions: (1) significant bleeding (more than 500 ml in ½ h); (2) recurrent extrasystoles; (3) persistent low quality of the arterial signal affecting hemodynamic monitoring measurements; (4) use of continuous infusion of vasopressors before the study protocol start (Fig. 1).
Fig. 1.
Flow of patients in the study. AF atrial fibrillation, FC fluid challenge, IO intraoperative, IBP invasive blood pressure
Perioperative management and hemodynamic monitoring
All patients received standard intraoperative monitoring including heart rate, peripheral oxygen saturation, continuous electrocardiography, invasive blood pressure monitoring. After pre-oxygenation, general anesthesia was induced with propofol, remifentanil and rocuronium (0.6 mg kg−1), and maintained with propofol (1.5–3.0 mg kg−1 h−1) plus remifentanil (0.1–0.5 mcg kg−1 min−1) to target the bispectral index (BIS monitor, Medtronic, Brooklyn Park, MN) between 40 and 60 throughout the surgical time. Neuromuscular transmission was monitored using train-of-four supramaximal stimulations. Patients were ventilated in volume-control mode with a Tidal Volume of 6–8 ml kg−1 of predicted body weight and positive end-expiratory pressure between 3 and 5 cm H2O. Intraoperatively, all patients received Ringer’s solution, at 4 ml kg−1 h−1, as maintenance fluid.
Invasive blood pressure monitoring was obtained by inserting a 20-G cannula into the radial artery and the pressure signal was then connected to the MostCare® device (Vyetech Health, Padua, Italy). The arterial waveform was optimized to exclude under or over-damping and a square-wave test was used in all patients to check the quality of the pressure signal [19]. A comprehensive description of the analysis of the arterial waveform for SV calculation by MostCare® is reported elsewhere [20–22]. Arterial pressures [SAP, diastolic, mean (MAP), dicrotic] were directly measured from the arterial pressure waveform, while the indexed values, including SV index (SVI) and cardiac index (CI), by using the patient’s anthropometric characteristics. Finally, the system calculates the arterial elastance (Ea), as dicrotic pressure/SV.
Study protocol and measurements
Eligible patients consecutively received both FCs using a computer-generated random sequence of infusion. Each assignment was designated in a consecutively numbered, sealed, opaque envelope. The obtained envelopes were finally distributed to each enrolling center and serially opened before each enrolment. Each patient received both the FCs consecutively, according to the randomization sequence (FC10/FC20 or FC20/FC10).
The study protocol start was triggered by the decision of the attending physician to administer the first FC, during a period of intraoperative hemodynamic stability after the induction of general anesthesia, defined as a change in mean arterial pressure < 10% over 5 min [23, 24]. Before the protocol start, an arterial blood gases analysis was obtained. We tested two infusion times of FC administration (Ringer Acetate): FC10 = 4 ml kg−1administered over 10 min; FC10 = 4 ml kg−1 administered over 20 min.
Each FC infusion was separated from the following by a 10-min period. For the patient’s safety, interruption of the protocol was at the discretion of the attending anesthetist, by using a rescue bolus of 5 mg of ephedrine, whenever needed. A constant FC infusion was ensured by using a peripheral dedicated venous line (16 or 14 gauge), two volumetric infusion pumps (Alaris® GP Volumetric Pump, Cardinal Health, Switzerland or Terumo® -Terufusion TE-171, Tokyo, Japan) (Supplemental Table 1 and Supplemental Fig. 1 in the Supplementary Information).
The beginning of FC10 and FC20 infusions were recorded electronically on MostCare®. MostCare® was set to record a standard set of hemodynamic measurements by automatically averaging the recorded values every 30 s (i.e. two values/min). Accordingly, baseline values of SAP, MAP, CI, SVI, Ea and HR were defined as the average of the two values recorded in minute before FC10 or FC20 administration.
The rough hemodynamic data recorded were finally exported into a dedicated EXCEL® (Microsoft, Redwood, MS, USA) spreadsheet for statistical elaboration and analysis.
Statistical analysis
The sample size calculation of this study was based on the expected proportions of responders after FC10 and FC20, retrieved from a database of FCs administered with different infusion times. We predicted a rate of fluid responsiveness, after FC10 and FC20, of 50% and 20%, respectively. An overall sample size of 48 FCs per single arm was estimated to find a difference of 30%, with an alpha and beta error of 5% and 10%, respectively [25].
Hemodynamic variables were summarised with median with interquartile (IQR 25th–75th) range or mean ± standard deviation (SD) and compared as appropriate. For dichotomous or categorical variables, the McNemar's test for comparison of proportions of dependent variables was applied, whereas paired t test or Wilcoxon Signed-Rank test were used for continuous variables, as appropriate. Fluid responsiveness was defined as an SVI increase ≥ 10% after either FC10 or FC20.
