Abstract
Evidence is emerging that exercise and physical activity provides protection against severe COVID-19 disease in patients infected with SARS-CoV-2, but it is not known how exercise affects immune responses to the virus. A healthy man completed a graded cycling ergometer test prior to and after SARS-CoV-2 infection, then again after receiving an adenovirus vector-based COVID-19 vaccine. Using whole blood SARS-CoV-2 peptide stimulation assays, IFN-γ ELISPOT assays, flow cytometry, ex vivo viral-specific T-cell expansion assays and deep T-cell receptor (TCR) β sequencing, we found that exercise robustly mobilized highly functional SARS-CoV-2 specific T-cells to the blood compartment that recognized spike protein, membrane protein, nucleocapsid antigen and the B.1.1.7 α-variant, and consisted mostly of CD3+/CD8+ T-cells and double-negative (CD4-/CD8-) CD3+ T-cells. The magnitude of SARS-CoV-2 T-cell mobilization with exercise was intensity dependent and robust when compared to T-cells recognizing other viruses (e.g. CMV, EBV, influenza). Vaccination enhanced the number of exercise-mobilized SARS-CoV-2 T-cells recognizing spike protein and the α-variant only. Exercise-mobilized SARS-CoV-2 specific T-cells proliferated more vigorously to ex vivo peptide stimulation and maintained broad TCR-β diversity against SARS-CoV-2 antigens both before and after ex vivo expansion. Neutralizing antibodies to SARS-CoV-2 were transiently elevated during exercise after both infection and vaccination. Finally, infection was associated with an increased metabolic demand to defined exercise workloads, which was restored to pre-infection levels after vaccination. This case study provides impetus for larger studies to determine if these immune responses to exercise can facilitate viral clearance, ameliorate symptoms of long COVID syndrome, and/or restore functional exercise capacity following SARS-CoV-2 infection.
Keywords: Exercise immunology, Long COVID syndrome, α-variant, TCR sequencing, Virus specific T-cells, Metabolic response, Respiratory gas exchange, Lactate, Catecholamines, Cortisol, Physical activity
Highlights
-
•
Exercise mobilizes SARS-CoV-2 T-cells and augments their ex vivo expansion.
-
•
SARS-CoV-2 T-cells are highly exercise-responsive compared to other viral T-cells.
-
•
Exercise mobilized and expanded SARS-CoV-2 T-cells maintain broad TCR-β diversity.
-
•
Exercise transiently elevates neutralizing antibodies to SARS CoV-2 in serum.
-
•
Vaccination restored the greater metabolic demand of exercise seen after infection.
1. Introduction
As of August 5th, 2021, the coronavirus disease of 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than ∼200 million people worldwide resulting in over 4.2 million deaths (Dong et al., 2020). The rapid development and distribution of vaccine candidates has seen more than 4.3 billion vaccine doses administered to date, lowering rates of infection and mortality. It has been reported, however, that 10–30% of COVID-19 survivors will develop so-called long COVID syndrome, experiencing continuing symptoms of disease such as fatigue, shortness of breath, “brain fog”, fever, anxiety, depression, sleep disorders and gastrointestinal problems (Logue et al., 2021; Sudre et al., 2021; Yong, 2021). The causes of long COVID syndrome are not yet known, but viral persistence and/or a prolonged inflammatory response are likely to be involved (Yong, 2021).
