Abstract
Background
Because the effectiveness of strengthening guideline-based therapy (GBT) to prevent heart failure (HF) rehospitalization of chronic HF patients remains unclear, this study investigated the characteristics of HF patients in the Kobe University Heart Failure Registry in Awaji Medical Center (KUNIUMI) acute cohort.
Methods and Results
We studied 254 rehospitalized HF patients from the KUNIUMI Registry. Optimized GBT was defined as a Class I or IIa recommendation for chronic HF based on the guidelines of the Japanese Circulation Society. The primary endpoint was all-cause death or first HF rehospitalization after discharge. Outcomes tended to be more favorable for patients who had rather than had not received optimized GBT (hazard ratio [HR] 0.82; 95% confidence interval [CI] 0.57–1.19; P=0.27). Similarly, among New York Heart Association (NYHA) Class IV patients, outcomes tended to be more favorable for those who had rather than had not undergone optimized GBT (HR 0.73; 95% CI 0.47–1.12; P=0.15). Importantly, outcomes were significantly more favorable among NYHA Class IV patients aged <79 years who had rather than had not undergone optimized GBT (HR 0.33; 95% CI 0.14–0.82; P=0.02). Multivariate Cox regression analysis showed that optimized GBT was the only independent factor for the prediction of the primary endpoint.
Conclusions
Optimized GBT can be expected to play an important role as the next move for chronic HF patients.
Key Words: Guideline-based therapy, Heart failure, Rehospitalization
Heart failure (HF) is a global public health problem. In Japan, the number of patients hospitalized due to HF is increasing as a result of the rapidly aging population,1–4 and this is one of the most important issues for the management of HF. Although there are regional differences in the characteristics, management, and outcomes of hospitalized HF patients,5 Japan is known to have the highest ratio of aged persons in the world, with 27.3% of the population aged >65 years in 2016. Pharmacological treatment of HF can be the first-line therapeutic strategy for HF, especially Stage C and D. The goals of the pharmacological treatment of HF patients are to improve their clinical status, functional capacity, and quality of life, as well as to prevent rehospitalization and reduce mortality. Specifically, angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARBs), β-blockers, mineralocorticoid receptor antagonists (MRAs), and diuretics are the key medications used for managing Stage C and D HF.6
A marked feature after Stage C HF is repeated acute exacerbation of chronic HF. In addition, a previous history of HF hospitalization has been reported to be a strong risk factor for death among patients admitted for acute HF.6–9 Bello et al showed that a previous history of HF hospitalization was a strong predictor of adverse cardiac outcomes for patients with HF with reduced ejection fraction (HFrEF) and patients with HF with preserved ejection fraction (HFpEF).7 Moreover, Akita et al reported that a history of multiple previous HF hospitalizations, rather than a history of a single or no previous HF hospitalization, was as an independent predictor for all-cause death and HF rehospitalization.8 Therefore, Stage C HF patients need treatment for both chronic HF and acute exacerbations of chronic HF, such that avoiding HF rehospitalization for patients with chronic HF could be one of the main issues for improving outcomes in what has been called the “HF pandemic” era.
Because the effectiveness of optimized guideline-based therapy to prevent HF rehospitalization of patients with chronic HF remains uncertain, we designed and conducted a retrospective, population-based study of the Kobe University Heart Failure Registry in Awaji Medical Center (KUNIUMI) acute cohort to investigate, in detail, the characteristics of hospitalized HF patients.
Methods
Study Design
This study is part of the KUNIUMI acute cohort, a population-based registry of acute HF on Awaji Island, Japan. The details of the KUNIUMI acute cohort have been described previously.9 In this study, we only used patient data from Awaji Medical Center, because some data was missing for patients from other institutions. Awaji Island is one of the largest islands in Japan. It has one of the highest ratios of elderly people in Japan and a low migration rate with a relatively stable population.10 Therefore, the higher-quality incidence and follow-up data used in this study can be compared with previous data from KUNIUMI.10
This study was approved by the Ethics Committee of Awaji Medical Center (No. 20-11) and was conducted in accordance with the Declaration of Helsinki.
