Abstract
Objectives
Axillary lymphadenopathy from coronavirus disease 2019 (COVID-19) vaccine is an emerging phenomenon during unprecedented mass vaccinations, which can be incidentally found on computed tomography (CT) scans. This study investigated the incidence, predisposing factors, and imaging characteristics of vaccine-related axillary lymphadenopathy in patients with thoracic malignancy who underwent CT scans before and after COVID-19 vaccinations.
Methods
The study included patients with thoracic malignancies who received two doses of mRNA-based COVID-19 vaccinations and had prevaccine and postvaccine chest CT scans. Postvaccine chest CT scan results were reviewed for increase in size of lymph nodes in the axilla and subpectoral areas, comparing with the prevaccine scan results. The cases with lymphadenopathy were further reviewed independently by two radiologists referring to clinical information to find whether lymphadenopathy was attributed to the vaccinations.
Results
Vaccine-related axillary lymphadenopathy was noted in 21 of 232 patients (9.0%). The median short-axis diameter of the largest node was 7 mm (range: 5–14 mm). The median number of increased nodes was 4 (range: 1–10). The median time to the postvaccine scan revealing lymphadenopathy was 1.7 weeks (range: −2.9 to 6.6) from the second dose. Vaccine-related lymphadenopathy was noted more often in women than in men (18 of 144, 12.5% versus 3 of 88, 3.4%, respectively; p = 0.019) and with mRNA-1273 vaccines than BNT162b2 vaccines (6 of 28, 21% versus 15 of 204, 7.4%, respectively; p = 0.026).
Conclusions
The incidence of lymphadenopathy was 9%, with a median onset time of 1.7 weeks after the second vaccine dose. Female sex and vaccine type (mRNA-1273 vaccine) were associated with higher frequency of lymphadenopathy, providing initial observations to inform further investigations in larger cohorts.
Keywords: COVID-19, Vaccinations, Lymphadenopathy, Computed tomography, mRNA vaccine
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has affected tens of millions of people worldwide, prompting urgent needs for effective vaccinations. The mRNA-based vaccines have been given widely since the end of 2020, leading to emerging vaccine-related observations, including axillary lymphadenopathy noted on imaging.1 Patients with cancer under active treatment or surveillance undergo frequent cross-sectional imaging, leading to the incidental detection of vaccine-related lymphadenopathy.1 , 2 Although sporadic reports exist for axillary lymphadenopathy on imaging after COVID-19 vaccinations, the details of this phenomenon remain to be investigated for its incidence, predisposing factors, and imaging characteristics.1, 2, 3 A recent report described the findings in patients who underwent 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) for various malignancies.4 CT scan is by far the most often used imaging modality for cancer monitoring, and patients with thoracic malignancy provide a unique opportunity to investigate this phenomenon on CT because they undergo frequent chest CT scans that provides detailed evaluations of the axilla.
The purposes of this report are to find the incidence of vaccine-related axillary lymphadenopathy on postvaccine CT scan results in patients with thoracic malignancy, identify predisposing factors, and describe imaging characteristics.
Materials and Methods
The study included patients with thoracic malignancies who received two doses of COVID-19 vaccinations (Pfizer-BioNtech BNT162b2 or Moderna mRNA-1273) between December 2020 and April 2021. All patients had at least one prevaccine chest CT within 6 months before the first vaccine dose and underwent at least one chest CT or FDG-PET/CT after the vaccination. Patients with existing axillary lymph node enlargement (≥10 mm in short axis) on prevaccine CT were excluded. The medical records and imaging studies were retrospectively reviewed in these patients who had consented to a correlative research study approved by the institutional review board at the Dana-Farber Cancer Institute.
Postvaccine chest CT scan results were reviewed by a board-certified thoracic radiologist (MN) for increase in size of lymph nodes in the axilla and subpectoral areas, based on side-by-side comparison with the prevaccine scan results. The increase of lymph nodes was visually assessed, and when noted, the short axis of the largest node was measured. The number of increased nodes was counted. The radiologist was aware of the vaccination dates but was not aware of the type or the side (right or left) of vaccinations at the time of the initial review.
After the initial review, the cases that were positive for lymphadenopathy on the postvaccine scan results were reviewed independently by two radiologists (MN, the first reader, and HH, another board-certified thoracic radiologist), referring to the side of vaccinations and the detailed clinical history, including the disease status and treatment information, to find whether lymphadenopathy was attributed to the COVID-19 vaccinations.