It has emerged that lifestyle factors are playing a role in disease severity following SARS-CoV-2 infection, with those who are obese and/or physically inactive more likely to die, become hospitalized and/or require ventilatory support following infection (Cummins et al., 2021; Sallis et al., 2021). Nevertheless, the mechanisms by which physical activity can protect against severe infections and potentially ameliorate the symptoms of long COVID remain unknown. Each bout of exercise evokes an instantaneous mobilization and redistribution of effector lymphocytes, including CD4+ and CD8+ T-cells that recognize viral antigens. For instance, memory subsets of CD8+ T-cells specific to cytomegalovirus (CMV), Epstein-Barr virus (EBV) and adenovirus (AdV) are immediately mobilized to the blood compartment in response to acute leg cycling exercise (Kunz et al., 2018, 2020; Spielmann et al., 2014, 2016). This response is driven by the release of catecholamines acting on β2 adrenergic receptors expressed by the antigen-specific T-cells and is purported to facilitate anti-viral immune surveillance in individuals who exercise regularly (Kunz et al., 2020). Moreover, exercise enhances lymphatic flow to transiently increase circulating levels of immunoglobulins (Nehlsen-Cannarella et al., 1991), but it is not known if acute exercise can increase circulating levels of neutralizing antibodies against certain viruses. It is possible that any positive changes in anti-SARS-CoV-2 immunity with exercise could play an important role facilitating viral clearance in those with long COVID syndrome. Indeed, observational studies are emerging to indicate that symptoms of long COVID syndrome improve after vaccination (Arnold et al., 2021), possibly due to vaccine-induced enhancements in neutralizing antibodies and SARS-CoV-2 specific T-cells to reduce the viral reservoir.
We demonstrate in an otherwise healthy man that acute exercise transiently increases circulating neutralizing antibodies and instantaneously mobilizes highly functional SARS-CoV-2 specific T-cells after natural infection and again following COVID-19 vaccination. We also report that natural infection increased the metabolic response to defined exercise workloads, which was reversed after COVID-19 vaccination. This case study provides impetus for future research to determine if the frequent mobilization and redistribution of SARS-CoV-2 specific T-cells and increased transportation of neutralizing antibodies with each bout of exercise can increase viral clearance, ameliorate symptoms of long COVID syndrome, and/or restore functional exercise capacity after infection.
2. Methods
2.1. Study participant and procedures
A 42-year-old healthy male (height: 180.5 cm; body mass: ∼73.5 kg) with a background in competitive endurance sports (e.g. running, cycling, duathlon) contracted SARS-CoV-2 on December 28th, 2020 and subsequently received the Johnson and Johnson (J&J) human adenovirus-vector COVID-19 vaccine on March 31st, 2021. He reported testing positive for SARS CoV-2 the day after his wife received a positive test by polymerase chain reaction (PCR) and described experiencing symptoms consistent with acute SARS CoV-2 infection (e.g. fever, chills, muscle aches, runny nose and a temporary loss of taste and smell) that lasted for 48–72 h. For the remainder of the study period (up to April 21st, 2021) he described experiencing increased levels of fatigue/malaise, particularly in the late afternoon when he would oftentimes have to nap for 30-60-min on most days. He reported participating in vigorous exercise 4–6 times/week for 45–90 min/session with no major changes in his exercise habits throughout the study period (May 22nd, 2020–April 21st, 2021). Body mass measured on 4 separate occasions across the study timepoints was recorded as 71.4, 73.9, 74.3 and 74.5 kg, respectively. As part of an ongoing IRB approved research study in our laboratory at the University of Arizona, he completed a graded exercise test to volitional exhaustion on a cycle ergometer (Velotron, RacerMate, USA) on May 22, 2020, to determine his peak oxygen consumption (V̇O2peak). Subsequently, he performed an incremental continuous cycling bout consisting of 4x5-minute stages at power outputs corresponding to 50% (50 W), 60% (95 W), 70% (150 W), and 80% (200 W) of his pre-determined V̇O2peak (20-min total) on November 18th, 2020, 5.7 weeks prior to contracting SARS-CoV-2 (pre-infection; Pre-I). This exercise test was repeated using identical absolute power outputs on February 12th, 2021 (6.6 weeks after infection; Post-I) and again on April 21st, 2021 (3.0-weeks after vaccination/16.3-weeks after infection; Post-V). During each exercise trial, he arrived at the laboratory at 08:00 following an overnight fast and an intravenous catheter was inserted to allow blood to be drawn at rest, during exercise at the 60% and 80% V̇O2peak stages, and at 1 h post (+1 h) exercise. Respiratory gas exchange (Quark CPET, COSMED, Italy) and heart rate (Garmin, USA) were monitored continuously at rest and during exercise by indirect calorimetry (breath-by-breath) and short-range telemetry, respectively.
