Abstract
In the present study, we investigated the regulatory mechanisms underlying sperm hyperactivation enhanced by 5-hydroxytryptamine (5-HT) in hamsters. First, we examined the types of 5-HT receptors that regulate hyperactivation. Hyperactivation was significantly enhanced by 5-HT2A and 5-HT4 receptor agonists. Moreover, the results of the motility assay revealed that 5-HT2A, 5-HT3, and 5-HT4 receptor agonists significantly decreased the velocity and/or amplitude of sperm. Under 5-HT2 receptor stimulation, hyperactivation was associated with phospholipase C (PLC), inositol 1,4,5-trisphosphate (IP3) receptor, soluble adenylate cyclase (sAC), and protein kinase A (PKA). In contrast, under 5-HT4 receptor stimulation, hyperactivation was associated with transmembrane adenylate cyclase (tmAC), sAC, PKA, and CatSper channels. Accordingly, under the condition that sperm are hyperactivated, 5-HT likely stimulates PLC/IP3 receptor signals via the 5-HT2A receptor and tmAC/PKA/CatSper channel signals via the 5-HT4 receptor. After sAC and PKA are activated by these stimulations, sperm hyperactivation is enhanced.
Keywords: 5-Hydroxytryptamine (5-HT), 5-HT2 receptor, 5-HT4 receptor, Hyperactivation, Sperm
Mammalian sperm are activated after ejaculation and are capacitated in the oviduct. Under in vitro capacitation conditions, sperm are reportedly capacitated after activation. During capacitation, sperm motility changes from activated to hyperactivated [1, 2] (Supplementary movie 1 and Supplementary movie 2). Activated sperm motility consists of a small-bend amplitude and linear swimming patterns. In contrast, hyperactivated sperm motility consists of a large amplitude and a substantial asymmetric beating pattern [1, 2] (Supplementary movie 1). Notably, hyperactivation allows sperm to move through the oocyte envelope [1, 2]. In addition, capacitated sperm exhibit an acrosome reaction that exposes proteases for digestion of the oocyte envelope [2].
Under in vitro capacitation conditions, albumin, Ca2+, and HCO3- play important roles [2]. Mammalian sperm are not hyperactivated in the absence of albumin [3, 4]. Albumin removes cholesterol from the sperm cell membrane [5] and induces Ca2+ influx via the CatSper channel [6]. Ca2+ and HCO3– activate soluble adenylate cyclase (sAC) and increase cAMP concentrations [7,8,9,10]. Moreover, Ca2+ and cAMP control phosphorylation, activating protein kinases and phosphatases [2, 10,11,12].
5-Hydroxytryptamine (5-HT) is a neurotransmitter formed by hydroxylation and decarboxylation of tryptophan. In several tissues and organs, 5-HT controls numerous functions via specific receptors [13, 14]. Notably, 5-HT receptors are composed of seven types (5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6, and 5-HT7). The 5-HT1 receptor consists of five subtypes (5-HT1A, 5-HT1B, 5-HT1C, 5-HT1D, and 5-HT1F), and the 5-HT2 receptor consists of three subtypes (5-HT2A, 5-HT2B, and 5-HT2C). 5-HT1 and 5-HT5 receptors inhibit transmembrane adenylate cyclase (tmAC) through a Gi-protein, decreasing cAMP concentrations; however, 5-HT4, 5-HT6, and 5-HT7 receptors activate tmAC through Gs-protein and increase cAMP concentrations. The 5-HT2 receptor activates phospholipase C (PLC) through a Gq-protein and increases the inositol 1,4,5-trisphosphate (IP3) concentration. IP3 binds to the IP3 receptor (IP3R) and releases Ca2+ from the Ca2+-store. The 5-HT3 receptor is a ligand-gated ion channel. As 5-HT and 5-HT receptors can be detected in mammalian reproductive organs such as ovaries, testes, oocytes, cumulus-oocyte complexes (COCs), follicular fluid, and embryos [13, 15,16,17,18], some studies have suggested that serotonergic signals are associated with the regulation of steroidogenesis, oocyte maturation, spermatogenesis, and embryonic development. Recently, it has been reported that 5-HT regulates sperm function in mammals. In hamster sperm, 5-HT was found to enhance hyperactivation and induce the acrosome reaction via 5-HT2 and 5-HT4 receptors [19, 20]. In human sperm, 5-HT increases straight-line velocity (VSL), curvilinear velocity (VCL), and average path velocity (VAP) [21]. Furthermore, 5-HT1B, 5-HT2A, and 5-HT3 receptors have been identified in human and stallion sperm [21, 22]. In mice, 5-HT reportedly increases sperm hyperactivation via the 5-HT2, 5-HT3, 5-HT4, and 5-HT7 receptors and improves the success rate of in vitro fertilization (IVF) [23]. In the present study, we attempted to determine the 5-HT receptor type involved in enhancing hyperactivation and examine how signals regulate hyperactivation in hamster sperm.
Materials and Methods
Chemicals
Sumatriptan succinate (sumatriptan), α-methylserotonin maleate (MS), 1-(3-chlorophenyl) biguanide hydrochloride (mCPBG), 5-methoxytryptamine (MT), WAY208466, LP12, 2’,3’-dideoxyadenosine (ddAdo), 2-hydroxyestradiol (2-CE), KH7, 2,4-dithenoyl-1,2,5-oxadiazone n2-oxide (HC-056456, HC), U73122, U73343, D609, ET-18-OCH3, neomycin, spermine, and bisindolylmaleimide 1 (Bis-1) were purchased from Merck KGaA (Darmstadt, Germany). TCB2, BW723C86, and MK212 were purchased from TOCRIS Bioscience (Bristol, UK). H-89, mibefradil (Mib), and NNC 55–0396 (NNC) were purchased from Cayman Chemical (Ann Arbor, MI, USA). Anti 5-HT2A receptor antibody (SR-2A (A-4); sc-166775) and anti-5-HT4 receptor antibody (SR-4 (G-3); sc-376158) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, Texas, USA). Polyvinylidene difluoride (PVDF) membranes were purchased from Millipore (Bedford, MA, USA). The molecular weight marker set was purchased from Bio-Rad Laboratories Inc. (Hercules, CA, USA). EzWestLumi Plus® was purchased from ATTO Corporation (Tokyo, Japan). Xestospongin C, anti-mouse IgG antibody conjugated peroxidase, bovine serum albumin (BSA), fraction V, and other reagent grade chemicals were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan).
Animals
Syrian hamsters (Mesocricetus auratus) were bred at the Research Center for Laboratory Animals, Dokkyo Medical University. The present study was approved by the Animal Care and Use Committee of the University (experimental permission numbers: 0107 and 1248), and all experiments were performed in accordance with the University’s Guidelines for Animal Experimentation.
Preparation of hyperactivated sperm
Sperm were collected from the cauda epididymis of male hamsters (10–20 weeks old). Hyperactivated sperm were prepared as described previously [11]. Modified Tyrode’s albumin lactate pyruvate medium [24] was used as the capacitation medium. A drop (~ 5 μl) of cauda epididymis sperm was placed on a culture dish (35 mm diameter; Iwaki, Asahi Glass Co., Ltd., Tokyo, Japan), and 3 ml of medium was added to the dish. The sperm were incubated for 5 min at 37°C for activation. Then, the supernatant containing motile sperm was placed in a new dish containing the vehicle or inhibitors. After incubation for 5 min, the supernatant was transferred to a new dish containing the vehicle or agonist. Sperm were incubated for 4 h at 37°C to induce hyperactivation under 5% CO2. As stock solutions, sumatriptan (100 μM), MS (100 pM), mCPBG (100 mM), WAY208466 (7.3 μM), LP12 (0.13 μM), TCB2 (0.75 μM), MK212 (0.3 μM), and ddAdo (100 mM) were dissolved in pure water. MT (10 nM), U73122 (1 mM), U73343 (1 mM), D609 (10 mM), ET-18-OCH3 (15 mM), neomycin (65 mM), spermine (1 M), and 2-CE (20 mM) were dissolved in ethanol. BW723C86 (2 mM), Bis-1 (10 μM), H-89 (100 mM), xestospongin C (1 mM), KH7 (10 mM), HC (30 mM), Mib (40 mM), and NNC (20 mM) were dissolved in dimethyl sulfoxide. For all experiments, the maximum concentration of the vehicle was 0.2%.