Demographics and disease characteristics were compared between patients with and without vaccine-related lymphadenopathy, using a Fisher’s exact test and chi-square test for categorical variables and a Wilcoxon ranked sum test for continuous variables.
Results
Among a total of 232 vaccinated patients with prevaccine and postvaccine CT scans without preexisting axillary adenopathy, the initial imaging review identified 28 patients with new unilateral lymphadenopathy in the axilla and subpectoral areas on the postvaccine scans. Lymphadenopathy was not considered to be attributed to the vaccinations in seven patients in the second review, owing to contralateral vaccine injections (n = 6) and because of a recent ipsilateral breast surgery with breast inflammation on the postvaccine PET/CT (n = 1). After excluding these seven patients, vaccine-related lymphadenopathy was noted in 21 of 232 patients (9.0%) (Table 1 ). The median short-axis diameter of the largest node was 7 mm (range: 5–14 mm) (Fig. 1 ). Only two of the 21 patients (9.5%) had nodes greater than or equal to 10 mm in the short axis (Fig. 1 A). The median number of increased nodes was 4 (range: 1–10). More than one node was noted in 17 patients (81%), and greater than or equal to five nodes were noted in six patients (28%; Fig. 1 B). Both axilla and subpectoral nodes were noted in 11 patients, whereas 10 patients had only axillary nodes. Lymphadenopathy was on the left in 20 patients, and on the right in one patient. Three of the 21 patients with lymphadenopathy had postvaccine FDG-PET/CT, and nodes were FDG-avid in two patients (Fig. 1 C). A total of 14 patients had advanced-stage thoracic malignancies at the time of vaccination and seven had early stage disease. A total of 11 patients were on systemic anticancer treatment and 10 were not on treatment.
Table 1.
Demographics, Clinical Characteristics, and Postvaccine Scan Details of Patients With and Without Vaccine-Related Lymphadenopathy
| Demographics, Clinical Characteristics, and Scan Details | With Adenopathy (n = 21) | Without Adenopathy (n = 211) | All Patients (N = 232) | p Value | |
|---|---|---|---|---|---|
| Age (y) | Median [range] | 69 [52–82] | 71 [40–96] | 71 [40–96] | 0.49 |
| Sex | Male | 3 | 85 | 88 | 0.019 |
| Female | 18 | 126 | 144 | ||
| Race | White | 19 | 185 | 204 | 0.54 |
| Asian | 2 | 11 | 13 | ||
| Black | 0 | 7 | 7 | ||
| Other | 0 | 8 | 8 | ||
| Smoking | Never | 6 | 67 | 73 | 0.21 |
| Former | 14 | 106 | 120 | ||
| Current | 1 | 38 | 39 | ||
| Tumor types | NSCLC | 18 | 182 | 200 | 0.90 |
| SCLC | 1 | 11 | 12 | ||
| Mesothelioma | 1 | 9 | 10 | ||
| Other | 1 | 9 | 10 | ||
| Tumor stage at vaccination | Advanced | 14 | 162 | 176 | 0.29 |
| Not advanced | 7 | 49 | 56 | ||
| Vaccine types | BNT162b2 | 15 | 189 | 204 | 0.026 |
| mRNA-1273 | 6 | 22 | 28 | ||
| Postvaccine scan type | CT only | 18 | 192 | 210 | 0.092 |
| PET/CT onlya | 0 | 10 | 10 | ||
| CT and PET/CTa | 3 | 9 | 12 | ||
| Postvaccine scan time point | 1 time point | 20 | 161 | 181 | 0.052 |
| 2–4 time points | 1 | 50 | 51 | ||
| Postvaccine scan timing | Between two dosesb only | 2 | 44 | 46 | 0.13 |
| After second dose only | 18 | 135 | 153 | ||
| Between two doses and after second dose | 1 | 32 | 33 | ||
| Median time between vaccine dose and scan (wk) | Pre-CT to first dose | 5.4 | 5.4 | 5.4 | 0.58 |
| First dose to post-CTc | 4.8 | 5.0 | 5.0 | 0.95 | |
| Second dose to post-CTc | 1.7 | 1.7 | 1.7 | 0.80 | |
CT, computed tomography; PET, positron emission tomography.
The rate of lymphadenopathy did not differ significantly between patients with and without postvaccine PET/CT (3 of 22 versus 18 of 210, respectively; p = 0.43).
“Between two doses” indicates the scans performed after the first vaccine dose before the second dose.
Time was calculated from each vaccine dose to the postvaccine scan, using the first postvaccine scan in patients with more than one postvaccine scans.
Figure 1.
