Abstract
Chromium in its trivalent form (chromium (III)) is an essential component of a balanced diet, and its deficiency disturbs glucose and lipid metabolism in humans and animals. The prevailing view is that chromium (III) is notably less toxic than chromium (VI), which is genotoxic and carcinogenic. Thus, the biotransformation of environmental chromium (VI) to chromium (III) is a promising and environmentally friendly detoxification method. However, increasing evidence suggests that chromium (III) induces considerable cytotoxicity. However, the toxicity of chromium (III) to early embryos remains largely unknown. In the present study, we used in vitro fertilization (IVF) to produce mouse embryos and identified the direct embryotoxicity of chromium (III). On exposure to high concentrations of CrCl3, blastocyst formation almost completely failed and a large proportion of embryos were arrested at the 2- to 4-cell stage. At low concentrations of CrCl3, IVF embryos showed a significant decrease in blastocyst formation, reduced total cell numbers, aberrant lineage differentiation, increased oxidative stress, and apoptosis. We also found that chromium (III) exposure during the preimplantation stage, even at low concentrations, led to impaired post-implantation development. Thus, our study substantiates the direct embryotoxicity of chromium (III) during preimplantation development and prolonged impairment of development potential. The results further highlight the potential adverse effects of chromium (III) on public reproductive health with respect to increased environmental enrichment of and dietary supplementation with chromium (III) complexes.
Keywords: Apoptosis, Blastocysts, Chromium (III), Mouse embryos, Toxicity
Heavy metal chromium, derived from electroplating and the tanning industry, is a common metal contaminant. Although organo-chromium complexes are essential for human health, highly enriched chromium in the human body, obtained via food resources or through polluted air and water, is thought to be toxic. Sustained increase in and inappropriate disposal of chromium wastes over recent decades have contributed significantly to the increase in chromium pollution in various environments that exceeds safe limits and negatively impacts human health and ecosystems [1, 2]. Chromium mainly exists in oxidation states II, III, and VI. Of these, chromium (VI) exhibits significant genotoxicity and carcinogenic effects, increasing the risk of various cancers [3,4,5,6]. In addition, embryotoxicity and fetotoxicity of chromium (VI) have been reported in rodent models. Oral administration of potassium dichromate has been reported to lead to a notable decrease in implantation and live births in a dose-dependent manner [7,8,9].
Notably, oxidation states are important variables that influence toxicity. The prevailing view is that chromium (III) is an essential element for carbohydrate and lipid metabolism and that it is noncarcinogenic and much less toxic to most organisms than chromium (VI) [10,11,12]. Thus, the biotransformation of chromium (VI) to chromium (III) via the metabolic pathways of plants and microorganisms has been considered a feasible and practical process to detoxify environmental chromium (VI) contaminants [13,14,15]. In addition, chromium (III) complexes have been recommended as dietary supplements because of their role in the maintenance of normal carbohydrate metabolism in mammals [16, 17]. However, increasing evidence has demonstrated that chromium (III) can also induce apoptosis in various cell types [18,19,20,21,22]. Thus, although less toxic than chromium (VI), chromium (III) also represents a potential health and safety hazard, and the evaluation of its toxicity is of great concern with respect to the substantial environmental enrichment of chromium (III) complexes and the increased use of chromium (III) complexes as dietary supplements [23,24,25].
Although a previous study based on long-term oral administration indicated that exposure of female mice to chromium (III) significantly reduced the number of implantation sites and viable fetuses [26], it could not exclude the possibility that reduced implantation and viability may be due to impaired oocyte quality or uterine receptivity. These considerations, together with the fact that early embryos are highly susceptible to spontaneous and induced apoptosis [27,28,29], led us to question whether chromium (III) has direct embryotoxicity and whether it impairs embryo development potential, as the potential results would enrich our current knowledge of the adverse effects of chromium (III) on reproductive health. The present study aimed to determine the toxic effects of chromium trichloride (CrCl3) exposure on preimplantation embryos in terms of embryo quality and development potential by assessing blastocyst development rate, apoptosis, cell proliferation, and lineage during blastocyst formation. In addition, given that the preimplantation stage is a critical developmental window wherein embryos gain implantation and differentiation competency, implantation success and development rate shortly after implantation were evaluated.
Materials and Methods
All reagents and chemicals used in this study, except chromium (III) chloride hexahydrate (CrCl3·6H2O) or other special supplements, were purchased from Millipore-Sigma (Burlington, MA, USA). CrCl3·6H2O was purchased from the China National Pharmaceutical Group Corporation (Beijing, China).
Animals
Two-month-old ICR female mice were fed ad libitum and housed under controlled lighting (12 light:12 dark) and specific pathogen-free conditions. Superovulation was induced by an intraperitoneal (i.p.) injection of 10 IU of pregnant mare serum gonadotropin (PMSG), and 48 h later, an i.p. injection of 10 IU of human chorionic gonadotropin (hCG). Oocyte–cumulus complexes were isolated from the oviducts of mice 14–16 h after the administration of hCG.