Measurements of motility and hyperactivation
Motility and hyperactivation were measured as previously described [25]. Motile sperm were recorded on a DVD recorder (RDR-HX50; Sony Corp., Tokyo, Japan) using a CCD camera (Progressive 3CCD, Sony) attached to a microscope (IX70, Olympus Corp., Tokyo, Japan) with phase-contrast illumination and a small CO2 incubator (MI-IBC, Olympus). Observations were performed at 37°C for 1 min. Visual analyses of the movies comprised manual counts of the number of total sperm, motile sperm, and hyperactivated sperm in ten different fields. For all experiments, visual analyses were performed in a blinded manner. Motile sperm exhibiting asymmetric and whiplash-like flagellar movements were defined as hyperactivated [1, 2] (Supplementary movie 1). The percentage of motility and hyperactivation were defined as the number of motile sperm/number of total sperm × 100 and the number of hyperactivated sperm/number of total sperm × 100, respectively. Each experiment was repeated four times using four different hamsters. If the proportion of motile sperm was equal to or below 80%, the experiment was repeated. Data were statistically analyzed using a repeated-measures ANOVA post-hoc test in Microsoft Excel (Microsoft Japan, Tokyo, Japan), with ystat2018 (Igakutosho Shuppan, Saitama, Japan) add-on. Statistical significance was set at P < 0.05.
Motility assay by the sperm motility analysis system (SMAS)
The motility assay was evaluated using SMAS for animals (Ver. 3.18) with the loaded parameter file mouse_BM10×_ 640 nm _Bright59_150fps-shutter200.ini (Ditect Co. Ltd., Tokyo, Japan) as previously described [23]. The suspension containing motile sperm (20 μl) was transferred to an observation chamber (0.1 mm deep, 18 mm wide, and 18 mm long) made of mending tape attached to the glass slide in two parallel strips, which were then covered with a cover glass. Sperm movement was recorded for 1 sec on the hard disk drive of SMAS via a high-speed digital camera (HAS-L2; Ditect) attached to a microscope (ECLIPSE E2000; Nikon Corp., Tokyo, Japan) with phase-contrast illumination, a 650 nm band-pass filter, and a warm plate (MP10DM; Kitazato Corp., Shizuoka, Japan). SMAS analyzed 150 consecutive images obtained from a single field at 10 × magnification in negative phase contrast. SMAS automatically calculated VSL (μm/sec), VCL (μm/sec), VAP (μm/sec), linearity (LIN), straightness (STR), amplitude of lateral head displacement (ALH; μm), and beat-cross frequency (BCF; Hz), with the wobbler coefficient (WOB; defined as VAP/VCL) manually calculated [26]. SMAS analysis was repeated five times using five different hamsters. In each experiment, ≥ 300 sperm were detected. Only motile sperm judged to be significant were analyzed. The effects of agonists were statistically analyzed by Student’s t-test performed using Microsoft Excel or by repeated-measures ANOVA post-hoc test, using Microsoft Excel with ystat2018. Statistical significance was set at P < 0.05.
Preparation of sperm protein extracts
Sperm proteins were extracted using the following method. In brief, sperm obtained from the epididymis were washed once with 0.9% (w/v) NaCl and collected by centrifugation at 4°C for 10 min at 15,000 × g. Sperm pellets were suspended at 100 mg/ml (w/v) in sodium dodecyl sulfate (SDS) buffer containing 5 M urea, 0.1% SDS, 1% 2-mercaptoethanol, and 75 mM Tris-HCl (pH 6.8) [27]. After pipetting, the suspension was incubated on ice for 10 min. Next, the suspension was centrifuged at 4°C for 20 min at 15,000 × g and the supernatant was used as the sperm protein extract.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was performed according to the method described by Laemmli [28] using a separating gel of 10% (w/v) polyacrylamide containing 0.1% (w/v) SDS. After the gel was stained with Coomassie Brilliant Blue (CBB), images of stained gels were scanned using a densitometer (GS-800 densitometer, Bio-Rad Laboratories).
Western blotting
Western blotting was performed according to a previously described method [11, 27] with some modifications. The blotted membrane was blocked with 5% (w/v) BSA in Tris-buffered saline (TBS) containing 0.15 M NaCl and 20 mM Tris-HCl (pH 7.5) for 1 h at 25°C. After washing three times with TBS, the membrane was incubated with the primary antibody (1:1000 dilution with 5% [w/v] BSA in TBS) for 1 h at 25°C. After washing three times with TBS, the membrane was incubated with secondary antibody conjugated peroxidase (1:5000 dilution with 5% [w/v] BSA in TBS). After the membrane was washed with Tween-TBS containing 0.05% (w/v) Tween-20 and TBS three times, the color reaction was performed using the EzWestLumi Plus® (ATTO). Western blotting was performed using Ez-Capture MG (ATTO).
Results
Effects of 5-HT receptors on hyperactivation and motility assay
Although a previous study [20] has suggested that 5-HT enhanced hyperactivation via 5-HT2 and 5-HT4 receptors in hamster sperm, it remains unknown whether 5-HT enhances hyperactivation via other receptors. As shown in Supplementary Fig. 1, the 5-HT receptor types affecting hyperactivation were examined. As shown in Supplementary Fig. 1A–F, 100 fM MS (5-HT2 receptor agonist) and 10 pM MT (5-HT4 receptor agonist) [20, 23] significantly increased hyperactivation, although sumatriptan (17 nM, 5-HT1B/1D receptor agonist; 100 nM, 5-HT1A receptor agonist) [23, 29], 100 μM mCPBG (5-HT3 receptor agonist) [23, 30], 7.3 nM WAY208466 (5-HT6 receptor agonist) [instruction manual] [23] , and 0.13 nM LP12 (5-HT7 receptor agonist) [instruction manual] [23] did not impact hyperactivation. Moreover, we examined which 5-HT2 receptor subtypes affected hyperactivation, as the 5-HT2 receptor consists of three known subtypes [13, 14] (Supplementary Fig. 1G–I). TCB2 (5-HT2A receptor agonist) at 0.75 nM [instruction manual] significantly increased hyperactivation; however, this effect was not observed with 2 μM BW723C86 (5-HT2B receptor agonist) [31] and 0.3 nM MK212 (5-HT2C receptor agonist) [instruction manual]. Furthermore, motility was not affected by 5-HT receptor agonists (Supplementary Fig. 1). As shown in Supplementary Fig. 2, the 5-HT2A receptor was detected as an approximately 55-kDa band from sperm protein extracts. Moreover, the 5-HT4 receptor was detected as an approximately 40-kDa band.
Following treatment with 5-HT receptor agonists, sperm motility was evaluated using SMAS (Supplementary Table 1). MS at 100 fM significantly decreased VSL and VAP. mCPBG at 100 μM significantly decreased VCL and ALH. MT at 10 pM significantly reduced ALH. TCB2 (0.75 nM) significantly decreased VSL. Other agonists did not affect these parameters.
