Abstract
This work investigated the clinical prognostic implications and biological function of plasma soluble programmed cell death ligand 1 in breast cancer patients. Plasma sPD-L1 levels of recurrent/metastatic breast cancer patients were determined, and the association of sPD-L1 levels and metastatic progression-free survival and metastatic overall survival was assessed. The PD-L1 expression on breast cancer cells was analyzed by flow cytometry, and the level of sPD-L1 in the supernatant of breast cancer cells was determined by enzyme-linked immunosorbent assay. Furthermore, the effect of sPD-L1 on the proliferation and apoptosis of T lymphocytes was detected by WST-1 assay and flow cytometry. The plasma sPD-L1 levels in 208 patients with recurrent/metastatic breast cancer before receiving first-line rescue therapy were measured. The optimal cutoff value of plasma sPD-L1 for predicting disease progression was 8.774 ng/ml. Univariate and multivariate analyses identified high sPD-L1 level (≥ 8.774 ng/ml) and visceral metastasis were independent factors associated with poor prognosis. Relevance analysis showed that the plasma sPD-L1 level was weakly
associated with some systemic inflammation markers, including white cell count (WBC), absolute monocyte
count, and absolute neutrophil count. Furthermore, we found sPD-L1 could be found in supernatant of culture with breast cancer cell line expressing PD-L1 on the cell surface and inhibit T lymphocyte function, playing a negative regulatory role in cellular immunity. sPD-L1 was a good tumor predictive maker in breast cancer and it may play a potentially important role in immune tolerance.
Supplementary information
The online version contains supplementary material available at 10.1007/s00262-021-02898-4.
Keywords: SPD-L1, Breast cancer, Clinical implication, Predictive marker, T lymphocyte
Introduction
Breast cancer is the most common malignancy in women and is the top cause of cancer-related death [1]. Early breast cancer can be curable, but the treatment of recurrent or metastatic breast cancer remains controversial. Early diagnosis, appropriate treatment and monitoring the treatment response during the treatment are of important prognostic significance for recurrent or metastatic breast cancer. Carcinoembryonic antigen (CEA) and breast cancer antigen (CA153) are the most widely used serum markers, which play an important role in the process of monitoring relapse or disease progression in breast cancer patients [2]. CEA and CA153 are the non-specific tumor markers, CA153 response paralleled disease in only approximately 50% of patients who receive anthracycline-based first-line treatment in prospective phrase of II and III trials [3]. Consequently, there is a need to identify biomarkers in breast cancer.
There is a growing body of evidence suggesting that cancer immune suppression and immune escape play essential roles in tumor progression [4, 5]. Thus, identification of the mechanisms involved in the escape of immune suppression might help to identify a novel prognostic biomarker. The programmed death receptor 1 (PD-1)/programmed death ligand 1 (PD-L1) pathway plays a critical role in regulating the endogenous immune response to cancer [6–9]. PD-1, an inhibitory immune checkpoint receptor is constitutively expressed on activated T cells, protects healthy cells from excessive inflammatory or autoimmune responses via combination with its ligands PD-L1 and PD-L2 [6, 10]. However, tumors can co-opt the PD-1/PD-L1 pathway to evade immune destruction. The binding of PD-L1 to PD-1 could inhibit T lymphocyte proliferation, cytokine production and promote T cell apoptosis, thus terminally evading the anti-tumor immune response and enabling neoplastic growth [11, 12]. Abundant studies have shown that PD-L1 is over-expressed on tumor cells and tumor-associated macrophages in multiple malignancies [6] and is negatively correlated with survival prognosis [13]. Recently, various clinical trials with anti-PD-1/PD-L1 drugs have shown improved outcomes and response rates in patients with non-small-cell lung cancer [14], gastric cancer [15], melanoma [16] and urothelial cancer [17].
Significantly, high expression of PD-L1 in tumor tissue is valuable, as is that of sPD-L1, a soluble form of PD-L1 in blood measured by enzyme-linked immunosorbent assay (ELISA) [18], which is a potential prognostic predictor in certain hematological malignancies and solid tumors [19–22]. Finkelmeier et al. [20] stated that high sPD-L1 levels could predict unfavorable outcomes in hepatocellular carcinoma patients and was positively correlated with the stages of disease. Rossille et al. [21] reported that the plasma sPD-L1 level could predict the treatment response and OS in patients with DLBCL. Similarly, in metastatic or recurrent gastric cancer, Takahashi et al. [22] also found that elevated serum sPD-L1 level was an independent predictor for poor overall survival. A study focused on immune regulatory molecules in peripheral blood mononuclear cells (PBMCs) examined PD-L1 mRNA expression in PBMCs with a significant fold change in metastatic breast cancer patients in contrast to healthy volunteers and primary breast cancer patients, indicating that PD-L1 was a specific gene related to disease progression [23]. Li Y et al. reported that serum levels of sPD-1 and sPD-L1 could be used as noninvasive biomarkers for evaluating the malignancy of TNBC before neoadjuvant chemotherapy and predicting neoadjuvant chemotherapy response in TNBC patients [24]. Nonetheless, the prognostic value of sPD-L1, as well as their association with clinicopathological factors in breast cancer, remains a matter of debate.
Thus, despite its potential importance, the regulatory roles, functions and biological significance of the sPD-L1 are still under investigation. Whether sPD-L1 are involved in immune regulation and disease progression of breast cancer has yet to be elucidated. It is well known that the PD-1/PD-L1 pathway is identified as the most critical mechanism of tumor evasion, inhibiting T cell proliferation, inducing T cell exhaustion and enhancing the activity of regulatory T cells. It remains to be clarified the biological function of sPD-L1 in regulating the functions of T lymphocyte in breast cancer.
In this study, the expression of sPD-L1 in the supernatant of breast cancer cell lines was detected, and the effect of sPD-L1 on the biological function of T lymphocytes in peripheral blood of healthy people was further tested. By exploring the effect of sPD-L1 on T lymphocyte function, it would provide evidence for the future use of sPD-L1 in breast cancer.
Materials and methods
Patients
Female patients at the Harbin Medical University Cancer Hospital who were newly diagnosed with recurrent/metastatic breast cancer between 2015 September and 2017 February were selected and consecutively recruited in the prospective study. The inclusion criteria were as follows: pathologically diagnosed breast cancer, clinical radiologically or pathologically confirmed recurrent or metastatic lesions without anti-tumor therapy since metastasis or relapse, Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 to 1, adequate hematological and organ function. Patients with a diagnosed second tumor in the previous 5 years and with severe life-threatening illness, receiving immunosuppressive medications or with HIV infection, autoimmunity disease, active infections, hematologic neoplasms, history of organ allograft and viral hepatitis were excluded. In addition, early breast cancer patients at diagnosis were enrolled as controls. A total of 208 recurrent/metastatic patients and 32 patients with preliminary confirmed diagnosis of primary early breast cancer formally entered our study after giving written informed consent for use of clinical data and materials. Two of them were lost to follow-up because of being unable to contact. This research was approved by the ethics committee of Harbin Medical University Cancer Hospital (KY2018-06).
Treatment and radiologic evaluation
Due to its heterogeneity, breast cancer is divided into different relevant molecular subtypes using IHC. Four different molecular subtypes are categorized as follows: Lumin A-like subtype (ER/PR positive, HER2 negative, low ki67); Lumin B-like subtype (ER/PR positive, HER2 negative, high ki67); HER2 subtype; non-luminal (ER and PR negative, HER2 positive) or luminal (ER/PR positive, HER2 positive); or basal-like subtype (ER and PR and HER2 negative, namely triple-negative breast cancer). Guided by these molecular subtypes from metastatic or primary tumor biology, systemic therapy involving endocrine therapy, chemotherapy and molecular targeted therapy is the first choice for recurrent/metastatic breast cancer [25]. Patients continued to receive current first-line rescue therapy until disease progression or intolerable adverse events.
Patients were regularly radiologically evaluated every six weeks via contrast-enhanced computed tomography (CT) according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1. The therapeutic responses were categorized as complete remission (CR), partial remission (PR), stable disease (SD), disease progression (PD) and non-evaluable (NE).
Blood sample collection
Plasma specimens were obtained from 32 patients with early breast cancer at diagnosis, 208 recurrent or metastatic breast cancer patients at baseline, and at the evaluation time (each 2 cycles of treatment).
Blood samples were collected into lithium heparin (LH) BD blood collection vials within 4 h, centrifuged at 3500 r/min for 10 min and stored in 1000 µl aliquots at −80 °C until measurement. Experiment was repeated for three sets using the same serum samples.
