Abstract
Background
Although the causative pathogens in cardiac implantable electronic device (CIED) infections are well known, the relationship between time after implantation and infection patterns has not been sufficiently investigated. This study investigated the microbiology and onset of CIED infections according to infection patterns.
Methods and Results
This retrospective study included 97 patients who underwent CIED removal due to device-related infections between April 2009 and December 2018. After device implantation, infections peaked in the first year and declined gradually over 10 years. Most infections (>60%) occurred within 5 years. Staphylococcal infections, the predominant form of CIED infections, occurred throughout the study period. CIED infections were categorized as systemic (SI; n=26) or local (LI; n=71) infections according to clinical presentation, and as CIED pocket-related (PR; n=85) and non-pocket-related (non-PR; n=12) infections according to the pathogenic pathway. The main causative pathogen in SI was Staphylococcus aureus, whereas coagulase-negative staphylococci were mainly related to LI. Both SI and LI peaked in the first year after implantation and then decreased gradually. There was no significant microbiological difference between PR and non-PR infections. PR infections showed the same temporal distribution as the overall cohort. However, non-PR infections exhibited a uniform temporal distribution after the first year.
Conclusions
The severity of CIED infections depends on the causative pathogen, whereas their temporal distribution is affected by the microbiological intrusion pathway.
Key Words: Devices, Endocarditis, Infection
The number of patients with cardiac implantable electronic devices (CIEDs) has increased in recent decades. With the increased use of implanted devices, an upsurge in CIED complications has also been reported.1–3 Of the complications associated with CIEDs, CIED infections are the most serious and can lead to life-threatening conditions.4,5 Removal of the infected device6 and antimicrobial treatment are the mainstays of therapeutic strategies for CIED infection.7 However, to develop a comprehensive therapeutic strategy for CIED infections requires an understanding of the microbiology and pathogenesis patterns of these infections.8
Regarding the microbiology of CIED infections, previous studies reported that the main causative organisms were staphylococcal species, such as those present in skin flora.1,4,6,7 Although the causative organisms in CIED infections are well known, the temporal distributions and infection patterns associated with these microbiological data have not been sufficiently elucidated. In particular, with respect to investigations of the temporal distribution of CIED infection, many reports have focused on the early phase (<12 months) after CIED surgical manipulation,9,10 with very few reports focusing on the temporal distribution of CIED infections.11 Investigations of the long-term temporal distribution and infection patterns may reveal new findings regarding CIED infections.
In this study we retrospectively reviewed the clinical records of patients with CIED infections and categorized them on the basis of infection type and pathogenic intrusion pathways. In addition, the long-term temporal distribution of CIED infections was assessed for a period of 10 years after the last CIED surgical manipulation. The purpose of this study was to investigate the relationship between infection patterns and microbiological temporal distribution in CIED infections.
Methods
The records of all patients with CIED infections who underwent device and transvenous lead extraction or removal between April 2009 and December 2018 at Nippon Medical School were examined retrospectively. All procedures were performed in accordance with the guidelines of the Declaration of Helsinki12 and were approved by the Ethics Committee of Nippon Medical School in Tokyo, Japan (Reference no. B-2019-067). The requirement for informed consent was waived by the Board because the researchers retrospectively accessed a deidentified database for analytical purposes.
All patients were managed by cardiologists, cardiovascular surgeons, and infectious disease control specialists. The indications for device removal and the procedures for lead and device removal were in accordance with the 2010 and 2017 Heart Rhythm Society consensus documents.4,6 Only cases with a clinical consensus for an infected CIED and any treatment were included in this study.
Culture Methods
Whenever possible, blood cultures were obtained from the patients before initiation of antibiotic therapy. Cultures were obtained from the device generators, lead tips and ends, device pocket tissue, purulent drainage in the pocket, and vegetation in the cardiac cavity. Vancomycin or cefazolin was chosen as the first-line antibiotic until the pathogen or sensitivity to antibiotics became clear.
Classification of CIED Infection Types
The microbiological profiles were reviewed from the clinical records of all patients. Patients were classified into 2 groups based on their clinical presentation. The first group consisted of patients showing systemic infection (SI), which was defined as the presence of systemic symptoms and/or positive blood culture findings and/or evidence of vegetation with or without CIED pocket infection. The second group consisted of patients showing local infection (LI), which was defined by the presence of device pocket-related (PR) infectious signs without systemic symptoms, evidence of positive blood cultures, and vegetation in the cardiac cavity at the time of CIED removal.
In a subanalysis, patients with CIED infections were also categorized as those showing CIED PR infection and non-PR infection. PR infection was defined as the presence of local erythema, swelling, pain, warmth, discharge from the device pocket, pocket erosion, and positive cultures of the device pocket, leads, or blood. Pocket erosion was defined as the migration of the device into the overlying skin with visible device parts.