Regulatory mechanisms of hyperactivation enhanced by 5-HT2 receptor stimulation
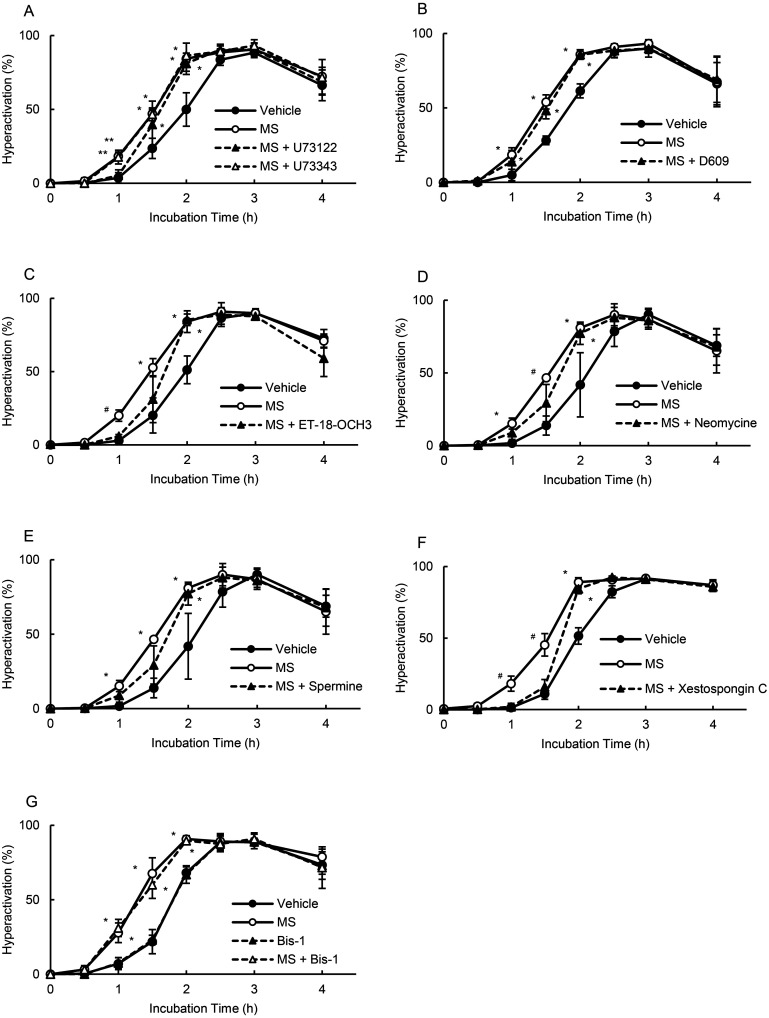
After 5-HT binds to the 5-HT2 receptor, it stimulates PLC and produces IP3 [13, 14]. As shown in Fig. 1, we examined whether PLC was associated with MS-enhanced hyperactivation. As shown in Fig. 1A, 1 μM U73122 (standard PLC inhibitor) [3] significantly suppressed the enhancement observed at 1 h, whereas no such effect was observed with 1 μM U73343 (control of U73122) [3]. Although 10 μM D609 (phosphatidylcholine-PLC inhibitor) [3] did not affect the enhancement, 15 μM ET-18-OCH3 (phosphatidylinositol (PI)-PLC inhibitor) [3] significantly inhibited the enhanced hyperactivation at 1 h (Fig. 1B and 1C). Neomycin at 65 μM (non-specific PLC inhibitor) [3] significantly inhibited the enhancement at 1.5 h; this finding was not observed with 1 mM spermine (PLCα inhibitor) [3] (Figs. 1D and 1E). PLC produces IP3 and diacylglycerol [13, 14]. IP3 binds to IP3R, and diacylglycerol activates protein kinase C (PKC). Xestospongin C at 1 μM (IP3R inhibitor) [32] significantly inhibited the enhancement at 1 and 1.5 h; these findings were not observed with 10 nM Bis-1 (PKC inhibitor) [32] (Figs. 1F and 1G).
Fig. 1.
Suppression of MS-enhanced hyperactivation by PLC, IP3R, and PKC inhibitors. Percentages of hyperactivation were detected after sperm were cultured for 4 h with 100 fM MS and inhibitors, including 1 μM U73122 and 1 μM U73343 (A), 10 μM D609 (B), 15 μM ET-18-OCH3 (C), 65 μM neomycin (D), 1 mM spermine (E), 1 μM xestospongin C (F), and 10 nM Bis-1 (G). Data represent the mean ± standard deviation (SD). (A) (Vehicle) medium with 0.1% (v/v) pure water and 0.1% (v/v) ethanol as vehicle; (MS) medium with 100 fM MS and vehicle; (MS + U73122) medium with 100 fM MS, 1 μM U73122, and vehicle; (MS + U73343) medium with 100 fM MS, 1 μM U73343, and vehicle. (B) (Vehicle) same as above; (MS) medium with 100 fM MS and vehicle; (MS + D609) medium with 100 fM MS, 10 μM D609, and vehicle. (C) (Vehicle) same as above; (MS) medium with 100 fM MS and vehicle; (MS + ET-18-OCH3) medium with 100 fM MS, 15 μM ET-18-OCH3, and vehicle. (D) (Vehicle) same as above; (MS) medium with 100 fM MS and vehicle; (MS + Neomycin) medium with 100 fM MS, 65 μM neomycin, and vehicle. (E) (Vehicle) same as above; (MS) medium with 100 fM MS and vehicle; (MS + Spermine) medium with 100 fM MS, 1 mM spermine, and vehicle. (F) (Vehicle) medium with 0.1% (v/v) pure water and 0.1% (v/v) dimethyl sulfoxide as vehicle; (MS) medium with 100 fM MS and vehicle; (MS + Xestospongin C) medium with 100 fM MS, 1 μM xestospongin C, and vehicle. (G) (Vehicle) same as above; (MS) medium with 100 fM MS and vehicle; (MS + Bis-1) medium with 100 fM MS, 10 nM Bis-1, and vehicle. * indicates significant differences compared with “Vehicle” (P < 0.05). ** indicates significant differences compared with “Vehicle,” “MS + U73122” (P < 0.05). # indicates significant differences compared with “Vehicle” and “MS + inhibitors” (P < 0.05). MS, α-methylserotonin maleate; Bis-1, bisindolylmaleimide 1; PLC, phospholipase C; PKC, protein kinase C; IP3, inositol 1,4,5-trisphosphate.
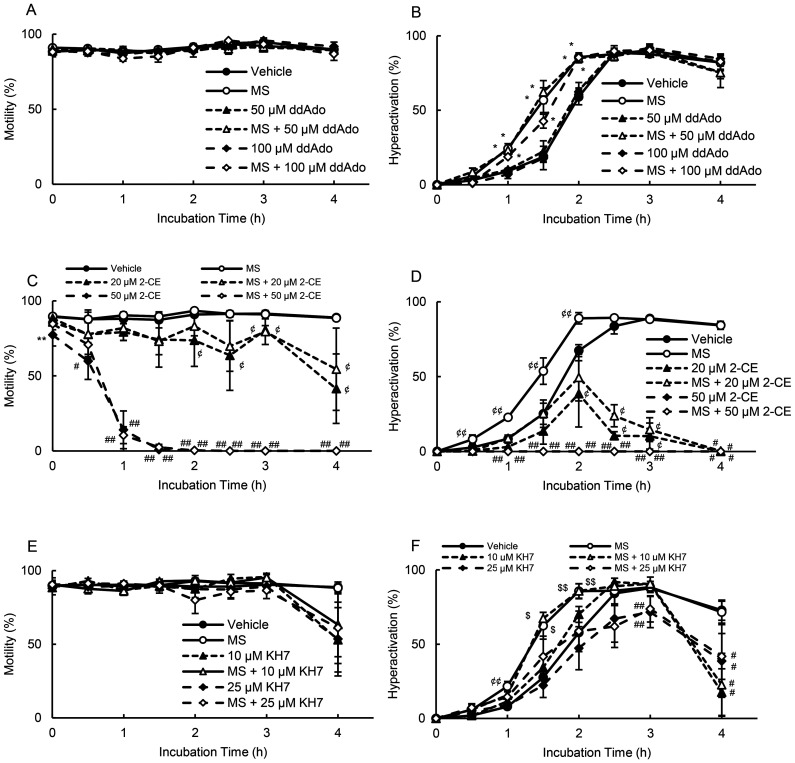
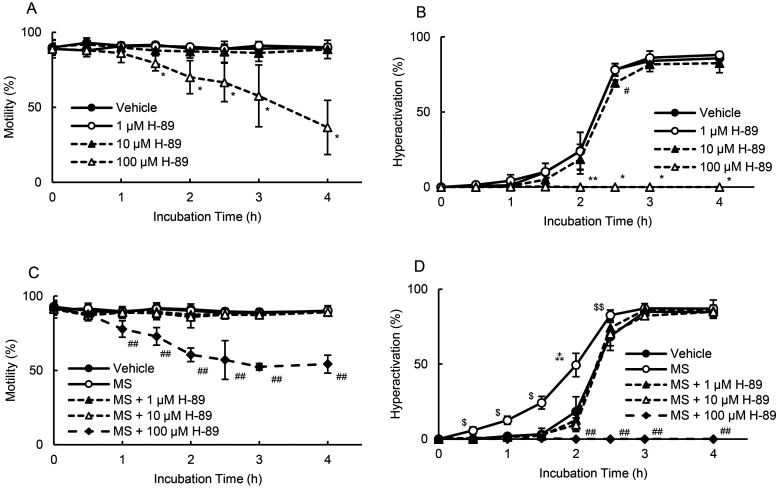
Hyperactivation is regulated by sAC [10], and in addition, it has been reported that tmAC and sAC exist in hamster sperm and produce cAMP [7]. Next, we examined whether adenylate cyclase was associated with MS-enhanced hyperactivation. As shown in Fig. 2A and 2B, 50 and 100 μM ddAdo (tmAC inhibitor) [33] did not affect motility and hyperactivation in the absence or presence of MS. In contrast, 20 μM 2-CE (sAC inhibitor) [33] significantly decreased motility after 2 h in the absence of MS and after 3 h in the presence of MS (Fig. 2C). Moreover, in the absence and presence of MS, 50 μM 2-CE [33] significantly decreased motility at 0, 0.5, and 1 h and did not allow sperm to swim after 1.5 h (Fig. 2C). In terms of hyperactivation (Fig. 2D), 20 μM 2-CE significantly inhibited hyperactivation after 2 h in the absence of MS and after 2.5 h in the presence of MS. Moreover, 50 μM 2-CE did not allow sperm hyperactivation in the absence and presence of MS (Fig. 2D). KH7, another sAC inhibitor, did not affect motility (Fig. 2E). In the absence and presence of MS, 10 μM KH7 [33] significantly inhibited hyperactivation at 4 h (Fig. 2F). Furthermore, 25 μM KH7 [33] significantly inhibited MS-enhanced hyperactivation at 1, 1.5, and 2 h, significantly suppressing hyperactivation at 3 and 4 h in the absence and presence of MS (Fig. 2F). As sAC produces cAMP and activates PKA [7, 10], we examined the effects of H-89 (a PKA inhibitor) on motility and hyperactivation in the absence and presence of MS (Fig. 3). In the absence of MS, 100 μM H-89 significantly inhibited motility after 1.5 h, whereas 1 and 10 μM H-89 did not affect motility (Fig. 3A). As for hyperactivation, 100 μM H-89 did not enable sperm hyperactivation, whereas 1 and 10 μM H-89 did not affect the ability of the sperm to be hyperactivated (Fig. 3B). In the presence of MS, 100 μM H-89 significantly inhibited motility after 1 h and did not allow sperm hyperactivation (Figs. 3C and 3D). In contrast, 1 and 10 μM H-89 significantly inhibited MS-enhanced hyperactivation without impacting motility (Figs. 3C and 3D).