Study Population and Eligibility Criteria
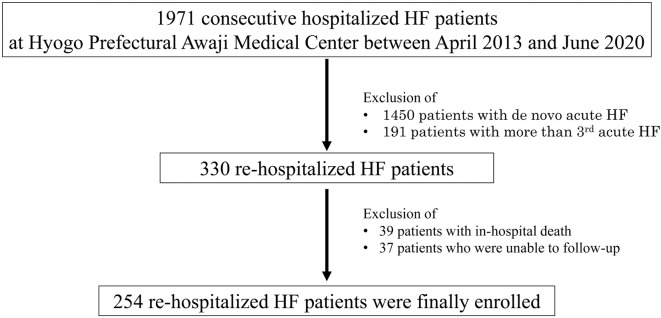
In all, 1,971 consecutive HF patients who met the Framingham criteria11 and were hospitalized on Awaji Island between April 2013 and March 2020 were retrospectively enrolled in this study (Figure 1). After exclusion of 1,450 patients with de novo acute HF and 191 patients with more than 3 acute HF hospitalizations, the remaining 330 rehospitalized HF patients included in the present study. Another 39 patients who died in hospital and 37 patients who could not be followed-up were also excluded, such 254 rehospitalized HF patients who had already received guideline-based medical therapy were enrolled in the present study.
Figure 1.
Flow chart of study recruitment in this study. HF, heart failure.
Decisions regarding in-hospital and post-discharge HF care were made by attending physicians, including senior cardiologists. Echocardiography was performed using commercially available ultrasound systems. Standard echocardiographic measurements were obtained in accordance with the current guidelines of the American Society of Echocardiography/European Association of Cardiovascular Imaging.12 All echocardiographic examinations were performed by senior echocardiologists or sonographers.
Definitions of Optimized Guideline-Based Therapy for Chronic HF
Optimized guideline-based therapy has been defined as a Class I or IIa recommendation for chronic HF based on the current guidelines of the Japanese Circulation Society (JCS)/Japanese Heart Failure Society (JHFS)6 during both the hospital stay and after discharge (Table 1). Specifically, optimized guideline-based medical therapy was defined as upward titration or introduction of ACEI/ARBs, β-blockers, MRAs, loop diuretics, and tolvaptan for patients with HFrEF, and as upward titration or the introduction of loop diuretics and tolvaptan for patients with HFpEF.6 Exercise therapy, the use of adaptive servo-ventilation (ASV), continuous positive airway pressure (CPAP), obstructive sleep apnea, and home oxygen therapy (HOT) were classified as optimized guideline-based non-pharmacological therapies for chronic HF.6 Other optimized guideline-based invasive therapies for chronic HF, including implantable cardioverter-defibrillator, cardiac resynchronization therapy, catheter ablation for atrial fibrillation, pacemaker treatment for bradyarrhythmia, percutaneous coronary intervention for ischemic heart disease, and surgical and catheter therapy for valvular heart disease, were not performed as part of this Registry.
Table 1.
Definition of Additional Guideline-Based Therapy for Chronic HF Based on the Japanese Circulation Society/Japanese Heart Failure Society Guidelines6
| Class of recommendation |
No. patients (%) with optimized GBT (n=193) |
|
|---|---|---|
| Pharmacological therapy for HFrEF | ||
| ACEI/ARBs | I | 19 (22) |
| β-blockers | I | 30 (35) |
| MRAs | I | 24 (28) |
| Loop diuretics | I | 27 (31) |
| Tolvaptan | IIa | 33 (38) |
| Digitalis | IIa | 2 (2) |
| Short-term use of oral inotropic drugs | IIa | 9 (10) |
| Amiodarone | IIa | 0 (0) |
| Pharmacological therapy for HFpEF | ||
| Diuretics | I | 39 (31) |
| Tolvaptan | IIa | 32 (26) |
| Pharmacological therapy for HFrEF with COPD or bronchial asthma | ||
| β1-Adrenergic receptor-selective antagonists for bronchial asthma | IIa | 0 (0) |
| Treatment of COPD or bronchial asthma | IIa | 0 (0) |
| Non-pharmacological therapy | ||
| ASV | ||
| Relieve symptoms of congestion in patients receiving optimized GBT for HF in the hospital setting |
IIa | 0 (0) |
| Continuous use to satisfy the above criterion for patients who respond well to ASV and are expected to worsen if ASV is discontinued |
IIa | 3 (2) |
| Exercise therapy | ||
| Combined with drug therapy to relieve symptoms and improve exercise capacity in patients with HFrEF |
I | 0 (0) |
| To improve QOL, reduce cardiac accidents, and improve life expectancy in patients with HFrEF |
IIa | 0 (0) |
| To improve exercise capacity in patients with HFpEF with low exercise capacity | IIa | 0 (0) |
| Supervised exercise therapy at experienced institutions to improve exercise capacity and QOL in patients with pulmonary hypertension whose symptoms are stable on drug therapy |
IIa | 0 (0) |
| Resistance training to improve ADL and QOL by increasing muscle strength and endurance in patients with advanced deconditioning and patients with reduced physical function |
IIa | 0 (0) |
| CPAP | ||
| Symptomatic OSA | I | 0 (0) |
| For patients with HFrEF and moderate or severe OSA to improve left ventricular function | IIa | 0 (0) |
| HOT | ||
| In patients with moderate or severe CSR-CSA and NYHA Class III or VI HFrEF with LVEF ≤45% to improve cardiac function and symptoms |
IIa | 2 (2) |
ACEI, angiotensin converting enzyme inhibitors; ADL, activities of daily living; ARBs, angiotensin II receptor blockers; ASV, adaptive servo-ventilation; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; CSR-CSA, central sleep apnea with Cheyne-Stokes respiration; GBT, guideline-based therapy; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HOT, home oxygen therapy; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; OSA, obstructive sleep apnea; QOL, quality of life.