Analysis of Temporal Distribution
Temporal distribution was examined per year from the first to ninth year and >10 years since the last CIED-related manipulation. The onset of CIED infection and/or the date of removal were reviewed in the clinical records and operative reports. The proportion of CIED infections per year was compared between the systemic and local infection groups, as well as between the PR and non-PR infection groups.
Statistical Analysis
All continuous variables are expressed as the mean±SD. All categorical variables are reported as the number and percentage of patients. Paired Student’s t-tests were used to compare all continuous variables. Categorical variables were analyzed using the Chi-squared test. Statistical significance was set at 2-tailed P<0.05. All statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). Bonferroni correction was used for multiple-comparison correction for several dependent or independent statistical tests.
Results
Baseline Characteristics
Between April 2009 and December 2018, 114 patients underwent device removal at Nippon Medical School. Ninety-seven patients (85.1%) underwent device removal due to CIED-related infections; all these patients were included in the present study. The baseline characteristics of all patients with CIED infections are presented in Table 1. The mean age of patients was 71.3±13.8 years, and most patients were male (n=69; 71.1%). The comorbidities included hypertension (43.3%), atrial fibrillation (20.6%), coronary artery disease (13.4%), chronic renal failure (13.4%), and a prior history of stroke (10.3%). Histories of prior open-heart surgery included both coronary bypass surgery and valve surgery (9.3%). Only 2.1% of patients in this cohort had been administered steroids.
Table 1.
Baseline Characteristics of the 97 Patients Who Underwent CIED Removal or Extraction for CIED-Related Infections Between 2009 and 2018
| Age (years) | 71.3±13.8 |
| Sex | |
| Female | 28 (28.9) |
| Male | 69 (71.1) |
| Coronary artery disease | 13 (13.4) |
| Congestive heart failure | 9 (9.3) |
| Hypertension | 42 (43.3) |
| Diabetes | 13 (12.4) |
| Hyperlipidemia | 9 (9.3) |
| Prior stroke | 10 (10.3) |
| Atrial fibrillation | 20 (20.6) |
| CRF (Cr >1.3 mg/dL) | 13 (13.4) |
| Hemodialysis | 3 (3.1) |
| Prior CABG | 9 (9.3) |
| Prior valve surgery | 9 (9.3) |
| Prior endocarditis | 7 (7.2) |
| COPD | 3 (3.1) |
| Steroid use | 2 (2.1) |
Values are the mean±SD or n (%). CABG, coronary artery bypass graft; CIED, cardiac implantable electronic device; COPD, chronic obstructive pulmonary disease; Cr, creatinine; CRF, chronic renal failure.
Overall Microbiology
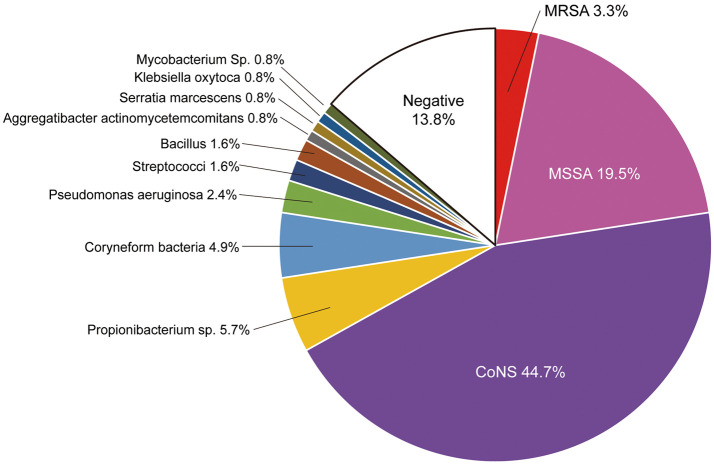
The distribution of pathogens in patients with CIED infections is shown in Figure 1. Pathogens were identified in most patients (86.2%), but some patients showed negative results for organisms from any culture source (13.8%; Figure 1). The primary pathogen was Staphylococcus (67.5%). Coagulase-negative staphylococci (CoNS) were detected in 44.7% of; patients. Staphylococcus aureus was detected in 22.8% of; patients. Of the S. aureus species detected, 3.3% were methicillin-resistant. The remaining pathogens identified were Propionibacterium sp. (5.7%), coryneform bacteria (4.9%), Pseudomonas aeruginosa (2.4%), and streptococci (1.6%).
Figure 1.
Microbiology of 97 patients who underwent cardiac implantable electronic device removal/extraction for cardiac implantable electronic device (CIED)-related infections from 2009 to 2018. CoNS, coagulase-negative Staphylococcus; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
Multiple pathogens were detected: 16 patients had a double-pathogen infection and 4 had a triple-pathogen infection. Staphylococcal species were present in all cases of multiple pathogens (S. aureus, n=7; CoNS, n=19).