Fig. 2.
Suppression of MS-enhanced hyperactivation by adenylate cyclase inhibitors. Percentages of motility (A, C, and E) and hyperactivation (B, D, and F) were detected after sperm were cultured for 4 h with 100 fM MS and inhibitors, including 50 and 100 μM ddAdo (A and B), 20 and 50 μM 2-CE (C and D), and 10 and 25 μM KH7 (E and F). Data represent the mean ± standard deviation (SD). (A and B) (Vehicle) medium with 0.2% (v/v) pure water as vehicle; (MS) medium with 100 fM MS and vehicle; (50 μM ddAdo) medium with 50 μM ddAdo and vehicle; (MS + 50 μM ddAdo) medium with 100 fM MS, 50 μM ddAdo, and vehicle; (100 μM ddAdo) medium with 100 μM ddAdo and vehicle; (MS + 100 μM ddAdo) medium with 100 fM MS, 100 μM ddAdo, and vehicle. (C and D) (Vehicle) medium with 0.1% (v/v) pure water and 0.1% (v/v) ethanol as vehicle; (MS) medium with 100 fM MS and vehicle; (20 μM 2-CE) medium with 20 μM 2-CE and vehicle; (MS + 20 μM 2-CE) medium with 100 fM MS, 20 μM 2-CE, and vehicle; (50 μM 2-CE) medium with 50 μM 2-CE and vehicle; (MS + 50 μM 2-CE) medium with 100 fM MS, 250 μM 2-CE, and vehicle. (E and F) (Vehicle) medium with 0.1% (v/v) pure water and 0.1% (v/v) dimethyl sulfoxide as vehicle; (MS) medium with 100 fM MS and vehicle; (10 μM KH7) medium with 10 μM KH7 and vehicle; (MS + 10 μM KH7) medium with 100 fM MS, 10 μM KH7, and vehicle; (25 μM KH7) medium with 25 μM KH7 and vehicle; (MS + 25 μM KH7) medium with 100 fM MS, 25 μM KH7, and vehicle. * indicates significant differences compared with “Vehicle” and “Inhibitors” (P < 0.05). ** indicates significant differences compared with “Vehicle,” “MS,” “Low concentration of inhibitor,” and “MS + inhibitors” (P < 0.05). # indicates significant differences compared with “Vehicle” and “MS” (P < 0.05). ## indicates significant differences compared with “Vehicle,” “MS,” “Low concentration of inhibitor,” and “MS + low concentration of inhibitor” (P < 0.05). ¢ indicates significant differences compared with “Vehicle,” “MS,” “High concentration of inhibitor,” and “MS + High concentration of inhibitor” (P < 0.05). ¢¢ indicates significant differences compared with “Vehicle,” “Inhibitors,” and “MS + Inhibitor” (P < 0.05). $ indicates significant differences compared with “Vehicle,” “Inhibitors,” and “MS + High concentration of inhibitor” (P < 0.05). $$ indicates significant differences compared with “Vehicle,” “High concentration of inhibitor,” and “MS + High concentration of inhibitor” (P < 0.05). MS, α-methylserotonin maleate; 2-CE, 2-hydroxyestradiol; ddAdo, 2’,3’-dideoxyadenosine.
Fig. 3.
Suppression of motility and hyperactivation by H-89 (PKA inhibitor). Percentages of motility (A and C) and hyperactivation (B and D) were determined after sperm were cultured with various concentrations of H-89 for 4 h in the absence and the presence of 100 fM MS. Data represent the mean ± standard deviation (SD). (A and B) (Vehicle) medium with 0.1% (v/v) dimethyl sulfoxide as vehicle; (1 µM H-89) medium with 1 µM H-89 and vehicle; (10 µM H-89) medium with 10 µM H-89 and vehicle; (100 µM H-89) medium with 100 µM H-89 and vehicle. (C and D) (Vehicle) medium with 0.1% (v/v) pure water and 0.1% (v/v) dimethyl sulfoxide as vehicle; (MS) medium with 100 fM MS and vehicle; (MS + 1 µM H-89) medium with 100 fM MS, 1 µM H-89, and vehicle; (MS + 10 µM H-89) medium with 100 fM MS, 10 µM H-89, and vehicle; (MS + 100 µM H-89) medium with 100 fM MS, 100 µM H-89, and vehicle. * indicates significant differences compared with “Vehicle,” “1 µM H-89,” and “10 µM H-89” (P < 0.05). ** indicates significant differences compared with “Vehicle” and “1 µM H-89” (P < 0.05). # indicates significant differences compared with “Vehicle” (P < 0.05). ## indicates significant differences compared with “Vehicle,” “MS,” “MS + 1 µM H-89,” and “MS + 10 µM H-89” (P < 0.05). $ indicates significant differences compared with “Vehicle” (P < 0.05). $$ indicates significant differences compared with “Vehicle” and “MS + 10 µM H-89” (P < 0.05). ⁂ indicates significant differences compared with “Vehicle,” “MS + 1 µM H-89,” and “MS + 10 µM H-89” (P < 0.05). MS, α-methylserotonin maleate.