Oocyte collection and in vitro fertilization (IVF)
Previous studies showed that chromium exposure led to a decreased number of ovulated oocytes, a disrupted estrus cycle, changes in placental morphology, and a decrease in sperm viability [7, 30, 31]; therefore, in the present study, we used mouse embryos generated under standardized IVF conditions, which excluded the possible influences of these variables, to evaluate the direct toxic effect of chromium (III) on early embryos during preimplantation development. All experiments involving embryo preparation were performed as described previously [32]. Sperm from male ICR mice were released in human tubal fluid (HTF) medium (SAGE, Bedminster, NJ, USA) supplemented with 4 mg/ml bovine serum albumin (BSA; Sigma-Aldrich, St Louis, MO, USA) and 0.25 mM glutathione (GSH; Sigma-Aldrich). The sperm were suspended in a 200-μl droplet of the IVF medium, covered with mineral oil, and incubated at 37°C for 1 h in a humidified atmosphere of 5% CO2 for capacitation. Oocyte–cumulus complexes were placed in a 200-μl droplet of HTF medium under mineral oil. Capacitated sperm were added to each droplet to obtain a concentration of 1–2 × 106 motile sperm/ml. After co-incubation for 6 h at 37°C, oocytes were removed and washed in fresh potassium simplex optimization medium containing amino acids (KSOM+AA; Millipore, Billerica, MA, USA) and placed at 37°C in 5% CO2. Zygotes were washed and cultured to the blastocyst stage in KSOM+AA medium under mineral oil at 37°C in a humidified atmosphere of 5% CO2 and 20% O2.
Experimental design
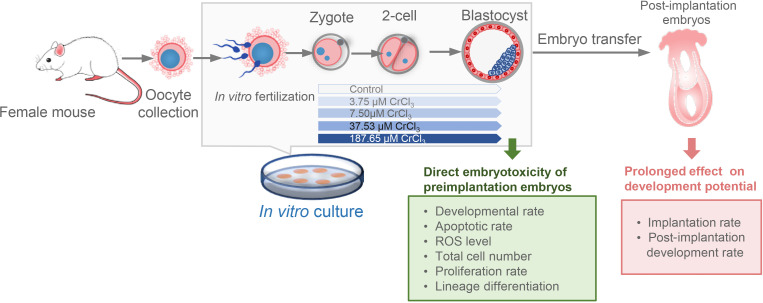
After IVF, zygotes were washed and randomly divided into different groups, which were further cultured in KSOM+AA medium supplemented with CrCl3 at different concentrations (1, 2, 10, and 50 μg/ml, equivalent to 3.75, 7.50, 37.53, and 187.65 μM, respectively) or without CrCl3 (control group), under mineral oil at 37°C in a humidified atmosphere of 5% CO2 and 20% O2. Given that the sensitivity to chromium (III)-induced toxicity is variable among cell types, and the toxic effect of chromium (III) on early embryos has not been reported under in vitro exposure conditions, we used a series of concentrations ranging from a low concentration (1 µg/ml, equivalent to 3.75 μM) to a high concentration (50 µg/ml, equivalent to 187.65 μM), which are generally comparable to the concentration ranges reported in previous studies [18, 20,21,22]. After 42–44 h and 106–119 h of hCG injection, 2-cell embryos and blastocysts were detected to evaluate their development rates. Blastocysts were collected to evaluate the direct embryotoxicity of preimplantation embryos. Implantation success and development rate shortly after implantation were also tested to evaluate the prolonged effect of chromium (III) exposure on embryo development potential (Fig. 1).
Fig. 1.
Schematic diagram of the experimental design. To evaluate the direct toxicity and prolonged effect of chromium (III) exposure on early embryos, mouse embryos were generated under standardized IVF conditions and cultured in medium supplemented with CrCl3 at different concentrations. Developmental and cellular characteristics were detected at preimplantation and post-implantation stages, respectively.
Measurement of reactive oxygen species (ROS)
The intracellular reactive oxygen species (ROS) levels of 2-cell and blastocysts were determined using the Reactive Oxygen Species Assay Kit (Beyotime Institute of Biotechnology, Nanjing, China), according to the manufacturer’s instructions. Fluorescent signals were acquired using an upright microscope (BX51; Olympus, Tokyo, Japan) with an attached digital microscope camera (DP72; Olympus). Fluorescence intensity was calculated using ImageJ software (https://imagej.nih.gov/ij/).
Apoptosis analysis
At the 2-cell and blastocyst stages, the embryos were washed three times with 0.1% polyvinyl alcohol (PVA)/phosphate-buffered saline (PBS) and transferred to PBS supplemented with 4% (v/v) paraformaldehyde and 0.5% Triton X-100 for simultaneous fixation and permeabilization at 37°C for 45 min. A terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was used to assess the presence of apoptotic nuclei (in situ cell death detection kit, TMR red; Roche, Mannheim, Germany). According to the manufacturer’s instructions, fixed embryos were incubated in TUNEL reaction medium for 1 h at 37°C in the dark. After the reaction was stopped, the embryos were washed, transferred into 2 μg/ml 4,6-diamidine-2-phenylindole dihydrochloride solution (DAPI, Roche), and mounted on slides.