Microbiological and Temporal Distribution of CIED Infections
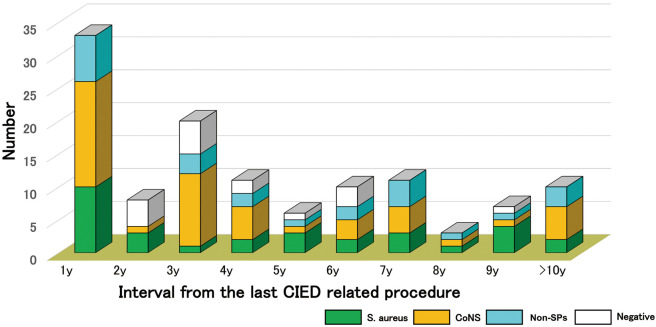
Infections peaked in the first year after the last CIED pocket manipulation and diminished gradually with the passage of time (Figure 2). Up to 5 years after the last CIED pocket manipulation, 63.4% of patients experienced a CIED infection. Moreover, approximately 8.4% of all CIED infections emerged even 10 years after the last CIED pocket procedure. S. aureus and CoNS infections occurred throughout the study period. Over 50% of causative organisms in every time period were Staphylococcus species; in particular, in the first year and >10 years after the last manipulation, 70% of causative pathogens were staphylococcal species.
Figure 2.
Temporal and microbiological distribution of cardiac implantable electronic device (CIED) infections. CoNS, coagulase-negative Staphylococcus; non-SP, non-staphylococcal species; S. aureus, Staphylococcus aureus.
Classification of CIED Infections
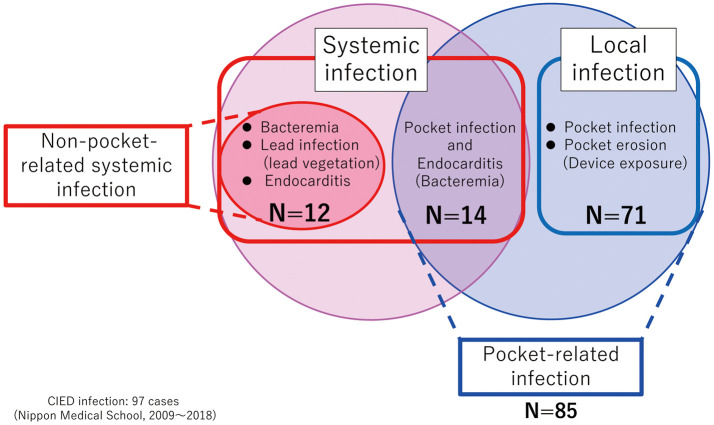
Of patients with CIED infections, 71 (73.2%) had local infections, and the remaining 26 (26.8%) had SIs (Figure 3). Among patients with SIs, 12 had bacteremia, lead infection, and endocarditis without CIED PR infection, whereas the remaining 14 patients had both bacteremia and CIED PR infections. Thus, 85 patients (87.6%) were classified as having PR infections. The remaining 12 patients (12.4%) were classified as having non-PR infections (Figure 3). Of patients with local infections, 39 had CIED PR infections and 32 had CIED pocket erosions with device exposure.
Figure 3.
Classification of patients with cardiac implantable electronic device (CIED) infections. CIED patients were classified as showing systemic infection (n=26) or local infection (n=71). CIED patients were also classified as having either pocket-related infections (n=85) or non-pocket-related infections (n=12).
Microbiology of SI and LI
The overall microbiological results were compared between the SI and LI groups (Table 2). In both groups, staphylococcal species were the major pathogens (responsible for 77.4% of SI and 60.9% of LI). The overall proportion of staphylococcal species did not differ between the SI and LI groups (P=0.379, Table 3). In assessments of the primary organism, S. aureus accounted for the highest proportion of pathogens in the SI group (48.4%). Furthermore, S. aureus was significantly more frequently detected in SI than in LI (15 [48.4%] vs. 16 [17.4%], respectively; P=0.002, Table 2). In contrast, CoNS was the most frequent pathogen in the LI group (43.5%). Although non-staphylococcal species were more likely in the LI than SI group, the difference was not statistically significant (25.0% vs. 6.5%, respectively; P=0.11). There was no significant difference between the LI and SI groups in the rate of negative cultures (14.1% vs. 16.1%, respectively; P=3.14; Table 2).
Table 2.
Microbiology of Systemic vs. Local Cardiac Implantable Electronic Device-Related Infections From 2009 to 2018
| Systemic infection (n=26) |
Local infection (n=71) |
P value (Bonferroni correction) |
|
|---|---|---|---|
| Total no. pathogens | 31 | 92 | |
| Staphylococcal species | 24 (77.4) | 56 (60.9) | 0.379 |
| Staphylococcus aureus | 15 (48.4) | 16 (17.4) | 0.002 |
| CoNS | 9 (29.0) | 40 (43.5) | 0.621 |
| Non-SPs | 2 (6.5) | 23 (25) | 0.106 |
| Negative culture | 5 (16.1) | 13 (14.1) | 3.14 |
Unless indicated otherwise, data are given as n (%). CoNS, coagulase-negative Staphylococcus; non-SPs, non-staphylococcal species.