As shown in Fig. 1, MS-enhanced hyperactivation is associated with Ca2+ signals. In addition, sACs are activated by Ca2+ [10]. During hyperactivation, Ca2+ signals are reportedly associated with a CatSper channel [34]; therefore, we examined whether a CatSper channel was associated with MS-enhanced hyperactivation (Supplementary Fig. 3). As shown in Supplementary Figs. 3A and 3C, 3 and 10 μM HC (a potent CatSper channel blocker) [35] did not affect motility in the absence or presence of MS. Regarding hyperactivation, 3 μM HC did not impact hyperactivation in the absence or presence of MS, although 10 μM HC significantly inhibited hyperactivation after 1.5 h in the absence of MS and after 2 h in the presence of MS (Supplementary Figs. 3B and 3D). Mib and NNC are typical T-type voltage-activated Ca2+ channel blockers [instruction manual] and are proven to be potent CatSper channel blockers in sperm studies [36,37,38]. As shown in Supplementary Fig. 3E, in the absence and presence of MS, 30 and 40 μM Mib [38] significantly suppressed motility after 2 h and 1.5 h, respectively, and did not enable sperm hyperactivation (Supplementary Fig. 3F). As shown in Supplementary Fig. 3G, in the absence and presence of MS, 10 and 20 μM NNC [38] significantly inhibited motility after 1.5 and 0.5 h, respectively; both doses did not allow sperm hyperactivation in the absence or presence of MS (Supplementary Fig. 3H).
Regulatory mechanisms of hyperactivation enhanced by stimulation of 5-HT4 receptor
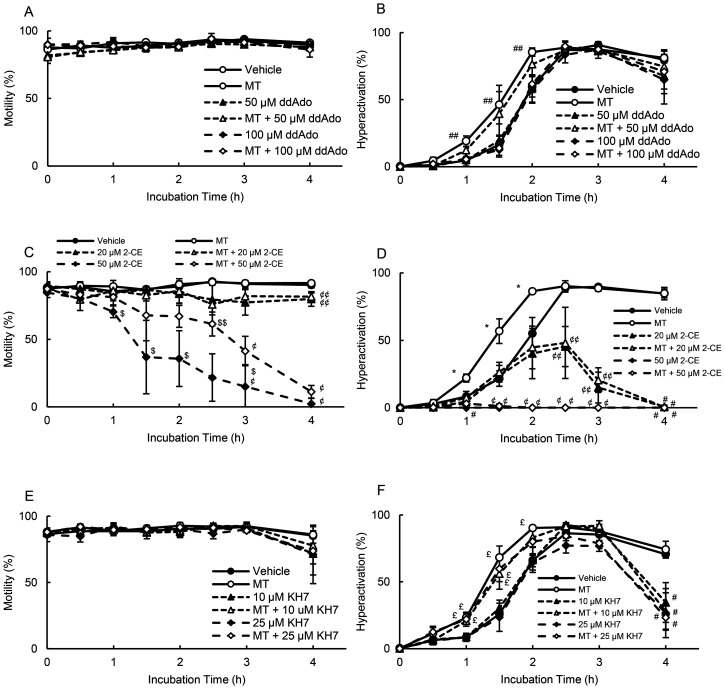
After 5-HT binds to the 5-HT4 receptor, it stimulates tmAC and induces cAMP production [13, 14]. Additionally, hyperactivation is regulated by sAC [10]. We examined whether tmAC and sAC were associated with MT-enhanced hyperactivation (Fig. 4). As shown in Fig 4A, 50 and 100 μM ddAdo did not affect motility in the absence or presence of MT and did not affect hyperactivation in the absence of MT. In terms of MT-enhanced hyperactivation, 100 μM ddAdo significantly suppressed this effect; however, 50 μM ddAdo demonstrated no such effect (Fig. 4B). As shown in Fig. 4C, 20 μM 2-CE significantly inhibited motility at 4 h, both in the absence and presence of MT. Moreover, 50 μM 2-CE significantly inhibited motility after 1 h in the absence of MT and after 2.5 h in the presence of MT (Fig. 4C). Moreover, 20 μM 2-CE significantly inhibited hyperactivation after 2.5 h in the absence and presence of MT (Fig. 4D). In addition, 50 μM 2-CE did not enable sperm hyperactivation in the absence or presence of MT (Fig. 4D). As shown in Fig. 4E and 4F, in the absence and presence of MT, 10 and 25 μM KH7 significantly inhibited hyperactivation at 4 h, with no impact on motility.
Fig. 4.
Suppression of MT-enhanced hyperactivation by adenylate cyclase inhibitors. Percentages of motility (A, C, and E) and hyperactivation (B, D, and F) were determined after sperm were cultured for 4 h with 10 pM MT and inhibitors, including 50 and 100 μM ddAdo (A and B), 20 and 50 μM 2-CE (C and D), and 10 and 25 μM KH7 (E and F). Data represent the mean ± standard deviation (SD). (A and B) (Vehicle) medium with 0.1% (v/v) pure water and 0.1% (v/v) ethanol as vehicle; (MT) medium with 10 pM MT and vehicle; (50 μM ddAdo) medium with 50 μM ddAdo and vehicle; (MT + 50 μM ddAdo) medium with 10 pM MT, 50 μM ddAdo, and vehicle; (100 μM ddAdo) medium with 100 μM ddAdo and vehicle; (MT + 100 μM ddAdo) medium with 10 pM MT, 100 μM ddAdo, and vehicle. (C and D) (Vehicle) medium with 0.2% (v/v) ethanol as vehicle; (MT) medium with 10 pM MT and vehicle; (20 μM 2-CE) medium with 20 μM 2-CE and vehicle; (MT + 20 μM 2-CE) medium with 10 pM MT, 20 μM 2-CE, and vehicle; (50 μM 2-CE) medium with 50 μM 2-CE and vehicle; (MT + 50 μM 2-CE) medium with 10 pM MT, 50 μM 2-CE, and vehicle. (E and F) (Vehicle) medium with 0.1% (v/v) ethanol and 0.1% (v/v) dimethyl sulfoxide as vehicle; (MT) medium with 10 pM MT and vehicle; (10 μM KH7) medium with 10 μM KH7 and vehicle; (MT + 10 μM KH7) medium with 10 pM MT, 10 μM KH7, and vehicle; (25 μM KH7) medium with 25 μM KH7 and vehicle; (MT + 25 μM KH7) medium with 10 pM MT, 25 μM KH7, and vehicle. * indicates significant differences compared with “Vehicle,” “Inhibitors,” and “MT + Inhibitors” (P < 0.05). # indicates significant differences compared with “Vehicle” and “MT” (P < 0.05). ## indicates significant differences compared with “Vehicle,” “Inhibitors,” and “MT + High concentration of inhibitor” (P < 0.05). $ indicates significant differences compared with “Vehicle,” “MT,” “Low concentration of inhibitor,” and “MT + Inhibitors” (P < 0.05). $$ indicates significant differences compared with “Vehicle,” “MT,” and “High concentration of inhibitor” (P < 0.05). ¢ indicates significant differences compared with “Vehicle,” “MT,” “Low concentration of inhibitor,” and “MT + Low concentration of inhibitor” (P < 0.05). ¢¢ indicates significant differences compared with “Vehicle,” “MT,” “High concentration of inhibitor,” and “MT + High concentration of inhibitor” (P < 0.05). £ indicates significant differences compared with “Vehicle” and “Inhibitors” (P < 0.05). MT, 5-methoxytryptamine; 2-CE, 2-hydroxyestradiol; ddAdo, 2’,3’-dideoxyadenosine.
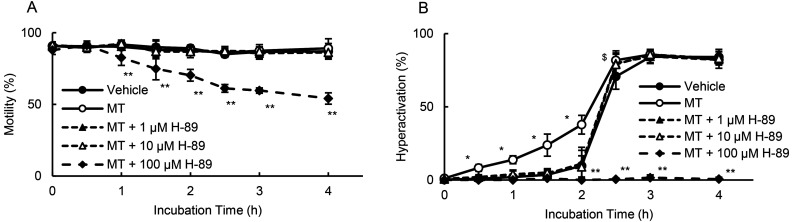
As tmAC produces cAMP and activates PKA [33], we examined the effects of H-89 on motility and hyperactivation in the presence of MT (Fig. 5). At 100 μM, H-89 significantly suppressed motility after 1 h and did not facilitate sperm hyperactivation. H-89 at 1 μM and 10 μM significantly inhibited MT-enhanced hyperactivation without impacting motility.