The pharmacodynamic effect of FC was assessed for the hemodynamic variables SAP, MAP, CI, SVI, Ea and HR by considering the percent change at each minute of the following variables, as compared to the baseline: the maximal percentage difference observed from baseline (dmax), time when the maximal value was observed (tmax), expressed as percentage of time over the whole FC administration period, and change from baseline at 1-min (d1) and 5-min (d5) after the end of the FC are also reported. Finally, the global effect has been quantified as the net AUC calculated using the trapezoidal rule [26] considering the overall percentage change from baseline to d5 (i.e. the percentage of increase of each single variables multiplied for the minutes of observation). The AUCs were then compared. The dmax and the AUC defined the magnitude of hemodynamic response. One-way analysis of variance (ANOVA) for repeated measures was performed to compare hemodynamic changes of all the considered variables at baseline, d1 and d5.
The effect of the duration of the FC and of the sequence of randomization on SVI changes was evaluated by means of a multilevel mixed-effects linear regression model, considering these two parameters as independent variables and the SVI changes from baseline of the entire population at the end of the FC and at d5, as dependent variables. Finally, we analyzed both the period and carry-over effects for the AUC of the flow-related variables (see Supplemental Methods section in the Supplementary Information for further details).
Statistical analyses were conducted using GraphPad PRISM V8 (GraphPad Software Inc., San Diego, CA, USA) and STATA version 16 (StatsCorp, Texas, USA). A p value of < 0.05 was considered statistically significant.
Results
From April 2019 to February 2020, 152 consecutive patients were considered eligible to participate. However, 95 were excluded before and eight after the enrolment (Fig. 1). Finally, 49 patients receiving both the FCs were analysed (98 FCs overall) [Milano, 25 patients (51%); Vicenza, 14 patients (29%); Novara, ten patients (20%)]. Demographic characteristics, co-morbidities, surgical procedures, risk scores and ventilatory variables of the enrolled population are reported in Table 1. The protocol started 42 ± 9 min after the induction of the general anesthesia in the FC10 group and after 45 ± 11 in the FC20 group (p = 0.40).
Table 1.
Patients’ characteristics at enrolment
| Variables | Whole population |
|---|---|
| General characteristics | |
| Age (years) | 55 (16) |
| Weight (kg) | 71 ± 13 |
| Sex (M, %) | 21 (42.8%) |
| BMI, kg m−2 | 25 (22–27) |
| NSQIP score for serious complication, (%) | 6.9 (5.2–9) |
| NSQIP score for all the complication, (%) | 7.7 (5.5–10.1) |
| ASA score | |
| 1 (%) | 10 (20.4%) |
| 2 (%) | 33 (67.3%) |
| 3 (%) | 6 (12.2%) |
| Preoperative comorbidities (%) | |
| Hypertension | 27% |
| Previous neoplasia | 24% |
| Psychiatric diseases | 15% |
| History of epilepsy | 13% |
| Pulmonary diseases | 6% |
| Endocrinological diseases | 5% |
| Diabetes | 5% |
| Gastrointestinal diseases | 3% |
| Other metabolic diseases | 2% |
| Duration of surgery, (min) | 282 (150) |
| Preoperative hemoglobin, (mg dl−1) | 14.1 (13.1–15.0) |
| Preoperative creatinine, (mg dl−1) | 0.7 (0.3–0.8) |
| Intervention, n (%) | |
| Primitive cerebral neoplasia | 86% |
| Cerebral metastasis | 10% |
| Epilepsy surgery | 2% |
| Vascular tumor | 2% |
| Intraoperative ventilator settings and blood gases at T0 | |
| VT, (ml) | 480 (435–520) |
| Total PEEP, (cmH2O) | 5 (4–5) |
| Driving pressure, (cmH2O) | 11 (3) |
| RR, (breaths min−1) | 12.7 (1.5) |
| PaO2/FiO2, (ratio) | 368 (277–436) |
| Ct, (ml cmH2O−1) | 46 (40–52) |
| pH | 7.42 (0.04) |
| PCO2, (mmHg) | 38.7 ± 4.2 |
| Lactate, (mmol l−1) | 0.98 (0.75–1.74) |
| Base excess, (mEq l−1) | 1.9 (0.9–3.3) |
Values are presented as absolute (percentage); mean (standard deviation) or median (25th–75th interquartile range), as appropriate
NSQIP national surgical quality improvement program, ASA American society of anesthesiologists classification, VT tidal volume, PEEP positive end-expiratory pressure, RR respiratory rate, PaO2/FiO2 arterial partial pressure of oxygen/fraction of inspired oxygen, Ct total respiratory compliance, PaCO2 arterial partial pressure of carbon dioxide
Assessment of baseline hemodynamic characteristics of the patients before FC10 and FC20
As shown in the Supplemental Table 2 in the Supplementary Information, the baseline values before FC10 and FC20 administration of all the considered values in either the entire population or responders/non-responders were comparable. The analyses of either the period effect (mean difference (95% CI) 7.16 (− 8.51; 22.83); p-value: 0.36) or the carry-over effect (mean difference (95% CI) 7.16 (− 10.30; 24.62); p-value: 0.4) for the flow variables, considering the sequence of randomization, did not show a significant effect.
Fluid responsiveness
The FC of 4 ml kg−1 was initially administered in 10 min (FC10) in 27 patients (55.1%) while in 20 min (FC20) in the other 22 patients (44.9%) (p = 0.42). After FC10, 25 of 49 patients (51.0%) were classified as fluid responders, while after FC20, 14 were classified as responders (28.5%) [difference 95%CI − 22.45% (− 34.13 to − 10.77); p = 0.001].