RNA isolation and quantitative real-time reverse transcription PCR (qRT-PCR)
Total RNA was extracted from mouse embryo blastocysts using TRIzol reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. Before reverse transcription (RT), RNA samples were digested with DNase I (EN0521; Fermentas, Hanover, CT, USA) to remove contaminating genomic DNA. The concentration and quality of the extracted total RNA (A 260 nm/A 280 nm and A 260 nm/A 230 nm ratio) were assessed using a DS-11 spectrophotometer (Denovix, Wilmington, DE, USA). Reverse transcription was performed using a commercially available first-strand cDNA synthesis kit (iScript cDNA Synthesis Kit, Bio-Rad Laboratories, Hercules, CA, USA). Real-time PCR was performed in a Bio-Rad CFX96 Real-Time PCR System using SsoFast EvaGreen Supermix (Bio-Rad Laboratories) and cDNA as a template. The following primers were used in the present study: caspase 3 (Casp3) (F/R: ACAGCACCTGGTTACTATTC/CAGTTCTTTCGT-GAGCAT), B-cell lymphoma 2 (Bcl2) (F/R: GTGGATGACTGAGTACCTGAA-CC/AGCCAGGAGAAATCAAACAGAG), glyceraldehyde-3-phosphate dehydrogenase (Gapdh) (F/R: TGCCCCCATGTTTGTGATG/TGTGGTCATGAGCC-CTTCC).
5-ethynyl-2'-deoxyuridine (EdU) assay
The proliferation of embryos was detected using a BeyoClick EdU-594 Cell Proliferation Kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Embryos were incubated in KSOM+AA medium containing 10 μM EdU at 37 °C for 2 h, and then fixed and permeabilized. Embryos were then incubated with the click reaction mixture for 1 min at room temperature before staining with DAPI.
Immunofluorescence analysis
Embryos were fixed with 4% paraformaldehyde in PBS–0.1% PVA at 4°C overnight. After permeabilization with 0.5% Triton X-100 in PBS–0.1% PVA (PBST–PVA) for 1 h at room temperature, embryos were blocked with 1% BSA in PBST–PVA at 4°C for 6 h. Embryos were incubated with primary anti-NANOG antibody (1:500; Abcam, Cambridge, MA, USA), and anti CDX2 antibody (1:200; BioGenex, Fremont, CA, USA) at 4°C overnight, followed by Alexa Fluor-488 (anti-mouse; Invitrogen) and Alexa Fluor-594 (anti-rabbit; Invitrogen)-labeled secondary antibodies for 1 h at room temperature. Finally, the embryos were counterstained with DAPI.
Embryo transfer
Pseudopregnant ICR females were mated with ICR males 3.5 days before embryo transfer. Blastocysts exposed to low concentrations of CrCl3 were transferred to the uterine horn of pseudopregnant female mice. Implantation was determined at E4.5 by intravenous injection of 0.1 ml of Chicago sky blue dye solution (1% in saline; Sigma) 5 min before sacrificing [33].
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used to compare the differences among groups using SPSS (version 25.0; IBM, Armonk, NY, USA). Statistical significance was set at P < 0.05.
Registration and ethics committee approval
All animal experiments were approved by and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of China Agricultural University (AW42210202-1). Oocyte collection, IVF, and developmental evaluation were conducted at the Laboratory Animal Research Center of China Agriculture University; gene expression detection, apoptosis analysis, and cell number quantification were conducted at the Key Laboratory of Animal Genetics, Breeding and Reproduction of the Ministry of Agriculture and Rural Affairs.
Results
High concentrations of chromium (III) block blastocyst formation
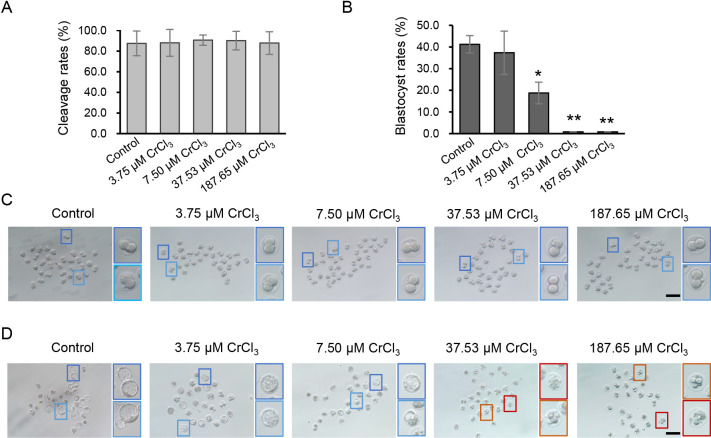
To test the direct embryotoxicity of chromium (III) during preimplantation development, IVF zygotes were cultured in KSOM supplemented with CrCl3 at different concentrations (3.75, 7.50, 37.53, and 187.65 μM). By the cleavage stage, there was no significant difference in the formation of 2-cell embryos between the control and treatment groups (Fig. 2A). By the blastocyst stage, however, complete failure of blastocyst formation was observed when high concentrations of CrCl3 (37.53 and 187.65 μM) were added to the culture medium (Fig. 2B). Detailed morphological analysis showed that a large proportion of embryos exposed to high concentrations of CrCl3 displayed developmental arrest between the 2-cell and 4-cell stages or embryo fragmentation (Fig. 2D). Notably, 7.50 μM CrCl3 did not block early embryonic cleavage (Figs. 2A, C) but decreased subsequent blastocyst formation significantly (P < 0.05) (Figs. 2B, D).