2.2. Blood processing & flow cytometry
Frozen serum samples from each exercise timepoint were analyzed for SARS-CoV-2 neutralizing antibodies (Cayman Chemical, Michigan, USA), cortisol (Invitrogen, MA, USA), lactate (Sigma-Aldrich, Missouri, USA), and catecholamines (2-CAT ELISA, LDN, Germany) using commercially available ELISA kits. Heparinized blood was utilized for 24 –hour whole blood peptide stimulation assays and to isolate peripheral blood mononuclear cells (PBMCs) using standard density gradient centrifugation procedures. PBMCs were used for IFN-γ ELISPOTs, IFN-γ capture assays, and the ex vivo expansion of SARS-CoV-2 virus-specific T-cells (VSTs). EDTA whole blood samples collected from each timepoint were stained and analyzed by 7-color flow cytometry (MACSQuant 10; Miltenyi Biotec Inc., Germany) using the following directly conjugated antibodies: CD8-VioBlue, CD14-VioGreen, CD3-FITC, CD4-PE, CD20-PerCP, CD45-APC, CD56-APC-Vio770 (Miltenyi). The percentage of all CD45+ lymphocytes expressing the surface markers of interest were multiplied by the total lymphocyte count (determined using a volumetric flow cytometry-based whole blood assay) to enumerate each lymphocyte subtype per unit of whole blood.
2.3. Whole blood peptide stimulation
Whole blood (1 mL) treated with lithium heparin and 2 mg glucose was stimulated with saline (1st negative control), PHA (10 μg/mL; Sigma Aldrich; positive control), an actin pepmix (1 μg/mL; JPT Peptide Technologies, Germany; 2nd negative control), or the SARS-CoV-2 spike (S), membrane (M), nucleocapsid (N), and α-variant pepmixes, each overlapping by 11 amino acids (10 μg/mL; Miltenyi). Whole blood was also stimulated with pooled S, M and N pepmixes, or with pooled pepmixes derived from other common viruses (10 μg/mL; Miltenyi) including, Epstein-Barr virus (EBV; consensus), cytomegalovirus (CMV; IE-1, pp65), adenovirus (ADV; hexon, penton), respiratory syncytial virus (RSV; nucleoprotein), BK virus (BKV; LT, VP-1), JC virus (JCV; LT, VP-1), herpes simplex virus-1 (HSV-1; Envelope Glycoprotein D), influenza (HA, NA, MP-1, NP), and varicella-zoster virus (VZV; IE-63) (5 μg/mL; JPT). After 24-h incubation at 37 °C, plasma was removed and stored at −80 °C until analysis. Interferon gamma (IFN-γ) concentration was then determined using a commercially available ELISA kit (R&D Systems, MN, USA).
2.4. Generation of SARS-CoV-2 virus-specific T-cells
SARS-CoV-2 VSTs were expanded from PBMCs in the Post-I trial only using a previously described VST expansion protocol (Keller et al., 2020). Briefly, 1 × 107 PBMCs were pulsed with 700 ng each of S, M, and N SARS-CoV-2 pepmix pools (JPT) in 100 μL RPMI culture medium consisting of 10% fetal bovine serum and 1% penicillin-streptomycin for 1 h at 37 °C with 5% CO2. After incubation, culture media supplemented with IL-4 (1000IU/mL) and IL-7 (10 ng/mL) was added to a final concentration of 1 × 106 cells/mL culture medium and cells were placed within gas permeable G-REX devices for 7-days. On day 7, cells were enumerated and resuspended at 1 × 106 cells/mL in media supplemented with IL-4 (1000IU/mL), IL-7 (10 ng/mL), and IL-15 (5 ng/mL) until day 10 whereby cells were harvested, enumerated, phenotyped, and used for functional assays.
