Abstract
Considering the COVID-19 pandemic where concomitant occurrence of ARDS and severe acute brain injury (sABI) has increasingly coemerged, we synthesize existing data regarding the simultaneous management of both conditions. Our aim is to provide readers with fundamental principles and concepts for the management of sABI and ARDS, and highlight challenges and conflicts encountered while managing concurrent disease. Up to 40% of patients with sABI can develop ARDS. Although there are trials and guidelines to support the mainstays of treatment for ARDS and sABI independently, guidance on concomitant management is limited. Treatment strategies aimed at managing severe ARDS may at times conflict with the management of sABI. In this narrative review, we discuss the physiological basis and risks involved during simultaneous management of ARDS and sABI, summarize evidence for treatment decisions, and demonstrate these principles using hypothetical case scenarios. Use of invasive or noninvasive monitoring to assess brain and lung physiology may facilitate goal-directed treatment strategies with the potential to improve outcome. Understanding the pathophysiology and key treatment concepts for comanagement of these conditions is critical to optimizing care in this high-acuity patient population.
Key Words: acute brain injury, ARDS, intensive care, intracranial hypertension
Abbreviations: CPP, cerebral perfusion pressure; CMRO2, cerebral metabolic rate of oxygen; CSF, cerebrospinal fluid; ECMO, extracorporeal membrane oxygenation; EVD, external ventricular drain; HD, hospital day; HT, hyperosmolar therapy; ICP, intracranial pressure; IH, intracranial hypertension; LTVMV, low tidal volume mechanical ventilation; NMB, neuromuscular blockade; PEEP, positive end-expiratory pressure; PP, prone positioning; sABI, severe acute brain injury; SAH, subarachnoid hemorrhage; TBI, traumatic brain injury
ARDS occurs in up to 40% of patients1 , 2 with severe acute brain injury (sABI), including acute ischemic stroke, subarachnoid hemorrhage (SAH), intracerebral hemorrhage, and traumatic brain injury (TBI), and is a major determinant of morbidity and mortality.1 , 3 With the increase in ARDS cases and reports of neurologic complications in COVID-19,4 , 5 there is an increasing need to manage concomitant severe ARDS and sABI. Standard treatment strategies for ARDS can conflict with management of elevated intracranial pressure (ICP) and reduced cerebral perfusion pressure (CPP). Here, we introduce evidence-based independent management of ARDS and sABI, then review challenges of concurrent management highlighting case scenarios with extrapolation of evidence-based management recommendations.
Methods
PubMed and Google Scholar were searched using ARDS and X, with X representing sABI (eg, ICP, hemorrhage, stroke, TBI), and included references known to authors. Abstracts were reviewed, and articles whose abstracts addressed ARDS and/or neurocritical care were evaluated in full. A minimum of 210 articles were reviewed in full, with 113 articles (1973-2021) ultimately deemed relevant for inclusion.
Management of the Brain-Lung Conflict
sABI can induce and worsen ARDS via multiple physiological pathways (Fig 1 ).6, 7, 8, 9 Many ARDS therapies can raise ICP or decrease CPP, potentiating secondary brain injury because of impaired cerebral autoregulation. In patients with concurrent ARDS-sABI, oxygenation, ventilation, and perfusion parameters considered standard ARDS care may insufficiently support the injured, hypermetabolic brain. Many ARDS studies excluded patients with sABI and therefore do not consider the physiological implications of ARDS management in patients with sABI. Some strategies for optimizing brain and lung physiology conflict; hence, nuanced management strategies are necessary (Fig 2 ).10 Here we discuss theoretic risks, discuss evidence-based treatment considerations, and propose management strategies extrapolated from existing data and expert opinion (Table 1 ).
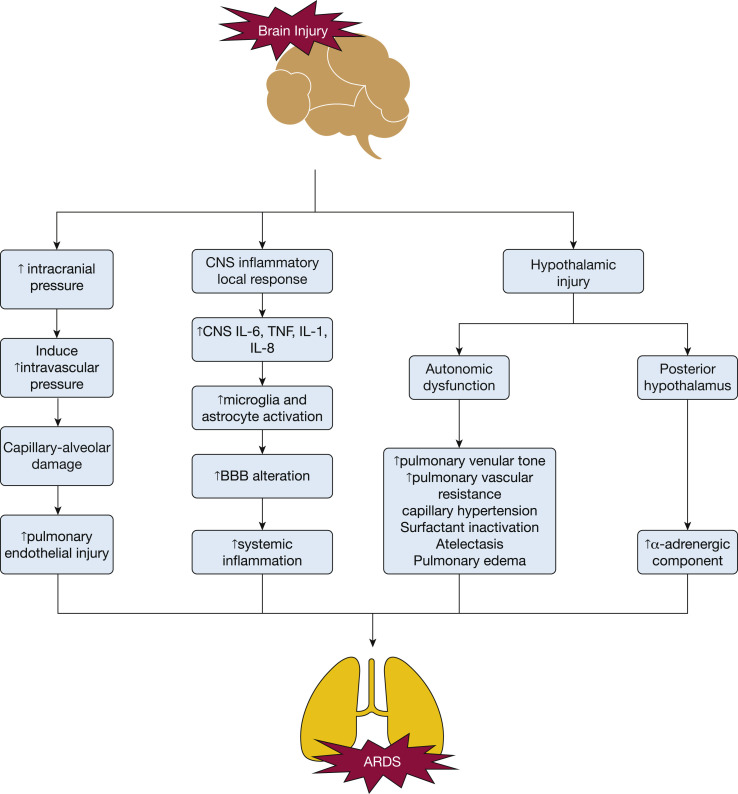
Figure 1.
CNS injury pathways to inducing ARDS. Several pathways have been hypothesized to be activated after a brain injury which can subsequently lead to the development or induction of ARDS, including (1) direct or indirect hypothalamic injury, (2) local CNS inflammatory response, and (3) increase in intracranial pressure.6, 7, 8, 9 BBB = blood-brain barrier; TNF = tumor necrosis factor.
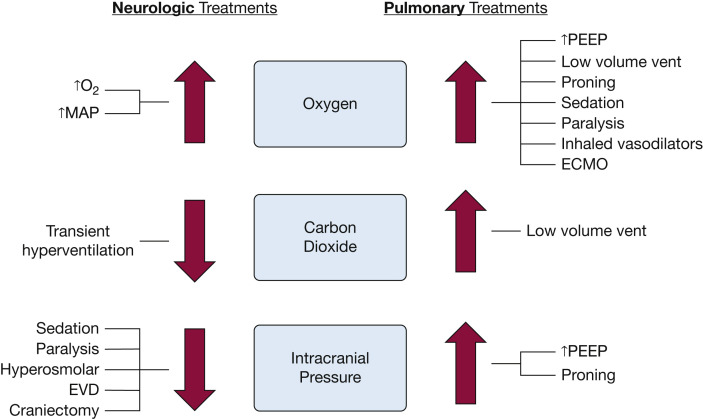
Figure 2.
Summary of potential conflicts in patients with concurrent severe acute brain injury and ARDS. Treatments targeting improved oxygenation benefit both neurologic and pulmonary physiology. However, other pulmonary treatments may lead to unintended secondary injury on the brain and vice versa. ECMO = extracorporeal membrane oxygenation; EVD = external ventricular drain; MAP = mean arterial pressure; PEEP = positive end-expiratory pressure; vent = ventilation.