Fig. 5.
Suppression of MT-enhanced hyperactivation by PKA inhibitors. Percentages of motility (A) and hyperactivation (B) were determined after sperm were cultured for 4 h with 10 pM MT and various concentrations of H-89. Data represent the mean ± standard deviation (SD). (A and B) (Vehicle) medium with 0.1% (v/v) ethanol and 0.1% (v/v) dimethyl sulfoxide as vehicle; (MT) medium with 10 pM MT and vehicle; (MT + 1 µM H-89) medium with 10 pM MT, 1 µM H-89, and vehicle; (MT + 10 µM H-89) medium with 10 pM MT, 10 µM H-89, and vehicle; (MT + 100 µM H-89) medium with 10 pM MT, 100 µM H-89, and vehicle. * indicates significant differences compared with “Vehicle,” “Inhibitors,” and “MT + Inhibitors” (P < 0.05). ** indicates significant differences compared with “Vehicle,” “MT,” “MT + 1 µM H-89,” and “MT + 10 µM H-89” (P < 0.05). $ indicates significant differences compared with “Vehicle,” “MT + 1 µM H-89,” and “MT + 10 µM H-89” (P < 0.05). MT, 5-methoxytryptamine; PKA, protein kinase A.
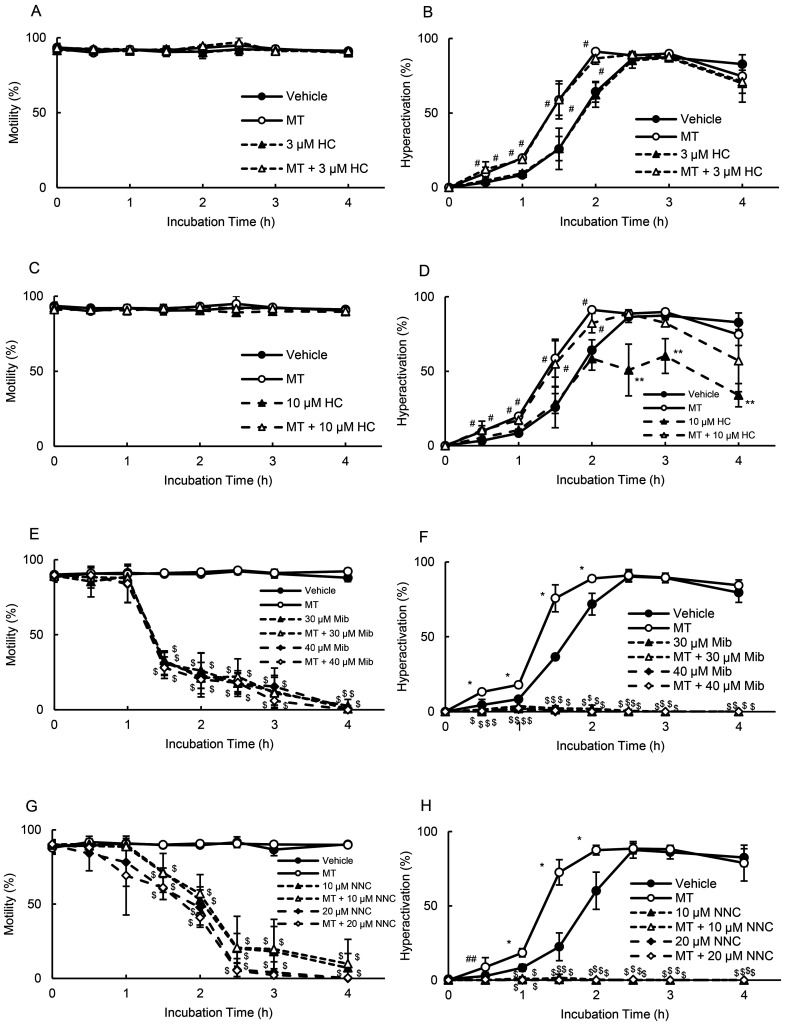
In mouse sperm, cAMP-induced Ca2+ influx possibly occurs through the CatSper channel [34]. Additionally, a recent mouse study suggested that the CatSper channel is activated by PKA [39]; thus, the present study examined whether the CatSper channel was associated with MT-enhanced hyperactivation (Fig. 6). As shown in Figs. 6A and 6C, 3 and 10 μM HC did not affect motility in the absence or presence of MT. As shown in Fig. 6B, 3 μM HC did not inhibit hyperactivation in the absence or presence of MT. In contrast, 10 μM HC did not affect MT-enhanced hyperactivation (Fig. 6D). Furthermore, 10 μM HC significantly inhibited hyperactivation after 2.5 h in the absence of MT; however, MT canceled the inhibition of hyperactivation (Fig. 6D). As shown in Figs. 6E and 6F, in the absence and presence of MT, 30 and 40 μM Mib significantly inhibited motility after 1.5 h and did not allow sperm hyperactivation. Moreover, in the absence and presence of MT, 10 and 20 μM NNC significantly inhibited motility after 1.5 h and did not enable sperm hyperactivation.
Fig. 6.
Suppression of MT-enhanced hyperactivation by CatSper inhibitors. Percentages of motility (A, C, E, and G) and hyperactivation (B, D, F, and H) were determined after sperm were cultured for 4 h with 10 pM MT and inhibitors such as 3 μM HC (A and B), 10 μM HC (C and D), 30 and 40 μM Mib (E and F), and 10 and 20 μM NNC (G and H). Data represent the mean ± standard deviation (SD). (A and B) (Vehicle) medium with 0.1% (v/v) ethanol and 0.1% (v/v) dimethyl sulfoxide as vehicle; (MT) medium with 10 pM MT and vehicle; (3 µM HC) medium with 3 µM HC and vehicle; (MT + 3 µM HC) medium with 10 pM MT, 3 µM HC, and vehicle. (C and D) (Vehicle) same as above; (MT) medium with 10 pM MT and vehicle; (10 µM HC) medium with 10 µM HC and vehicle; (MT + 10 µM HC) medium with 10 pM MT, 10 µM HC, and vehicle. (E and F) (Vehicle) same as above; (MT) medium with 10 pM MT and vehicle; (30 µM Mib) medium with 30 µM Mib and vehicle; (MT + 30 µM Mib) medium with 10 pM MT, 30 µM Mib, and vehicle; (40 µM Mib) medium with 40 µM Mib and vehicle; (MT + 40 µM Mib) medium with 10 pM MT, 40 µM Mib, and vehicle. (G and H) (Vehicle) same as above; (MT) medium with 10 pM MT and vehicle; (10 µM NNC) medium with 10 µM NNC and vehicle; (MT + 10 µM NNC) medium with 10 pM MT, 10 µM NNC, and vehicle; (20 µM NNC) medium with 20 µM NNC and vehicle; (MT + 20 µM NNC) medium with 10 pM MT, 20 µM NNC, and vehicle. * indicates significant differences compared with “Vehicle,” “inhibitors,” and “MT + inhibitors” (P < 0.05). ** indicates significant differences compared with “Vehicle,” “MT,” and “MT + inhibitors” (P < 0.05). # indicates significant differences compared with “Vehicle” and “inhibitors” (P < 0.05). ## indicates significant differences compared with “inhibitors” and “MT + inhibitors” (P < 0.05). $ indicates significant differences compared with “Vehicle” and “MT” (P < 0.05). MT, 5-methoxytryptamine; HC, 2,4-dithenoyl-1,2,5-oxadiazone n2-oxide; Mib, mibefradil.