2.5. Enumeration of SARS-CoV-2 specific T-cells by IFN-γ ELISPOT
The number of functional SARS-CoV-2 specific T-cells producing IFN-γ in response to virus-specific peptide stimulation were determined by enzyme-linked immunospot (ELISPOT) assays (Mabtech AB, Sweden) as previously described (Keller et al., 2020). Frozen PBMCs (100,000 cells/well) and expanded SARS-CoV-2 VSTs (100,000 cells/well) were stimulated with media only (1st negative control), (1 μg/mL), actin (2nd negative control), 1 μg/mL anti-CD3 mAb (1st positive control), 2 μg/mL PHA (2nd positive control), or 2 μg/mL each of the individual SARS-CoV-2 (JPT) pepmixes in separate wells. The number of IFN-γ spot-forming-cells (SFCs) were enumerated by Zellnet Consulting (NJ, USA) and, for the PBMCs collected on Day 0, adjusted for the total blood T-cell count (SFC/ml).
2.6. SARS-COV2 IFN-γ assay methods
SARS-CoV-2 specific T-cells and expanded VSTs were assessed for CD3, CD4 and CD8 surface marker expression using an IFN-γ Secretion Assay Kit (Miltenyi) and flow cytometry. Briefly, PBMCs or VSTs were washed and resuspended in RPMI+5% human serum at a final concentration of 1 × 106 cells/mL; 200IU/mL IL-2 was then added to the culture medium to rest overnight. On day 2, the cells were resuspended in culture medium at a concentration of 1 × 107 cells/mL in a 96-well plate (150 μL/well). Each sample received 3 μL of cytostim (positive control), 1 μL of MOG (negative control), and 10 μLS, M or N SARS-CoV-2 pepmixes followed by 3 h incubation at 37 °C with 5% CO2. Cells were then harvested, washed, and IFN-γ capture reagent was added. After 45 min at 37 °C with 5% CO2, the cells were washed and monoclonal antibodies were added. After 30 min in the dark, cells were washed, and phenotyped by flow cytometry.
2.7. TCR-β sequencing
The DNeasy blood Extraction Kit (Qiagen, USA) was used to extract genomic DNA from PBMCs collected at rest, during-exercise (80% intensity only), and 1 h post-exercise in the Post-I and Post-V trials only. Genomic DNA was also extracted from the SARS-CoV-2 VSTs that were expanded after these trials. T-cell receptor (TCR)-β deep sequencing was performed by Adaptive Biotechnologies (Seattle, WA, USA) and TCR-β rearrangements specific to SARS-CoV-2 were determined using the immunoSEQ T-MAP COVID ImmuneCODE database (Adaptive Biotechnologies, USA).
3. Results
3.1. Exercise mobilizes diverse SARS-CoV-2 specific T-cells to the blood compartment and transiently elevates neutralizing antibodies