Fig. 2.
Effects of different concentrations of CrCl3 on development rate of preimplantation embryos. (A and B) Cleavage rate (A) and blastocyst development rate (B) of embryos exposed to different concentrations of CrCl3. (C and D) Representative images of cleavage embryos exposed to different concentrations of CrCl3 at E1.5 (C) and E4.0 (D). Higher magnification of light and dark blue box regions indicates well-developing 2-cell embryos or blastocysts in each group. Higher magnification of orange and red box regions indicates embryos arrested at the 4-cell stage or embryos showing a representative fragmentation pattern. * P < 0.05; ** P < 0.01. Three independent experiments were performed.
Low concentrations of chromium (III) decrease blastocyst quality
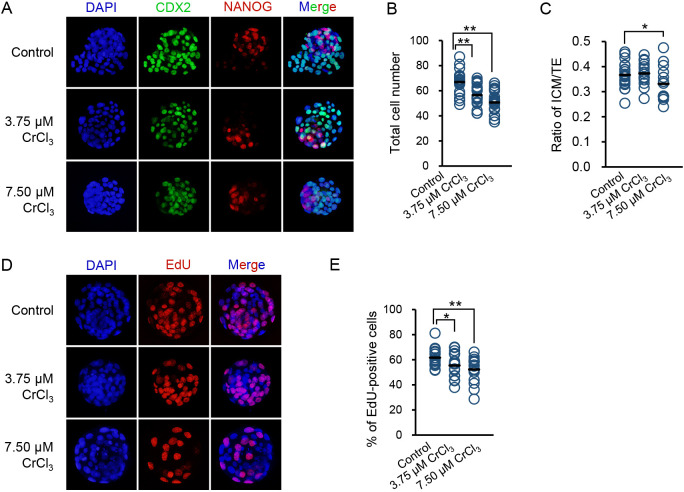
Preimplantation embryos appeared to be resistant to low concentrations of chromium (III), because 3.75 μM CrCl3 did not decrease the blastocyst development rate. However, we noticed that the developmental progression of embryos exposed to 3.75 μM CrCl3 was delayed, because only a few hatching blastocysts could be observed on embryonic day (E) 4.0, whereas a substantial proportion of embryos had hatched in the control group (Fig. 2D). Thus, we speculated that low concentrations of chromium (III) impair blastocyst quality. To test this, we measured the total cell number, lineage differentiation, and proliferation rate, which are thought to be tightly associated with the implantation and development potential, in blastocysts exposed to low concentrations of chromium (III). Compared with blastocysts in the control group, blastocysts exposed to either 3.75 μM or 7.50 μM CrCl3 displayed a significant decrease in the total cell number (Figs. 3A, B). In addition, 7.50 μM CrCl3 changed the ICM:TE ratio of blastocysts, an indicator of preimplantation lineage differentiation (Figs. 3A, C). In line with the reduced total cell number, we also found that exposure to both 3.75 μM and 7.50 μM CrCl3 significantly inhibited the proliferation of blastocysts, as indicated by the reduced percentage of EdU-positive cells (Figs. 3D, E).
Fig. 3.
Effects of low concentrations of CrCl3 on the quality of blastocysts. (A) Representative images of CDX2, NANOG, and DAPI staining of blastocysts exposed to low concentrations of CrCl3 or no CrCl3. (B and C) Quantification of total cell number (B) and ICM:TE ratio (C) of detected blastocysts in each group. (D) Representative images of EdU staining of blastocysts exposed to low concentrations of CrCl3 or no CrCl3. (E) Quantification of EdU-positive cells of detected blastocysts in each group. * P < 0.05; ** P < 0.01. Three independent experiments were performed.