Other clinical laboratory tests were examined before initiation of first-line palliative chemotherapy, including white cell count (WBC), absolute monocyte count, absolute neutrophil count, and absolute lymphocyte count. The lymphocyte-to-monocyte ratio (LMR) was calculated by dividing the lymphocyte count by the monocyte count, and the neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the neutrophil count by the lymphocyte count.
Measurement of plasma sPD-L1
An ELISA kit was used to measure the protein concentration of sPD-L1 (PDCD1LG1 ELISA kit, USCN Life Science, Wuhan, China) in blood collected from early patient controls and recurrent/metastatic breast cancer patients. The minimum detectable dose of plasma sPD-L1 was 0.057 ng/ml. Following the manufacturer instructions, samples were measured in triplicate, and the intra- and inter-assay variations were less than 20%. The same assay and procedure were used to measure sPD-L1 levels for all studied cohorts. In our study, each sample was tested in triplicate for sPD-L1, with the medians used for analysis.
Tissue sample collection and IHC
Biopsy of the first metastatic or recurrent lesion was proposed to verify breast cancer histology and reassess the tumor biology if clinically feasible because histology might vary from the primary site due to heterogeneity. We retrospectively 86 consecutively recruited patients with adequate metastatic tissues for PD-L1 and CD8 measurement.
PD-L1 (clone ab58810, Abcam, Paris, France) and CD8 (clone ab66868, Abcam, Paris, France) were assessed in formalin-fixed, paraffin-embedded archival tumor samples by IHC. The positive and negative controls were supplied by the manufacturer. Slides, each having 1000 tumor cells and 1000 adjacent non-tumor cells, were scored by two pathologists who did not participate in the clinical data study. Positivity was defined as PD-L1 and CD8 expression in the stroma or ≥ 1% in the tumor cells.
In this study, slides of full-face hematoxylin and eosin-stained sections from primary tumors were retrieved for the evaluation of TILs by light microscopy and we set the cutoff value at 20% and defined high TILD.
Cell culture
Human breast cancer cell lines (MDA-MB-231, T47D, MCF-7, MDA-MB-453) were obtained from the Heilongjiang Cancer Institute (Harbin, China). MDA-MB-231 cells were cultured in L15 (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco). MCF-7and T47D cells were cultured in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco). MDA-MB-453 cells were cultured in RPMI1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco); both cell lines were then incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Preparation of T lymphocytes in human peripheral blood
Fresh peripheral blood of patients with heparin sodium anticoagulant was centrifuged by Ficoll density gradient centrifugation for 30 min at 1800 r/min to obtain PBMCs. The cell concentration was adjusted to 2 × 106 cells/ml, further separation and purification of human T lymphocytes from PBMC using the EasySep Human Monocyte Enrichment Kit, purity > 90%; placing the cells in RPMI 1640 medium, 5% CO2, 37 ℃ were cultured to the logarithmic growth stage.
Analysis of PD-L1 on breast cancer cell surface
Different human breast cancer cell lines were incubated with PE-mouse anti-human PD-L1 mAb (clone ab270652, Abcam, Paris, France) at 37 ℃ for 20 min. After washing with PBS, labeled cells were detected by flow cytometry and analyzed by Beckman-Coulter’s Expo32 MULTICOMP software.
Detection of sPD-L1 in breast cancer cell culture supernatant
MDA-MB-231, T47D, MCF-7 and MDA-MB-453 cellular supernatants were centrifuged at 1000 g/min for 20 min, and the cell-free supernatants were stored at −20 °C for the ELISA assay. sPD-L1 in cell culture supernatants was measured using the PathScan total PD-L1 Sandwich ELISA Kit (Cell Signaling Technology).
Cell viability assay
The viability of the T cells was measured by WST-1 assay. To generate activated T cells, T lymphocyte cells were treated with 10 µg/ml PHA for 3 days. The experiment was divided into 10 groups: resting T lymphocyte, activated T lymphocyte, activated T lymphocyte plus MDA-MB-231 cell supernatant, activated T lymphocyte plus T47D cell supernatant, activated T lymphocyte plus MCF-7 cell supernatant, activated T lymphocyte plus MDA-MB-453 cell supernatant, activated T lymphocyte plus MDA-MB-231 cell supernatant plus anti-PD-L1 atezolizumab (ATE 100 μg/ml), activation T lymphocyte plus T47D cell supernatant plus ATE, activated T lymphocyte plus MCF-7 cell supernatant plus ATE, activated T lymphocyte plus MDA-MB-453 cell supernatant plus ATE.
In brief, as the confluence of the MDA-MB-231, T47D, MCF-7 and MDA-MB-453 reached around 70%, they were seeded in 96-well plates. After 72-h incubation co-cultured with T lymphocyte cells, WST-1 solution (20 μL, 0.5 mg/mL in PBS) was added to each well and the plates were incubated at 37 °C for 4 h. Finally, the medium was removed and dimethyl sulfoxide (DMSO, 150μL) was added to each well for 10 min to dissolve the purple formazan crystals. Absorbance was measured at 570 nm using a microplate reader. All assays were performed in three independent experiments.
Flow cytometry
The apoptosis of T lymphocyte was determined by flow cytometry using the Annexin V-FITC and propidium iodide (PI) staining. The experimental group was the same as above, the purified T lymphocytes were added to the 24-well culture plate at 1 × 106 cells/ml, and the culture plate was placed in an incubator for 3 days, and then the cell suspension was transferred to a centrifuge tube. Then, 1 × 106cells were collected and washed twice with ice-cold PBS. Cells were dual-stained using a FITC Annexin V Apoptosis Detection Kit I (BD Biosciences, Franklin Lakes, NJ) according to the manufacturer's protocol. Stained cells were immediately analyzed using a flow cytometer (BD Biosciences).
Statistical analysis
Receiver operating characteristic (ROC) curve analysis was used to define the discriminating cutoff value with maximized sensitivity and specificity for sPD-L1 concentration.
Metastatic progression-free survival (PFS) was defined the period from newly confirmed metastatic or recurrent breast cancer to disease progression or death because of disease progression. Metastatic OS was defined the period from newly confirmed metastatic or recurrent breast cancer to death because of disease progression. Median PFS and OS were computed by the Kaplan–Meier method, and the comparisons of variance were assessed using log-rank tests. Potential survival prognostic factors for PFS and OS were assessed by univariate analysis at first, and factors with p value less than 0.05 were accessed to multivariate for further validation. Hazard ratio (HR) and 95% confidence interval (95% CI) for all variables were calculated in the regression model.
Comparisons of clinical data between groups were made using the χ2 test, the Mann–Whitney t-test or the Wilcoxon-matched test, as appropriate. Relevance between plasma sPD-L1 levels and other laboratory examinations was analyzed using the Spearman or Pearson correlation analysis calculating the coefficient. A two-sided verified p value less than 0.05 was unified as statistical significance. SPSS version 17.0 statistical software and GraphPad Prism 5.01 (GraphPad Software, La Jolla, CA, USA) were used for statistical analysis.
Results
Patient characteristics
A total of 208 patients with recurrent or metastatic breast cancer were enrolled in our study. The expected baseline characteristics are shown in Table 1. Among the 208 cases, according to biological tumor subtypes, 33 (15.9%) had triple-negative breast cancer, 99 (47.6%) had HER2-positive disease, and the remaining 76 (36.5%) had luminal disease. A total of 181 patients (87.0%) relapsed after surgery and received necessary adjuvant treatment according to routine clinical practice in the early-stage setting. The location of metastatic occurrence was primarily liver, lung, or both (70.7%), and the remainder were located in bone, nodal and soft tissues (29.3%).
Table 1.