Table 1.
Brain-Lung Conflict and Recommendations Based on Current Literature Review
| Lung-Focused ARDS Therapy | Brain-Focused ABI Therapy | Recommendation |
|---|---|---|
| Low tidal volume mechanical ventilation with permissive hypercarbia |
|
|
| High PEEP |
|
|
| Recruitment maneuvers |
|
|
| Prone positioning |
|
|
| ECMO |
|
|
| Pulmonary vasodilator therapy |
|
|
| Fluid conservation strategies |
|
|
| Sedation and neuromuscular blockade |
|
|
| Steroids |
|
|
ABI = acute brain injury; CPP = cerebral perfusion pressure; ECMO = extracorporeal membrane oxygenation; HoB = head of bed; ICP = intracranial pressure; PEEP = positive end expiratory pressure.
Low Tidal Volume Mechanical Ventilation
Challenge
Low tidal volume mechanical ventilation (LTVMV) improves mortality in ARDS, but may cause hypercarbia and hypoxemia, which may lead to raised ICP and brain hypoxia.
Physiology
High tidal volumes are associated with ARDS development during mechanical ventilation (OR, 1.3 per mL > 6 mL/kg).11, 12, 13, 14 The ARDS Clinical Trials Network Low-Tidal-Volume Trial (ARMA) demonstrated that ventilation with low tidal volumes (4-6 mL/kg ideal body weight) led to a 9% absolute reduction in mortality,15 and LTVMV has shown to increase ventilator-free days.15 , 16 LTVMV targets are often Pao 2 of 55 to 80 mm Hg15 , 17 and pH > 7.15, with permissive hypercapnia.
Theoretical Risk in sABI
ARDS protocols often allow hypercapnia and mild hypoxemia, but the resulting cerebral arteriole vasodilation and increased ICP, and brain tissue hypoxia, is poorly tolerated after sABI. Cerebral vasculature is highly responsive to CO2 levels. Increasing Paco 2—as permitted in LTVMV—can lead to hypercapnia, cerebral vasodilation, and a rise in ICP. In contrast, hyperventilation can decrease Paco 2 and induce alkalemia and transient ICP reduction. However, this occurs via cerebral arteriole vasoconstriction,18 which may worsen cerebral ischemia.
Evidence
Evidence supports hypercapnia-induced cerebral vasodilation and increased ICP.19 However, prolonged hypocapnia (Paco 2 < 25 mm Hg for > 30 min) is also no longer recommended.20
Hyperventilation should only be a temporizing measure (< 30 min) while awaiting definitive intracranial hypertension (IH) treatment20 , 21 to avoid cerebral ischemia.21 Therefore, a target Paco 2 of 35 to 45 mm Hg or a graded hypoventilation strategy is recommended.22 Although mild hypoxemia is well tolerated in patients without brain injury, patients with sABI have up to a 50% higher odds of death with a PaO2 < 110 mm Hg.23, 24, 25
Summary
The benefit of LTVMV must be balanced with risks of hypercarbia and hypoxemia. An individualized target, based on direct measures of brain physiology (eg. invasive ICP, brain tissue oxygen monitoring), should be implemented.15 , 26 , 27 If direct measurement is not feasible, normal Paco 2 (35-45 mm Hg) and Pao 2 > 110 mm Hg should be targeted as much as tolerated from a lung compliance standpoint.
High Positive End-Expiratory Pressure
Challenge
High positive end-expiratory pressure (PEEP) may improve oxygenation but reduce cerebral perfusion by raising ICP.
Physiology
PEEP prevents alveolar collapse to maintain oxygenation, increases functional residual capacity, and improves ventilation-perfusion matching.28, 29, 30 PEEP may be optimized to individual patient physiology via (1) empirical PEEP and Fio 2 titration tables, (2) pressure-volume loops or other dynamic physiological parameters, (3) esophageal manometry to estimate transpulmonary pressure, and (4) titration of PEEP to optimize driving pressure.31 Lower driving pressure is associated with reduced mortality.32
Theoretical Risk in sABI
PEEP increases intrathoracic pressure which can increase right atrial pressure and decrease cerebral venous drainage resulting in elevated ICP.28, 29, 30 , 33, 34, 35, 36 PEEP may trigger compensatory vasodilation, thereby increasing ICP when cerebral autoregulation is intact and intracranial compliance is decreased. Conversely, if cerebral autoregulation is impaired, increased PEEP may reduce CPP causing cerebral ischemia.37, 38, 39
Evidence
In a trial assessing the effect of PEEP on cerebral autoregulation in patients with ARDS without known sABI,28 ≥ 50% of patients had impaired cerebral autoregulation with increased PEEP, but no clinical significance was seen at PEEP < 14 cm H2O.28 Studies in sABI reveal conflicting opinions regarding the theoretical concerns of IH with increased PEEP.39 , 40 Studies in SAH, acute ischemic stroke, and TBI have shown that increasing PEEP up to 12 cm H2O had no significant change on ICP.41, 42, 43 However, systemic and cerebral hemodynamics may be more dramatically affected by changes in PEEP in patients with high respiratory system compliance compared with patients with low compliance.41
Summary
High PEEP carries a theoretical risk of worsening ICP, but the effect is minimal when PEEP is ≤ 12 cm H2O and in patients with low respiratory system compliance. Adequate volume resuscitation along with high PEEP may mitigate adverse effects.43 A recent consensus panel44 recommended using the same PEEP in patients with sABI as in patients with non-brain injury, unless IH was noted to be linked to increased PEEP. ICP monitoring in patients with sABI-ARDS may aid in PEEP titration. Oxygenation targets can be titrated to brain tissue oxygen measures or targeted to a Pao 2 > 110 mm Hg, as tolerated based on lung compliance, if monitoring is not possible.
Recruitment Maneuvers
Challenge
Recruitment maneuvers may open collapsed alveoli and improve oxygenation but can cause hypotension and impair brain perfusion.
Physiology
Recruitment maneuvers open collapsed alveoli using sustained or stepwise increase/decrease inflation for a short duration. A randomized trial found that aggressive recruitment maneuvers followed by high PEEP increased mortality, hemodynamic collapse, and barotrauma in ARDS.45
Theoretical Risk in sABI
Sudden high distending pressures used with recruitment maneuvers can be hazardous for systemic and cerebral hemodynamics.
Evidence
Evidence supports that recruitment maneuvers (20-35 cm H2O) may significantly decrease CPP and elevate ICP.46 , 47 Modified pressure controlled recruitment maneuvers may be tolerated in patients with sABI without baseline IH.46
Summary
Recruitment maneuvers using PEEP ≥ 20 cm H2O should be avoided when maintaining cerebral perfusion is of critical importance, or ICP control is a major concern.
Pulmonary Vasodilator Therapy
Challenge
Inhaled pulmonary vasodilators may improve ventilation-perfusion matching and hypoxemia but could inhibit platelet function.
Physiology
Inhaled vasodilators selectively dilate pulmonary capillaries in well-ventilated alveoli to improve ventilation-perfusion matching and oxygenation.