Low concentrations of chromium (III) induce oxidative stress and activate caspase3 signaling
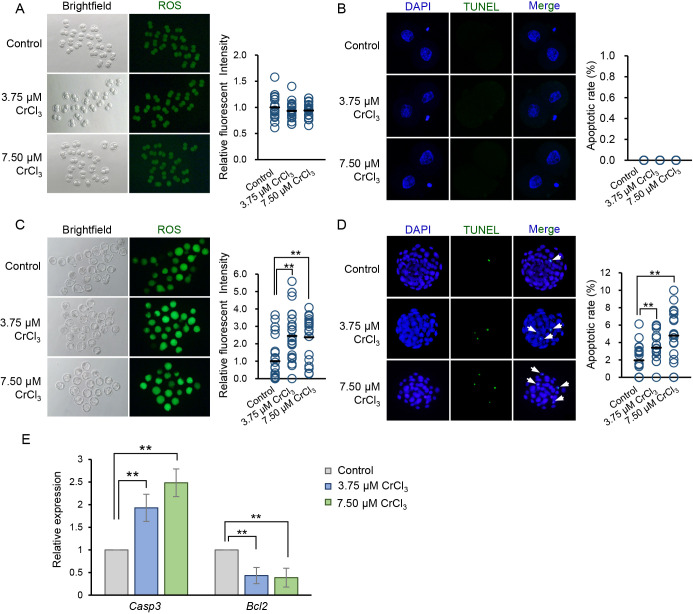
Since previous evidence demonstrated that oxidative stress and induced apoptosis may underlie the toxicity of chromium (III) [18, 20,21,22], we next detected ROS accumulation and apoptosis in cleavage embryos and blastocysts, respectively. In line with the results of preimplantation development rate (Figs. 2A, B), we found that neither 3.75 μM nor 7.50 μM CrCl3 exposure induced ROS elevation and apoptosis in 2-cell embryos (Figs. 4A, B). However, ROS levels and the apoptotic rate of blastocysts exposed to 3.75 μM or 7.50 μM CrCl3 were significantly higher than those in the control group (Figs. 4C, D).
Fig. 4.
Effects of low concentrations of CrCl3 on ROS accumulation and apoptosis occurrence in cleavage embryos and blastocysts. (A and C) Representative fluorescent images of ROS in 2-cell embryos (A) and blastocysts (C) exposed to low concentrations of CrCl3 or no CrCl3. Right panel: quantification of ROS levels of embryos in each group. (B and D) Representative images of apoptosis detected using the TUNEL assay in 2-cell embryos (B) and blastocysts (D). TUNEL-positive apoptotic nuclei are indicated by arrows. Right panel: quantification of the apoptotic rate of embryos in each group. (E) Effect of low concentrations of CrCl3 on the expression levels of representative pro-apoptotic and anti-apoptotic genes in blastocysts. Data are presented as the mean ± SD. ** P < 0.01. Three independent experiments were performed, and at least 20 embryos were evaluated in each group for each replicate.
Apoptosis is regulated by a variety of pro-apoptotic and anti-apoptotic proteins. To understand the involvement of these proteins in low concentrations of chromium (III)-induced apoptosis, we detected the influence of 3.75 μM or 7.50 μM CrCl3 exposure on the expression of Casp3 and Bcl2, which are representative pro-apoptotic and anti-apoptotic genes, respectively, in IVF blastocysts. Exposure to 3.75 μM or 7.50 μM CrCl3 increased the expression levels of Casp3 significantly (P < 0.01), in a dose-dependent manner; by contrast, the expression of Bcl2 was inhibited significantly in CrCl3-treated blastocysts (P < 0.01) (Fig. 4E).
Exposure to low concentrations of chromium (III) during the preimplantation stage impairs post-implantation development
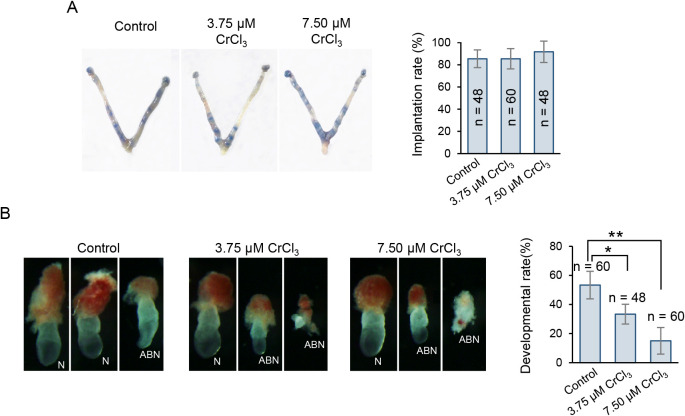
Given that the quality of blastocysts was significantly reduced owing to low-dose chromium (III) exposure, we next attempted to determine whether exposure would further impair the implantation process or post-implantation development. To this end, we transferred blastocytes exposed to 3.75 μM or 7.50 μM CrCl3 into pseudopregnant recipient females and detected the implantation success and development rate shortly after implantation. Although exposed embryos could normally complete the implantation process (Fig. 5A), a greater incidence of morphological abnormalities, including delayed gastrulation and disorganized or degraded embryonic/extraembryonic tissues, could be observed shortly after implantation (Fig. 5B), suggesting prolonged impairment of development potential due to prior chromium (III) exposure. In addition, similar to the dose-dependent decrease in blastocyst formation (Fig. 2B), chromium (III) exposure during the preimplantation stage also tended to impair post-implantation development in a dose-dependent manner.