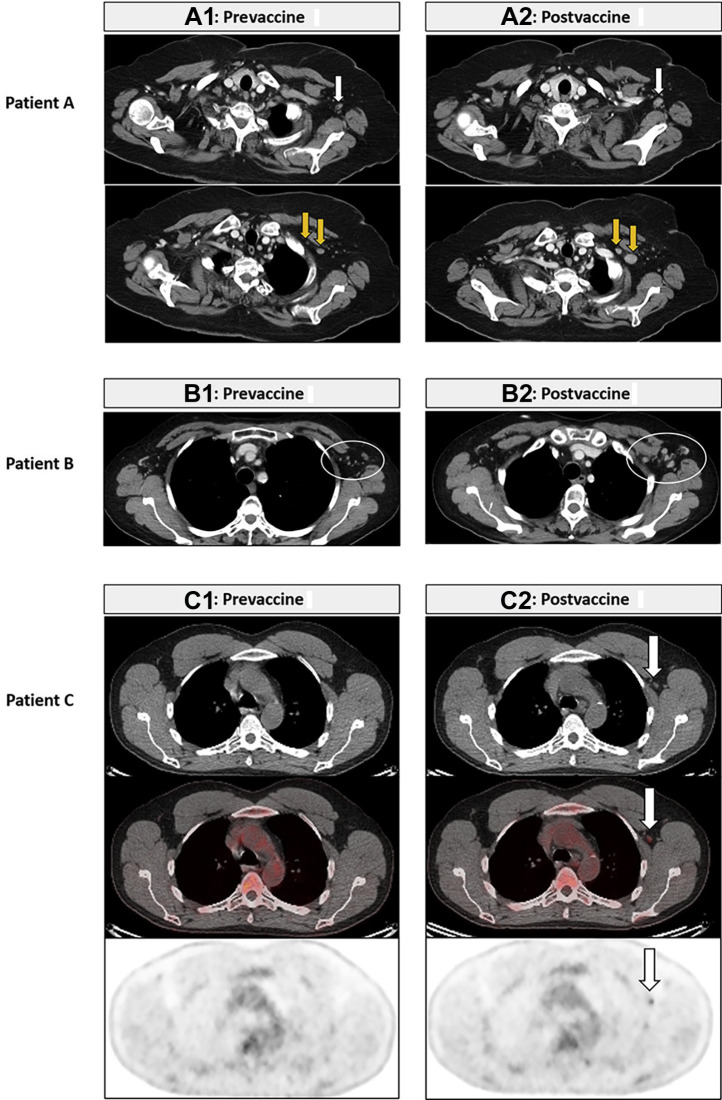
COVID-19 vaccine-related axillary and subpectoral lymphadenopathy on CT and FDG-PET/CT. Patient A. A 67-year-old woman with SCLC who received two doses of COVID-19 vaccine in the left deltoid and underwent a prevaccine CT (4.4 wk before the first dose) and a postvaccine CT (0.6 wk after the second dose) for tumor surveillance. Postvaccine CT results revealed increased lymph nodes in the left axilla (A2, white arrow) and the subpectoral region measuring up to 14 mm in the short axis (A2, yellow arrows), compared with the prevaccine CT results (A1, arrows). There was no increase of other thoracic nodes or lung lesions on the chest CT. The patient underwent PET/CT at the outside institution 7 weeks after the postvaccine CT, which reportedly revealed decrease of these nodes without FDG uptake (the images are not available and not illustrated). Patient B. A 57-year-old woman with stage IV NSCLC undergoing systemic therapy who received two doses of COVID-19 vaccine in the left deltoid and underwent a prevaccine CT (8.7 wk before the first dose) and a postvaccine CT (0.3 wk after the second dose) for treatment monitoring. Postvaccine CT results revealed increase of the left axillary and subpectoral lymph nodes (B2, white oval) measuring up to 8 mm in the short axis, compared with the prevaccine CT results (B1). The CT scan results otherwise revealed stable disease without new or increasing lesions. Patient C. A 52-year-old man with stage IV NSCLC who received two doses of COVID-19 vaccine in the left deltoid and underwent a prevaccine CT (4.4 wk before the first dose), PET/CT between two doses (3.4 wk after the first dose), and CT after the second dose (3.6 wk after the second dose) for tumor surveillance. Postvaccine PET/CT results revealed an increased left axillary lymph node with FDG uptake (SUVmax: 2.2) (C2, arrows), compared with the prevaccine CT results (C1). The PET/CT results revealed tumor response to therapy with decreased right lower lobe lesion, without any other FDG-avid lymph nodes. COVID-19, coronavirus disease 2019; CT, computed tomography; FDG, 18F-fluorodeoxyglucose; PET, positron emission tomography; SUVmax, maximum standardized uptake value.