Table 3.
Comparison of CIED-Related Factors Between Systemic and Local Infections
| Systemic infection (n=26) |
Local infection (n=71) |
P value | |
|---|---|---|---|
| Type of CIED | 0.511 | ||
| Pacer | 18 (69.3) | 46 (64.8) | |
| Non-pacer (ICD, CRT, S-ICD) | 7 (26.9) | 25 (35.2) | |
| Unknown | 1 (3.8) | 0 | |
| Last operation | 0.205 | ||
| Initial operation | 10 (38.5) | 36 (50.7) | |
| Reopen operation | 14 (53.8) | 34 (47.9) | |
| Unknown | 2 (7.7) | 1 (1.4) | |
| Time from the last device-related procedure to infection (years) | 3.4±2.8 | 4.1±4.0 | 0.397 |
Unless indicated otherwise, data are given as the mean±SD or n (%). CIED, cardiac implantable electronic device; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; S-CID, subcutaneous implantable cardioverter defibrillator.
Of the 20 patients with multiple-pathogen CIED infections, 2 had SI and 18 had LI S. aureus was detected in 2 SIs and 5 LIs. CoNS was detected in 1 SI and 18 LIs. Triple-pathogen infections appeared only as LIs (n=4 patients).
Microbiological and Temporal Distribution: SI vs. LI
The mean interval from the last device-related procedure to the occurrence of CIED did not differ significantly between the SI and LI groups (3.4±2.8 vs. 4.1±4.0 years, respectively; P=0.40; Table 3). There was also no significant difference in other CIED-related data between the 2 groups.
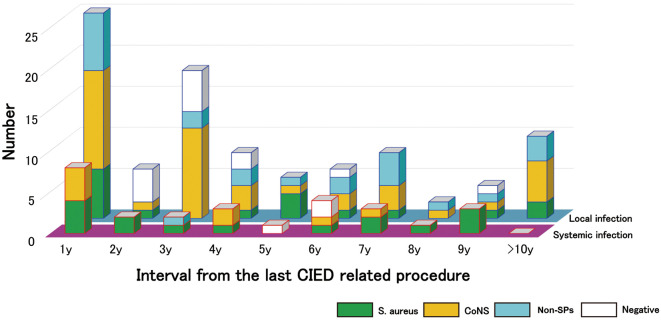
In the temporal analysis, the peak of CIED infections appeared in the first year after the last device-related procedure in both the SI and LI groups (Figure 4). From the second year, there was a uniformly low distribution of SIs in the population (mean case rate from the second year to >10 years: 2.1±4.7 cases/year). However, the rate of LIs gradually decreased until the tenth year. Over half the LIs occurred during the first to fourth years (62.0%). However, even after the fifth year, the rate of LIs was more than double that of SIs (mean case rate from the fifth year to >10 years: 6.3±3.1 cases/year). In both systemic and local infections, S. aureus and CoNS infections occurred throughout all periods (Figure 4). Non-staphylococcal species were detected only in LIs in almost all periods throughout the study.
Figure 4.
Temporal and microbiological distribution: systemic infections vs. local infection. CoNS, coagulase-negative Staphylococcus; non-SP, non-staphylococcal species; S. aureus, Staphylococcus aureus.
Microbiology of PR vs. Non-PR Infections
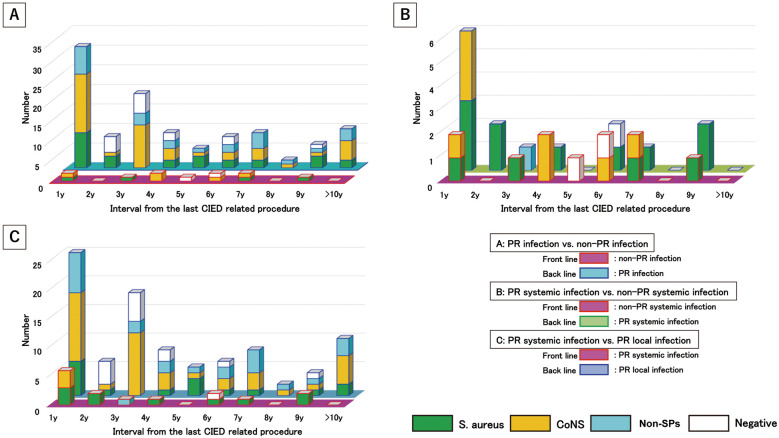
Temporal microbiological distribution was compared between PR infections (n=85 in total, 110 pathogens) and non-PR infections (n=12 in total, 13 pathogens). PR infections peaked during the first year after the last CIED manipulation. Most cases of PR infections (>60%) occurred within 1–4 years after the last CIED manipulation. In contrast, non-PR infections showed a uniform distribution from the first year in a small population (Figure 5A). In the overall microbiological analysis, there was no difference between PR and non-PR infections (Supplementary Table 1).
Figure 5.