Fig. 5.
Effect of low-dose CrCl3 exposure during the preimplantation stage on subsequent implantation success and post-implantation development. (A) Representative pictures of uterine morphology and implantation sites. Twelve blastocytes exposed to low concentrations of CrCl3 or no CrCl3 were transferred into each pseudopregnant recipient female. Implantation was determined at E4.5 by the blue dye method; each blue band indicates an implantation site. Right panel: quantification of implantation rate in each group. (B) Representative images of E7.5 embryos with normal (N) or abnormal (ABN) morphologies. Right panel: quantification of development rate of embryos with normal morphology in each group. The number (n) of embryos examined in each group is indicated.
Discussion
Chromium (VI) and chromium (III) are the most stable and common forms of chromium. Unlike genotoxic and carcinogenic chromium (VI), chromium (III) is thought to be an essential trace element known for its role in the maintenance of normal carbohydrate metabolism in mammals [17]. Moreover, it has been suggested that chromium (III) is involved in maintaining the tertiary structure of proteins and inducing DNA conformational changes [34, 35]. Thus, biotransformation of chromium (VI) to chromium (III) via microbial or plant metabolism has been considered a feasible and practical process for the detoxification of environmental chromium (VI) contaminants [13,14,15]. However, increasing evidence suggests that chromium (III), although much less toxic than chromium (VI), can also induce cytotoxicity via increased apoptosis and oxidative stress [18,19,20,21,22]. However, the toxic effects of chromium (III) on early embryos have not been determined yet. In particular, given that early embryos are highly susceptible to spontaneous and induced apoptosis [27, 28], evaluating the embryotoxicity of chromium (III) would be of great significance with regard to the increased environmental enrichment of and dietary supplementation with chromium (III) complexes.
Previous studies have reported that chromium exposure has toxic effects on the maternal uterus, hormone levels, and sperm viability [7, 30, 31]. In particular, a previous study based on long-term oral administration showed a reduction in the number of implantation sites and viable fetuses owing to chromium (III) exposure [26]. However, it also could not exclude the possible involvement of impaired oocyte quality or uterine receptivity. Therefore, we used IVF mouse embryos as a model to exclude the possible influence of these variables. We showed that chromium (III) induces direct embryotoxicity in a time- and dose-dependent manner. High-dose exposure led to severe embryotoxicity and an almost complete failure of blastocyst formation; a large proportion of the embryos were arrested at the 2- to 4-cell stage or showed a representative pattern of fragmentation. However, at high concentrations, short-term exposure did not impair embryonic cleavage; in contrast, extended exposure at a low concentration could also significantly reduce blastocyst formation. These findings are consistent with the results of ROS accumulation and apoptosis. The time- and dose-dependent toxic effects were similar to those reported in other cell types in previous studies [18, 20, 22]. An alternative possible explanation for these observations is that, compared with cleavage embryos, embryos at later stages of preimplantation development may be more sensitive to chromium (III)-induced embryotoxicity.
In addition to reduced blastocyst formation, we showed that developmental progression, total cell number, lineage differentiation, and blastocyst proliferation rate were significantly decreased, and the apoptosis rate increased, all of which are indicators of poor blastocyst quality. Results following embryo transfer also support these findings: chromium (III) exposure during the preimplantation stage, even at low concentrations, led to the prolonged impairment of development potential. Our results also indicated that activated caspase-3 signaling might be involved in chromium (III)-induced apoptosis in blastocysts, which was similar to that reported in lymphocytes [20]. In addition to its effects in increasing pro-apoptotic casp3 expression, we found that low-dose chromium (III) exposure significantly inhibited the expression of anti-apoptotic bcl2, implying that cellular apoptotic homeostasis is disrupted by chromium (III) exposure. Further to chromium (III)-induced apoptosis, the decrease in blastocyst cell numbers suggested that chromium (III) exposure, even at low concentrations, can inhibit embryonic cell proliferation. This is in line with previous observations of other cell types [21, 36].
Compared with those of chromium (VI), which is reported to pass through the placental barrier and accumulate in the fetus [8, 37], the distribution, transport, and accumulation of chromium (III) remain unclear. However, our results raise concerns over the safety of chromium (III), especially in terms of its long-term intake via contaminated food or water or as a long-term nutritional supplement [38].
Collectively, although the underlying mechanism remains to be determined, we provide direct evidence of the embryotoxicity of chromium (III). Chromium (III) exposure during the preimplantation stage not only is directly toxic to the blastocyst quality, but also has a prolonged adverse effect on subsequent post-implantation development. Our study highlights safety concerns over the long-term intake of chromium (III) and challenges the existing detoxification strategy for chromium (VI), which might result in the further environmental enrichment of chromium (III), thus threatening public reproductive health.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by grants from the National Key R&D Program (2017YFD0501905 and 2017YFD0501901) and the National Transgenic Major Program (2009ZX08006-008B and 2013ZX08006-002).