Definition of the Primary Endpoint
The primary endpoint was defined as all-cause death or first HF rehospitalization after discharge over a median follow-up of 400 days (interquartile range 346–455 days).
Statistical Analysis
Continuous variables are expressed as the mean±SD if they were normally distributed and as the median with interquartile range (IQR) if they were not normally distributed. Categorical variables are expressed as frequencies and percentages. Parameters in 2 subgroups were compared using Student’s t-test or the Mann-Whitney U test depending on data distribution. Differences in proportions were evaluated with Fisher’s exact test. Survival curves of freedom from all-cause death and HF rehospitalization were determined with the Kaplan-Meier method, and cumulative event rates were compared using the log-rank test. Associations of parameters with cardiovascular death were identified using a Cox proportional hazards model for univariate and multivariate analyses. Variables with a univariate value of P<0.05 were incorporated into the stepwise selection. For all steps, P<0.05 was considered statistically significant. All analyses were performed using MedCalc version 19.0.7 (MedCalc Software, Mariakerke, Belgium).
Results
Patient Characteristics
The baseline characteristics of the 254 rehospitalized chronic HF patients are summarized in Table 2. Mean patient age was 81.2±10 years and 142 (56%) were female. Thirteen patients (5%) were classified as New York Heart Association (NYHA) Class II, 62 (22%) were classified as NYHA Class III, and 179 (70%) were classified as NYHA Class IV. In accordance with the JCS/JHFS guidelines,6 86 patients (34.1%) were diagnosed with HFrEF, 41 (16.3%) were diagnosed with HF with mid-range ejection fraction (HFmrEF), 125 (49.6%) were diagnosed with HFpEF, and 2 (0.4%) had unknown HF phenotypes.
Table 2.
Baseline Characteristics of Patients
| All patients (n=254) |
Patients with optimized GBT (n=193) |
Patients without optimized GBT (n=61) |
P value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 81.2±10.0 | 81.2±9.3 | 81.1±12.5 | 0.93 |
| Male sex | 142 (56) | 110 (57) | 32 (52) | 0.53 |
| BMI (kg/m2) | 21.0±3.7 | 20.9±3.4 | 21.4±4.5 | 0.43 |
| NYHA class | ||||
| II | 13 (5) | 10 (5) | 3 (5) | 0.98 |
| III | 62 (24) | 49 (25) | 13 (21) | 0.51 |
| IV | 179 (70) | 134 (69) | 45 (74) | 0.52 |
| HF classification | ||||
| HFrEF | 86 (34) | 72 (37) | 14 (23) | 0.04 |
| HFmrEF | 41 (16) | 31 (16) | 10 (16) | 0.92 |
| HFpEF | 125 (50) | 89 (46) | 36 (59) | 0.07 |
| Unknown | 2 (0.8) | 1 (0.5) | 1 (1.6) | 0.39 |
| Comorbidities | ||||
| Hypertension | 182 (72) | 145 (75) | 37 (61) | 0.03 |
| Diabetes | 82 (32) | 67 (35) | 15 (25) | 0.16 |
| Atrial fibrillation | 139 (55) | 105 (54) | 34 (56) | 0.89 |
| Ischemic heart disease | 93 (37) | 77 (40) | 16 (26) | 0.05 |
| Valvular disease | 71 (30) | 51 (26) | 20 (33) | 0.35 |
| Lung disease | 49 (19) | 35 (18) | 14 (23) | 0.36 |