Theoretical Risk in sABI
Pulmonary vasodilators are well tolerated in patients with sABI, likely from improved cerebral oxygenation.48 One theoretical complication of prostacyclins is an inhibitory impact on platelet function and/or synergism with P2Y12 inhibitors.49 , 50
Evidence
Case reports and studies suggest pulmonary vasodilators may improve ICP and cerebral oxygenation.51, 52, 53, 54 Potential complications include hypotension if systemically absorbed, and bleeding because of platelet inhibition.30
Summary
Limited evidence suggests pulmonary vasodilators are safe and potentially beneficial in sABI and ARDS. The minor concern of antiplatelet effects has yet to be validated and should be weighed based on bleeding risks in the individual patients.
Fluid Management
Challenge
A fluid conservative strategy reduces duration of ventilation in ARDS, but hypovolemia may reduce cerebral perfusion and aggressive hyperosmolar therapy (HT) may induce hypervolemia.
Physiology
HT (eg, mannitol, hypertonic saline) is the standard treatment for IH.18 , 55 HT induces movement of fluid from interstitial/intracellular space to intravascular space, reducing cerebral edema and ICP.56 Recent guidelines suggest favoring hypertonic saline,21 but evidence remains limited. The choice is mainly guided by factors accounting for comorbidities (eg, heart failure, renal failure), serum values (eg, sodium concentration, osmolality), and clinical factors (eg, hypovolemia, central venous access).
Theoretical Risk in sABI
Although aggressive diuresis is commonly recommended in ARDS, euvolemia is essential for maintaining adequate CPP after sABI. Additionally, hypertonic saline may counter diuresis efforts especially in concurrent heart failure or valvular disease and mannitol may worsen septic physiology or renal failure.
Evidence
The Fluid and Catheter Treatment Trial (FACTT) established that conservative fluid management in ARDS leads to more ventilator-free days and improved gas exchange.57 After initial resuscitation, early, aggressive diuresis is often implemented. However, hypovolemia and resultant hypotension reduces CPP and worsens outcome after sABI.58 Similarly, hypervolemia may worsen outcomes in sABI.59 , 60
Summary
Fluid strategy should be tailored to the individual patient. Careful assessment of fluid status and judicious use of fluids and HT is critical and may be best guided by simultaneous hemodynamic and cerebral perfusion/oxygenation monitoring.61 Special considerations include avoiding hypotension in TBI and maintaining cerebral perfusion in SAH to reduce vasospasm risk.
Sedation and Neuromuscular Blockade
Challenge
Deep sedation and neuromuscular blockade (NMB) are used to improve ventilator synchrony and reduce oxygen consumption in ARDS but limit the ability to perform a neurologic examination.
Physiology
Sedation and NMB decrease global oxygen consumption, improve patient-ventilator synchrony, and optimize chest wall viscoelasticity. However, minimizing their use reduces delirium, promotes mobility, and reduces duration of mechanical ventilation.62 Although one randomized trial demonstrated improved mortality and oxygenation among patients with moderate-to-severe ARDS treated with NMB,63 the Reevaluation of Systemic Early Neuromuscular Blockade (ROSE) trial found no benefit in 90-day mortality.64 It did, however, demonstrate that NMB is safe, well tolerated, and may be considered for patients with refractory hypoxemia or ventilator dyssynchrony.64
Propofol and benzodiazepines are example anesthetics used in refractory IH management. They can reduce seizures (which elevate ICP and cerebral metabolic rate of oxygen [CMRO2]).65 , 66 Managing pain decreases ICP elicited by Valsalva maneuver.67 NMB reduces ICP by reducing airway and intrathoracic pressure often related to biting the endotracheal tube, shivering, posturing, or breathing against the ventilator, which facilitates cerebral venous outflow. In addition, NMB reduces metabolic demand secondary to skeletal muscle contraction therefore decreasing CMRO2. Barbiturates also lower CMRO2 but may reduce ICP by additional mechanisms.68
Theoretical Risk in sABI
Loss of neurologic examination to monitor for neurologic deterioration increases risk of delirium and risk of ICU-acquired weakness which further complicate neurologic examination, management decision, and prognostication during lung recovery.69
Evidence
Although the neurologic examination is critical, patients with sABI with IH may have increased ICP and metabolic crisis with daily awakenings which may contribute to secondary brain injury.70 , 71
Summary
Sedation and paralytics can improve both ICP and oxygenation in sABI and ARDS and improve ventilator dyssynchrony. Their use may outweigh the risk of a temporary loss in neurologic examination. However, minimal effective dose and duration should be used to reduce hypotension, delirium, and sustained loss of neurologic examination. In cases of high concern for neurologic deterioration, alternative approaches to neurologic assessment (eg, pupillometry, continuous EEG, surveillance CT scans, other noninvasive or invasive monitoring) may be considered in patients receiving deep sedation or NMB.
Steroids
Challenge
Corticosteroids may be helpful in ARDS, especially in cases such as COVID-19, but may be harmful in some types of brain injury.
Physiology
Corticosteroids may reduce pulmonary and systemic inflammation in ARDS, and may have antifibrotic properties; however, ARDS is a heterogenous syndrome with many etiologies and both hypoinflammatory and hyperinflammatory phenotypes.72 Corticosteroids can aid in vasogenic edema by reducing permeability of the blood-brain barrier, but is not effective in injuries which induce cytotoxic edema.73
Theoretical Risk in sABI
Steroids worsen outcomes in ischemic stroke, intracranial hemorrhage, and TBI. They are also an independent risk factor for ICU-acquired weakness which further complicates neurologic examination and recovery in sABI.69
Evidence
Glucocorticoid use for cerebral edema is common and has shown benefits in brain tumors,56 , 74 TB, and bacterial meningitis.21 , 75 However, there is evidence suggesting that steroids are potentially harmful in cerebral edema associated with intracerebral hemorrhage,21 ischemic stroke,76 or TBI.21 , 77
Corticosteroids in ARDS are controversial, except when the etiology is COVID-19. A randomized trial demonstrated early administration of dexamethasone in patients with moderate-to-severe ARDS improved mortality.78 , 79 However, older literature suggests late initiation (> 14 days) of methylprednisolone increases mortality.80 Regarding the current COVID-19 pandemic, the use of steroids decreases mortality in patients with respiratory failure because of COVID-19 pneumonia.81 , 82 Although steroids may be beneficial in ARDS,78 , 81 , 82 steroids in multiple forms of sABI are detrimental to recovery.21 , 76 , 77
Summary
Although steroids may be beneficial in ARDS, individual patient with sABI risk-benefit should be considered.
Prone Positioning
Challenge
In addition to the potential for increased ICP, there is added complexity in proning a patient with one or more intracranial drains or invasive ICP monitors.
Physiology
Prone positioning (PP) improves gas exchange through recruitment of dependent lung regions and reduces ventilator-induced lung injury by creating more uniform ventilation.83 A meta-analysis suggested a survival benefit for severe ARDS,84 and the Proning Severe ARDS Patients (PROSEVA) trial found a mortality benefit if PP is performed ≥ 16 h/d.85 A synergistic mortality reduction was seen with PP and LTVMV. However, these trials excluded patients with sABI.