Discussion
A recent human study has suggested that the capacity for sperm hyperactivation can be correlated with IVF success [40]. Some hormones induce sperm hyperactivation [41]; thus, the success of IVF might be controlled by artificial regulation of hyperactivation. Progesterone (P4) is a popular inducer of hyperactivation [3, 36, 37]. In human sperm, P4 induces hyperactivation via activation of the CatSper channel [36, 37]. In hamster sperm, P4 binds to a membrane progesterone receptor and enhances hyperactivation via signals related to PLC, IP3R, PKA, and PKC [3, 33]. Reportedly, 5-HT and melatonin enhance hyperactivation via specific receptors in hamster sperm [4, 20]. Estrogen suppresses the enhancement of hyperactivation mediated by P4 and melatonin via membrane estrogen receptors [42,43,44]. γ-Aminobutyric acid (GABA) suppressed the enhancement of hyperactivation mediated by P4 and 5-HT via a GABAA receptor in hamster sperm [25, 45], but it induced hyperactivation via the GABAA receptor in human sperm [46]. In a human study, the regulation of hyperactivation by P4 was not correlated with IVF success [40], although 5-HT increased hyperactivation and the success of IVF in rodents [20, 23]. It remains unknown whether melatonin, estrogen, and GABA affect IVF success.
Previous studies [20, 23], as well as the present study, have shown that 5-HT enhanced hyperactivation via several types of 5-HT specific receptors. Typically, 5-HT receptors are comprised of seven major types [13, 14]. The 5-HT2 and 5-HT4 receptor-specific agonists significantly enhanced hamster sperm hyperactivation [20] (Supplementary Fig. 1). Moreover, the enhancement of hyperactivation mediated by 5-HT was suppressed by 5-HT2 and 5-HT4 receptor-specific antagonists [20]. In addition, 5-HT2 receptors consist of three subtypes [13, 14]. The 5-HT2A receptor-specific agonist significantly enhanced the hyperactivation of hamster sperm (Supplementary Fig. 1). As 5-HT2A and 5-HT4 receptors were detected in hamster sperm, it appears that 5-HT enhanced hamster sperm hyperactivation via 5-HT2A and 5-HT4 receptors [20] (Supplementary Figs. 1 and 2). A previous study [23] using receptor-specific agonists and antagonists has revealed that 5-HT increased hyperactivation and the success of IVF via 5-HT2, 5-HT3, 5-HT4, and 5-HT7 receptors. As the 5-HT2A receptor was detected in human sperm [21], the 5-HT2 receptor is considered a vital receptor for artificially regulating the success of IVF.
As shown in Supplementary Table 1, stimulation with the 5-HT2 or 5-HT2A receptors decreased VSL. In mouse sperm, stimulation of the 5-HT2 receptor decreases VSL [23]. As stimulation of the 5-HT2 receptor enhanced hyperactivation [20, 23] (Supplementary Fig. 1), the decrease in VSL by MS can likely be correlated with MS-enhanced hyperactivation. Stimulation of the 5-HT3 receptor decreased VCL and ALH (Supplementary Table 1), but the 5-HT3 receptor was not associated with hyperactivation (Supplementary Fig. 1). In mouse sperm, stimulation of the 5-HT3 receptor did not impact VCL and ALH, but stimulation increased hyperactivation [23]. These findings indicate that stimulation of the 5-HT3 receptor can be associated with hyperactivation in a species-specific manner. Moreover, stimulation of the 5-HT4 receptor decreased ALH levels (Supplementary Table 1). In mouse sperm, stimulation of the 5-HT4 receptor decreases ALH [23]. Stimulation of the 5-HT4 receptor enhances hyperactivation [20, 23] (Supplementary Fig. 1), and thus, it is likely that the decrease in ALH by MT can be correlated with MT-enhanced hyperactivation.
When MS enhanced hyperactivation, PI-PLC, IP3R, sAC, and PKA were associated with enhanced hyperactivation (Figs. 1, 2, and 3). Therefore, it is likely that stimulation of the 5-HT2 receptor activates sAC and PKA via Ca2+ signals, which are known to be related to PLC and IP3R. Moreover, sAC appears to be associated with hyperactivation regulation, as sAC inhibitors inhibit hyperactivation in the absence of MS (Fig. 2). These findings suggest that sAC is associated with the basal regulatory mechanism of hyperactivation. The CatSper channel is an important channel for regulating hyperactivation and induces an increase in Ca2+ [34, 36, 37]. Although 3 μM HC did not inhibit hyperactivation in the absence and presence of MS, 10 μM HC inhibited hyperactivation in the absence and presence of MS (Supplementary Fig. 3B and 3D). As HC did not inhibit motility (Supplementary Figs. 3A and 3C), the results suggest that the CatSper channel is associated with a basal regulatory mechanism of hyperactivation. When hyperactivation occurs through the basal regulatory mechanism, MS possibly enhances hyperactivation by activating the basal regulatory mechanism without CatSper (Supplementary Fig. 4). Conversely, 100 μM H-89, Mib, and NNC suppressed motility and did not allow sperm hyperactivation in the absence and presence of MS (Fig. 3 and Supplementary Fig. 3). In addition, hyperactivation was suppressed after a decrease in motility induced by the sAC inhibitor (Fig. 2). These observations reveal that motility is an important event when sperm are hyperactivated. HC, which is a CatSper inhibitor, did not affect motility (Supplementary Fig. 3). Mib and NNC are typical inhibitors of T-type Ca2+ channels, and it appears that T-type Ca2+ channels are associated with the maintenance of motility.
Based on the observed effects of MT, ddAdo, and H-89 on motility and hyperactivation (Figs. 4 and 5), it is likely that stimulation of the 5-HT4 receptor activates tmAC and PKA. Moreover, the results of MT and sAC inhibition (Fig. 4) suggest that stimulation of the 5-HT4 receptor is associated with the activation of sAC. sAC is activated by Ca2+ [10], and thus, stimulation of the 5-HT4 receptor is possibly associated with Ca2+ signals. In mouse sperm, it has been suggested that cAMP-induced Ca2+ influx occurs through the CatSper channel, which is activated by PKA [34, 39]. Inhibition of hyperactivation by HC was abolished by stimulation of the 5-HT4 receptor (Fig. 6); therefore, it is likely that stimulation of the 5-HT4 receptor activates PKA via tmAC and activates sAC via the CatSper channel, which is activated by PKA.
Herein, we propose a hypothesis regarding the regulatory mechanisms of 5-HT-enhanced hyperactivation in hamster sperm (Supplementary Fig. 4). In hamster sperm, 5-HT enhances hyperactivation through 5-HT2A and 5-HT4 receptors. Stimulation of the 5-HT2 receptor is associated with PI-PLC/IP3R/Ca2+ signals. Stimulation of the 5-HT4 receptor can be associated with tmAC/cAMP/PKA/CatSper/Ca2+ signals. Both stimulations activate sAC/PKA signaling and enhance hyperactivation.
Conflict of interests
The authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
Supplementary
Acknowledgments
This work was partially supported by a Grant-in-Aid for Scientific Research (C) (No. 18K09204 to MF) from the Japan Society for the Promotion of Science (JSPS).