References
- 1.Garg UK, Kaur MP, Garg VK, Sud D. Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J Hazard Mater 2007; 140: 60–68. [DOI] [PubMed] [Google Scholar]
- 2.Dhal B, Thatoi HN, Das NN, Pandey BD. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: a review. J Hazard Mater 2013; 250–251: 272–291. [DOI] [PubMed] [Google Scholar]
- 3.Davidson T, Kluz T, Burns F, Rossman T, Zhang Q, Uddin A, Nadas A, Costa M. Exposure to chromium (VI) in the drinking water increases susceptibility to UV-induced skin tumors in hairless mice. Toxicol Appl Pharmacol 2004; 196: 431–437. [DOI] [PubMed] [Google Scholar]
- 4.National Toxicology Program. Toxicology and carcinogenesis studies of sodium dichromate dihydrate (Cas No. 7789-12-0) in F344/N rats and B6C3F1 mice (drinking water studies). Natl Toxicol Program Tech Rep Ser 2008; 546: 1–192. [PubMed] [Google Scholar]
- 5.Beveridge R, Pintos J, Parent ME, Asselin J, Siemiatycki J. Lung cancer risk associated with occupational exposure to nickel, chromium VI, and cadmium in two population-based case-control studies in Montreal. Am J Ind Med 2010; 53: 476–485. [DOI] [PubMed] [Google Scholar]
- 6.Welling R, Beaumont JJ, Petersen SJ, Alexeeff GV, Steinmaus C. Chromium VI and stomach cancer: a meta-analysis of the current epidemiological evidence. Occup Environ Med 2015; 72: 151–159. [DOI] [PubMed] [Google Scholar]
- 7.Junaid M, Murthy RC, Saxena DK. Embryo- and fetotoxicity of chromium in pregestationally exposed mice. Bull Environ Contam Toxicol 1996; 57: 327–334. [DOI] [PubMed] [Google Scholar]
- 8.Junaid M, Murthy RC, Saxena DK. Embryotoxicity of orally administered chromium in mice: exposure during the period of organogenesis. Toxicol Lett 1996; 84: 143–148. [DOI] [PubMed] [Google Scholar]
- 9.Kanojia RK, Junaid M, Murthy RC. Embryo and fetotoxicity of hexavalent chromium: a long-term study. Toxicol Lett 1998; 95: 165–172. [DOI] [PubMed] [Google Scholar]
- 10.Bagchi D, Stohs SJ, Downs BW, Bagchi M, Preuss HG. Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 2002; 180: 5–22. [DOI] [PubMed] [Google Scholar]
- 11.Sivakumar S, Subbhuraam CV. Toxicity of chromium(III) and chromium(VI) to the earthworm Eisenia fetida. Ecotoxicol Environ Saf 2005; 62: 93–98. [DOI] [PubMed] [Google Scholar]
- 12.Vignati DA, Dominik J, Beye ML, Pettine M, Ferrari BJ. Chromium(VI) is more toxic than chromium(III) to freshwater algae: a paradigm to revise? Ecotoxicol Environ Saf 2010; 73: 743–749. [DOI] [PubMed] [Google Scholar]
- 13.Lytle CM, Lytle FW, Yang N, Qian J-H, Hansen D, Zayed A, Terry N. Reduction of Cr(VI) to Cr(III) by Wetland Plants: Potential for In Situ Heavy Metal Detoxification. Environ Sci Technol 1998; 32: 3087–3093. [Google Scholar]
- 14.Jobby R, Jha P, Yadav AK, Desai N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere 2018; 207: 255–266. [DOI] [PubMed] [Google Scholar]
- 15.Chang J, Deng S, Liang Y, Chen J. Cr(VI) removal performance from aqueous solution by Pseudomonas sp. strain DC-B3 isolated from mine soil: characterization of both Cr(VI) bioreduction and total Cr biosorption processes. Environ Sci Pollut Res Int 2019; 26: 28135–28145. [DOI] [PubMed] [Google Scholar]
- 16.Stearns DM, Belbruno JJ, Wetterhahn KE. A prediction of chromium(III) accumulation in humans from chromium dietary supplements. FASEB J 1995; 9: 1650–1657. [DOI] [PubMed] [Google Scholar]
- 17.Debski B, Zalewski W, Gralak MA, Kosla T. Chromium-yeast supplementation of chicken broilers in an industrial farming system. J Trace Elem Med Biol 2004; 18: 47–51. [DOI] [PubMed] [Google Scholar]
- 18.Balamurugan K, Rajaram R, Ramasami T, Narayanan S. Chromium(III)-induced apoptosis of lymphocytes: death decision by ROS and Src-family tyrosine kinases. Free Radic Biol Med 2002; 33: 1622–1640. [DOI] [PubMed] [Google Scholar]
- 19.Rudolf E, Cervinka M. Chromium (III) produces distinct type of cell death in cultured cells. Acta Med (Hradec Kralove) 2003; 46: 139–146. [PubMed] [Google Scholar]