Clinical characteristics of advanced breast cancer according to optimal sPD-L1 level
| Characteristics | Total patients (n = 208)% | Low sPD-L1 (<8.774 ng/ml) | High sPD-L1 (≥ 8.774 ng/ml) | p value |
|---|---|---|---|---|
| Age, years | ||||
| ≤ 60 | 160(76.9) | 98(83.8) | 62(68.1) | 0.005 |
| > 60 | 48(23.1) | 19(16.2) | 29(31.9) | |
| Menopause status | ||||
| Premenopausal | 104(50.0) | 58(49.6) | 46(50.5) | 0.889 |
| Postmenopausal | 104(50.0) | 59(50.4) | 45(49.5) | |
| IHC profile | ||||
| Triple-negative | 33(15.9) | 16(13.7) | 17(18.6) | 0.202 |
| HER-2-positive | 99(47.6) | 62(53.0) | 37(40.7) | |
| Luminal | 76(36.5) | 39(33.3) | 37(40.7) | |
| Location of metastases | ||||
| Visceral | 147(70.7) | 86(73.5) | 61(67.0) | 0.309 |
| Non-visceral | 61(29.3) | 31(26.5) | 30(33.0) | |
| TNM staging | ||||
| I-II | 101(48.6) | 60(51.3) | 41(45.1) | 0.373 |
| III-IV | 107(51.4) | 57(48.7) | 50(54.9) | |
| Previous chemotherapy | ||||
| Yes | 159(87.8) | 89(88.1) | 70(87.5) | 0.899 |
| No | 22(12.2) | 12(11.9) | 10(12.5) | |
| Previous endocrine therapy | ||||
| Yes | 87(48.0) | 51(50.5) | 36(45.0) | 0.462 |
| No | 94(52.0) | 50(49.5) | 44(55.0) | |
| Previous radiotherapy | ||||
| Yes | 120(66.3) | 59(58.4) | 61(76.2) | 0.012 |
| No | 61(33.7) | 42(41.6) | 19(23.8) | |
| First-line therapy | ||||
| Targeted therapy | 0.635 | |||
| Trastuzumab + taxane | 84(84.8) | 51(82.3) | 29(60.7) | |
| Lapatinib + capecitabine | 15(15.2) | 11(17.7) | 8(60.0) | |
| Endocrine therapy | 0.589 | |||
| Fulvestrant | 4(17.4) | 3(17.7) | 1(10.0) | |
| AIs ± deprivation therapy | 23(85.2) | 14(82.3) | 9(90.0) | |
| Chemotherapy | 0.605 | |||
| Capecitabine | 5(5.7) | 1(2.6) | 4(9.1) | |
| Gemcitabine ± platinum | 29(33.0) | 13(34.2) | 16(36.4) | |
| Vinorelbine ± platinum | 7(19.3) | 3(7.9) | 4(9.1) | |
| Taxane ± epirubicin/platinum | ||||
| /capecitabine | 41(46.6) | 21(55.3) | 20(45.4) | |
Abbreviations: sPD-L1, soluble programmed death-ligand 1; IHC, immunohistochemical; AIs, aromatase inhibitors
sPD-L1 level with clinical characteristics
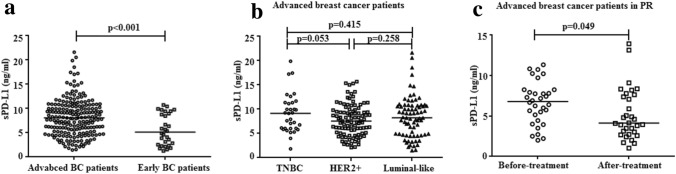
The median sPD-L1 level in plasma collected from all recurrent/metastatic breast cancer cases before first-line rescue therapy was 7.964 ng/ml (range: 1.442–21.618), apparently higher than that of control cohorts of 30 early breast cancer patients at diagnosis (median: 4.891 ng/ml, range: 1.249–10.718, p < 0.001, Fig. 1a). The baseline characteristics of these early breast cancer patients are shown in Supplementary Table 1.
Fig. 1.
Comparison of plasma sPD-L1 level between different patients cohorts. a Plasma sPD-L1 protein measurement in 208 patients of our cohort before first-line treatment and 30 early breast cancer patients at diagnosis. b Plasma sPD-L1 protein measurement in three molecular subtypes cohort. c Plasma sPD-L1 protein measurement in 32 patients of our cohort in PR (partial remission) with sPD-L1 collected before first-line treatment and within 1 week after treatment. Statistical analysis was performed using the Mann–Whitney t-test or the Wilcoxon-matched test
The optimal cutoff value of plasma sPD-L1 defined by the ROC curve in prediction of disease progression was 8.774 ng/ml (AUC = 0.676, p < 0.001, Supplementary Fig. 1). According to the optimal cutoff level, 91 (43.8%) patients were allocated to the high sPD-L1 (≥ 8.774 ng/ml) group, and 171 (56.2%) were allocated to the low-sPD-L1 (< 8.774 ng/ml) group. The high-sPD-L1 group were significantly associated with older age (over 60 years) and previous adjuvant radiotherapy history (p = 0.005 and p = 0.012, respectively, Table 1). In addition, the mean plasma sPD-L1 level showed no visible difference in patients with triple-negative subtype, HER2-positive subtype and luminal subtype (mean: 9.111, 7.765, and 8.402, p > 0.05, respectively, Fig. 1b). However, no significant correlation was noted between plasma sPD-L1 level and menopausal status, location of metastases, previous chemotherapy, endocrinotherapy history and different IHC profiles.
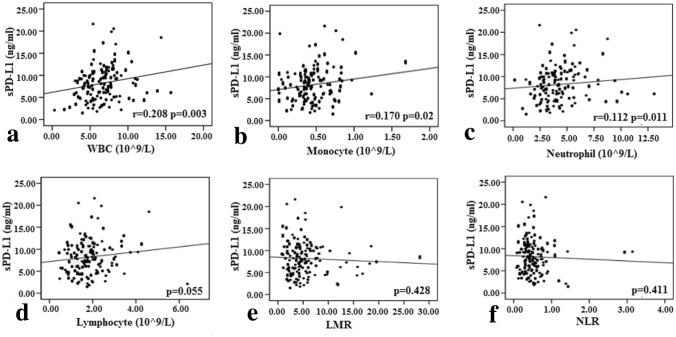
In addition, the correlations of plasma sPD-L1 level with systemic inflammation markers were analyzed, including white cell count (WBC), absolute monocyte, neutrophil and lymphocyte count; lymphocyte-to-monocyte ratio (LMR); and neutrophil-to-lymphocyte ratio (NLR). Weak correlation was found between plasma sPD-L1 level and WBC (r = 0.208, p = 0.003, Fig. 2a), absolute monocyte count (r = 0.170, p = 0.020, Fig.2b), and absolute neutrophil count (r = 0.112, p = 0.011, Fig. 2c). No relevance was found for other markers, including absolute lymphocyte count, LMR and NLR (p = 0.055, p = 0.428, and p = 0.411, respectively, Fig. 2d-f).
Fig. 2.
Correlation between sPD-L1 level and systemic inflammation marker. a WBC (white cell count), b monocyte count, c neutrophil count, d lymphocyte count, e LMR (lymphocyte-to-monocyte ratio), f NLR (neutrophil-to-lymphocyte). Statistical analysis was performed using the Pearson correlation b or Spearman correlation b–f.
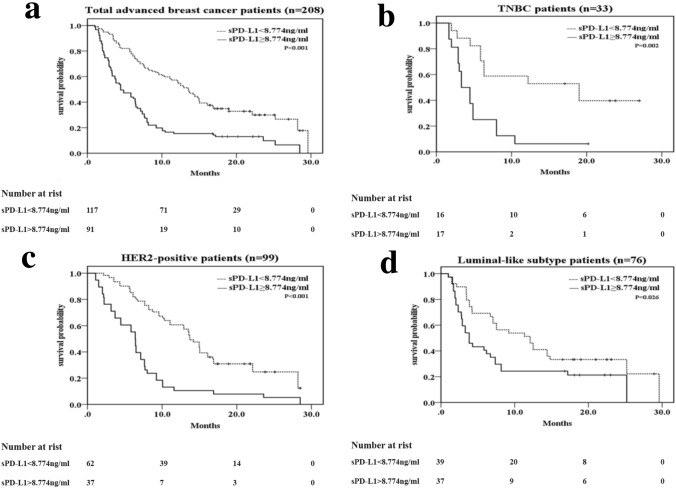
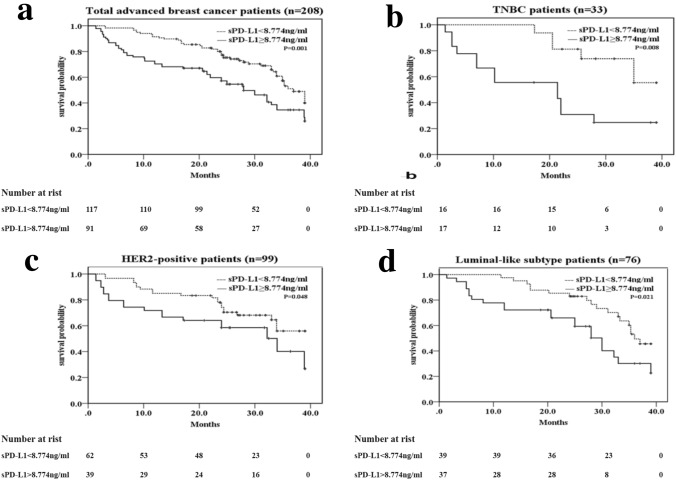
Progress analysis
The median follow-up was 25.2 months (range: 1.5–39.0), at which 166 (79.3%) cases exhibited disease progression and 99 patients died of breast cancer. As shown in Fig. 3, 4, high-sPD-L1 patients had poorer prognosis than low-sPD-L1 patients. Of all of the recurrent/metastatic patients, Kaplan–Meier analysis showed that patients with sPD-L1 ≥ 8.774 ng/ml had poorer PFS and OS than the patients with sPD-L1 < 8.774 ng/ml (PFS 7.2 m vs. 13.6 m, p < 0.001; OS 21.4 m vs 28.0 m, p = 0.001; Figs. 3a, 4a). For triple-negative breast cancer patients, patients with sPD-L1 ≥ 8.774 ng/ml had a significantly poorer outcome than those with sPD-L1 < 8.774 ng/ml (PFS 5.1 m vs. 13.9 m, p = 0.002; OS 17.4 m vs 26.6, p = 0.008; Figs. 3b, 4b). Similarly, for the HER2-positive subtype (PFS 7.2 m vs. 13.7 m, p < 0.001; OS 21.7 m vs 26.2 m, p = 0.048; Figs. 3c, 4c) and luminal subtype (PFS 8.0 m vs. 12.3 m, p = 0.026; OS 21.9 m vs 29.9 m, p = 0.021; Figs. 3d, 4d) breast cancers, high sPD-L1 level remained significantly associated with poor PFS and OS.