References
- 1.Mohri H, Inaba K, Ishijima S, Baba SA. Tubulin-dynein system in flagellar and ciliary movement. Proc Jpn Acad B 2012; 88: 397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD (ed.), The Physiology of Reproduction Vol. 2, 2nd ed. New York: Raven Press; 1994: 189–317. [Google Scholar]
- 3.Noguchi T, Fujinoki M, Kitazawa M, Inaba N. Regulation of hyperactivation of hamster spermatozoa by progesterone. Reprod Med Biol 2008; 7: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujinoki M. Melatonin-enhanced hyperactivation of hamster sperm. Reproduction 2008; 136: 533–541. [DOI] [PubMed] [Google Scholar]
- 5.Langlais J, Roberts KD. A molecular membrane model of sperm capacitation and the acrosome reaction of mammalian spermatozoa. Gamete Res 1985; 12: 183–224. [Google Scholar]
- 6.Xia J, Ren D. The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reprod Biol Endocrinol 2009; 7: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visconti PE, Muschietti JP, Flawia MM, Tezon JG. Bicarbonate dependence of cAMP accumulation induced by phorbol esters in hamster spermatozoa. Biochim Biophys Acta 1990; 1054: 231–236. [DOI] [PubMed] [Google Scholar]
- 8.Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, Gossen JA, Esposito G, van Duin M, Conti M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol 2006; 296: 353–362. [DOI] [PubMed] [Google Scholar]
- 9.Wertheimer E, Krapf D, de la Vega-Beltran JL, Sánchez-Cárdenas C, Navarrete F, Haddad D, Escoffier J, Salicioni AM, Levin LR, Buck J, Mager J, Darszon A, Visconti PE. Compartmentalization of distinct cAMP signaling pathways in mammalian sperm. J Biol Chem 2013; 288: 35307–35320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harayama H. Flagellar hyperactivation of bull and boar spermatozoa. Reprod Med Biol 2018; 17: 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujinoki M, Suzuki T, Takayama T, Shibahara H, Ohtake H. Profiling of proteins phosphorylated or dephosphorylated during hyperactivation via activation on hamster spermatozoa. Reprod Med Biol 2006; 5: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki T, Fujinoki M, Shibahara H, Suzuki M. Regulation of hyperactivation by PPP2 in hamster spermatozoa. Reproduction 2010; 139: 847–856. [DOI] [PubMed] [Google Scholar]
- 13.Dubé F, Amireault P. Local serotonergic signaling in mammalian follicles, oocytes and early embryos. Life Sci 2007; 81: 1627–1637. [DOI] [PubMed] [Google Scholar]
- 14.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev 1994; 46: 157–203. [PubMed] [Google Scholar]
- 15.Campos MB, Vitale ML, Calandra RS, Chiocchio SR. Serotonergic innervation of the rat testis. J Reprod Fertil 1990; 88: 475–479. [DOI] [PubMed] [Google Scholar]
- 16.Tinajero JC, Fabbri A, Ciocca DR, Dufau ML. Serotonin secretion from rat Leydig cells. Endocrinology 1993; 133: 3026–3029. [DOI] [PubMed] [Google Scholar]
- 17.Frungieri MB, Gonzalez-Calvar SI, Rubio M, Ozu M, Lustig L, Calandra RS. Serotonin in golden hamster testes: testicular levels, immunolocalization and role during sexual development and photoperiodic regression-recrudescence transition. Neuroendocrinology 1999; 69: 299–308. [DOI] [PubMed] [Google Scholar]
- 18.Gerendai I, Banczerowski P, Csernus V, Halász B. Innervation and serotoninergic receptors of the testis interact with local action of interleukin-1beta on steroidogenesis. Auton Neurosci 2007; 131: 21–27. [DOI] [PubMed] [Google Scholar]
- 19.Meizel S, Turner KO. Serotonin or its agonist 5-methoxytryptamine can stimulate hamster sperm acrosome reactions in a more direct manner than catecholamines. J Exp Zool 1983; 226: 171–174. [DOI] [PubMed] [Google Scholar]
- 20.Fujinoki M. Serotonin-enhanced hyperactivation of hamster sperm. Reproduction 2011; 142: 255–266. [DOI] [PubMed] [Google Scholar]
- 21.Jiménez-Trejo F, Tapia-Rodríguez M, Cerbón M, Kuhn DM, Manjarrez-Gutiérrez G, Mendoza-Rodríguez CA, Picazo O. Evidence of 5-HT components in human sperm: implications for protein tyrosine phosphorylation and the physiology of motility. Reproduction 2012; 144: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiménez-Trejo F, Coronado-Mares I, Boeta M, González-Santoyo I, Vigueras-Villaseñor R, Arriaga-Canon C, Herrera LA, Tapia-Rodríguez M. Identification of serotoninergic system components in stallion sperm. Histol Histopathol 2018; 33: 951–958. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama Y, Fujinoki M, Shibahara H. Effects of 5-hydroxytryptamine on spermatozoal hyperactivation and in vitro fertilization in mice. J Reprod Dev 2019; 65: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maleszewski M, Kline D, Yanagimachi R. Activation of hamster zona-free oocytes by homologous and heterologous spermatozoa. J Reprod Fertil 1995; 105: 99–107. [DOI] [PubMed] [Google Scholar]
- 25.Fujinoki M, Takei GL. γ-Aminobutyric acid suppresses enhancement of hamster sperm hyperactivation by 5-hydroxytryptamine. J Reprod Dev 2017; 63: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortimer ST. A critical review of the physiological importance and analysis of sperm movement in mammals. Hum Reprod Update 1997; 3: 403–439. [DOI] [PubMed] [Google Scholar]
- 27.Fujinoki M, Tomiyama T, Ishimoda-Takagi T. Tropomyosin isoforms present in the sea anemone, Anthopleura japonica (Anthozoa, Cnidaria). J Exp Zool 2002; 293: 649–663. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy BG, Peroutka SJ. Comparative neuropharmacology of dihydroergotamine and sumatriptan (GR 43175). Headache 1989; 29: 420–422. [DOI] [PubMed] [Google Scholar]
- 30.Zhang LN, Su SW, Guo F, Guo HC, Shi XL, Li WY, Liu X, Wang YL. Serotonin-mediated modulation of Na+/K+ pump current in rat hippocampal CA1 pyramidal neurons. BMC Neurosci 2012; 13: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niebert M, Vogelgesang S, Koch UR, Bischoff AM, Kron M, Bock N, Manzke T. Expression and function of serotonin 2A and 2B receptors in the mammalian respiratory network. PLoS One 2011; 6: e21395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujinoki M. Progesterone-enhanced sperm hyperactivation through IP3-PKC and PKA signals. Reprod Med Biol 2012; 12: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bitterman JL, Ramos-Espiritu L, Diaz A, Levin LR, Buck J. Pharmacological distinction between soluble and transmembrane adenylyl cyclases. J Pharmacol Exp Ther 2013; 347: 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature 2001; 413: 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson AE, Burnett LA, del Camino D, Quill TA, Hille B, Chong JA, Moran MM, Babcock DF. Pharmacological targeting of native CatSper channels reveals a required role in maintenance of sperm hyperactivation. PLoS One 2009; 4: e6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011; 471: 387–391. [DOI] [PubMed] [Google Scholar]
- 37.Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011; 471: 382–386. [DOI] [PubMed] [Google Scholar]
- 38.Tamburrino L, Marchiani S, Minetti F, Forti G, Muratori M, Baldi E. The CatSper calcium channel in human sperm: relation with motility and involvement in progesterone-induced acrosome reaction. Hum Reprod 2014; 29: 418–428. [DOI] [PubMed] [Google Scholar]
- 39.Orta G, de la Vega-Beltran JL, Martín-Hidalgo D, Santi CM, Visconti PE, Darszon A. CatSper channels are regulated by protein kinase A. J Biol Chem 2018; 293: 16830–16841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alasmari W, Barratt CLR, Publicover SJ, Whalley KM, Foster E, Kay V, Martins da Silva S, Oxenham SK. The clinical significance of calcium-signalling pathways mediating human sperm hyperactivation. Hum Reprod 2013; 28: 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujinoki M, Takei GL, Kon H. Non-genomic regulation and disruption of spermatozoal in vitro hyperactivation by oviductal hormones. J Physiol Sci 2016; 66: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujinoki M. Suppression of progesterone-enhanced hyperactivation in hamster spermatozoa by estrogen. Reproduction 2010; 140: 453–464. [DOI] [PubMed] [Google Scholar]
- 43.Fujinoki M. Regulation and disruption of hamster sperm hyperactivation by progesterone, 17β-estradiol and diethylstilbestrol. Reprod Med Biol 2014; 13: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujinoki M, Takei GL. Estrogen suppresses melatonin-enhanced hyperactivation of hamster spermatozoa. J Reprod Dev 2015; 61: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kon H, Takei GL, Fujinoki M, Shinoda M. Suppression of progesterone-enhanced hyperactivation in hamster spermatozoa by γ-aminobutyric acid. J Reprod Dev 2014; 60: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calogero AE, Hall J, Fishel S, Green S, Hunter A, D’Agata R. Effects of γ-aminobutyric acid on human sperm motility and hyperactivation. Mol Hum Reprod 1996; 2: 733–738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.