Pharmacodynamic effect of the FC10 and FC20
Magnitude of hemodynamic effect: AUC and dmax
With the exception of the AUCs of SAP and MAP (both greater after FC10 as compared to FC20) all the other AUCs were comparable (Table 2; see also Figs. 3 and 4).
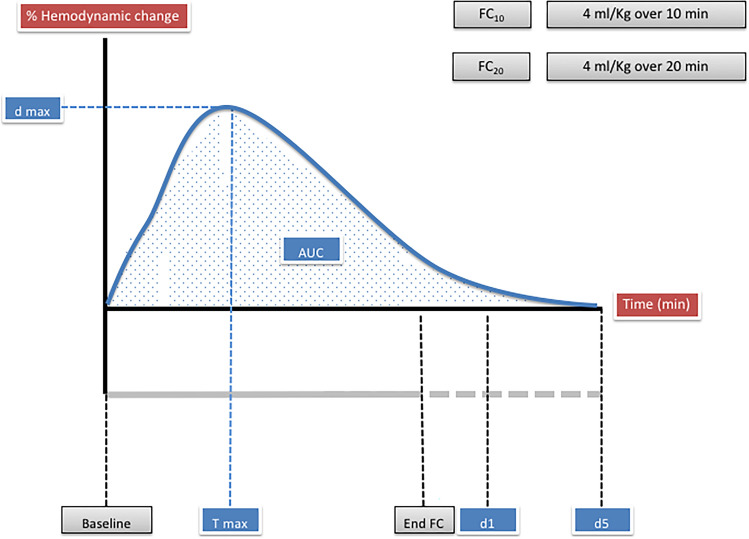
Fig. 2.
Schematic representation of the pharmacodynamic assessment of the hemodynamic changes related to fluid challenge (FC) administration (FC10 4 ml kg−1 over 10 min; FC20 4 ml kg−1 over 20 min). The dmax is the maximal percentage difference from baseline observed of the considered variable. The tmax is the time when dmax is observed. We also considered the changes from baseline at 1-min (d1) and 5-min (d5). The area under the curve (AUC), is calculated for each variable considering the percentage change from baseline to d5
Table 2.
Pharmacodynamic effects of FC10 (4 ml kg−1 over 10 min) and FC20 (4 ml kg−1 over 20 min)
| Hemodynamic variable | FC10 | FC20 | p-value |
|---|---|---|---|
| AUC SAP (%) | 62 ± 32 | 31 ± 38 | < 0.0001 |
| AUC MAP (%) | 59 ± 31 | 41 ± 35 | 0.009 |
| AUC SVI (%) | 47 ± 28 | 51 ± 32 | 0.49 |
| AUC CI (%) | 31 ± 25 | 26 ± 27 | 0.45 |
| AUC Ea (%) | 12 ± 24 | 22 ± 52 | 0.22 |
| AUC HR (%) | 9 ± 16 | 11 ± 20 | 0.46 |
| dmax SAP (%) | 18 ± 18 | 16 ± 11 | 0.96 |
| dmax MAP (%) | 15 ± 17 | 18 ± 11 | 0.15 |
| dmax SVI (%) | 23 ± 14 | 26 ± 8 | 0.12 |
| dmax CI (%) | 19 ± 14 | 22 ± 11 | 0.15 |
| dmax Ea (%) | − 14 ± 10 | − 17 ± 6 | 0.36 |
| dmax HR (%) | 10 ± 16 | 8 ± 12 | 0.80 |
| tmax SAP (%) | 65 ± 30 | 68 ± 30 | 0.77 |
| tmax MAP (%) | 64 ± 34 | 74 ± 24 | 0.33 |
| tmax SVI (%) | 83 ± 31 | 76 ± 30 | 0.49 |
| tmax CI (%) | 80 ± 32 | 77 ± 32 | 0.76 |
| tmax Ea (%) | 70 ± 38 | 60 ± 41 | 0.51 |
| tmax HR (%) | 43 ± 43 | 44 ± 45 | 0.96 |
| d1 SAP (%) | 7 ± 17 | 2 ± 10 | 0.09 |
| d1 MAP (%) | 6 ± 15 | 3 ± 10 | 0.45 |
| d1 SVI (%) | 3 ± 14 | 3 ± 10 | 0.81 |
| d1 CI (%) | 3 ± 13 | 2 ± 9 | 0.93 |
| d1 Ea (%) | 0 ± 14 | − 1 ± 18 | 0.94 |
| d1 HR (%) | 0 ± 7 | 0 ± 6 | 0.52 |
| d5 SAP (%) | 2 ± 10 | 1 ± 10 | 0.21 |
| d5 MAP (%) | 4 ± 11 | 2 ± 9 | 0.22 |
| d5 SVI (%) | 0 ± 10 | 0 ± 8 | 0.78 |
| d5 CI (%) | 1 ± 8 | 2 ± 8 | 0.36 |
| d5 Ea (%) | 1 ± 12 | − 1 ± 19 | 0.55 |
| d5 HR (%) | 3 ± 11 | 1 ± 8 | 0.65 |