Temporal and microbiological analysis according to pathogenic pathways. (A) pocket-related (PR) infections (n=12) vs. non-PR infections (n=85); (B) PR systemic infections (n=12) vs. non-pocket-related systemic infections (n=14); (C) PR systemic infections (n=14) vs. PR local infections (n=71). CoNS, coagulase-negative Staphylococcus; non-SP, non-staphylococcal species; S. aureus, Staphylococcus aureus.
Microbiology of PR SI vs. Non-PR SI
Temporal microbiological distribution was compared between PR SIs (n=14 in total, 18 pathogens) and non-PR SIs (n=12 in total, 13 pathogens). CIED PR SIs peaked in the first year after the last CIED operation. In contrast, non-PR SIs occurred throughout the study period (Figure 5B). PR SI was associated with a high prevalence of S. aureus; however, there were no significant differences between the 2 groups in overall analyses (Supplementary Table 2).
Microbiology of PR SI vs. PR LI
Temporal microbiological distributions was compared between PR SIs (n=14 in total, 18 pathogens) and PR LIs (n=71 in total, 92 pathogens). In the overall analysis, S. aureus was present in a significantly higher proportion of cases PR SIs than PR LIs (11 [61.1%] vs. 16 [17.4%], respectively; P=0.0003; Supplementary Table 3). In the microbiological analysis, 27 patients were infected with S. aureus through CIED pocket sites. Of these 27 patients, 14 (52%) had an SI.
In the temporal distribution analysis, both groups showed the same temporal trend in the distribution of CIED infections (Figure 5C). The proportion of S. aureus cases among patients with PR SI was higher than that among those with PR LI, but there was no significant difference between the 2 groups (50.0±47.1% vs. 18.7±16.9%, respectively; P=0.07; Figure 5C). In the temporal analysis, 31.7% of S. aureus infections were PR SIs occurring every year for 10 years. In contrast, 30–60% of S. aureus infections were systemic and occurred within the second year after the last CIED pocket manipulation (Figure 5C). The proportion of CoNS infections was significantly higher in the case of PR LIs than PR SIs (37.9±14.5% vs. 5.0±15.8%, respectively; P=0.00013; Figure 5C).
Outcomes
Four in-hospital deaths were recorded (mean age 72.5±4.9 years; 2 females and 2 males). The causes of death were multiple organ failure (n=3) and pneumonia (n=1). Infection types included 3 SIs and 1 LI. Blood cultures were positive for all patients with SIs. Patients with LIs had multiple-pathogen infections. The causative pathogens were S. aureus (n=3, all SIs), CoNS (n=1 LI), P. aeruginosa (n=1 LI), and Micrococcus sp. (n=1 LI).
Discussion
In the present cohort of CIED infections, staphylococcal species were the predominant pathogens. In the temporal distribution analysis, CIED infection peaked in the first year after the last CIED manipulation, and half the infections were detected during the fourth year.
Classification Analysis
S. aureus is the predominant pathogen in systemic CIED infections. In contrast, local CIED infections were mainly caused by CoNS. Even within the same pathogenic pathway groups, which compared PR SIs and PR LIs, the incidence of S. aureus was higher in PR SIs than PR LIs. S. aureus caused serious CIED infections.
In temporal distribution analysis, temporal differences between PR and non-PR infections were recognized in relation to the different pathogenic pathways. Even in comparisons between the same infection patterns (i.e., between PR SIs and non-PR SIs), temporal differences were observed according to different pathogenic intrusion routes. PR CIED infections peaked immediately after CIED pocket surgeries.
Temporal Distribution of CIED Infections
Previous reports on CIED infections have reported the duration since the last pocket surgical procedures.13,14 However, very few reports have focused on the temporal distribution of CIED infections.11 We investigated both the microbiology and temporal distribution of CIED infections.
Features of the Temporal Distribution of CIED Infections
Previous studies have focused on the early phase after the last surgical manipulation,11 such as CIED infections after 6 months and/or 1 year. In our cohort, the yearly distribution of CIED infections was examined up to 10 years after the last CIED surgical manipulation. Figure 2 shows the features of the temporal distribution of CIED infections. CIED infections peaked during the first year after the last CIED pocket manipulation. The rate of these infections decreased gradually until the fifth year. Most cases occurred during the first to fourth years (>60%). After the sixth year, there was a uniform trend in CIED infections. In their study, Harper et al11 found a similar long-term distribution of CIED infections as in the present study.
Two phases of CIED infections can been seen on the temporal distribution graph: (1) from the first to the fourth year, the number of CIED infections gradually decreased, thus, this time period constituted the early phase of CIED infections; and (2) from the fifth to the tenth year, CIED infections were still observed in a certain number of CIED patients and this time period constituted the late phase of CIED infections. During the acute period (<12 months), the causative pathogens responsible for lead-related endocarditis were reported to differ for infections occurring within and after 6 months.15 However, in the present long-term cohort, there was no microbiological difference between the early (first–fourth years) and late (fifth–tenth years) phases (data not shown). We speculate that the microbiological characteristics of early phase infections may be directly affected by surgical manipulation. In addition, the findings for the late phase may be secondarily affected by CIED manipulations, such as pocket erosion and/or device exposure.9 To confirm our speculations, we first classified CIED infections into SIs and LIs according to clinical presentation. Second, we classified CIED infections into PR and non-PR infections.