Fig. 3.
Kaplan–Meier curves of PFS for all patients and different IHC profile patients based on the cutoff sPD-L1 level (< 8.774 vs. ≥ 8.774 ng/ml). a All patients (n = 208), b TNBC (triple-negative breast cancer) (n = 33) patients, c HER2-positive subtype patients (n = 99), d luminal subtype patients (n = 76). p value was from Log-rank test according to the cutoff value of sPD-L1 level
Fig. 4.
Kaplan–Meier curves of OS for all patients and different IHC profile patients based on the cutoff sPD-L1 level (< 8.774 vs. ≥ 8.774 ng/ml). a All patients (n = 208), b TNBC (triple-negative breast cancer) (n = 33) patients, c HER2-positive subtype patients (n = 99), d luminal subtype patients (n = 76). p value was from Log-rank test according to the cutoff value of sPD-L1 level
Univariate analysis and multivariate analysis identified a high sPD-L1 level and visceral metastasis as factors associated with poor prognosis (Table 2). A high plasma sPD-L1 level (≥ 8.774 ng/ml) was an independent risk factor that more significantly affected PFS (HR = 3.358, 95%CI: 2.425–4.650, p < 0.001) and OS (HR = 2.792, 95%CI: 1.863–4.184, p < 0.001).
Table 2.
Univariate and multivariate Cox analyses for mPFS and mOS
| mPFS | mOS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR | 95%CI | p value | HR | 95%CI | p value | HR | 95%CI | p value | HR | 95%CI | p value | |
| Age,years | ||||||||||||
| ≤ 60 vs > 60 | 1.178 | 0.181–1.712 | 0.384 | 0.930 | 0.568–1.525 | 0.930 | ||||||
| Menopausal status | ||||||||||||
| Pre- vs postmenopausal | 1.170 | 0.861–1.589 | 0.316 | 0.989 | 0.664–1.472 | 0.956 | ||||||
| IHC profile | ||||||||||||
| Triple-negative vs Luminal | 1.059 | 0.661–1.699 | 0.811 | 1.128 | 0.846–1.504 | 0.411 | ||||||
| HER2-positive vs Luminal | 1.030 | 0.735–1.443 | 0.863 | 0.980 | 0.633–1.518 | 0.929 | ||||||
| Location of metastases | ||||||||||||
| Visceral vs non-visceral | 2.728 | 1.862–3.997 | < 0.001 | 3.786 |
2.545 –5.633 |
< 0.001 | 4.066 | 2.113–7.826 | < 0.001 | 4.976 | 2.575–9.618 | < 0.001 |
| Postoperative TNM staging | ||||||||||||
| I-II vs III | 0.763 | 0.550–1.058 | 0.104 | 0.836 | 0.552–1.266 | 0.396 | ||||||
| Previous chemotherapy | ||||||||||||
| Yes vs No | 1.359 | 0.795–2.322 | 0.262 | 0.980 | 0.520–1.844 | 0.949 | ||||||
| Previous endocrine therapy | ||||||||||||
| Yes vs No | 0.973 | 0.702–1.349 | 0.871 | 0.812 | 0.536–1.230 | 0.325 | ||||||
| Previous radiotherapy | ||||||||||||
| Yes vs No | 1.264 | 0.894–1.786 | 0.185 | 1.507 | 0.944–2.405 | 0.086 | ||||||
| Plasma sPD-L1 level | ||||||||||||
| ≥ 8,774 vs <8.774 | 2.342 | 1.720–3.189 | < 0.001 | 3.358 |
2.425– 4.650 |
< 0.001 | 2.286 | 1.530–3.415 | < 0.001 | 2.792 | 1.863–4.184 | < 0.001 |
Abbreviations: sPD-L1, soluble programmed death-ligand 1; IHC, immunohistochemical; HR, hazard ratio; CI, confidence interval; mPFS, metastatic progression-free survival
Bold values indicate univariate and multivariate analyses identified high sPD-L1 level (≥ 8.774 ng/ml) and visceral metastasis were independent factors associated with poor prognosis
Decreased sPD-L1 level in partial remission patients
All of the recurrent/metastatic patients received rescue first-line treatment in accordance with patient preference, tumor biology and disease clinical features. A total of 99 (47.6%) HER2-positive metastatic patients received trastuzumab plus taxane chemotherapy or lapatinib plus capecitabine chemotherapy. A total of 27 (13.0%) HER2-negative luminal metastatic patients received aromatase inhibitors (AIs) combined medicine or surgical deprivation therapy or fulvestrant therapy. A total of 82 (39.4%) received capecitabine monotherapy, gemcitabine monotherapy or combined platinum, vinorelbine monotherapy or combined platinum, and taxane monotherapy or taxane-based combined therapy.
Of these, 32 patients achieved partial remission (PR) according to RESIST criteria version 1.1. The plasma sPD-L1 levels of these patients were found to significantly decrease compared with their corresponding levels in plasma collected before first-line treatment (p = 0.049, Fig. 1c). Nevertheless, 11 (34.4%) of the 32 patients showed no disease progression at the time of the last follow-up (median mPFS: 15.0 m).
Correlation between tumoral PD-L1 and sPD-L1 level in breast cancer patients
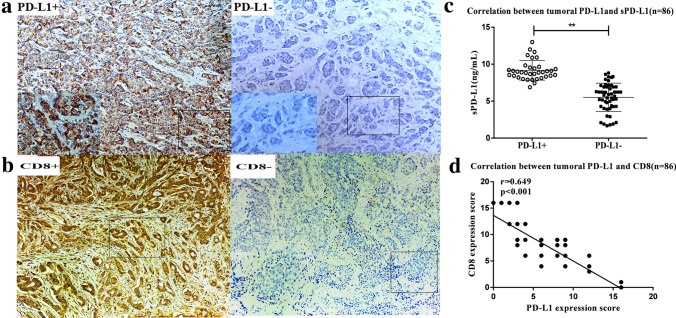
The direct comparison of the clinical burden between tissue and serum sPD-L1 had not been examined. Of the 86 patients with recurrent/metastatic breast cancer whose tumor samples were screened for PD-L1 expression (77 in nodal and soft tissue, 9 in liver tissue), 35 (40.7%) presented PD-L1 expression in stroma or at least 1% of tumor cells (as shown in Fig. 5a). In the analysis of tumoral PD-L1 expression and the corresponding plasma sPD-L1 level, a significant correlation was observed (p < 0.01, Fig. 5c).
Fig. 5.