The area under the curve (AUC) has been quantified using the trapezoidal rule as the net overall percentage of increase (or reduction) of the considered variable from baseline to d5; dmax is the maximal percentage difference from baseline; tmax is the minutes when dmax has been recorded (please note that this variable is expressed as percentage of time (from FC start to FC end) since the two tests have different durations—i.e. the 50% of tmax is 5 min for FC10 and 10 min for FC20); d1 and d5 the changes observed from baseline at 1-min time and 5-min after the end of the FC (see text and Fig. 2 for further explanations)
FC fluid challenge, SAP systolic arterial pressure, MAP mean arterial pressure, SVI stroke volume index, CI cardiac index, Ea arterial elastance, HR heart rate
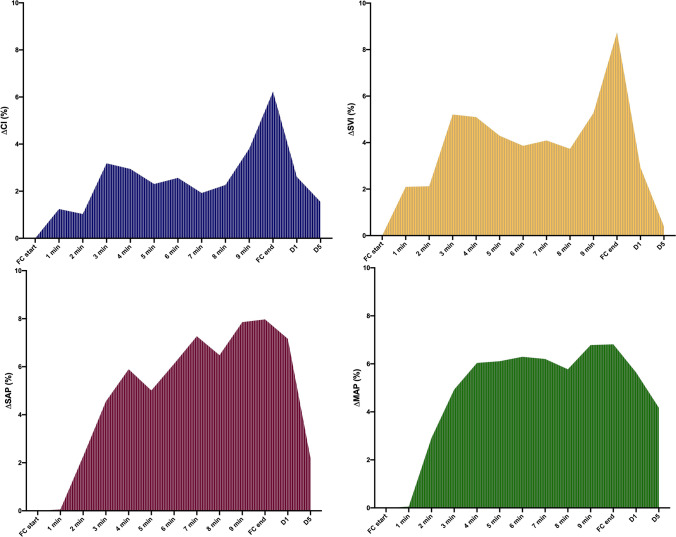
Fig. 3.
Area under the curve of cardiac index (CI; blue), stroke volume index (SVI; yellow); systolic arterial pressure (SAP; violet) and mean arterial pressure (MAP; green) calculated during FC10 administration. FC10 = fluid challenge of 4 ml kg−1 over 10 min; FC, fluid challenge; d1, 1-min after FC20 end; d5, 5-min after FC10 end
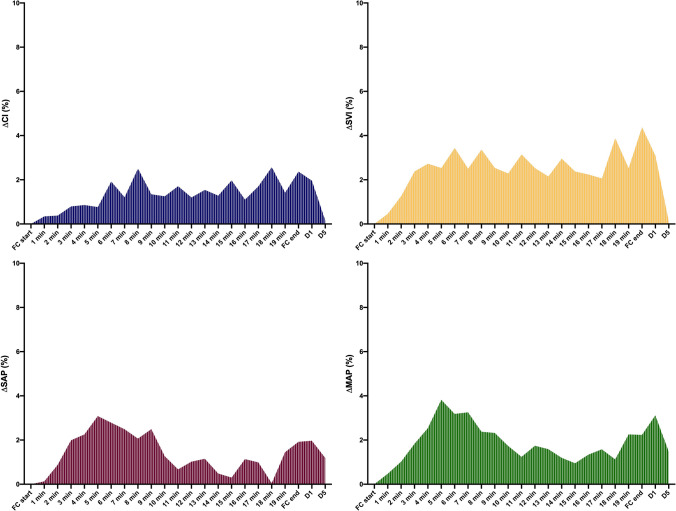
Fig. 4.
Area under the curve of cardiac index (CI; blue), stroke volume index (SVI; yellow); systolic arterial pressure (SAP; violet) and mean arterial pressure (MAP; green) calculated during FC20 administration. FC20 = fluid challenge of 4 ml kg−1 over 20 min; FC, fluid challenge; d1, 1-min after FC20 end; d5, 5-min after FC20 end
The dmax of pressure variables after FC10 and FC20 was comparable [SAP increase of 18% ± 18% vs. 16% ± 11%, respectively (p = 0.96); MAP increase of 15% ± 17% vs. 18% ± 11%, respectively (p = 0.15)]. Also, the dmax of flow variables after FC10 and FC20 was comparable [SVI increase of 23% ± 14% vs. 26% ± 8%, respectively (p = 0.12); CI increase of 19% ± 14% vs. 22% ± 11%, respectively (p = 0.15)] (Table 2; see also Figs. 3 and 4). The maximal reduction of the Ea was − 14% ± 10 after the FC10 and − 17% ± 6 after the FC20 (p = 0.36). The dmax of the HR was 10% ± 16% after the FC10 and 8% ± 12% after the FC20.
Timing of maximal effect: tmax
The tmax was overall comparable after FC10 and FC20. Specifically, the tmax of pressure variables was reached after 64–74% of FC infusion, whereas the tmax of flow variables after 76–83% (Table 2; see also Figs. 3 and 4). The tmax of Ea and HR was reached after 60–70% and 43–44% of FC infusion, respectively.