| Blood examination | ||||
| Hemoglobin (mg/dL) | 10.9±1.9 | 11.0±1.87 | 10.9±2.1 | 0.70 |
| Albumin (mg/dL) | 3.0±0.5 | 3.1±0.5 | 3.0±0.5 | 0.42 |
| Blood urea nitrogen (mg/dL) | 32.3±17.5 | 31.9±17.9 | 33.6±16.4 | 0.52 |
| Creatinine (mg/dL) | 1.6±1.2 | 1.66±1.27 | 1.59±1.00 | 0.72 |
| B-type natriuretic peptide (pg/mL) | 407 [505–692] | 652 [537–767] | 394 [307–481] | 0.03 |
| Medications | ||||
| ACEI/ARBs | 188 (74) | 145 (75) | 43 (70) | 0.78 |
| β-blockers | 196 (77) | 156 (81) | 40 (66) | 0.04 |
| MRAs | 105 (42) | 76 (39) | 29 (48) | 0.18 |
| Loop diuretics | 204 (81) | 156 (81) | 48 (79) | 0.87 |
| Tolvaptan | 62 (25) | 45 (23) | 17 (28) | 0.38 |
| Unknown | 1 | |||
| Echocardiographic data | ||||
| LV end-diastolic dimension (mm) | 50.7±9.9 | 51.5±10.1 | 47.3±8.2 | 0.01 |
| LV end-systolic dimension (mm) | 38.3±11.0 | 39.2±11.3 | 34.4±8.7 | 0.008 |
| LVEF (%) | 47.0±14.2 | 46.3±14.3 | 49.2±13.9 | 0.16 |
| Left atrial diameter (mm) | 46.5±8.0 | 46.5±7.6 | 46.7±9.7 | 0.85 |
Unless indicated otherwise, data are given as the mean±SD for normally distributed data, the median [interquartile range] for non-normally distributed data, or n (%). BMI, body mass index; HFmrEF, heart failure with mid-range ejection fraction; LV, left ventricular. Other abbreviations as in Table 1.
Effect of Optimized Guideline-Based Therapy on Outcomes
Of the 254 rehospitalized chronic HF patients, 193 were classified as having received optimized guideline-based therapy; the remaining 61 patients were classified as the non-optimized guideline-based therapy group. The baseline characteristics of patients in these 2 groups were similar except that the optimized guideline-based therapy group had a higher prevalence of hypertension (75% vs. 61%; P=0.03), enlarged left ventricle (left ventricular [LV] end-diastolic dimension 51.5±10.1 vs. 47.3±8.2 mm [P=0.01]; LV end-systolic dimension 39.2±11.3 vs. 34.4±8.7 mm [P=0.008]), β-blocker use (79% vs. 61%; P=0.005), and tolvaptan use (50% vs. 31%; P=0.01), as well as higher B-type natriuretic peptide concentrations (652 [537–767] vs. 394 [307–481] pg/mL). Details of the optimized guideline-based therapy are provided in Table 1. The upward titration or introduction of established cardioprotective medications, such as ACEI/ARBs, β-blockers, and MRAs, was used for 120 patients; the upward titration or introduction of loop diuretics and tolvaptan was used for 137 patients; and HOT and ASV were used for 5 patients.
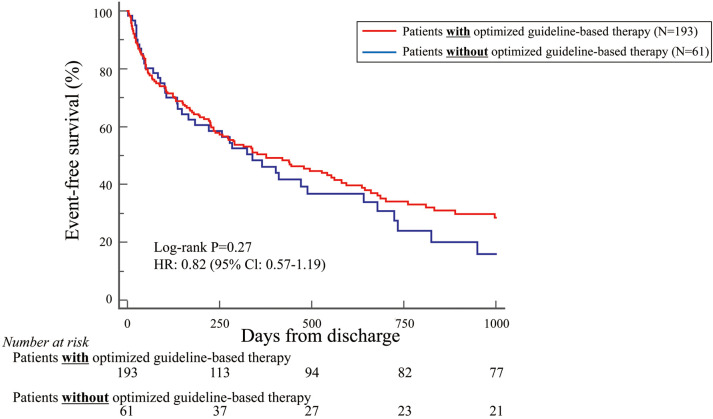
During the mean follow-up period of 400 days, the primary endpoint of all-cause death or first HF rehospitalization after discharge was registered for 166 patients (65.3%). Patients who had undergone optimized guideline-based therapy tended to have more favorable outcomes than those who had not, but the difference was not statistically significant (hazard ratio [HR] 0.82; 95% confidence interval [CI] 0.57–1.19; P=0.27; Figure 2).