Immunohistochemical staining of tumoral PD-L1 expression and correlation with corresponding plasma sPD-L1 level. a Immunohistochemical staining of PD-L1 expression in advanced breast cancer (10 × HPF and 40 × HPF), PD-L1 staining in ≥ 1% of tumor cells or in stroma cells(left), PD-L1 staining in < 1% of tumor cells (right). b Immunohistochemical staining of CD8 expression in advanced breast cancer (10 × HPF and 40 × HPF), PD-L1 staining in ≥ 1% of tumor cells or in stroma cells(left), PD-L1 staining in < 1% of tumor cells (right). c Correlation between tumoral PD-L1 expression and corresponding plasma sPD-L1 level (n = 86). Statistical analysis was performed using the Mann–Whitney U test. **, p < 0.01. d A statistically significant inverse correlation between tumoral PD-L1 and CD8 expression score in 86 cases of breast cancer tissues
Immunohistochemical analysis of CD8-positive T cells
We evaluated the correlation between the numbers of CD8-positive T cells and tissue PD-L1 expression by IHC (Fig. 4a, b). We found CD8-positive T cells were significantly decreased with elevated tissue PD-L1 expression in the breast cancer tissues (Fig. 5d).
Correlation between TILD and sPD-L1 level in breast cancer patients
We analyzed the correlation between TILD (tumor-infiltrating lymphocyte density) and sPD-L1 level in breast cancer patients. Of the 86 patients with recurrent/metastatic breast cancer whose tumor samples were screened for TILD expression, 50 (58.1%) presented TILD expression at least 20% (as shown in (Supplementary Fig. 2). In the analysis of TILD expression and the corresponding plasma sPD-L1 level, a significant correlation was observed (p < 0.001, Supplementary Fig. 2).
sPD-L1 expression in supernatant of breast cancer cells
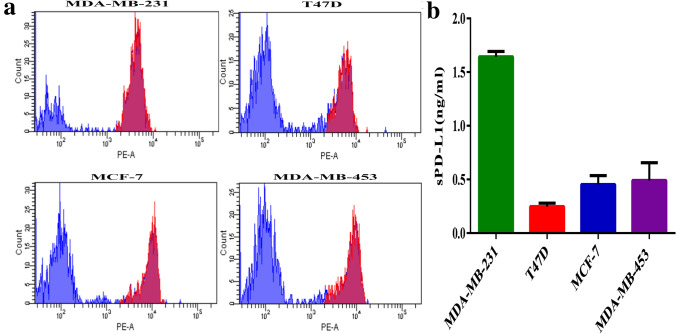
To reveal whether breast cancer cell lines expressing mPD-L1 can produce soluble PD-L1, PD-L1 expression on breast cancer cells was analyzed by flow cytometry and the level of sPD-L1 in the supernatant of breast cancer cells was determined with an ELISA kit (PDCD1LG1 ELISA kit, USCN Life Science, Wuhan, China). It showed that sPD-L1 could be detected in the supernatant of the culture of mPD-L1 ( +) breast cancer cell lines (Fig. 6a, b).
Fig. 6.
sPD-L1 expression in supernatant of breast cancer cells: a flow cytometry was analyzed with mPD-L1 expression on breast cancer cells. b The cell-free supernatants of MDA-MB-231, T47D, MCF-7 and MDA-MB-453 cells were collected to determine the levels of sPD-L1 by the ELISA method
sPD-L1 inhibits the proliferation of T lymphocytes
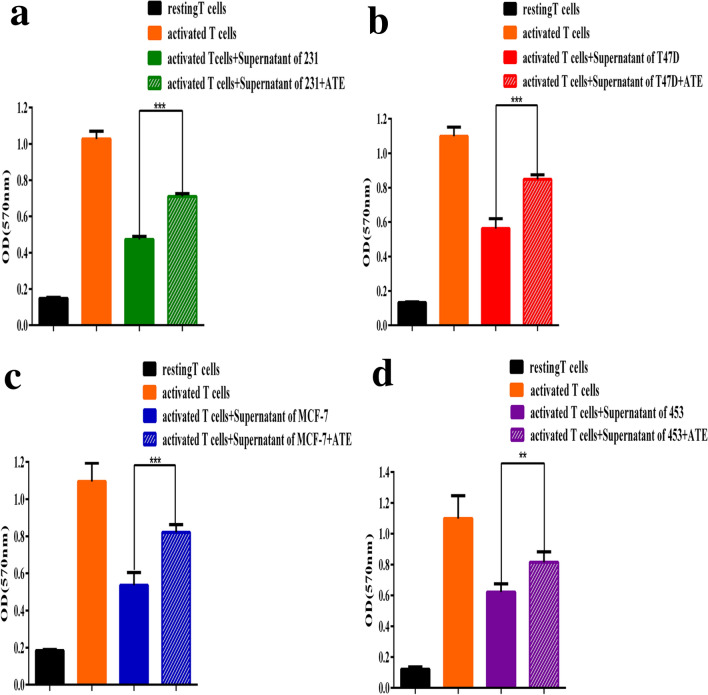
We next assessed the effect of the inhibition of T lymphocyte after co-culture with the supernatant of MDA-MB-231, T47D, MCF-7, MDA-MB-453 breast cancer cell lines expressing sPD-L1 by WST-1. T cell proliferation was inhibited by co-culture with MDA-MB-231, T47D, MCF-7, and MDA-MB453 breast cancer cell lines producing sPD-L1, whereas sPD-L1 could effectively restore the inhibitory effect of PD-1/PD-L1 on T lymphocytes after the addition of anti-PD-L1 antibody (atezolizumab). The results showed that sPD-L1 could inhibit the proliferation of T lymphocytes (p < 0.001, p < 0.001, p < 0.001 and p < 0.01, respectively, Fig. 7a–d).
Fig. 7.
sPD-L1 inhibits proliferation of T lymphocytes. a–d WST-1 analysis of cell proliferation T lymphocyte when it was co-cultured with 231, T47D, MCF-7 and 453 cells or ATE (anti-PD-1 mAb (100 μg/ml)). **, p < 0.01; ***, p < 0.001. These data are representative of three independent experiments
sPD-L1 increases the apoptosis rate of T lymphocytes
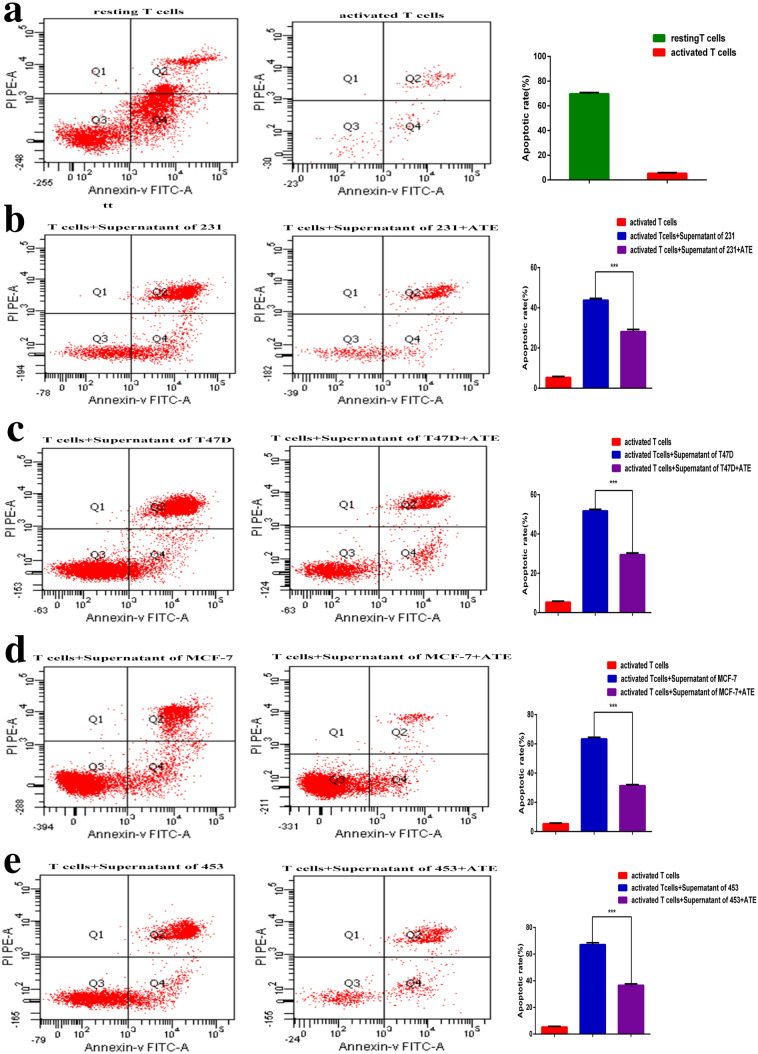
We performed flow cytometry assays to investigate the effect of sPD-L1 on regulating the apoptosis of T lymphocytes. Compared with the group without anti-PD-L1 antibody, the apoptosis rate of containing anti-PD-L1 antibody group was significantly decreased, the same results were observed in MDA-MB-231, T47D, MCF-7, MDA-MB-453 breast cancer cell lines (p < 0.001 all in Fig. 8a–e). We found that sPD-L1 could promote apoptosis of activated T lymphocytes, whereas the effect could also be reversed by the adding of antibody against PD-L1.