Dissipation of FC infusion: d1 and d5
The dissipation of the hemodynamic effect was overall comparable after FC10 and FC20. In fact, with respect to the baseline, at d1 the Ea and the HR were comparable, whereas all the other considered variables were significantly higher (Supplemental Table 3 in the Supplementary Information). On the contrary, at d5 only the MAP was significantly higher, as compared to the baseline, after both after FC10 and FC20 (Supplemental Table 3 in the Supplementary Information). At d5, the percentage changes of all the considered variables were comparable after FC10 and FC20, and, overall, below the 5% of increase, with respect to the baseline values (Table 2).
Effect of sequence of randomization and FC infusion time on SVI changes
As shown in the Supplemental Table 4 in the Supplementary Information, the sequence of randomization and the infusion time did not impact the SVI changes considered either at the end of FC10 and FC20 or at d5.
Discussion
To the best of our knowledge, this is the first trial exploring the pharmacodynamic effect of different infusion times of FC administration for a fixed dose of fluid. The main results of this trial performed in a selected population undergoing elective neurosurgery are: (1) the infusion times of FC administration affects the rate of fluid responsiveness, moving from 51.0% after FC10 to 28.5% after FC20; (2) the magnitude of the hemodynamic effect on flow variables is overall dissipated within 5 min after FC end.
Pharmacodynamic data interpretation
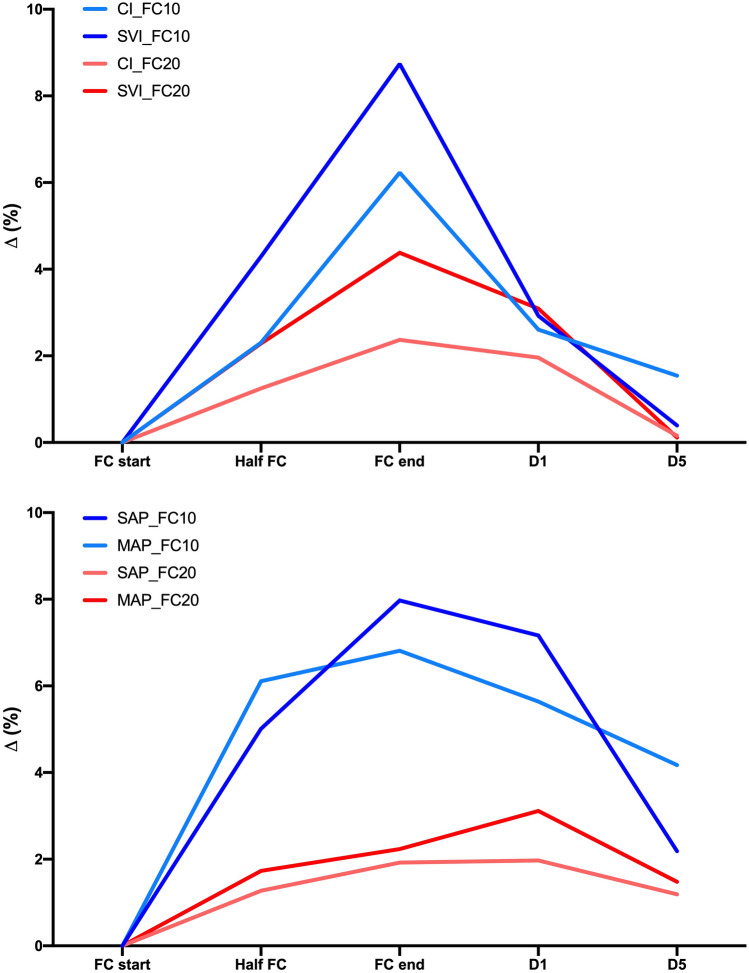
The hemodynamic effect of the two FCs is depicted in the Figs. 3, 4 and 5.
Fig. 5.
Flow and pressure variables’ change (percentage of variation with respect to baseline values) at predefined time-point of FC administration. FC10 = 4 ml kg−1 over 10 min; FC20 = 4 ml kg−1 over 20 min; SAP systolic arterial pressure, MAP mean arterial pressure, SVI stroke volume index, CI cardiac index; d1, 1-min after FC end; d5, 5-min after FC end
During FC10 infusion, the marked pressure response (more evident) and volume variables may be depicted as a “classic” bell-shaped pharmacodynamic curve, implying a sudden increase of the response until the peak is reached, and then a quick dissipation of the effect when the infusion is stopped. On the contrary, the hemodynamic response after FC20 depicts roughly squared curves, implying a plateau of the response reached after about 3–4 min which is maintained until the end of the infusion.
The AUC is affected by the different duration of the infusion since this computation is performed employing a sum of the percentages of increase of the considered variable multiplied by the minutes of observation. For this reason, the AUC comparison between the two tests should be interpreted with caution. The AUC analysis, however, showed that the magnitude of the pressure changes after a FC10 is significantly higher as compared to FC20 (Fig. 5). This result suggests that the coupling between flow and pressure variables in hemodynamically stable surgical patients may also be influenced by the infusion times of the FC, a field of potential interest for future researches.