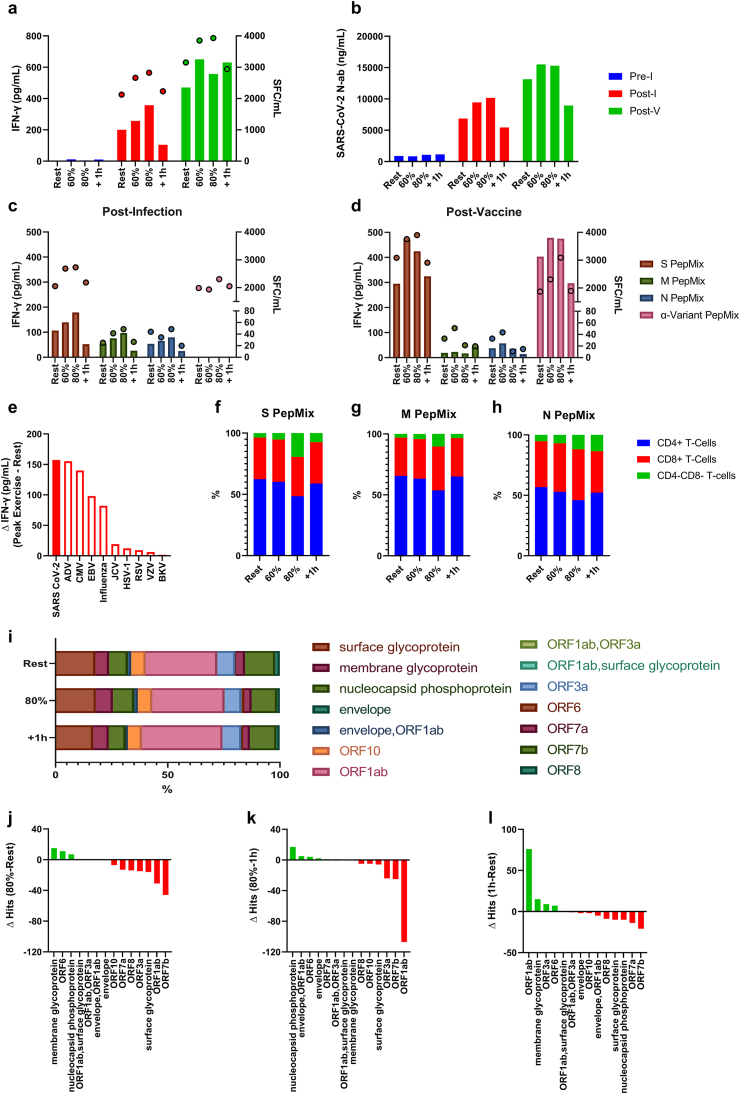
Changes in the numbers of circulating monocytes, lymphocytes and the major lymphocyte subsets in response to each exercise trial are shown in Table 1. The effects of exercise on the mobilization of SARS-CoV-2 specific T-cells after infection and vaccination are depicted in Fig. 1 Specifically, exercise increased the whole blood IFN-γ response to pooled SARS-CoV-2 pepmix stimulation in an intensity dependent manner (Fig. 1a). These results were corroborated by the IFN-γ ELISPOT assay (Fig. 1a) (the alpha-variant pepmix was not used to stimulate whole blood in the Post-I trial as they were not available at this time). There was an enhanced IFN-γ response after exercise Post-V, but not in an intensity dependent manner (Fig. 1a). A similar exercise response was observed with SARS-CoV-2 specific neutralizing antibodies (Fig. 2b). The IFN-γ response to peptide stimulation (whole blood and ELISPOT) was increased after exercise in the Post-I trial with the strongest effect seen for T-cells recognizing S protein (and α-variant), followed by M, and then N structural proteins (Fig. 1c). The IFN-γ response to peptide stimulation (whole blood and ELISPOT) increased at the 60% intensity Post-V, with the strongest effect seen for T-cells recognizing S protein (and α-variant), followed by N, and then M structural proteins (Fig. 1d). In the Post-I trial, the absolute change in IFN-γ secretion to combined SARS-CoV-2 whole blood peptide stimulation was greater than that observed after stimulating with pooled peptides derived from nine other viruses (Fig. 1e). Phenotypic characterization of T-cells responding to SARS-COV2 peptides among PBMCs Post-I revealed a relative dominance of CD4+ T-cells, regardless of exercise time point or SARS-CoV-2 antigen specificity (S, M, N) (Fig. 1 f/g/h). The percentages of CD4+ and CD8+ T-cells remained relatively stable across time. Interestingly, the percentage of ‘double negative’ (CD4−CD8−) T-cells responsive to each individual pepmix, increased at 80% V̇O2peak; CD4−CD8− T-cells increased from 3.61% to 19.46% (S), 3.06%–10.3% (M), and 5.22%–11.84% from (N) rest to 80% V̇O2peak (Fig. 1 f/g/h). Deep TCR-β sequencing in the Post-I trial revealed that exercise mobilized cells maintain diversity (Fig. 1i), although there was a discernible increase in the number of unique clones recognizing M glycoprotein, ORF6 and N phosphoprotein in PBMCs collected during exercise compared to PBMCs collected at rest and 1 h post-exercise (Fig. 1i/j/k/l).
Table 1.