Fig. 8.
sPD-L1 increase the apoptosis rate of T lymphocytes. a–e Flow cytometric analysis of T cell apoptosis in 231, T47D, MCF-7 and 453 cells, in which cancer cells were co-cultured with activated T cells alone or activated T cells with ATE at 100 µg/ml concentrations for 72 h time; ***, p < 0.001
Discussion
Recent studies indicated that cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs) and regulatory T cells (Tregs) within the tumor microenvironment were involved in the process of breast cancer growth, invasion and metastasis [26–28]. Immune checkpoint molecules such as PD-1, PD-L1 and CTLA4 also play a pivotal role in immunosuppression of the tumor microenvironment [6]. Immunotherapy with immune checkpoint inhibitors is a promising and rapidly growing field of interest in many solid tumors; to date, pembrolizumab and atezolizumab, two checkpoint inhibitors, have been most extensively studied in breast cancer [29–33].
Except for the expression of immune checkpoint molecules in the tissues, sPD-L1 detection also drew attention. Recently, research has shown that high sPD-L1 levels were proved as prognostic for poor treatment response and survival prognosis in hepatocellular carcinoma [34], renal cell cancer [35], ovarian cancer [36], lung cancer [37], gastric cancer [38], melanoma [39] and extranodal NK/T cell lymphoma [40]. To the best of our knowledge, the current work is the first to investigate the clinical prognostic complications of plasma sPD-L1 in recurrent or metastatic breast cancer patients before receiving first-line rescue therapy. In our study, it suggested that the plasma sPD-L1 level was comparatively higher in recurrent/metastatic patients than early-stage patients.
Several studies have shown that PD-1/PD-L1 play an important role in the occurrence and development of breast cancer. IHC studies showed that PD-L1 expression in breast cancer was an unfavorable indicator associated with poor DFS and OS [41]. A genomic analysis on mRNA expression of immune regulatory molecules in PBMCs from primary and metastatic breast cancer patients suggested a correlation between PD-L1 gene and disease progression [23]. In our study, we found that sPD-L1 level greater than 8.774 ng/ml measured in the peripheral blood before first-line treatment was significantly associated with a short PFS of recurrent or metastatic breast cancer. Consistent with a report in the 2017 ASCO meeting that higher serum sPD-L1 levels were prognostic for poor PFS and OS in HER2-positive metastatic breast cancer patients treated with first-line trastuzumab. Luminal-like subtypes patients corresponded to 36.5% of our cohort, which would get a long survival if they received an useful treatment regimen. Hence, we did not compare the correlation between sPD-L1 levels and OS of these breast cancer patients. Because breast cancer was a heterogeneous disease, different subtypes have different molecular and immunohistochemical profiles, prognoses, and responses to treatment [29–32]. However, in our study, plasma sPD-L1 expression showed no difference in different subgroups of breast cancer (triple-negative, HER2-positive, and luminal-like).
Good tumor biomarkers were expected to predict the therapeutic effect of anti-tumor drugs. A clinical trial reported that a significant decrease of the sPD-L1 level in DLBCL patients achieved CR of at least half year compared with the corresponding levels at diagnosis [19]. Additionally, we found that elevated levels of sPD-L1 were associated with poorer prognosis, regardless of the assigned treatment, and that sPD-L1 levels decreased dramatically for patients who achieved PR, especially those with high sPD-L1 levels. These results indicated that sPD-L1 might be representative of an anti-tumor immunosuppression state and an improvement in intrinsic adaptive anti-tumor immunity via effective therapy. These results cleared the way for the further studies aimed at determining whether monitoring the alternation of sPD-L1 level before and after therapy in the peripheral blood can identify patients who are most likely to benefit from conventional treatments.
Circulating inflammatory cells, including lymphocytes, monocytes, neutrophils, and their combined index (LMR, NLR), reflect a systemic inflammatory response to cancer [42], which indicates that systemic inflammatory response is an important prognostic factor in tumor development and progression [43]. Lymphocytes inhibit tumor cell proliferation and migration, induce cytotoxic cell death and subsequently eradicate cancer, and in contrast, monocytes release cytokines and free radicals related to angiogenesis, tumor growth and distant spread. Neutrophils mediate tissue damage via certain biochemical mechanisms such as the release of arachidonic acid metabolites, oxidative free radicals and other hydrolytic enzymes. The current study shows significant relationships between selected inflammatory markers (monocyte count, neutrophil count) and plasma sPD-L1 levels. He et al. found that the ratio of PD-L1( +) neutrophils to PD-1( +) T cells was higher in peritumoral tissue and better predicted the disease-free survival of patients with HCC [42]. Compared with healthy subjects, interferon-γ, IL-6 and IL-10 were significantly increased in the plasma of high-sPD-L1 patients [44]. These data support the hypothesis that sPD-L1 could indicate the anti-immune response of the disease and cooperatively impact tumor progression.
Furthermore, findings also showed that soluble PD-L1 associated immune suppression via regulation the function of T lymphocyte. Shi B. et al. [45] stated that sPD-L1 may contribute to the proliferation of T cells and the development of diabetic macrovascular diseases. Pan X. et al. [46] suggested that the immune mechanism of sPD-L1 and the PD-1/PD-L1 pathway is associated with immune response in tuberculous pleural effusion. Avendaño-Ortiz J. et al. [47] demonstrated that elevated concentration of sPD-L1 in sepsis results in the lowest rate of T cell proliferation by PD-L1/PD-1 cross talk. Blinova E. et al. [48] showed negative correlation of sPD-L1 serum concentration and CD8 + tumor expression in subgroups of Durvalumab-treated mice that carried both primary and relapsed non-muscular invasive bladder cancer of GATA 3 and KRT 5/6 expressed subtypes. Wu D. et al. [49] reported that activation of the PD-1/PD-L1 pathway using sPD-L1 could improve the imbalance of Th1/Th2 and Treg/Th17 immune cells in ITP patients. Li Y. et al. [50] stated that by controlling the expression of sPD-L1, it may be possible to block the inhibitory effect of the PD-1/PD-L1 signaling pathway and improve the function of effector T cells in cystic echinococcosis. Orme et al. [51] showed that sPD-L1 from tumor cells induces CD8 + T cell death and inhibits anti-tumor immunity. Although sPD-Ll have been recognized as naturally existing regulators of PD-1/PD-L1 membrane signaling pathways in various disease systems, the biological activity of sPD-L1 remains incompletely understood, clearly, further work is needed to better understand the implications of sPD-L1 levels on immunotherapy efficacy in breast cancer. The results of this study indicate that, like other soluble factors with immunoregulatory functions, sPD-L1 was present in a functional form in breast cancer cells. In vitro, MDA-MB-231, T47D, MCF-7, MDA-MB-453 breast cancer cell lines produced sPD-L1, the supernatant of breast cancer cells containing sPD-L1 significantly inhibited the proliferation of PHA-stimulated T cells. Atezolizumab blocked the interaction of PD-1/sPD-L1 and effectively restored the proliferative capacity of T cells. It can be speculated that with the increased of sPD-L1, more sPD-L1 bound to PD-1 on the surface of activated lymphocytes, which limited the activation and proliferation of T lymphocytes, leading to the killing effect of breast cancer cells on immune cells [37]. Studies had shown that the expression of soluble costimulatory molecules was significantly correlated with clinicopathological features such as lymph node metastasis, tumor size and multiple organ metastasis.
To summarize, sPD-L1 is a good tumor maker in recurrent or metastatic breast cancer patients before receiving first-line rescue therapy, and high plasma levels of sPD-L1 are associated with a shorter PFS. This is the first study to confirm the negative impact of sPD-L1 on the progress of recurrent or metastatic breast cancer in the patient population. Abnormality of soluble factors plays an important role in the long-term and early immune regulation of the body, and it is beneficial to the tumor cells to resist the killing and elimination of lymphocytes in the tumor microenvironment. Specific anti-PD-L1 antibody can reduce the expression of sPD-L1 and remove its blocking effect on PD-1/PD-L1 negative signaling pathway, which may help to improve T cell viability and enhance the killing for breast cancer cells.
Supplementary information
Below is the link to the electronic supplementary material.