Theoretical Risk in sABI
Traditional PP can result in a significant elevation of ICP given the reduced head elevation and potential inhibition of cerebral venous drainage because of compression of neck veins.86, 87, 88 Also, invasive brain monitors (eg, external ventricular drain [EVD]) can be accidentally displaced.18 , 19 , 24, 25, 26 , 58 , 89 Because of these risks, patients with sABI have been excluded from PP studies.85 , 90 , 91 Transient IH may occur particularly during and immediately after PP. Proper preparation by optimizing ICP prior to PP may help minimize ICP fluctuations. This includes premedication (HT, sedation/NMB), temperature management, and optimal cerebrospinal fluid (CSF) drainage. Once prone, use of reverse Trendelenburg to achieve head of bed elevation and ensuring midline head positioning are simple maneuvers to help decrease ICP by improving cerebral venous return92 , 93 and CSF redistribution.94 , 95 ICP-CPP balance appears optimized around 30° to 45°.96, 97, 98 Pillows and wedges can help with head elevation and maintenance of midline position while reducing the impact of abdominal pressure on ICP.
Evidence
There is no clear evidence of when and how long to prone patients with sABI. Based on small studies, PP benefits on gas exchange and cerebral tissue oxygenation may outweigh risk of IH and CPP reduction in specific populations.99 , 100 Other studies suggest that changes in ICP and CPP during proning in sABI are clinically insignificant.87 , 101, 102, 103
Summary
Although existing evidence is not strong,44 PP is a challenging but feasible option in patients with concurrent ARDS and IH. ICP monitors, via EVD or intraparenchymal monitor, are recommended to optimize management of patients in PP. However, special care planning is needed to ensure proper bedside management and prevent dislodgement of invasive brain monitors during pronation/supination. Mispositioning of the EVD system can lead to erroneous interpretation of ICPs and overdrainage or underdrainage of CSF. In PP, reverse Trendelenburg head should be used to maintain a goal head of bed elevation approximating 30°.
Alternative Strategy: Supine Chest Compression
When PP is contraindicated, supine chest compression with the use of weights on the anterior chest wall yields similar physiological effects. Splinting of the anterior chest leads to a change in chest wall elastance and modifies regional ventilation to redistribute tidal volume and PEEP toward dependent lung regions. Dialysis (2 L saline) bags, sandbags, and weight bars have been used as chest weights. Although there are no evidence-based studies currently, chest weights have been used in neuro-ICUs in low resource settings for years (David Menon,MD and Aarti Sarwal, MD, personal communication, May 19, 2020).104 Chest weights are typically left for 8 to 12 h initially with close monitoring to avoid pressure injury. Head of the bed position at 30° is maintained to optimize ICP management. If a patient shows improving oxygenation, this strategy can be continued for longer periods with periodic breaks akin to PP. An actively enrolling trial Artificial increase in chest wall elastance as an altrenative to prone positioning in moderate-to-severe ARDS (ALTERPRONE)104 uses 100 g/kg weight on the anterior chest wall for 3 h in supine position and 30° head up, when PP is contraindicated or not feasible. Patient with ICP > 30 mm Hg or CPP < 60 mm Hg are included.
Extracorporeal Membrane Oxygenation
Challenge
Extracorporeal membrane oxygenation (ECMO) can improve oxygenation and perhaps outcome in severe ARDS but may increase risk of intracranial hemorrhage and impede cerebral venous drainage.
Physiology
Veno-venous ECMO can improve oxygenation in severely refractory ARDS cases with preserved cardiac function.30 The Conventional ventilation or ECMO for severe adult respiratory failure (CESAR) trial105 reported that patients referred to a specialty ECMO center had higher survival rates with decreased 6-month disability. The ECMO to Resue Lung Injury in Severe ARDS (EOLIA) trial did not replicate these findings, but numerous secondary end points demonstrated promise, including reduced treatment failure at 60 days, PP, and renal replacement therapy.
Theoretical Risk in sABI
ECMO therapy poses serious potential complications for patients with sABI, including hemorrhage, ischemic stroke, air emboli, hypoperfusion, and elevated ICP.106 Large venous cannulas, often placed in the internal jugular veins, may impede venous drainage. Additionally, the optimal CPP target in patients with sABI on ECMO is unknown.
Evidence
Neurologic complications include hemorrhage, ischemia, impaired cerebral venous drainage, and catheter-associated infection, among others.106 Although ECMO is an important salvage therapy in patients with ARDS, patients with sABI are usually not considered ECMO candidates. However, technology eliminating the need for anticoagulation exists and has been used in trauma patients.107 , 108 Case reports have indicated success in using modified anticoagulation protocols in patients with severe TBI undergoing ECMO for ARDS. Similarly, decompressive craniectomy has been performed while on ECMO with moderate-to-good outcome.106 Still, acquired coagulopathy and risk of spontaneous intracranial hemorrhage exists on ECMO.106
Summary
ECMO may be used in selected patients with sABI. Technology and approaches eliminating or reducing anticoagulation (eg, pumpless extracorporeal lung assist devices, femoral cannulation to ensure cerebral venous drainage) enable ECMO to be considered as a more accessible treatment option in patients with sABI.109 Multiple case series support their use by showing optimal ventilation and oxygenation while maintaining CPP and avoiding IH.109
Patient Scenarios
We present hypothetical case scenarios highlighting challenges of sABI and ARDS comanagement based on real patients, to exemplify comanagement challenges and approaches.
Case Presentation 1
A 24-year-old man presented after falling off a cliff, with severe TBI and a C1 arch fracture requiring a cervical collar.
Neurologic Management
ICP Treatment
An intraparenchymal ICP monitor was placed for a Glasgow Coma Scale score of 4 and compressed cisterns on CT scan; he subsequently required frequent HT therapy. Sedation was titrated to Richmond Agitation Sedation Scale score 5, and an EVD was placed on hospital day (HD) 3 and intermittently opened for CSF diversion. Paralytics and pentobarbital were added on HD 4 because of refractory IH. He required vasopressors with an elevated mean arterial pressure goal of ≥ 75 mm Hg to maintain CPP. By HD 7, no further ICP treatments were required.
Pulmonary Management
On HD 4, he developed septic shock and worsening hypoxemic respiratory failure because of Staphylococcus aureus pneumonia, with progressive bilateral opacities on chest radiograph.
ARDS Treatment
LTVMV was initiated but led to permissive hypercapnia (Pco 2 50s), which subsequently increased ICP. Increasing tidal volumes back to 7 cc/kg was not tolerated because of elevated static pressure. Sedation and paralysis were initially started for elevated ICP, but also helped achieve vent synchrony. Increasing PEEP to 16 cm H20 improved oxygenation but resulted in refractory ICP elevations. On HD 5, he was proned for 3 days. The spine team was at the bedside during the proning; a soft massage pillow was placed under his shoulders so the cervical collar could remain.
ICP transiently rose to 40 mm Hg during the first proning but decreased after treatment with HT. Reverse Trendelenburg and the addition of pillows under chest and hips to relieve abdominal pressure further improved his ICP. Prior to subsequent proning, HT was given and 20 cc of CSF was drained from his EVD. No further ICP elevations were noted. Hypoxemia and hypercapnia improved substantially with proning.