Figure 2.
Kaplan-Meier curve indicating that, based on the primary endpoint, patients who had been treated with optimized guideline-based therapy tended to show more favorable outcomes than those who had not, but the difference was not statistically significant. CI, confidence interval; HR, hazard ratio.
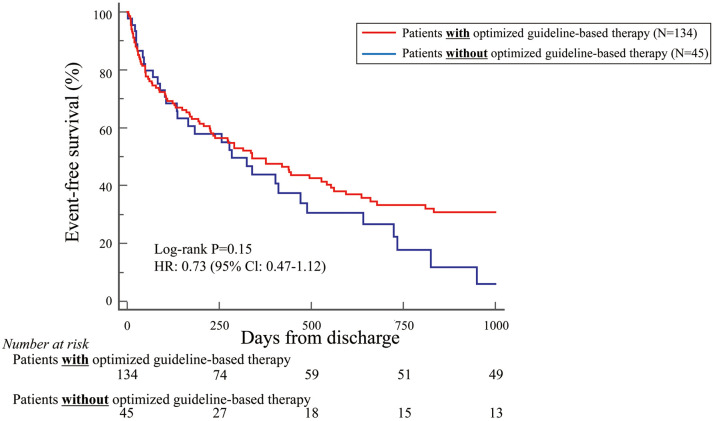
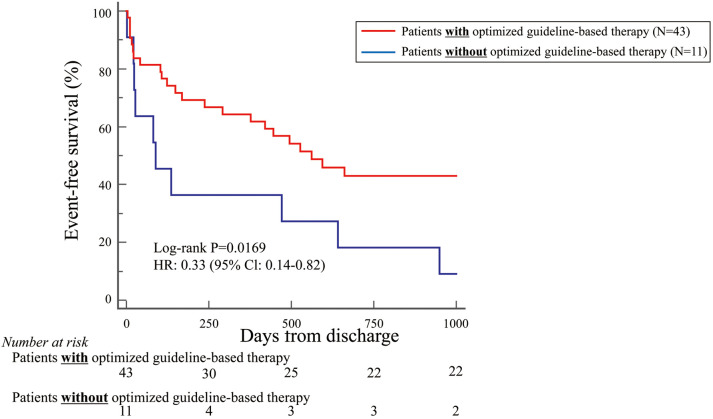
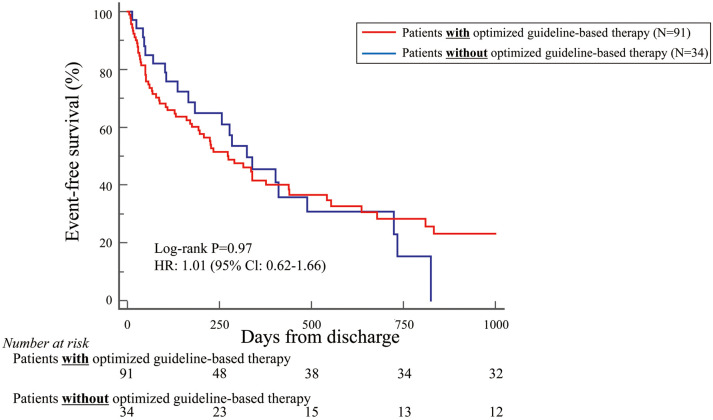
Because most patients in this study were NYHA Class IV (70%), the primary endpoint was also examined in these patients separately. Patients with NYHA Class IV and treated with optimized guideline-based therapy tended to have more favorable outcomes than those without this treatment, but the difference was not statistically significant (HR 0.73; 95% CI 0.47–1.12; P=0.15; Figure 3). It was of note that NYHA Class IV patients aged <79 years and treated with optimized guideline-based therapy had significantly more favorable outcomes than those without this treatment (HR 0.33; 95% CI 0.14–0.82; P=0.02; Figure 4). Conversely, this difference was not observed for NYHA Class IV patients aged >80 years treated with and without optimized guideline-based therapy (HR 1.01; 95% CI 0.62–1.66; P=0.97; Figure 5).
Figure 3.
Kaplan-Meier curve indicating that, based on the primary endpoint, New York Heart Association Class IV patients who had been treated with optimized guideline-based therapy tended to have more favorable outcomes than those who had not, but the difference was not statistically significant. CI, confidence interval; HR, hazard ratio.