However, our study shows that this type computation is feasible and may be added to the “standard” analysis of a FC, to define the hemodynamic profile of the infusion.
On the contrary, the interpretation of the other pharmacodynamic variables is more intuitive. The effect in terms of peak and timing was overall comparable, and may be summarized as a maximal increase of 15–20% in pressure variables and of 20–25% of flow variables, reached after about 70–80% of the FC infusion. These data suggest that when the FC administered can challenge the system appropriately, the hemodynamic response is consistent, irrespective of the infusion time adopted. However, the maximum effect is reached close to the end of FC administration, or immediately after, as previously shown by Aya et al. (1 min after FC end) [13].
Finally, in line with the results of Aya et al. [13], the hemodynamic effect of both FC10 and FC20 was overall dissipated within 5 min after the end of the infusion. Several different mechanisms could be implied in the dissipation of the effect of FC. First of all, many years ago, Prather et al. described a stress-relaxation mechanism allowing a rapid return to intravascular pressure baseline in response to a rapid increase in intravascular volume [27]. Moreover, crystalloids are redistributed from the central circulation to the rest of the cardiovascular system, and particularly to the compliant veins [28]. On the contrary, the persistence of the MAP effect (Supplemental Table 3 in the Supplementary Information) may be related to the interplay between the heart and vessels after FC administration. Since flow and pressures variations are not precisely correlated in the cardiovascular system; the two system components would return to the steady-state at different timings. However, the MAP increase at the d5 is below 5% of baseline values, which should be considered clinically irrelevant. Also, it is important to underline that our results have been obtained by infusing crystalloids, and further studies in this field are warranted to assess the pharmacodynamic profile of colloids’ infusion.
The overall analysis of MAP and SVI changes in our study may also be affected by the mathematical coupling between pressure and flow variables changes after the FCs, since the MostCare® system is based on the high sample rate analysis of the arterial waveform and is also very dependent on the quality of the arterial waveform signal [29]. The ability to track SVI changes by the hemodynamic monitoring adopted, which is usually uncalibrated in the operating room [30, 31], is crucial to assess the real effect of FC administration. However, very recently our group published a multicentric study showing that the least significant change of SVI detected by the MostCare® system (4.5%) is largely below the threshold used to define the responsiveness to each FC.
Limitations of the study
This study has some limitations. First, the present study protocol did not exactly follow the trial registration, which reports the use of a “mini-FC” before administering the entire aliquot. This adjunctive test has been planned but never performed because, before the enrolment of the first participant, the authors recognized technical limitations in the described infusion pump systems to manage both the mini-FC and the fixed infusion times of two FC10 and FC20. Data regarding the “mini-FC” have never been recorded, however, this discrepancy should be acknowledged.
Second, extrapolating pharmacodynamic evaluation from surgical patients is challenging. We deliberately aimed at selecting patients during a period of hemodynamic stability in neurosurgery, limiting the external validity of our results in different surgical settings or in critically ill patients. It is uncertain whether these results could also be applied in critically ill unstable patients, encouraging further researchers in this field, aiming at individualizing the modality of FC administration in different settings. Moreover, we adopted a standardized and reproducible electronic infusion set, ensuring a fixed infusion time and reducing the bias related to manual infusion at the different steps of the protocol. Again, this setting is not always available.
Third, the insertion of a central line is not considered a standard clinical practice in the involved centers. We therefore could not obtain hemodynamic preload filling values related to “stressed volume” and venous compliance, according to Guyton’s physiology [32, 33], such as central venous pressure and mean filling pressure analogous. This bias may lead to false-negative FCs. We assumed a 4 ml kg−1 FC volume to be adequate to challenge the system, only considering data from a single-center small-sized study, performed on postoperative cardiac patients [13].
Fourth, as shown in Fig. 1 the enrolment was limited by the availability in the operating room of the hemodynamic tool for the measurements since each center is equipped with only one MostCare®. This technical limitation could have partially biased the selection of those patients simultaneously eligible for the study, implying a choice that was at the discretion of the principal investigator of each center. Moreover, we excluded patients with cardiac dysfunctions. These two limitations may limit the external validity of our results.
Finally, we cannot exclude a carry-over hemodynamic effect of the first FC on the second, potentially biasing our results. In fact, the adopted protocol inevitably led to “clustered” observations, despite the adoption of a randomization of the FC sequence and comparable baseline hemodynamic values before FC administration.