Teaching Points
-
•
Hypercapnea because of LTVMV can result in increased ICP, resulting in the need to further escalate other ARDS treatments.
-
•
Increased PEEP can result in ICP spikes.
-
•
To maintain CPP, vasopressors may be required to meet adjusted mean arterial pressure goals.
-
•
Proning may be feasible in patients with spine injury; assessment of cervical spine stability and risks of proning should be assessed in consultation with the spine team.
-
•
Proning may be feasible in patients with elevated ICP and ICP monitors. Increased ICP may be observed during proning and can be mitigated with conventional interventions for IH and positioning maneuvers.
Case Presentation 2
A 26-year-old man presented after a high-speed motorcycle crash, with severe TBI, multiple rib fractures, pulmonary contusions, and unstable open pelvis fracture requiring emergent external fixation. He required massive blood transfusions on admission, and internal iliac embolization followed by external fixation of his pelvis.
Neurologic Management
ICP Treatment
A parenchymal ICP and brain tissue oxygen monitor was placed on HD 1 for a Glasgow Coma Scale score of 3. ICP was treated with HT, deep sedation, and paralysis.
Pulmonary Management
On HD 3, he became progressively hypoxemic, presumed because of worsening pulmonary contusions and/or transfusion-related acute lung injury. His brain tissue oxygen measures decreased to < 20 mm Hg on HD 4.
ARDS Treatment
LTVMV with PEEP titration was initiated in addition to deep sedation, paralysis, and inhaled epoprostenol. On HD 5, after multidisciplinary conversations, veno-venous ECMO was initiated given his persistent hypoxemia (including brain tissue hypoxia), and inability to prone because of pelvic fractures. Systemic anticoagulation was deferred because of concerns his large frontal hemorrhagic contusions would blossom. ECMO support was provided for 7 days during which no further ICP treatment was needed. Contusions remained stable on head CT scan.
Teaching Points
-
•
ECMO can be used in patients with concurrent sABI and ARDS, with appropriate modifications.
-
•
Femoral-femoral cannulation circumvents the risk of cerebral venous drainage impedance that may occur with internal jugular cannulation, despite the higher risk of recirculation.
-
•
Use of veno-venous ECMO for > 7 days without systemic anticoagulation using modern, heparin-bonded circuits has been successfully reported and should be considered in cases of concurrent sABI and ARDS.
Special Considerations in the COVID-19 Era
Interactions between sABI and ARDS are complex. Prevalence of ARDS is on the rise because of COVID-19, and given the neurologic dysfunctions associated with COVID-19,5 physicians may have to frequently treat these coexistent pathologies. The main goals in managing these patients are adequate oxygenation and perfusion while avoiding secondary end-organ injury. Understanding which treatments are safe or need modification is critical to optimizing care, particularly given the potential benefit of early proning in patients with COVID-19.89 , 110 There are few, if any, trials directly addressing concurrent management of patients with both ARDS and sABI. A recent international expert consensus panel for the European Society of Intensive Care Medicine44 on mechanical ventilation in acute brain injury emphasized that evidence is largely lacking for this population, highlighting the need for further research in this area.
Our current recommendations are extrapolated based on available data and expert opinion. By increasing utilization of invasive and noninvasive monitoring devices that directly measure brain and lung physiology , we can titrate treatment strategies to individualized targets.22 , 111 , 112 Prospective observational studies (eg, the newly enrolling Multicenter observational Study on Practice of Ventilation in Brain Injuried Patients [VENTIBRAIN] study113) may help inform future guidance and prospective clinical trials. Thoughtful multispecialty discussions optimizing these targets are paramount to maximizing good outcomes.
Acknowledgments
Author contributions: All authors contributed to the conceptualization of the manuscript. J. A. K. is the guarantor of the content of the manuscript. J. A. K., S. W., J. N. L., C. O. S. N., N. J. J., C. R., D. M. and E. J. G. contributed to writing the manuscript. D. M., K. H. O., S. M., A. S., S. W., and N. J. J. provided input regarding case scenarios. All authors provide critical feedback and helped shape the final version of the manuscript. J. A. K. and E. J. G. were in charge of the overall direction and planning.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. M. receives grant support from the Center for Clinical and Translational Science at The Ohio State University, sponsored by a National Center for Advancing Translational Sciences Award [Grant UL1TR002733], outside the submitted work. N. J. J. receives funding from the National Institutes of Health (NHLBI and NINDS) and Medic One Foundation for unrelated work. J. A. K. receives funding from the National Institutes of Health, AAN, and Swebilius Foundation for unrelated work. E. J. G. receives National Institutes of Health funding for unrelated work. None declared (S. W., J. N. L., C. O. S. N., C. R., D. M., K. H. O., A. S.).
Other contributions: We thank a social media network of female neurointensivists for the inspiration and advice in compiling this review.
References
- 1.Mascia L. Acute lung injury in patients with severe brain injury: a double hit model. Neurocrit Care. 2009;11(3):417–426. doi: 10.1007/s12028-009-9242-8. [DOI] [PubMed] [Google Scholar]
- 2.Veeravagu A., Chen Y.R., Ludwig C., et al. Acute lung injury in patients with subarachnoid hemorrhage: a nationwide inpatient sample study. World Neurosurg. 2014;82:e235–e241. doi: 10.1016/j.wneu.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Holland M.C., Mackersie R.C., Morabito D., et al. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma. 2003;55(1):106–111. doi: 10.1097/01.TA.0000071620.27375.BE. [DOI] [PubMed] [Google Scholar]
- 4.Needham E.J., Chou S.H.Y., Coles A.J., et al. Neurological implications of COVID-19 infections. Neurocrit Care. 2020;32(3):667–671. doi: 10.1007/s12028-020-00978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zubair A.S., McAlpine L.S., Gardin T., et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moss G. Shock, cerebral hypoxia, and pulmonary vascular control: the centrineurogenic etiology of the respiratory distress syndrome. Bull N Y Acad Med. 1973;49:689. [PMC free article] [PubMed] [Google Scholar]
- 7.de Oloveira G.G., Antonio M.P. Role of the central nervous system in the adult respiratory distress syndrome. Crit Care Med. 1987;15:844–849. doi: 10.1097/00003246-198709000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Blanch L., Quintel M. Lung-brain cross talk in the critically ill. Intensive Care Med. 2017;43(4):557–559. doi: 10.1007/s00134-016-4583-1. [DOI] [PubMed] [Google Scholar]
- 9.Mrozek S., Constantin J.-M., Geeraerts T. Brain-lung crosstalk: implications for neurocritical care patients. World J Crit Care Med. 2015;4(3):163–178. doi: 10.5492/wjccm.v4.i3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McHugh G.S., Engel D.C., Butcher I., et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:287–293. doi: 10.1089/neu.2006.0031. [DOI] [PubMed] [Google Scholar]