Lymphadenopathy was noted on the scans performed after the second vaccine dose in 18 patients, whereas three patients developed lymphadenopathy on the scans performed between the first and second doses. The median time to the postvaccine scan revealing lymphadenopathy in the 21 patients was 4.9 weeks (range: 1.0–9.6) from the first dose and 1.7 weeks (range: −2.9 to 6.6) from the second dose. Only one lymphadenopathy-positive patient had more than one postvaccine scans, and lymphadenopathy persisted on the second scan four weeks after the first scan (6.3 wk after the first dose and 2.3 wk after the second dose).
Vaccine-related lymphadenopathy was significantly more common in women than men (18 of 144, 12.5% versus 3 of 88, 3.4%, respectively; p = 0.019) and in those who received the mRNA-1273 vaccine than those who received the BNT162b2 vaccine (6 of 28, 21% versus 15 of 204, 7.4%, respectively; p = 0.026). No significant differences were noted between patients with and without lymphadenopathy for other factors, including age, race, smoking, tumor types, and stages (Table 1). Postvaccine scan timing, number of time points, scan types, and intervals between scans and vaccines did not significantly differ between the two groups (Table 1). Undergoing postvaccine PET/CT scan had no significant impact on the incidence of lymphadenopathy (p = 0.43).
Discussion
Vaccine-related lymphadenopathy was noted in 9% of the patients with thoracic malignancy on postvaccine CT scan results. Female sex and vaccine type (mRNA-1273) were associated with higher rates of lymphadenopathy. The study provides the first report on the incidence, predisposing factors, and imaging characteristics of vaccine-related lymphadenopathy on CT using the currently available cohort during the mass vaccinations for COVID-19, which can provide a basis for further studies.
Clinically, “axillary swelling or tenderness” was noted as a local reaction in 16.0% in patients aged 18 to 64 years and in 8.4% of patients aged more than 65 years in a trial of mRNA-1273 vaccine.5 The rate of lymphadenopathy identified on CT (9.0%) in this study is comparable with the rate of clinically noted axillary swelling/tenderness in the trial. In a recent study of 169 patients who underwent FDG-PET/CT after BNT162b2 vaccinations, 29% of the patients had FDG uptake in the axillary nodes,6 which is higher than 9% on the basis of CT size increase. This is expected because PET/CT can detect FDG-avid nodes without size increase. The incidence of lymphadenopathy among those who underwent PET/CT in our cohort is lower (3 of 21; 14.2%), which can be due to a small number of patients evaluated by PET/CT.
The median short-axis diameter of the largest node was 7 mm, ranging from 5 to 14 mm. Knowledge on the size of the affected nodes is important to establish imaging criteria for this new phenomenon.1 No size criteria were available to define vaccine-related adenopathy, and thus this study was specifically designed to carefully compare pre- and postvaccine CT scan results to identify visually notable increase of axillary and subpectoral nodes, rather than applying criteria developed for other purposes, such as the Response Evaluation Criteria in Solid Tumors (RECIST).7 The present cohort with thoracic malignancies at an academic cancer center with frequent serial chest CT scans provided unique advantages, with pre-CT scans with a median of 5.4 weeks before the first dose. The focus on thoracic malignancy was also advantageous as unilateral axillary lymphadenopathy is not a frequent manifestation of metastatic disease in this population, compared with other diseases such as breast cancer, melanoma, and lymphoma.6 Most patients (19 of 21, 90%) who developed new lymphadenopathy had nodes less than 10 mm in the short axis which would be considered nonpathologic by RECIST,7 consistent with the recent PET/CT report that described “normal size” of most FDG-avid nodes after vaccinations with a mean short axis of 5 mm (range: 1–16 mm).6 Nevertheless, in a few patients, the nodes can be bigger, measuring up to 14 mm in the short axis in one patient of our study, who reportedly had decrease of the nodes on follow-up PET/CT. The maximal short axis in the PET/CT study was 16 mm, which is similar to the upper range of our observation.6 As the differentiation of reactive and metastatic nodes is crucial for patients with cancer, further studies with a larger cohort with longer follow-up are needed to identify robust size criteria for vaccine-related lymphadenopathy.