The total number (cells/μL) of lymphocytes, CD3+ T-cells, CD4+ T-cells, CD8+ T-cells, ‘double-negative’ T-cells, NK-cells, B-Cells, and monocytes found in peripheral blood before (rest), during (60% and 80%), and 1 h after each exercise trial.
| Leukocyte Subsets (cells/μL) | Rest | 60% | 80% | +1 h |
|---|---|---|---|---|
| Lymphocytes | ||||
| Pre-I | 1744.54 | 2218.37 | 2735.60 | 2167.97 |
| Post-I | 1587.88 | 1832.95 | 2806.57 | 1512.49 |
| Post-V | 1710.38 | 1893.01 | 2387.63 | 1291.64 |
| CD3+T-cells | ||||
| Pre-I | 1295.67 | 1576.15 | 1856.38 | 1677.79 |
| Post-I | 1190.91 | 1288.01 | 1729.13 | 1098.82 |
| Post-V | 1271.33 | 1274.75 | 1443.08 | 964.08 |
| CD4+T-cells | ||||
| Pre-I | 875.09 | 1021.66 | 1203.49 | 1144.59 |
| Post-I | 761.47 | 812.87 | 1033.85 | 727.64 |
| Post-V | 817.08 | 754.91 | 821.98 | 623.86 |
| CD8+T-cells | ||||
| Pre-I | 387.41 | 509.10 | 605.55 | 491.43 |
| Post-I | 391.33 | 436.76 | 607.10 | 344.26 |
| Post-V | 421.06 | 486.57 | 581.27 | 315.45 |
| CD4−CD8−T-cells | ||||
| Pre-I | 27.47 | 33.41 | 46.97 | 34.90 |
| Post-I | 29.42 | 31.30 | 44.09 | 20.88 |
| Post-V | 28.35 | 29.19 | 34.63 | 21.79 |
| NK-cells | ||||
| Pre-I | 195.74 | 338.30 | 454.66 | 168.23 |
| Post-I | 249.46 | 336.71 | 662.91 | 243.81 |
| Post-V | 304.79 | 429.71 | 709.84 | 201.50 |
| B-cells | ||||
| Pre-I | 94.73 | 176.58 | 205.17 | 189.91 |
| Post-I | 116.55 | 161.12 | 227.05 | 159.87 |
| Post-V | 161.29 | 162.80 | 175.25 | 137.17 |
| Monocytes | ||||
| Pre-I | 204.26 | 300.09 | 352.17 | 318.71 |
| Post-I | 213.78 | 272.79 | 354.13 | 256.97 |
| Post-V | 219.8 | 257.39 | 310.80 | 174.79 |
Fig. 1.
The effect of exercise on the mobilization of SARS-CoV-2 specific T-cells and neutralizing antibodies. (a) IFN-γ response of SARS-CoV-2 specific T-cells (S/M/N Pepmix combined) to exercise Pre-I, Post-I, and Post-V as measured by whole blood peptide stimulation (bars) and ELISPOT (dots). (b) Neutralizing antibody responses to exercise Pre-I, Post-I, and Post-V. (c) Post-I and (d) Post-V IFN-γ response, specific to S, M, N, and α-variant Pepmix individually, to exercise Pre-I, Post-I, and Post-V as measured by whole blood peptide stimulation (bars) and ELISPOT (dots). (e) The absolute change in IFN- γ (peak exercise –rest) levels in response to stimulation with pepmixes derived from SARS-CoV-2 and 9 other common viruses to which this individual demonstrated immunity to. Proportions of T-cell subsets among IFN- γ secreting PBMCs stimulated with SARS-CoV-2 (f) S, (g) M, and (h) N pepmixes, respectively. (i) SARS-CoV-2 specific TCR-diversity in response to exercise (Post-I). The absolute change of SARS-CoV-2 unique clones, (j) 80% relative to rest. (k) 80% relative to 1 h, and (l) 1 h relative to rest.