Conclusions
In selected hemodynamically stable surgical patients, the number of fluid responders differs according to the infusion times of FC, being higher, for a fixed dose, after a 10-min infusion, as compared to a 20-min infusion. The effect on flow variables of either FCs fades 5 min after the end of infusion.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
AM: This author conceived the idea for the manuscript, and drafted, wrote, and approved the final version of the manuscript. CP: This author helped in data collection and interpretation and made critical revisions of the manuscript for important intellectual content. SDR: This author helped in data collection and interpretation and made critical revisions of the manuscript for important intellectual content. VD: This author helped in data collection and interpretation and made critical revisions of the manuscript for important intellectual content. EB: This author helped in data collection and interpretation and made critical revisions of the manuscript for important intellectual content. CM: This author helped in data collection and interpretation and made critical revisions of the manuscript for important intellectual content. SB: This author helped in data collection and interpretation and made critical revisions of the manuscript for important intellectual content. FV: This author helped in data collection and interpretation and made critical revisions of the manuscript for important intellectual content. PZ: This author helped in data collection and interpretation and made critical revisions of the manuscript for important intellectual content. KN: This author helped in data interpretation and English revision and made critical revisions of the manuscript for important intellectual content. FDC: This author helped in data interpretation and made critical revisions of the manuscript for important intellectual content. GC: This author helped in data interpretation and made critical revisions of the manuscript for important intellectual content. LS: This author helped in data analysis and interpretation and made critical revisions of the manuscript for important intellectual content. GS: This author performed the data analysis, and drafted, wrote, and approved the final version of the manuscript. MIMG: This author helped in the study design, in data interpretation, made critical revisions of the manuscript for important intellectual content and wrote the draft of the manuscript. MC: This author helped in the study design, in data interpretation, made critical revisions of the manuscript for important intellectual content and wrote the draft of the manuscript.
Funding
This work has not been funded by an external source.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
For the present study: The authors declare that they have no conflict of interest. Unrelated to the present study in the last 36 months: Dr. Messina received travel expenses and registration for meetings, congresses, and courses and lecture fees from Vygon, Edwards and Philips. Dr. Monge Garcia has received Honoraria and/or Travel Expenses from Edwards Lifesciences and Deltex Medical. He also received supply medical equipment (Doppler probes) in return for carrying out research works for Deltex Medical. Prof. Cecconi is a consultant for Edwards Lifesciences, LiDCO and Cheetah Medical.
Ethical approval and consent to participate
This prospective multicenter randomized trial was carried-out in the operating rooms of three Italian tertiary hospitals: the Humanitas Research Hospital (Rozzano, Milano), the University hospital “Maggiore della Carità” (Novara) and the San Bortolo Hospital (Vicenza). The protocol was designed in accordance with the principles outlined in the Declaration of Helsinki; the study was approved by all the local institutional ethics committees [Ethical Committee of the Coordinator Center—Humanitas Research Hospital, Rozzano (Milano; Italy); Protocol Number 92/19; 19 February 2019], and prospectively registered (NCT03810118).
Informed consent
Informed consent was obtained from all the participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. World J Surg. 2013;37(2):259–284. doi: 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 2.Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378(24):2263–2274. doi: 10.1056/NEJMoa1801601. [DOI] [PubMed] [Google Scholar]
- 3.Miller TE, Myles PS. Perioperative fluid therapy for major surgery. Anesthesiology. 2019;130(5):825–832. doi: 10.1097/ALN.0000000000002603. [DOI] [PubMed] [Google Scholar]
- 4.Wrzosek A, Jakowicka-Wordliczek J, Zajaczkowska R, Serednicki WT, Jankowski M, Bala MM, et al. Perioperative restrictive versus goal-directed fluid therapy for adults undergoing major non-cardiac surgery. Cochrane Database Syst Rev. 2019;12:CD012767. doi: 10.1002/14651858.CD012767.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toscani L, Aya HD, Antonakaki D, Bastoni D, Watson X, Arulkumaran N, et al. What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Crit Care. 2017;21(1):207. doi: 10.1186/s13054-017-1796-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marik PE. Perioperative hemodynamic optimization: a revised approach. J Clin Anesth. 2014;26(6):500–505. doi: 10.1016/j.jclinane.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Makaryus R, Miller TE, Gan TJ. Current concepts of fluid management in enhanced recovery pathways. Br J Anaesth. 2018;120(2):376–383. doi: 10.1016/j.bja.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Miller TE, Raghunathan K, Gan TJ. State-of-the-art fluid management in the operating room. Best Pract Res Clin Anaesthesiol. 2014;28(3):261–273. doi: 10.1016/j.bpa.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Miller TE, Roche AM, Mythen M. Fluid management and goal-directed therapy as an adjunct to Enhanced Recovery After Surgery (ERAS) Can J Anaesth/Journal canadien d'anesthesie. 2015;62(2):158–68. doi: 10.1007/s12630-014-0266-y. [DOI] [PubMed] [Google Scholar]