The number of nodes in vaccine-related lymphadenopathy has not been previously reported and ranged from one to 10 with the median of four nodes in this study. In the setting of lymphadenopathy, an increase in the number of nodes can be noted along with size increase, and thus the attention to the number of nodes is important for documenting this phenomenon. The axilla was involved in all patients, and the subpectoral region was also involved in a half of the cases. It remains to be investigated whether the size, number, and extent of lymphadenopathy are related to the degree of immune response to vaccines.
Three patients had vaccine-related lymphadenopathy on CT scans performed after the first dose before receiving the second dose, as early as 1 week after the first dose. Though the timing of lymphadenopathy detection largely depends on the timing of scans, it seems that lymphadenopathy occurs shortly after vaccination, and even with one dose. The maximum time from the second dose to the positive CT scan result was 6.6 weeks, with two patients having positive CT results after 6 weeks since the second dose. The recent article recommended postponing imaging at least 6 weeks after the final vaccination dose,1 which is intuitively reasonable but is not evidence based owing to a lack of data. The recent study reported persistence of FDG-avid nodes beyond 6 weeks in one-third of the vaccinated patients assessed by PET/CT.6 These accumulating data are indicative of longer duration of vaccine-related lymphadenopathy beyond 6 weeks. Considering the needs of frequent serial scanning in patients with cancer especially for those on active treatment, it is likely more realistic to precisely define the imaging characteristics of vaccine-related lymphadenopathy and establish criteria for accurate diagnosis of the phenomenon, rather than delaying imaging studies that are necessary for treatment decisions. A follow-up study is planned once a larger number of patients with longer postvaccine follow-up become available.
This study identified the factors associated with vaccine-related lymphadenopathy, including female sex and mRNA-1273 vaccine. The difference of the incidence rates between women and men was quite notable (12.5% versus 3.4%; p = 0.019), which may aid diagnosis when imaging findings are equivocal. Given the small number of patients who received the mRNA-1273 vaccines (n = 28), the significance of the differences between the two types of mRNA-based vaccines remains to be validated. The incidence of lymphadenopathy from other COVID-19 vaccines, such as viral vector vaccines, along with predisposing factors and imaging characteristics needs to be addressed as well.
In conclusion, vaccine-related axillary lymphadenopathy was noted in 9% on postvaccine CT scan results in patients with thoracic malignancy who received mRNA-based vaccinations. The imaging features were characterized and candidates for predisposing factors were identified, providing the initial data for further investigations of this emerging phenomenon amid mass vaccinations against the COVID-19 pandemic.
CRediT Authorship Contribution Statement
Mizuki Nishino: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Hiroto Hatabu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—review and editing.
Biagio Ricciuti, Victor Vaz, Kesi Michael: Data curation, Investigation, Methodology, Writing—review and editing.
Mark M. Awad: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing—review and editing.
Footnotes
Disclosure: Dr. Nishino reports serving as a consultant to Daiichi Sankyo and AstraZeneca and receiving research grants from Merck, Canon Medical Systems, AstraZeneca, and Daiichi Sankyo. Dr. Hatabu reports receiving research funding from Canon Inc., Canon Medical Systems, and Konica-Minolta and serving as a consultant to Canon Medical Systems and Mitsubishi Chemical Inc. Dr. Awad reports serving as a consultant/advisory board for Genentech, Bristol-Myers Squibb, Merck, AstraZeneca, Maverick, Blueprint Medicine, Syndax, Ariad, Nektar, Gritstone, ArcherDx, Mirati, NextCure, EMD Serono, and Hengrui. The remaining authors declare no conflict of interest.
References
- 1.Becker A.S., Perez-Johnston R., Chikarmane S.A., et al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: radiology scientific expert panel. Radiology. 2021;300:E323–E327. doi: 10.1148/radiol.2021210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn R.W., Mootz A.R., Brewington C.C., Abbara S. Axillary lymphadenopathy after mRNA COVID-19 vaccination. Radiol Cardiothorac Imaging. 2021;3 doi: 10.1148/ryct.2021210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Özütemiz C., Krystosek L.A., Church A.L., et al. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncology patients. Radiology. 2021;300:E296–E300. doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen D., Krauthammer S.H., Wolf I., Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA COVID-19 vaccine: incidence assessed by [18F]FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging. 2021;48:1854–1863. doi: 10.1007/s00259-021-05314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
- 6.Eshet Y., Tau N., Alhoubani Y., Kanana N., Domachevsky L., Eifer M. Prevalence of increased FDG PET/CT axillary lymph node uptake beyond 6 weeks after mRNA COVID-19 vaccination. Radiology. 2021;300:E345–E347. doi: 10.1148/radiol.2021210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]