- 11.Elmer J., Hou P., Wilcox S.R., et al. Acute respiratory distress syndrome after spontaneous intracerebral hemorrhage. Crit Care Med. 2013;41:1992–2001. doi: 10.1097/CCM.0b013e31828a3f4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oddo M., Citerio G. ARDS in the brain-injured patient: what’s different? Intensive Care Med. 2016;42:790–793. doi: 10.1007/s00134-016-4298-3. [DOI] [PubMed] [Google Scholar]
- 13.Mascia L., Zavala E., Bosma K., et al. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med. 2007;35:1815–1820. doi: 10.1097/01.CCM.0000275269.77467.DF. [DOI] [PubMed] [Google Scholar]
- 14.Wrigge H., Uhlig U., Zinserling J., et al. The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anesth Analg. 2004;98:775–781. doi: 10.1213/01.ane.0000100663.11852.bf. [DOI] [PubMed] [Google Scholar]
- 15.Brower R.G., Matthay M.A., Morris A., et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 16.Slutsky A., Ranieri M. Ventilator-induced lung injury. N Engl J Med. 2014;369(22):2126–2162. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 17.Brain Taruma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons, et al. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007;24(suppl 1):S59–S64. doi: 10.1089/neu.2007.9987. [DOI] [PubMed] [Google Scholar]
- 18.Ropper A.H. Hyperosmolar therapy for raised intracranial pressure. N Engl J Med. 2012;367:746–752. doi: 10.1056/NEJMct1206321. [DOI] [PubMed] [Google Scholar]
- 19.Godoy D.A., Seifi A., Garza D., et al. Hyperventilation therapy for control of posttraumatic intracranial hypertension. Front Neurol. 2017;8:250. doi: 10.3389/fneur.2017.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carney N., Totten A.M., O’Reilly C., et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80:6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 21.Cook A.M., Morgan Jones G., Hawryluk G.W.J., et al. Guidelines for the acute treatment of cerebral edema in neurocritical care patients. Neurocrit Care. 2020;32:647–666. doi: 10.1007/s12028-020-00959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okonkwo D.O., Shutter L.A., Moore C., et al. Brain oxygen optimization in severe traumatic brain injury phase-II: a phase II randomized trial. Crit Care Med. 2017;45:1907–1914. doi: 10.1097/CCM.0000000000002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Mackle D., Bellomo R., et al. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020;382(11):989–998. doi: 10.1056/NEJMoa1903297. [DOI] [PubMed] [Google Scholar]
- 24.Schjørring O.L., Klitgaard T.L., Perner A., et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. 2021;384(14):1301–1311. doi: 10.1056/NEJMoa2032510. [DOI] [PubMed] [Google Scholar]
- 25.Davis D.P., Meade W., Sise M.J., et al. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009;26:2217–2223. doi: 10.1089/neu.2009.0940. [DOI] [PubMed] [Google Scholar]
- 26.Picetti E, Pelosi P, Taccone FS, et al. VENTILatOry strategies in patients with severe traumatic brain injury: the VENTILO Survey of the European Society of Intensive Care Medicine (ESICM). Crit Care. 2020:24(1). Article:158. [DOI] [PMC free article] [PubMed]
- 27.Brower R.G., Lanken P.N., MacIntyre N., et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 28.Schramm P., Closhen D., Felkel M., et al. Influence of PEEP on cerebral blood flow and cerebrovascular autoregulation in patients with acute respiratory distress syndrome. J Neurosurg Anesthesiol. 2013;25(2):162–167. doi: 10.1097/ANA.0b013e31827c2f46. [DOI] [PubMed] [Google Scholar]
- 29.Rozet I., Domino K.B. Respiratory care. Best Pract Res Clin Anaesthesiol. 2007;21(4):465–482. doi: 10.1016/j.bpa.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Modock J. Complex care: ventilation management when brain injury and acute lung injury coexist. J Neurosci Nurs. 2014;46(2):71–78. doi: 10.1097/JNN.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 31.Sahetya S.K., Goligher E.C., Brower R.G. Setting positive end-expiratory pressure in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1429–1438. doi: 10.1164/rccm.201610-2035CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amato M.B.P., Maureen D., Meadle O. Driving pressure and survival in the ARDS. New Engl J Med. 2015;372(8):747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 33.Huseby J.S., Luce J.M., Cary J.M., et al. Effects of positive end-expiratory pressure on intracranial pressure in dogs with intracranial hypertension. J Neurosurg. 1981;55:704–707. doi: 10.3171/jns.1981.55.5.0704. [DOI] [PubMed] [Google Scholar]
- 34.Huseby J.S., Pavlin E.G., Butler J. Effect of positive end-expiratory pressure on intracranial pressure in dogs. J Appl Physiol Respir Environ Exerc Physiol. 1978;44:25–27. doi: 10.1152/jappl.1978.44.1.25. [DOI] [PubMed] [Google Scholar]
- 35.Luce J.M., Huseby J.S., Kirk W., et al. A Starling resistor regulates cerebral venous outflow in dogs. J Appl Physiol Respir Environ Exerc Physiol. 1982;53:1496–1503. doi: 10.1152/jappl.1982.53.6.1496. [DOI] [PubMed] [Google Scholar]
- 36.Lowe G.J., Ferguson N.D. Lung-protective ventilation in neurosurgical patients. Curr Opin Crit Care. 2006;12(1):3–7. doi: 10.1097/01.ccx.0000198055.29600.4b. [DOI] [PubMed] [Google Scholar]
- 37.Rosner M.J., Rosner S.D., Johnson A.H. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg. 1995;83:949–962. doi: 10.3171/jns.1995.83.6.0949. [DOI] [PubMed] [Google Scholar]
- 38.Doblar D.D., Santiago T.V., Kahn A.U., et al. The effect of positive end-expiratory pressure ventilation (PEEP) on cerebral blood flow and cerebrospinal fluid pressure in goats. Anesthesiology. 1981;55:244–250. doi: 10.1097/00000542-198109000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Videtta W., Villarejo F., Cohen M., et al. Effects of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure. Acta Neurochir Suppl. 2002;81:93–97. doi: 10.1007/978-3-7091-6738-0_25. [DOI] [PubMed] [Google Scholar]
- 40.McGuire G., Crossley D., Richards J., et al. Effects of varying levels of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure. Crit Care Med. 1997;25:1059–1062. doi: 10.1097/00003246-199706000-00025. [DOI] [PubMed] [Google Scholar]
- 41.Caricato A., Conti G., Della Corte F., et al. Effects of PEEP on the intracranial system of patients with head injury and subarachnoid hemorrhage: the role of respiratory system compliance. J Trauma. 2005;58(3):571–576. doi: 10.1097/01.ta.0000152806.19198.db. [DOI] [PubMed] [Google Scholar]
- 42.Huynh T., Messer M., Sing R.F., et al. Positive end-expiratory pressure alters intracranial and cerebral perfusion pressure in severe traumatic brain injury. J Trauma. 2002;53(3):488–492. doi: 10.1097/00005373-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Georgiadis D., Schwarz S., Baumgartner R.W., et al. Influence of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure in patients with acute stroke. Stroke. 2001;32:2088–2092. doi: 10.1161/hs0901.095406. [DOI] [PubMed] [Google Scholar]