- 10.Thacker JK, Mountford WK, Ernst FR, Krukas MR, Mythen MM. Perioperative fluid utilization variability and association with outcomes: considerations for enhanced recovery efforts in sample US surgical populations. Ann Surg. 2016;263(3):502–510. doi: 10.1097/SLA.0000000000001402. [DOI] [PubMed] [Google Scholar]
- 11.Cecconi M, Parsons AK, Rhodes A. What is a fluid challenge? Curr Opin Crit Care. 2011;17(3):290–295. doi: 10.1097/MCC.0b013e32834699cd. [DOI] [PubMed] [Google Scholar]
- 12.Messina A, Pelaia C, Bruni A, Garofalo E, Bonicolini E, Longhini F, et al. Fluid challenge during anesthesia: a systematic review and meta-analysis. Anesth Analg. 2018;127(6):1353–1364. doi: 10.1213/ANE.0000000000003834. [DOI] [PubMed] [Google Scholar]
- 13.Aya HD, Ster IC, Fletcher N, Grounds RM, Rhodes A, Cecconi M. Pharmacodynamic analysis of a fluid challenge. Crit Care Med. 2016;44(5):880–891. doi: 10.1097/CCM.0000000000001517. [DOI] [PubMed] [Google Scholar]
- 14.Holte K, Kehlet H. Fluid therapy and surgical outcomes in elective surgery: a need for reassessment in fast-track surgery. J Am Coll Surg. 2006;202(6):971–989. doi: 10.1016/j.jamcollsurg.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM, et al. Clinical review: goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013;17(2):209. doi: 10.1186/cc11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112(6):1392–1402. doi: 10.1213/ANE.0b013e3181eeaae5. [DOI] [PubMed] [Google Scholar]
- 17.Lobo SM, de Oliveira NE. Clinical review: what are the best hemodynamic targets for noncardiac surgical patients? Crit Care. 2013;17(2):210. doi: 10.1186/cc11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voldby AW, Brandstrup B. Fluid therapy in the perioperative setting—a clinical review. J Intensive Care. 2016;4:27. doi: 10.1186/s40560-016-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romagnoli S, Ricci Z, Quattrone D, Tofani L, Tujjar O, Villa G, et al. Accuracy of invasive arterial pressure monitoring in cardiovascular patients: an observational study. Crit Care. 2014;18(6):644. doi: 10.1186/s13054-014-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romagnoli S, Franchi F, Ricci Z, Scolletta S, Payen D. The Pressure Recording Analytical Method (PRAM): technical concepts and literature review. J Cardiothorac Vasc Anesth. 2017;31(4):1460–1470. doi: 10.1053/j.jvca.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Scolletta S, Franchi F, Romagnoli S, Carla R, Donati A, Fabbri LP, et al. Comparison between Doppler-echocardiography and uncalibrated pulse contour method for cardiac output measurement: a multicenter observational study. Crit Care Med. 2016;44(7):1370–1379. doi: 10.1097/CCM.0000000000001663. [DOI] [PubMed] [Google Scholar]
- 22.Romano SM. Cardiac cycle efficiency: a new parameter able to fully evaluate the dynamic interplay of the cardiovascular system. Int J Cardiol. 2012;155(2):326–327. doi: 10.1016/j.ijcard.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Biais M, Larghi M, Henriot J, de Courson H, Sesay M, Nouette-Gaulain K. End-expiratory occlusion test predicts fluid responsiveness in patients with protective ventilation in the operating room. Anesth Analg. 2017;125(6):1889–1895. doi: 10.1213/ANE.0000000000002322. [DOI] [PubMed] [Google Scholar]
- 24.Biais M, Lanchon R, Sesay M, Le Gall L, Pereira B, Futier E, et al. Changes in stroke volume induced by lung recruitment maneuver predict fluid responsiveness in mechanically ventilated patients in the operating room. Anesthesiology. 2017;126(2):260–267. doi: 10.1097/ALN.0000000000001459. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Chow S-C. Sample size calculation for comparing proportions. In: D'Agostino RB, Sullivan L, Massaro J, editors. Wiley Encyclopedia of clinical trials. Hoboken: Wiley; 2007. [Google Scholar]
- 26.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prather JW, Taylor AE, Guyton AC. Effect of blood volume, mean circulatory pressure, and stress relaxation on cardiac output. Am J Physiol. 1969;216(3):467–472. doi: 10.1152/ajplegacy.1969.216.3.467. [DOI] [PubMed] [Google Scholar]
- 28.Hahn RG, Lyons G. The half-life of infusion fluids: an educational review. Eur J Anaesthesiol. 2016;33(7):475–482. doi: 10.1097/EJA.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scolletta S, Franchi F, Romagnoli S, Carla R, Donati A, Fabbri LP, et al. Comparison between Doppler-echocardiography and uncalibrated pulse contour method for cardiac output measurement: a multicenter observational study. Crit Care Med. 2016 doi: 10.1097/CCM.0000000000001663. [DOI] [PubMed] [Google Scholar]
- 30.Messina A, Dell'Anna A, Baggiani M, Torrini F, Maresca GM, Bennett V, et al. Functional hemodynamic tests: a systematic review and a metanalysis on the reliability of the end-expiratory occlusion test and of the mini-fluid challenge in predicting fluid responsiveness. Crit Care. 2019;23(1):264. doi: 10.1186/s13054-019-2545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsingh D, Alexander B, Cannesson M. Clinical review: does it matter which hemodynamic monitoring system is used? Crit Care. 2013;17(2):208. doi: 10.1186/cc11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyton AC, Jones CE. Central venous pressure: physiological significance and clinical implications. Am Heart J. 1973;86(4):431–437. doi: 10.1016/0002-8703(73)90132-4. [DOI] [PubMed] [Google Scholar]
- 33.Guyton AC, Richardson TQ, Langston JB. Regulation of cardiac output and venous return. Clin Anesth. 1964;3:1–34. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.