Figure 4.
Kaplan-Meier curve indicating that, based on the primary endpoint, New York Heart Association Class IV patients aged <79 years old who had been treated with optimized guideline-based therapy had significantly more favorable outcomes than those who had not. CI, confidence interval; HR, hazard ratio.
Figure 5.
Kaplan-Meier curve indicating that, based on the primary endpoint, New York Heart Association Class IV patients aged >80 years with and without optimized guideline-based therapy showed similar outcomes. CI, confidence intervalx; HR, hazard ratio.
Table 3 shows the univariate and multivariate Cox regression analyses for primary endpoint prediction for the 54 patients <79 years old with NYHA Class IV. The most important finding of these analyses was that optimized guideline-based therapy was identified as the only independent factor for predicting the primary endpoint (HR 0.57; 95% CI 0.10–0.97; P=0.04).
Table 3.
Univariate and Multivariate Cox Proportional Hazards Analysis for Predicting the Primary Endpoint
| Covariate | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 0.02 | 0.94–1.00 | 0.09 | |||
| Female sex | 0.34 | 0.30–1.13 | 0.11 | |||
| BMI | 0.05 | 0.90–1.09 | 0.85 | |||
| Atrial fibrillation | 0.33 | 0.37–1.38 | 0.32 | |||
| LVEF | 0.01 | 0.97–1.02 | 0.56 | |||
| Albumin | 0.41 | 0.22–1.10 | 0.09 | |||
| Optimized GBT | 0.36 | 0.21–0.87 | 0.03 | 0.57 | 0.10–0.97 | 0.04 |
BMI, body mass index; CI, confidential interval; GBT, guideline-based therapy; HR, hazard ratio.
Discussion
The findings of our study indicate that chronic HF patients who had received optimized guideline-based therapy tended to have more favorable outcomes than those who had not. Furthermore, outcomes were significantly more favorable for chronic NYHA Class IV HF patients age <79 years who had been treated with optimized guideline-based therapy compared with those who had not, but this was not the case for patients aged >80 years. Moreover, optimized guideline-based therapy was the only independent factor for the prediction of the primary endpoint among NYHA Class IV HF patients <79 years old.
The KUNIUMI acute cohort shows the actual and current situation concerning the rehospitalization of chronic HF patients on Awaji Island, whose mean age is 81.2±10.0 years, which is higher than the age of participants in most commonly referenced HF registries. Because the ratio of the population >65 years of age on Awaji Island in 2015 is similar to that predicted for Japan as a whole in 2035, other regions in Japan may show similar trends in the future. Thus, KUNIUMI may represent the future for chronic HF patients in Japan.
Importance of Optimized Guideline-Based Therapy for Preventing Rehospitalization of Chronic HF Patients
HF is associated with high mortality and prolonged and frequent hospitalizations, such that HF places a significant economic burden on the healthcare system.1,13 Established cardioprotective medications, such as ACEI, ARBs, β-blockers, and MRAs, are the mainstays of guideline-based medical therapy to improve the prognosis for chronic HF patients, especially patients with HFrEF, as recommended in various guidelines.6,14,15 In addition, some diuretics, including loop diuretics and tolvaptan, have been recommended for both HFrEF and HFpEF patients.6,14,15 Although there is no evidence that these diuretics improve the prognosis for every phenotype of chronic HF, diuretics are recommended to relieve symptoms of congestion in HF, such as dyspnea on exertion and edema.6,14,15 In addition, it is anticipated that guideline-based medical therapy and non-pharmacological therapies, such as ASV, CPAP, and HOT, could be useful to avoid rehospitalization due to acute exacerbation of chronic HF.