- 44.Robba C., Poole D., McNett M., et al. Mechanical ventilation in patients with acute brain injury: recommendations of the European Society of Intensive Care Medicine consensus. Intensive Care Med. 2020;46:2397–2410. doi: 10.1007/s00134-020-06283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavalcanti A.B., Suzumura É.A., Laranjeira L.N., et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome - a randomized clinical trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nemer S.N., Caldeira J.B., Azeredo L.M., et al. Alveolar recruitment maneuver in patients with subarachnoid hemorrhage and acute respiratory distress syndrome: a comparison of 2 approaches. J Crit Care. 2011;26(1):22–27. doi: 10.1016/j.jcrc.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 47.De Rosa S., Franchi P., Mancino A., et al. Impact of positive end expiratory pressure on cerebral hemodynamic in paediatric patients with post-traumatic brain swelling treated by surgical decompression. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0196980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siobal M.S., Kallet R.H., Pittet J.F., et al. Description and evaluation of a delivery system for aerosolized prostacyclin. Respir Care. 2003;48(8):742–753. [PubMed] [Google Scholar]
- 49.Cavallini L., Coassin M., Borean A., et al. Prostacyclin and sodium nitroprusside inhibit the activity of the platelet inositol 1,4,5-trisphosphate receptor and promote its phosphorylation. J Biol Chem. 1996;271(10):5545–5551. doi: 10.1074/jbc.271.10.5545. [DOI] [PubMed] [Google Scholar]
- 50.Menitove J.E., Frenzke M., Aster R.H. Use of prostacyclin to inhibit activation of platelets during preparation of platelet concentrates. Transfusion. 1984;24(6):528–531. doi: 10.1046/j.1537-2995.1984.24685066818.x. [DOI] [PubMed] [Google Scholar]
- 51.Papadimos T.J., Medhkour A., Yermal S. Successful use of inhaled nitric oxide to decrease intracranial pressure in a patient with severe traumatic brain injury complicated by acute respiratory distress syndrome: a role for an anti-inflammatory mechanism? Scand J Trauma Resusc Emerg Med. 2009;17(5) doi: 10.1186/1757-7241-17-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanhoonacker M., Roeseler J., Hantson P. Reciprocal influence of refractory hypoxemia and high intracranial pressure on the postoperative management of an urgent neurosurgical procedure. Respir Care. 2012;57(7):1186–1190. doi: 10.4187/respcare.01322. [DOI] [PubMed] [Google Scholar]
- 53.Khan M.F., Azfar M.F., Khurshid S.M. The role of inhaled nitric oxide beyond ARDS. Indian J Crit Care Med. 2014;18(6):392–395. doi: 10.4103/0972-5229.133931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gritti P., Lanterna L.A., Re M., et al. The use of inhaled nitric oxide and prone position in an ARDS patient with severe traumatic brain injury during spine stabilization. J Anesth. 2013;27(2):293–297. doi: 10.1007/s00540-012-1495-2. [DOI] [PubMed] [Google Scholar]
- 55.Weed L.H., McKibben P.S. Pressure changes in the cerebro-spinal fluid following intravenous injection of solutions of various concentrations. Am J Physiol Content. 1919;48:512–530. [Google Scholar]
- 56.Changa A.R., Czeisler B.M., Lord A.S. Management of elevated intracranial pressure: a review. Curr Neurol Neurosci Rep. 2019;19(12):99. doi: 10.1007/s11910-019-1010-3. [DOI] [PubMed] [Google Scholar]
- 57.Wiedemann H.P., Wheeler A.P., Bernard G.R., et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 58.Chesnut R.M., Chesnut R.M., Marshall L.F., et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Lennihan L., Mayer S.A., Fink M.E., et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2000;31:383–391. doi: 10.1161/01.str.31.2.383. [DOI] [PubMed] [Google Scholar]
- 60.Solomon R.A., Fink M.E., Lennihan L. Prophylactic volume expansion therapy for the prevention of delayed cerebral ischemia after early aneurysm surgery: results of a preliminary trial. Arch Neurol. 1988;45:325–332. doi: 10.1001/archneur.1988.00520270107028. [DOI] [PubMed] [Google Scholar]
- 61.van der Jagt M. Fluid management of the neurological patient: a concise review. Crit Care. 2016;20:1–11. doi: 10.1186/s13054-016-1309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah F.A., Girard T.D., Yende S. Limiting sedation for patients with acute respiratory distress syndrome-time to wake up. Curr Opin Crit Care. 2017;23:45–51. doi: 10.1097/MCC.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papazian L., Forel J.M., Gacouin A., et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 64.Moss M., Huang D.T., Brower R.G., et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alnemari A.M., Krafcik B.M., Mansour T.R., et al. A comparison of pharmacologic therapeutic agents used for the reduction of intracranial pressure after traumatic brain injury. World Neurosurg. 2017;106:509–528. doi: 10.1016/j.wneu.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 66.Freeman W.D. Management of intracranial pressure. Contin Lifelong Learn Neurol. 2015;21:1299–1323. doi: 10.1212/CON.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 67.Roytowski D., Figaji A. Raised intracranial pressure: what it is and how to recognise it. Continuing Medical Education. 2013;31(3):85–90. [Google Scholar]
- 68.Steen P.A., Michenfelder J.D. Cerebral protection with barbiturates: relation to anesthetic effect. Stroke. 1978;9(2):140–142. doi: 10.1161/01.str.9.2.140. [DOI] [PubMed] [Google Scholar]
- 69.Vanhorebeek I., Latronico N., Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020;46(4):637–653. doi: 10.1007/s00134-020-05944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skoglund K., Enblad P., Marklund N. Effects of the neurological wake-up test on intracranial pressure and cerebral perfusion pressure in brain-injured patients. Neurocrit Care. 2009;11:135–142. doi: 10.1007/s12028-009-9255-3. [DOI] [PubMed] [Google Scholar]
- 71.Helbok R., Kurtz P., Schmidt M.J., et al. Effects of the neurological wake-up test on clinical examination, intracranial pressure, brain metabolism and brain tissue oxygenation in severely brain-injured patients. Crit Care. 2012;16:R226. doi: 10.1186/cc11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson J.G., Calfee C.S. ARDS subphenotypes: understanding a heterogeneous syndrome. Crit Care. 2020;24:1–8. doi: 10.1186/s13054-020-2778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Witt K.A., Sandoval K.E. Steroids and the blood–brain barrier: therapeutic implications. Adv Pharmacol. 2014;71:361–390. doi: 10.1016/bs.apha.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 74.Galicich J.H., French L.A. Use of dexamethasone in the treatment of cerebral edema resulting from brain tumors and brain surgery. Am Pract Dig Treat. 1961;12:169–174. [PubMed] [Google Scholar]
- 75.Muzumdar D., Jhawar S., Goel A. Brain abscess: an overview. Int J Surg. 2011;9:136–144. doi: 10.1016/j.ijsu.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 76.Wijdicks E.F.M., Sheth K.N., Carter B.S., et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(4):1222–1238. doi: 10.1161/01.str.0000441965.15164.d6. [DOI] [PubMed] [Google Scholar]
- 77.Baigent C., Bracken M., Chadwick D., et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury - outcomes at 6 months. Lancet. 2005;365(9475):1957–1959. doi: 10.1016/S0140-6736(05)66552-X. [DOI] [PubMed] [Google Scholar]