- 20.Balamurugan K, Rajaram R, Ramasami T. Caspase-3: its potential involvement in Cr(III)-induced apoptosis of lymphocytes. Mol Cell Biochem 2004; 259: 43–51. [DOI] [PubMed] [Google Scholar]
- 21.Shrivastava HY, Ravikumar T, Shanmugasundaram N, Babu M, Unni Nair B. Cytotoxicity studies of chromium(III) complexes on human dermal fibroblasts. Free Radic Biol Med 2005; 38: 58–69. [DOI] [PubMed] [Google Scholar]
- 22.Horie M, Nishio K, Endoh S, Kato H, Fujita K, Miyauchi A, Nakamura A, Kinugasa S, Yamamoto K, Niki E, Yoshida Y, Iwahashi H. Chromium(III) oxide nanoparticles induced remarkable oxidative stress and apoptosis on culture cells. Environ Toxicol 2013; 28: 61–75. [DOI] [PubMed] [Google Scholar]
- 23.Levina A, Lay PA. Chemical properties and toxicity of chromium(III) nutritional supplements. Chem Res Toxicol 2008; 21: 563–571. [DOI] [PubMed] [Google Scholar]
- 24.Staniek H, Krejpcio Z, Iwanik K. Evaluation of the acute oral toxicity class of tricentric chromium(III) propionate complex in rat. Food Chem Toxicol 2010; 48: 859–864. [DOI] [PubMed] [Google Scholar]
- 25.Staniek H, Krejpcio Z, Iwanik K, Szymusiak H, Wieczorek D. Evaluation of the acute oral toxicity class of trinuclear chromium(III) glycinate complex in rat. Biol Trace Elem Res 2011; 143: 1564–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elbetieha A, Al-Hamood MH. Long-term exposure of male and female mice to trivalent and hexavalent chromium compounds: effect on fertility. Toxicology 1997; 116: 39–47. [DOI] [PubMed] [Google Scholar]
- 27.Jurisicova A, Varmuza S, Casper RF. Programmed cell death and human embryo fragmentation. Mol Hum Reprod 1996; 2: 93–98. [DOI] [PubMed] [Google Scholar]
- 28.Fabian D, Makarevich AV, Chrenek P, Bukovská A, Koppel J. Chronological appearance of spontaneous and induced apoptosis during preimplantation development of rabbit and mouse embryos. Theriogenology 2007; 68: 1271–1281. [DOI] [PubMed] [Google Scholar]
- 29.Jurisicova A, Latham KE, Casper RF, Casper RF, Varmuza SL. Expression and regulation of genes associated with cell death during murine preimplantation embryo development. Mol Reprod Dev 1998; 51: 243–253. [DOI] [PubMed] [Google Scholar]
- 30.Murthy RC, Junaid M, Saxena DK. Ovarian dysfunction in mice following chromium (VI) exposure. Toxicol Lett 1996; 89: 147–154. [DOI] [PubMed] [Google Scholar]
- 31.Yoisungnern T, Das J, Choi YJ, Parnpai R, Kim JH. Effect of hexavalent chromium-treated sperm on in vitro fertilization and embryo development. Toxicol Ind Health 2016; 32: 1700–1710. [DOI] [PubMed] [Google Scholar]
- 32.Behringer R, Gertsenstein M, Nagy KV, Nagy A. Manipulating the Mouse Embryo: A Laboratory Manual, Fourth Edition. United States of America: Cold Spring Harbor Press; 2014. [Google Scholar]
- 33.Liang XH, Deng WB, Li M, Zhao ZA, Wang TS, Feng XH, Cao YJ, Duan EK, Yang ZM. Egr1 protein acts downstream of estrogen-leukemia inhibitory factor (LIF)-STAT3 pathway and plays a role during implantation through targeting Wnt4. J Biol Chem 2014; 289: 23534–23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zetić VG, Stehlik-Tomas V, Grba S, Lutilsky L, Kozlek D. Chromium uptake by Saccharomyces cerevisiae and isolation of glucose tolerance factor from yeast biomass. J Biosci 2001; 26: 217–223. [DOI] [PubMed] [Google Scholar]
- 35.Zayed AM, Terry N. Chromium in the environment: factors affecting biological remediation. Plant Soil 2003; 249: 139–156. [Google Scholar]
- 36.Rajaram R, Nair BU, Ramasami T. Chromium(III) induced abnormalities in human lymphocyte cell proliferation: evidence for apoptosis. Biochem Biophys Res Commun 1995; 210: 434–440. [DOI] [PubMed] [Google Scholar]
- 37.Shmitova LA. [Content of hexavalent chromium in the biological substrates of pregnant women and puerperae engaged in the manufacture of chromium compounds]. Gig Tr Prof Zabol 1980; 2: 33–35. [PubMed] [Google Scholar]
- 38.Speetjens JK, Collins RA, Vincent JB, Woski SA. The nutritional supplement chromium(III) tris(picolinate) cleaves DNA. Chem Res Toxicol 1999; 12: 483–487. [DOI] [PubMed] [Google Scholar]