Optimized Guideline-Based Therapy for Preventing Rehospitalization of Younger Chronic HF Patients
In this study we showed that HF patients who had been treated with optimized guideline-based therapy tended to have more favorable outcomes than those who had not. Interestingly, among NYHA Class IV HF patients <79 years old, outcomes were significantly more favorable for those who had received optimized guideline-based therapy than for those who had not, but this difference was not seen among those aged >80 years of age. Moreover, optimized guideline-based therapy was identified as the only independent factor for predicting the primary endpoint for NYHA Class IV HF patients <79 years of age. Most clinical trials have shown the utility of guideline-based medical therapy for chronic HF patients who were younger and had fewer comorbidities than real-world patients. Thus, improvements in the prognosis for or rehospitalization of elderly HF patients have remained limited, while data on the use of guideline-based medical therapy for elderly HF patients remains insufficient. Akita et al reported that the use of guideline-based medical therapy was associated with a reduction in cardiovascular death and rehospitalization of younger HFrEF patients (<79 years old), but not with a reduction in cardiovascular death and rehospitalization of elderly HFrEF patients (aged >80 years).16 They also showed that the number of prescriptions for guideline-based medical therapy at discharge was significantly lower for elderly (>80 years old) than for younger (<79 years old) HFrEF patients.16 Furthermore, Dobre et al reported that use of β-blockers was associated with a reduction in mortality for HF patients aged <75 but not ≥75 years.17
Previous investigators have discussed possible reasons why guideline-based medical therapy is less effective for elderly HF patients. The pharmacokinetics and pharmacodynamics specific to cardiovascular medications may change with increasing patient age. The pharmacokinetic changes may lead to changes in drug distribution, metabolism, and elimination, while age-related pharmacodynamic changes affect end-organ responsiveness, and may thus be likely to result in side effects.18,19 In addition, elderly HF patients often suffer from more and more severe comorbidities, including renal dysfunction, which could increase the risk of drug-related diseases and drug-drug interactions, so that such interactions could aggravate a patient’s clinical status and offset the hoped for benefits.18,20 Although there are studies, as mentioned above, in which guideline-based medical therapy was not associated with a reduction in cardiovascular death and rehospitalization for elderly HF patients, guideline-based medical therapy should be performed even in elderly HF patients because of the lack of clear evidence. In fact, optimized guideline-based therapy was effective for some specific elderly patients in the present study.
Clinical Implications
The number of patients with HF has been rapidly increasing worldwide, which has led to what has been termed the “HF pandemic”.1 It is known that a history of HF hospitalization is a high risk factor for HF rehospitalization.21 Furthermore, multiple previous HF hospitalizations are strongly associated with all-cause death and HF rehospitalization.8 Thus, there is an urgent need to prevent HF rehospitalization using various treatments, including guideline-based therapy. According to our findings, optimized guideline-based therapy may be useful for all chronic HF patients, and we suggest that non-elderly advanced chronic HF patients in particular should be treated with guideline-based therapy to prevent rehospitalization due to acute exacerbations of chronic HF. Therefore, attending physicians should administer not only temporary treatments, such as diuretics and inotropic drugs to provide relief of congestion in HF due to acute exacerbation of chronic HF during hospital stay, but also optimized guideline-based therapy.
Optimal guideline-based medical therapy is clearly essential to prevent rehospitalization due to acute exacerbations of chronic HF, but several recent large-scale clinical studies have proved to be inadequate in reality.22–26 For example, the prescription rate of MRA for patients with HFrEF has remained roughly 60–70%.22–24 Moreover, treatment with β-blockers for HFrEF patients should be titrated upwards for LV reverse remodeling,27 or better outcome,28 but HFrEF patients receiving at least 50% of the target daily dose of β-blockers was only 50%.25,26 Therefore, attending physicians still have the scope to strengthen guideline-based therapy for chronic HF patients in real-world practice.
Study Limitations
The present study was a retrospective study, so that some data are missing. Thus, further studies, both prospective and retrospective, with low rates of missing data are needed to validate our findings. The patients in this registry were enrolled between April 2013 and June 2020 so that novel cardioprotective medications, such as ivabradine, sacubitril/valsartan, and sodium–glucose cotransporter 2 inhibitors, could not be included in the optimized guideline-based therapy used for this study. Finally, the sample size was not calculated to be epidemiologically valid before the study started, so that optimal sample size calculations will be necessary for further prospective studies.
Conclusions
Optimized guideline-based therapy was found to be associated with avoiding HF rehospitalization of chronic HF patients, especially NYHA Class IV patients aged <79 years. Optimized guideline-based therapy can be expected to play an important role as the next move for chronic HF patients.
Sources of Funding
This study did not receive any specific funding.
Disclosures
H.T. is a consultant for AstraZeneca and Ono Pharmaceutical. K.H. has received research funding from Daiichi Sankyo, Actelion Pharmaceuticals Japan, Terumo, Abbott Vascular Japan, Otsuka Pharmaceutical, Kowa, Takeda Pharmaceutical, Nihon Medi-Physics, Novartis Pharma Company, Bayer, Biotronic Japan, FUJIFILM Toyama Chemical, Medtronic Japan, and Sysmex. The remaining authors have no conflicts of interest to declare.