SI vs. LI
Microbiology of SI vs. LI Microbiological differences between SIs and LIs were investigated. Staphylococcal species are the primary pathogens in both SIs and LIs. S. aureus caused a significantly higher proportion of SIs than LIs (48.4% vs. 17.9%, respectively; P=0.023; Table 2). S. aureus has unique characteristics owing to its virulence potential and ubiquitous occurrence. Therefore, S. aureus has the potential to cause both local and disseminated infections.16 Furthermore, strong virulence can lead to tissue invasiveness and bacteremia.17,18 These pathogenic features may explain why S. aureus was the common causative pathogen for SIs in the present cohort. In other studies, S. aureus was reported to account for 14.4% of all bacteremia patients.16,19–21 In the present study, 22 patients in the SI group (22.7%) had bacteremia, and 24 pathogens were detected in blood cultures. The main pathogen was S. aureus (50%). Among patients with bacteremia in the present cohort, the percentage of S. aureus infections was similar to that reported in other studies. In contrast, the most common pathogen in LIs was CoNS (47.7%). Previous studies have reported similar trends for LIs.22
Temporal Distributions of SIs vs. LIs The mean duration since the last CIED pocket surgical procedure did not differ between the SI and LI groups (Table 3). In addition, both types of infections showed a similar temporal distribution, with peaks appearing during the first year (Figure 4). One of the reasons for these early peaks in both groups was that both SIs and LIs may be caused by the same pathway of pathogenic entry during the first year after the last CIED pocket manipulation. More specifically, these CIED infections may be caused by pathogens intruding from the CIED pocket sites. In fact, 6 of the 8 SIs (75%) were caused by CIED PR infection, and all 25 cases in the first year were related to CIED PR infections. In the present cohort, both SIs and LIs included PR infections because these infections were distinguished by clinical presentations and/or positive blood cultures. Other studies have reported that pocket manipulation is a risk factor for early CIED infections.5
After the first year, there was a difference between SIs and LIs in their temporal course. A uniform distribution was observed for SIs, but the rate of LIs decreased steadily until the tenth year (Figure 4). Temporal differences may be caused by causative pathogens. S. aureus was present in a higher proportion of patients with SIs than LIs (Table 2). In addition, CoNS accounted for a higher proportion of LIs than SIs overall (Figure 4). As mentioned above, S. aureus has extensive toxicity. Because of this pathogenic feature, SIs were concentrated in the first year (29.6%). The rate of LIs decreased gradually after the first year because CoNS was the main causative pathogen of LIs.
Classification According to CIED Pocket-Related Infection
Fourteen patients with SIs (53.8%) and all 71 patients with an LI were categorized as showing PR infections. In this study, 87.6% of all CIED infections were PR infections. The remaining 12 patients with SIs had non-PR infections. All non-PR patients had bacteremia and/or lead vegetation and/or endocarditis without CIED pocket infection. We speculated that a comparison of PR and non-PR infections may reveal the effects of the CIED pocket site on CIED infection. CIED pocket sites are susceptible to surgical manipulations, such as initial CIED implantation and generator exchange.5,11 In addition, comparisons among these groups may reveal how different pathogenic pathways affect CIED infections.
PR vs. Non-PR Infection This subanalysis provided a comparison between different pathogenic pathways in patients with CIED infections. PR infection peaked during the first year after the last CIED manipulation. After the second year, PR infections decreased steadily. Conversely, non-PR infections showed a uniform distribution without any peaks throughout the study period (Figure 5A). The differences in temporal distribution between PR and non-PR infections may be caused by differences in the intrusion routes of pathogens. The CIED pocket site was found to be an intrusion pathway for pathogens in PR infections. However, in non-PR infections, other pathogen-intrusion routes were found (i.e., any kind of sepsis, sick teeth, or spondyloarthritis). In this cohort, all non-PR patients had bacteremia and/or lead vegetation and/or endocarditis without CIED pocket infection. The temporal distribution of PR infections was similar to that of the overall cohort and LIs. Patients with PR infections accounted for over 80% of patients in our series. Therefore, the PR group may have had a significant effect on the trends in temporal distribution for all patients. Regarding the temporal distribution of non-PR infections, there were very few occurrences of SI without CIED pocket infection per year. Surprisingly, although there were very few cases of pure CIED SI, a certain number of patients presented every year until 10 years after the last CIED manipulation.