ROC curve analysis for the optimal cutoff value of plasma sPD-L1 concentration. (TIF 11906 KB)
Correlation between TILD and sPD-L1 level in breast cancer patients, ***, p<0.001. (TIF 345630 KB)
Acknowledgments
Thanks are due to Harbin Medical University Cancer Hospital patients who provided samples.
Abbreviations
- Ais
aromatase inhibitors
- CAFs
cancer-associated fibroblasts
- CEA
carcinoembryonic antigen
- CI
confidence interval
- CR
complete remission
- DFS
disease-free survival
- DLBCL
diffuse large B cell lymphoma
- ELISA
enzyme-linked immunosorbent assay
- HR
hazard ratio
- IHC
immunohistochemistry
- NE
non-evaluable
- OS
overall survival
- PBMCs
peripheral blood mononuclear cells
- PD
disease progression
- PFS
progression-free survival
- PR
partial remission
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
stable disease
- sPD-L1
soluble programmed cell death ligand 1
- TAMs
tumor-associated macrophages
- TILD
tumor-infiltrating lymphocyte density
- Tregs
regulatory T cells
Author contributions
JW, QZ, BH and LD designed experiments. BH and LD performed experiments. BH, LD, JG JZ and YY acquired data; BH, LD, JX and KG analyzed data and generated figures. BH and LD wrote the manuscript. JX, ZX, WL, JW and QZ supervised the analysis. JW, QZ, BH, LD, JZ, YY and QX discussed the results and conclusions and commented on the manuscript at all stages. All authors read and approved the final manuscript.
Funding
The research was supplied by the National Natural Science Foundation of China (Grant No. 81472490 and 81772813). It was done in the Oncology Key Lab of the Heilongjiang Province Institution of Higher Education.
Declarations
Conflict of interest
The authors have no conflict of interest.
Ethics approval
This study was reviewed and approved by the Ethical Board of the Cancer Hospital of Harbin Medical University, China.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Baojuan Han and Lina Dong have contributed equally to this work and should be considered as co-first authors.
Contributor Information
Jingxuan Wang, Email: wjxilywendy@126.com.
Qingyuan Zhang, Email: zqywsci@163.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Gao Q, Xu G, Shi B, Ma X, Liu H, Li Q, Tian T, Tang J, Niu H. Postoperative recurrence analysis of breast cancer patients based on clinical serum markers using discriminant methods. Cancer Biomark. 2017;19:403–409. doi: 10.3233/CBM-160322. [DOI] [PubMed] [Google Scholar]
- 3.Tampellini M, Berruti A, Bitossi R, Gorzegno G, Alabiso I, Bottini A, Farris A, Donadio M, Sarobba MG, Manzin E, Durando A, Defabiani E, De Matteis A, Ardine M, Castiglione F, Danese S, Bertone E, Alabiso O, Massobrio M, Dogliotti L. Prognostic significance of changes in CA 15–3 serum levels during chemotherapy in metastatic breast cancer patients. Breast Cancer Res Treat. 2006;98(3):241–248. doi: 10.1007/s10549-005-9155-y. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Li M, Wang X, Dang Z, Jiang Y, Wang X, Kong Y, Yang Z. PD-1 TIGIT CD8 T cells are associated with pathogenesis and progression of patients with hepatitis B virus-related hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68(12):2041–2054. doi: 10.1007/s00262-019-02426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veldman J, Visser L, Berg AVD, Diepstra A. Primary and acquired resistance mechanisms to immune checkpoint inhibition in Hodgkin lymphoma. Cancer Treat Rev. 2020;82:101931. doi: 10.1016/j.ctrv.2019.101931. [DOI] [PubMed] [Google Scholar]
- 6.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Yao H, Li C, Shi H, Lan J, Li Z, Zhang Y, Liang L, Fang JY, Xu J. HIP1R targets PD-L1 to lysosomal degradation to alter T cell-mediated cytotoxicity. Nat Chem Biol. 2019;15(1):42–50. doi: 10.1038/s41589-018-0161-x. [DOI] [PubMed] [Google Scholar]
- 8.Sugiura D, Maruhashi T, Okazaki IM, Shimizu K, Maeda TK, Takemoto T, Okazaki T. Restriction of PD-1 function by -PD-L1/CD80 interactions is required for optimal T cell responses. Science. 2019;364(6440):558–566. doi: 10.1126/science.aav7062. [DOI] [PubMed] [Google Scholar]
- 9.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, Vale RD. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355(6332):1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 13.Sunshine JC, Nguyen PL, Kaunitz GJ, Cottrell TR, Berry S, Esandrio J, Xu H, Ogurtsova A, Bleich KB, Cornish TC, Lipson EJ, Anders RA, Taube JM. PD-L1 Expression in melanoma: a quantitative immunohistochemical antibody comparison. Clin Cancer Res. 2017;23(16):4938–4944. doi: 10.1158/1078-0432.CCR-16-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM. KEYNOTE-407 investigators Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and efficacy of Pembrolizumab monotherapy in patients with previously treated advanced gastric and Gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamid O, Puzanov I, Dummer R, Schachter J, Daud A, Schadendorf D, Blank C, Cranmer LD, Robert C, Pavlick AC, Gonzalez R, Hodi FS, Ascierto PA, Salama AKS, Margolin KA, Gangadhar TC, Wei Z, Ebbinghaus S, Ibrahim N, Ribas A. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37–45. doi: 10.1016/j.ejca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, Savage MJ, Perini RF, Keefe SM, Bajorin D, Bellmunt J. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, Zhang X. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine. 2011;56(2):231–238. doi: 10.1016/j.cyto.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda T, Kamai T, Masuda A, Nukui A, Abe H, Arai K, Yoshida K. Higher preoperative serum levels of PD-L1 and B7–H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF-targeted therapy and unfavorable prognosis of renal cell carcinoma. Cancer Med. 2016;5(8):1810–1820. doi: 10.1002/cam4.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finkelmeier F, Canli Ö, Tal A, Pleli T, Trojan J, Schmidt M, Kronenberger B, Zeuzem S, Piiper A, Greten FR, Waidmann O. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer. 2016;59:152–159. doi: 10.1016/j.ejca.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, Le Gouill S, Haioun C, Tarte K, Lamy T, Milpied N, Fest T; Groupe Ouest-Est des Leucémies et Autres Maladies du Sang; Groupe Ouest-Est des Leucémies et Autres Maladies du Sang High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28(12):2367–2375. doi: 10.1038/leu.2014.137. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi N, Iwasa S, Sasaki Y, Shoji H, Honma Y, Takashima A, Okita NT, Kato K, Hamaguchi T, Yamada Y. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol. 2016;142(8):1727–1738. doi: 10.1007/s00432-016-2184-6. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi K, Suzuki E, Yamaguchi A, Yamamoto M, Morita S, Toi M. Altered expression of major immune regulatory molecules in peripheral blood immune cells associated with breast cancer. Breast Cancer. 2017;24(1):111–120. doi: 10.1007/s12282-016-0682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Cui X, Yang YJ, Chen QQ, Zhong L, Zhang T, Cai RL, Miao JY, Yu SC, Zhang F (2019) Serum sPD-1 and sPD-L1 as Biomarkers for Evaluating the Efficacy of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Patients. Clin Breast Cancer 19(5):326–332 10.1016/j.clbc.2019.03.008 [DOI] [PubMed]
- 25.Lüftner D, Bauerfeind I, Braun M, Brucker SY, Fasching PA, Felberbaum R, Hagemann F, Haidinger R, Harbeck N, Hönig A, Huober J, Jackisch C, Kolberg HC, Kolberg-Liedtke C, Kühn T, Maass N, Reimer T, Schneeweiss A, Schumacher-Wulf E, Schütz F, Thomssen C, Untch M, Wuerstlein R, Thill M. Treatment of early breast cancer patients: evidence, controversies, consensus: focusing on systemic Therapy - German experts' opinions for the 16th international St. Gallen consensus conference (Vienna 2019) Breast Care (Basel) 2019;14(5):315–324. doi: 10.1159/000502603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres S, Bartolomé RA, Mendes M, Barderas R, Fernandez-Aceñero MJ, Peláez-García A, Peña C, Lopez-Lucendo M, Villar-Vázquez R, de Herreros AG, Bonilla F, Casal JI. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin Cancer Res. 2013;19(21):6006–6019. doi: 10.1158/1078-0432.CCR-13-1130. [DOI] [PubMed] [Google Scholar]