IRB Information
This study was approved by the Ethics Committee of Hyogo Prefectural Awaji Medical Center (No. 20-11).
References
- 1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al.. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 2. Roger VL.. Epidemiology of heart failure. Circ Res 2013; 113: 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanaka H, Nabeshima Y, Kitano T, Nagumo S, Tsujiuchi M, Ebato M, et al.. Optimal timing of echocardiography for heart failure inpatients in Japanese institutions: OPTIMAL study. ESC Heart Fail 2020; 7: 4213–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seo Y, Ishizu T, Ieda M, Ohte N, J-LONG Study Investigators.. Elderly Japanese standard data of echocardiography; From J-LONG study. J Echocardiogr 2020; 18: 175–182. [DOI] [PubMed] [Google Scholar]
- 5. Blair JE, Zannad F, Konstam MA, Cook T, Traver B, Burnett JC Jr, et al.. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol 2008; 52: 1640–1648. [DOI] [PubMed] [Google Scholar]
- 6. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, et al; on behalf of the Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group.. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure: Digest version. Circ J 2019; 83: 2084–2184.31511439 [Google Scholar]
- 7. Bello NA, Claggett B, Desai AS, McMurray JJ, Granger CB, Yusuf S, et al.. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail 2014; 7: 590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akita K, Kohno T, Kohsaka S, Shiraishi Y, Nagatomo Y, Goda A, et al.. Prognostic impact of previous hospitalization in acute heart failure patients. Circ J 2019; 83: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 9. Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, et al.. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007; 116: 1482–1487. [DOI] [PubMed] [Google Scholar]
- 10. Fujimoto W, Toh R, Takegami M, Hayashi T, Kuroda K, Hatani Y, et al.. Estimating incidence of acute heart failure syndromes in Japan: An analysis from the KUNIUMI Registry. Circ J, doi:10.1253/circj.CJ-20-1154. [DOI] [PubMed] [Google Scholar]
- 11. McKee PA, Castelli WP, McNamara PM, Kannel WB.. The natural history of congestive heart failure: The Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al.. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 13. Christ M, Stork S, Dorr M, Heppner HJ, Muller C, Wachter R, et al.. Heart failure epidemiology 2000–2013: Insights from the German Federal Health Monitoring System. Eur J Heart Fail 2016; 18: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al.. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 15. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al.. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 16. Akita K, Kohno T, Kohsaka S, Shiraishi Y, Nagatomo Y, Izumi Y, et al.. Current use of guideline-based medical therapy in elderly patients admitted with acute heart failure with reduced ejection fraction and its impact on event-free survival. Int J Cardiol 2017; 235: 162–168. [DOI] [PubMed] [Google Scholar]
- 17. Dobre D, DeJongste MJ, Lucas C, Cleuren G, van Veldhuisen DJ, Ranchor AV, et al.. Effectiveness of beta-blocker therapy in daily practice patients with advanced chronic heart failure; is there an effect-modification by age? Br J Clin Pharmacol 2007; 63: 356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rossello X, Pocock SJ, Julian DG.. Long-term use of cardiovascular drugs: Challenges for research and for patient care. J Am Coll Cardiol 2015; 66: 1273–1285. [DOI] [PubMed] [Google Scholar]
- 19. Mangoni AA, Jackson SH.. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br J Clin Pharmacol 2004; 57: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jhund PS, Tavazzi L.. Has the “epidemic” of heart failure been replaced by a tsunami of co-morbidities? Eur J Heart Fail 2016; 18: 500–502. [DOI] [PubMed] [Google Scholar]
- 21. Setoguchi S, Stevenson LW, Schneeweiss S.. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J 2007; 154: 260–266. [DOI] [PubMed] [Google Scholar]
- 22. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al.. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 23. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al.. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 24. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al.. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 25. Tsutsui H, Momomura SI, Yamashina A, Shimokawa H, Kihara Y, Saito Y, et al.. Efficacy and safety of ivabradine in Japanese patients with chronic heart failure: J-SHIFT study. Circ J 2019; 83: 2049–2060. [DOI] [PubMed] [Google Scholar]
- 26. Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, et al.. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010; 376: 875–885. [DOI] [PubMed] [Google Scholar]
- 27. Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, et al.. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 1996; 94: 2807–2816. [DOI] [PubMed] [Google Scholar]
- 28. Sin DD, McAlister FA.. The effects of beta-blockers on morbidity and mortality in a population-based cohort of 11,942 elderly patients with heart failure. Am J Med 2002; 113: 650–656. [DOI] [PubMed] [Google Scholar]