In the microbiological analysis, there was no difference between PR and non-PR infections (Supplementary Table 1). Both PR and non-PR infections were mainly caused by staphylococcal species. The proportion of infections caused by S. aureus and CoNS was similar between the PR and non-PR groups. These results suggest that different pathogen intrusion pathways may not affect the microbiology of CIED infections. Previous studies also reported that the main causative pathogens of CIED infections are staphylococcal species.23 However, previous studies did not discuss the pathogenic pathways, unlike the present study.
PR SI vs. Non-PR SI We compared the temporal and microbiological distributions among SIs caused by different pathogenic intrusion. This subanalysis provided a comparison between different pathogenic pathways (PR vs. non-PR infection) with the same clinical presentation (SI). Differences in the temporal distribution were observed between PR and non-PR SIs (Figure 5B). Non-PR SIs showed a uniform distribution over all time periods. In contrast, systemic PR infections peaked in the first year after the last CIED manipulation. This peak may be associated with surgery of the CIED pocket sites. However, both infection types showed a similar trend in temporal distribution after the second year. Pathogenic pathways affected the temporal differences between the 2 groups. The causative pathogens were mainly staphylococcal species in both groups (Supplementary Table 2). Although there was no significant difference between the 2 groups, infection with S. aureus was 2-folde higher in PR SI than non-PR SI. This suggests that the main pathogenic pathway of S. aureus was the CIED pocket site. However, it is possible that S. aureus induces SI even from other types of pathogenic intrusion. Therefore, regarding the comparison between SIs and LIs in the present study, this was one of the reasons for a higher proportion of SIs than LIs.
PR SI vs. PR LI This subanalysis compared findings between different infection patterns (SI vs. LI) with the same pathogenic pathway (CIED PR infection) with respect to temporal distribution and microbiological proportions. Both groups showed similar temporal trends. Specifically, infections peaked in both groups during the first year. All PR infections showed the same temporal distribution because all infections were associated with surgical CIED pocket sites. Surprisingly, approximately 50% of S. aureus infections on CIED pocket sites deteriorated into SI (Supplementary Table 3). In the temporal analysis, 30–60% of S. aureus infections caused SIs within the second year after the last CIED pocket manipulation (Figure 5C). Even after 5 years, PR SIs caused by S. aureus were detected in our cohort. Among the cases of PR S. aureus infections, 37.5% worsened to SI (mean 37.5%; range 0–66.7%). Surprisingly, even after the fifth year and up to 10 years, 27.8% of S. aureus pocket infections deteriorated to SI as a serious condition (mean 27.8%; range 0–66.7%). This study revealed the risks associated with CIED PR S. aureus infection not only in the early phase, but also in the late phase after CIED surgery. In addition, our data indicated that there is a high possibility of PR infections progressing to a serious condition, even during the late phase of infection.
Clinical Perspective
The present study showed differences in pathogenic patterns and temporal distributions according to the type of infectious agent and pathogenic pathway in CIED infections. This study will contribute to our understanding of the microbiology and pathogenic intrusions for CIED infections, and these data will help determine the early phase antibiotic treatment and facilitate prompt diagnosis of CIED infections before isolating the causative pathogens.
Study Limitations
This retrospective study has several limitations. Due to the limited population in the present study, future studies are needed to confirm the present findings. In addition, this study included patients who were referred from outside institutions for the removal of infected devices. As a form of referral bias, many patients received antibiotic therapy before being transferred to Nippon Medical School. Therefore, some of the microbiological results had the potential to include results from patients who had received prior antibiotic therapy. The present cohort included only patients who underwent lead extraction of their devices. This series excluded patients who did not undergo device removal due to comorbidities.
Conclusions
The severity of CIED infection depends on the causative pathogen. The temporal distribution of CIED infection was affected by the microbiological intrusion pathway. Close clinical observation is needed for the early diagnosis of CIED infections and causative pathogens up to 4 years after CIED pocket manipulation.
Acknowledgments / Disclosures
None declared.
Sources of Funding
This study did not receive any specific funding.
IRB Information
The study protocol was approved by the Ethics Committee of the Nippon Medical School in Tokyo, Japan (Reference no. B-2019-067).
Supplementary Files
Supplementary Table 1 Supplementary Table 2 Supplementary Table 3
Data Availability
The deidentified participant data will be shared upon reasonable request. It will become available immediately after publication and will be available indefinitely.