- 27.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212(4):435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai C, August S, Behar R, Polak M, Ardern-Jones M, Theaker J, Al-Shamkhani A, Healy E. Characteristics of immunosuppressive regulatory T cells in cutaneous squamous cell carcinomas and role in metastasis. Lancet. 2015;385(Suppl 1):S59. doi: 10.1016/S0140-6736(15)60374-9. [DOI] [PubMed] [Google Scholar]
- 29.Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, Maiya V, Husain A, Winer EP, Loi S, Emens LA. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 30.Araki K, Ito Y, Fukada I, Kobayashi K, Miyagawa Y, Imamura M, Kira A, Takatsuka Y, Egawa C, Suwa H, Ohno S, Miyoshi Y. Predictive impact of absolute lymphocyte counts for progression-free survival in human epidermal growth factor receptor 2-positive advanced breast cancer treated with pertuzumab and trastuzumab plus eribulin or nab-paclitaxel. BMC Cancer. 2018;18(1):982. doi: 10.1186/s12885-018-4888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, Campone M, Biganzoli L, Bonnefoi H, Jerusalem G, Bartsch R, Rabaglio-Poretti M, Kammler R, Maibach R, Smyth MJ, Di Leo A, Colleoni M, Viale G, Regan MM, André F. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019;20(3):371–382. doi: 10.1016/S1470-2045(18)30812-X. [DOI] [PubMed] [Google Scholar]
- 32.Rugo HS, Delord JP, Im SA, Ott PA, Piha-Paul SA, Bedard PL, Sachdev J, Le Tourneau C, van Brummelen EMJ, Varga A, Salgado R, Loi S, Saraf S, Pietrangelo D, Karantza V, Tan AR. Safety and antitumor activity of Pembrolizumab in patients with Estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res. 2018;24(12):2804–2811. doi: 10.1158/1078-0432.CCR-17-3452. [DOI] [PubMed] [Google Scholar]
- 33.Kim HM, Lee J, Koo JS. Clinicopathological and prognostic significance of programmed death ligand-1 expression in breast cancer: a meta-analysis. BMC Cancer. 2017;17(1):690. doi: 10.1186/s12885-017-3670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang B, Huang T, Wei H, Shen L, Zhu D, He W, Chen Q, Zhang H, Li Y, Huang R, Li W, Wu P. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68(3):353–363. doi: 10.1007/s00262-018-2271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kushlinskii NE, Gershtein ES, Morozov AA, Goryacheva IO, Filipenko ML, Alferov AA, Bezhanova SD, Bazaev VV, Kazantseva IA. Soluble ligand of the immune checkpoint receptor (sPD-L1) in blood serum of patients with renal cell carcinoma. Bull Exp Biol Med. 2019;166(3):353–357. doi: 10.1007/s10517-019-04349-8. [DOI] [PubMed] [Google Scholar]
- 36.Buderath P, Schwich E, Jensen C, Horn PA, Kimmig R, Kasimir-Bauer S, Rebmann V. Soluble programmed death receptor ligands sPD-L1 and sPD-L2 as liquid biopsy markers for prognosis and platinum response in epithelial ovarian cancer. Front Oncol. 2019;9:1015. doi: 10.3389/fonc.2019.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jovanović D, Roksandić-Milenković M, Kotur-Stevuljević J, Ćeriman V, Vukanić I, Samardžić N, Popević S, Ilić B, Gajić M, Simon M, Simon I, Spasojević-Kalimanovska V, Belić M, Mirkov D, Šumarac Z, Milenković V. Soluble sPD-L1 and serum amyloid A1 as potential biomarkers for lung cancer. J Med Biochem. 2019;38(3):332–341. doi: 10.2478/jomb-2018-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shigemori T, Toiyama Y, Okugawa Y, Yamamoto A, Yin C, Narumi A, Ichikawa T, Ide S, Shimura T, Fujikawa H, Yasuda H, Hiro J, Yoshiyama S, Ohi M, Araki T, Kusunoki M. Soluble PD-L1 expression in circulation as a predictive marker for recurrence and prognosis in gastric cancer: direct comparison of the clinical burden between tissue and serum PD-L1 expression. Ann Surg Oncol. 2019;26(3):876–883. doi: 10.1245/s10434-018-07112-x. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Mahoney KM, Giobbie-Hurder A, Zhao F, Lee S, Liao X, Rodig S, Li J, Wu X, Butterfield LH, Piesche M, Manos MP, Eastman LM, Dranoff G, Freeman GJ, Hodi FS. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res. 2017;5(6):480–492. doi: 10.1158/2326-6066.CIR-16-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, Ueda S, Takahara M, Kumai T, Ishibashi K, Kosaka A, Aoki N, Oikawa K, Uno Y, Akiyama N, Sado M, Takei H, Celis E, Harabuchi Y, Kobayashi H. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother. 2017;66(7):877–890. doi: 10.1007/s00262-017-1987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang W, Ran R, Shao B, Li H. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: a meta-analysis. Breast Cancer Res Treat. 2019;178(1):17–33. doi: 10.1007/s10549-019-05371-0. [DOI] [PubMed] [Google Scholar]
- 42.He G, Zhang H, Zhou J, Wang B, Chen Y, Kong Y, Xie X, Wang X, Fei R, Wei L, Chen H, Zeng H. Peritumoural neutrophils negatively regulate adaptive immunity via the PDL1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;18(34):141. doi: 10.1186/s13046-015-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grivennikov SI, Greten FR, Karin M. Immunity inflammation and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Wang L, Liu WJ, Xia ZJ, Huang HQ, Jiang WQ, Li ZM, Lu Y. High post-treatment serum levels of soluble programmed cell death ligand 1 predict early relapse and poor prognosis in extranodal NK/T cell lymphoma patients. Oncotarget. 2016;7(22):33035–33045. doi: 10.18632/oncotarget.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi B, Du X, Wang Q, Chen Y, Zhang X. Increased PD-1 on CD4(+) CD28(-) T cell and soluble PD-1 ligand-1 in patients with T2DM: association with atherosclerotic macrovascular diseases. Metabolism. 2013;62(6):778–785. doi: 10.1016/j.metabol.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Pan X, Zhong A, Xing Y, Shi M, Qian B, Zhou T, Chen Y, Zhang X. Increased soluble and membrane-bound PD-L1 contributes to immune regulation and disease progression in patients with tuberculous pleural effusion. Exp Ther Med. 2016;12(4):2161–2168. doi: 10.3892/etm.2016.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avendaño-Ortiz J, Maroun-Eid C, Martín-Quirós A, Lozano-Rodríguez R, Llanos-González E, Toledano V, Gómez-Campelo P, Montalbán-Hernández K, Carballo-Cardona C, Aguirre LA, López-Collazo E. Oxygen saturation on admission is a predictive biomarker for PD-L1 expression on circulating monocytes and impaired immune response in patients with sepsis. Front Immunol. 2018;9:2008. doi: 10.3389/fimmu.2018.02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blinova E, Roshchin D, Kogan E, Samishina E, Demura T, Deryabina O, Suslova I, Blinov D, Zhdanov P, Osmanov U, Nelipa M, Kaprin A. Patient-derived non-muscular invasive bladder cancer Xenografts of main molecular subtypes of the tumor for Anti-Pd-l1 treatment assessment. Cells. 2019;8(6):526. doi: 10.3390/cells8060526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu D, Liu Y, Pang N, Sun M, Wang X, Haridia Y, Zhao F, Qin Y, Fan W, Guo X, Ding J. PD-1/PD-L1 pathway activation restores the imbalance of Th1/Th2 and treg/Th17 cells subtypes in immune thrombocytopenic purpura patients. Medicine. 2019;98(43):e17608. doi: 10.1097/MD.000000000001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Xiao Y, Su M, Zhang R, Ding J, Hao X, Ma Y. Role of soluble programmed death-1 (sPD-1) and sPD-ligand 1 in patients with cystic echinococcosis. Exp Ther Med. 2016;11(1):251–256. doi: 10.3892/etm.2015.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orme JJ, Jazieh KA, Xie T, Harrington S, Liu X, Ball M, Madden B, Charlesworth MC, Azam TU, Lucien F, Wootla B, Li Y, Villasboas JC, Mansfield AS, Dronca RS, Dong H. ADAM10 and ADAM17 cleave PD-L1 to mediate PD-(L)1 inhibitor resistance. Oncoimmunology. 2020;9(1):1744980. doi: 10.1080/2162402X.2020.1744980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ROC curve analysis for the optimal cutoff value of plasma sPD-L1 concentration. (TIF 11906 KB)
Correlation between TILD and sPD-L1 level in breast cancer patients, ***, p<0.001. (TIF 345630 KB)