- 78.Villar J., Ferrando C., Martínez D., et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 79.Meduri G.U., Golden E., Freire A.X., et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131(4):954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 80.Steinberg K.P., Hudson L.D., Goodman R.B., et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 81.Sterne J.A.C., Murthy S., et al. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson N.J., Luks A.M., Glenny R.W. Gas exchange in the prone posture. Respir Care. 2018;62:1097–1110. doi: 10.4187/respcare.05512. [DOI] [PubMed] [Google Scholar]
- 84.Sud S., Friedrich J.O., Taccone P., et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med. 2010;36(4):585–599. doi: 10.1007/s00134-009-1748-1. [DOI] [PubMed] [Google Scholar]
- 85.Guérin C., Reignier J., Richard J.-C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 86.Scholten E.L., Beitler J.R., Prisk G.K., et al. Treatment of ARDS with prone positioning. Chest. 2017;151(1):215–224. doi: 10.1016/j.chest.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roth C., Ferbert A., Deinsberger W., et al. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care. 2014;21(2):186–191. doi: 10.1007/s12028-014-0004-x. [DOI] [PubMed] [Google Scholar]
- 88.Bein T., Kuhr L.P., Bele S., et al. Lung recruitment maneuver in patients with cerebral injury: effects on intracranial pressure and cerebral metabolism. Intensive Care Med. 2002;28(5):554–558. doi: 10.1007/s00134-002-1273-y. [DOI] [PubMed] [Google Scholar]
- 89.Paul V., Patel S., Royse M., et al. Proning in non-intubated (PINI) in times of COVID-19: case series and a review. J Intensive Care Med. 2020;35(8):818–824. doi: 10.1177/0885066620934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blanch L., Mancebo J., Perez M., et al. Short-term effects of prone position in critically ill patients with acute respiratory distress syndrome. Intensive Care Med. 1997;23:1033–1039. doi: 10.1007/s001340050453. [DOI] [PubMed] [Google Scholar]
- 91.Johannigman J.A., Davis K., Miller S.L., et al. Prone positioning for acute respiratory distress syndrome in the surgical intensive care unit: who, when, and how long? Surgery. 2000;128:708–716. doi: 10.1067/msy.2000.108225. [DOI] [PubMed] [Google Scholar]
- 92.Magnaes B. Body position and cerebrospinal fluid pressure. J Neurosurg. 1976;44:698–705. doi: 10.3171/jns.1976.44.6.0698. [DOI] [PubMed] [Google Scholar]
- 93.Magnaes B. Body position and cerebrospinal fluid pressure. Neurobiol Cerebrospinal Fluid. 1983;2:629–642. [Google Scholar]
- 94.Fan J.-Y. Effect of backrest position on intracranial pressure and cerebral perfusion pressure in individuals with brain injury. J Neurosci Nurs. 2004;36:278–288. doi: 10.1097/01376517-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 95.Kenning J.A., Toutant S.M., Saunders R.L. Upright patient positioning in the management of intracranial hypertension. Surg Neurol. 1981;15:148–152. doi: 10.1016/0090-3019(81)90037-9. [DOI] [PubMed] [Google Scholar]
- 96.Hawryluk G.W.J., Aguilera S., Buki A., et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC) Intensive Care Med. 2019;45(12):1783–1794. doi: 10.1007/s00134-019-05805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ng I., Lim J., Wong H.B., et al. Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery. 2004;54:593–598. doi: 10.1227/01.neu.0000108639.16783.39. [DOI] [PubMed] [Google Scholar]
- 98.Anderson C.S., Olavarría V.V. Head positioning in acute stroke. Stroke. 2019;50:224–228. doi: 10.1161/STROKEAHA.118.020087. [DOI] [PubMed] [Google Scholar]
- 99.Reinprecht A., Greher M., Wolfsberger S., et al. Prone position in subarachnoid hemorrhage patients with acute respiratory distress syndrome: effects on cerebral tissue oxygenation and intracranial pressure. Crit Care Med. 2003;31:1831–1838. doi: 10.1097/01.CCM.0000063453.93855.0A. [DOI] [PubMed] [Google Scholar]
- 100.Beuret P., Carton M.J., Nourdine K., et al. Prone position as prevention of lung injury in comatose patients: a prospective, randomized, controlled study. Intensive Care Med. 2002;28:564–569. doi: 10.1007/s00134-002-1266-x. [DOI] [PubMed] [Google Scholar]
- 101.Thelandersson A., Cider Å., Nellgård B. Prone position in mechanically ventilated patients with reduced intracranial compliance. Acta Anaesthesiol Scand. 2006;50:937–941. doi: 10.1111/j.1399-6576.2006.01037.x. [DOI] [PubMed] [Google Scholar]
- 102.Nekludov M., Bellander B.M., Mure M. Oxygenation and cerebral perfusion pressure improved in the prone position. Acta Anaesthesiol Scand. 2006;50:932–936. doi: 10.1111/j.1399-6576.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- 103.Kayani A.S., Feldman J.P. Prone ventilation in a patient with traumatic brain injury, bifrontal craniectomy and intracranial hypertension. Trauma. 2015;17(3):224–228. [Google Scholar]
- 104.National Institutes of Health Clinical Center Artificial increase in chest wall elastance as an alternative to prone positioning in moderate-to-severe ARDS. NCT03719937. ClinicalTrials.gov. 2018. Updated January 23, 2020. https://clinicaltrials.gov/ct2/show/NCT03719937
- 105.Peek G.J., Mugford M., Tiruvoipati R., et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 106.Sutter R., Tisljar K., Marsch S. Acute neurologic complications during extracorporeal membrane oxygenation: a systematic review. Crit Care Med. 2018;46:1506–1513. doi: 10.1097/CCM.0000000000003223. [DOI] [PubMed] [Google Scholar]
- 107.Kurihara C., Walter J.M., Karim A., et al. Feasibility of venovenous extracorporeal membrane oxygenation without systemic anticoagulation. Ann Thorac Surg. 2020:1209–1215. doi: 10.1016/j.athoracsur.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Juthani B., Hilaire C.S.T., Auvil B., et al. Outcomes of adult venovenous extracorporeal membrane oxygenation patients without anticoagulation: a retrospective review at a tertiary level referral center. J Am Coll Surg. 2016;223:S24. [Google Scholar]
- 109.Munoz-Bendix C., Beseoglu K., Kram R. Extracorporeal decarboxylation in patients with severe traumatic brain injury and ARDS enables effective control of intracranial pressure. Crit Care. 2015;19(381) doi: 10.1186/s13054-015-1088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Caputo N.D., Strayer R.J., Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375–378. doi: 10.1111/acem.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lara L.R., Püttgen H.A. Multimodality monitoring in the neurocritical care unit. Continuum (Minneap Minn) 2018;24(6):1776–1788. doi: 10.1212/CON.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 112.Corradi F., Robba C., Tavazzi G., et al. Combined lung and brain ultrasonography for an individualized “brain-protective ventilation strategy” in neurocritical care patients with challenging ventilation needs. Crit Ultrasound J. 2018;10(1):24. doi: 10.1186/s13089-018-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.National Institutes of Health Clinical Center. Multicenter observational study on practice of ventilation in brain injured patients. NCT04459884. ClinicalTrials.gov. 2020. Updated September 8, 2021. https://clinicaltrials.gov/ct2/show/NCT04459884