References
- 1. Wilkoff BL, Love CJ, Byrd CL, Bongiorni MG, Carrillo RG, Crossley GH, et al.. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: This document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009; 6: 1085–1104. [DOI] [PubMed] [Google Scholar]
- 2. Cabell CH, Heidenreich PA, Chu VH, Moore CM, Stryjewski ME, Corey GR, et al.. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999. Am Heart J 2004; 147: 582–586. [DOI] [PubMed] [Google Scholar]
- 3. Voigt A, Shalaby A, Saba S.. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: Temporal trends and causative insights. Pacing Clin Electrophysiol 2010; 33: 414–419. [DOI] [PubMed] [Google Scholar]
- 4. Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, et al.. Update on cardiovascular implantable electronic device infections and their management: A scientific statement from the American Heart Association. Circulation 2010; 121: 458–477. [DOI] [PubMed] [Google Scholar]
- 5. Tarakji KG, Wazni OM, Harb S, Hsu A, Saliba W, Wilkoff BL.. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: The impact of the infection type and the presence of vegetation on survival. Europace 2014; 16: 1490–1495. [DOI] [PubMed] [Google Scholar]
- 6. Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, et al.. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017; 14: e503–e551. [DOI] [PubMed] [Google Scholar]
- 7. Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, et al.. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections: Endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace 2020; 22: 515–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai V, Chen H, Hsia H, Zei P, Wang P, Al-Ahmad A.. Cardiac device infections complicated by erosion. J Interv Card Electrophysiol 2007; 19: 133–137. [DOI] [PubMed] [Google Scholar]
- 9. Greenspon AJ, Prutkin JM, Sohail MR, Vikram HR, Baddour LM, Danik SB, et al.. Timing of the most recent device procedure influences the clinical outcome of lead-associated endocarditis results of the MEDIC (Multicenter Electrophysiologic Device Infection Cohort). J Am Coll Cardiol 2012; 59: 681–687. [DOI] [PubMed] [Google Scholar]
- 10. Sohail MR, Hussain S, Le KY, Dib C, Lohse CM, Friedman PA, et al.. Risk factors associated with early- versus late-onset implantable cardioverter-defibrillator infections. J Interv Card Electrophysiol 2011; 31: 171–183. [DOI] [PubMed] [Google Scholar]
- 11. Harper MW, Uslan DZ, Greenspon AJ, Baddour LM, Carrillo RG, Danik SB, et al.. Clinical presentation of CIED infection following initial implant versus reoperation for generator change or lead addition. Open Heart 2018; 5: e000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stockhausen K.. The Declaration of Helsinki: Revising ethical research guidelines for the 21st century. Med J Aust 2000; 172: 252–253. [DOI] [PubMed] [Google Scholar]
- 13. Uslan DZ, Gleva MJ, Warren DK, Mela T, Chung MK, Gottipaty V, et al.. Cardiovascular implantable electronic device replacement infections and prevention: Results from the REPLACE Registry. Pacing Clin Electrophysiol 2012; 35: 81–87. [DOI] [PubMed] [Google Scholar]
- 14. Prutkin JM, Reynolds MR, Bao H, Curtis JP, Al-Khatib SM, Aggarwal S, et al.. Rates of and factors associated with infection in 200 909 Medicare implantable cardioverter-defibrillator implants: Results from the National Cardiovascular Data Registry. Circulation 2014; 130: 1037–1043. [DOI] [PubMed] [Google Scholar]
- 15. Greenspon AJ, Le KY, Prutkin JM, Sohail MR, Vikram HR, Baddour LM, et al.. Influence of vegetation size on the clinical presentation and outcome of lead-associated endocarditis: Results from the MEDIC registry. JACC Cardiovasc Imaging 2014; 7: 541–549. [DOI] [PubMed] [Google Scholar]
- 16. Grundmann H, Aanensen DM, van den Wijngaard CC, Spratt BG, Harmsen D, Friedrich AW, et al.. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: A molecular-epidemiological analysis. PLoS Med 2010; 7: e1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lowy FD.. Staphylococcus aureus infections. N Engl J Med 1998; 339: 520–532. [DOI] [PubMed] [Google Scholar]
- 18. Morgan M.. Methicillin-resistant Staphylococcus aureus and animals: Zoonosis or humanosis. J Antimicrob Chemother 2008; 62: 1181–1187. [DOI] [PubMed] [Google Scholar]
- 19. Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM.. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med 2000; 133: 604–608. [DOI] [PubMed] [Google Scholar]
- 20. Uslan DZ, Sohail MR, St Sauver JL, Friedman PA, Hayes DL, Stoner SM, et al.. Permanent pacemaker and implantable cardioverter defibrillator infection: A population-based study. Arch Intern Med 2007; 167: 669–675. [DOI] [PubMed] [Google Scholar]
- 21. Korkerdsup T, Ngarmukos T, Sungkanuparph S, Phuphuakrat A.. Cardiac implantable electronic device infection in the cardiac referral center in Thailand: Incidence, microbiology, risk factors, and outcomes. J Arrhythm 2018; 34: 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang R, Li X, Wang Q, Zhang Y, Wang H.. Microbiological characteristics and clinical features of cardiac implantable electronic device infections at a tertiary hospital in China. Front Microbiol 2017; 8: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Da Costa A, Lelièvre H, Kirkorian G, Célard M, Chevalier P, Vandenesch F, et al.. Role of the preaxillary flora in pacemaker infections: A prospective study. Circulation 1998; 97: 1791–1795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Supplementary Table 2 Supplementary Table 3
Data Availability Statement
The deidentified participant data will be shared upon reasonable request. It will become available immediately after publication and will be available indefinitely.