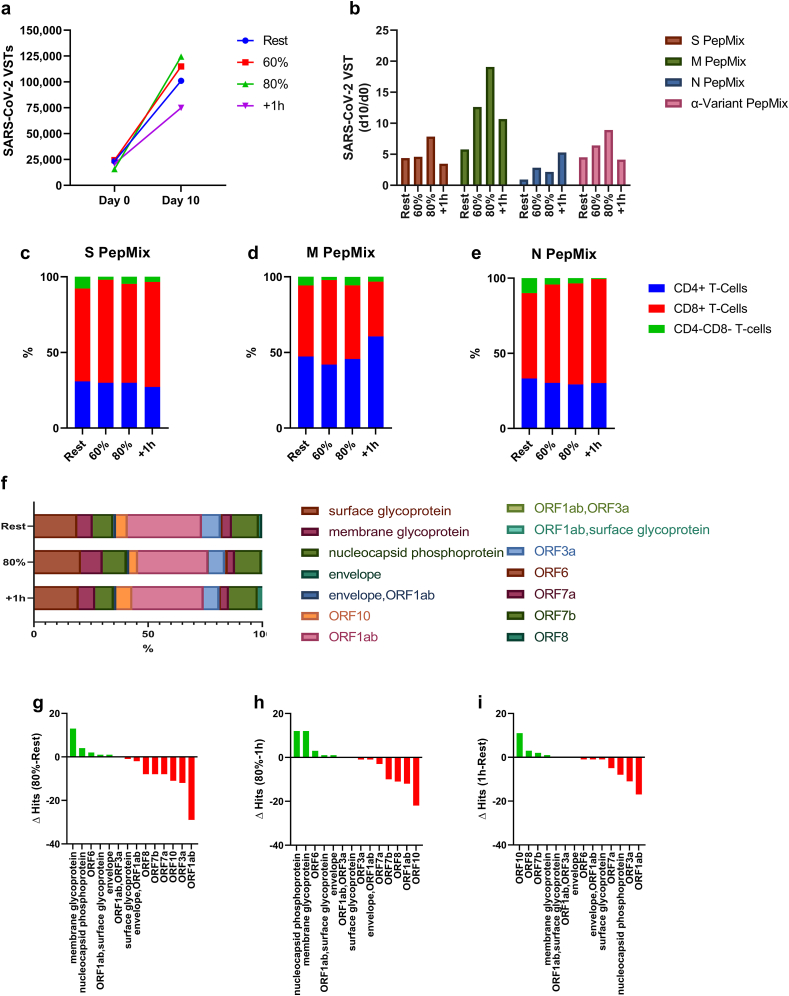
Fig. 2.
The effect of exercise on the ex vivo expansion of SARS-CoV-2 VSTs. (a) The total number of SARS-CoV-2 T-cells at the start of culture (day 0) and the total number of SARS-CoV-2 VSTs generated after 10 days of ex vivo expansion. (b) The number of SARS-CoV-2 VSTs generated at Day 10 divided by the number of SARS-CoV-2 T-cells in the PBMC fractions at Day 0, specific to each individual pepmix. Proportions of T-cell subsets among IFN- γ secreting expanded VSTs stimulated with SARS-CoV-2 (c) S, (d) M, and (e) N antigen pepmixes, respectively. (f) SARS-CoV-2 specific TCR-diversity in response to exercise after ex vivo expansion (Post-I). The absolute change in SARS-CoV-2 unique clones among the expanded VSTs, (j) 80% relative to rest. (k) 80% relative to 1 h, and (l) 1 h relative to rest.
3.2. Exercise mobilized SARS-CoV-2 specific T-cells are more responsive to ex vivo peptide stimulation and maintain broad TCR diversity
The effect of exercise on the ex vivo expansion of SARS-CoV-2 VSTs in the Post-I trial is depicted in Fig. 2. Exercise increased the ex vivo expansion of SARS-CoV-2 VSTs, with the greatest response seen at the 80% intensity (Fig. 2a). After adjusting for the number of VSTs stimulated on Day 0, VSTs responding to the M structural protein, had the greatest relative expansion, followed by S protein (including α-variant) then the N structural protein. In most cases, this response occurred in an exercise intensity dependent manner (Fig. 2b). Ex vivo expansion preferentially increased the proportion of CD8+ T-cells that recognize the S protein (Fig. 2c), M structural protein (Fig. 2d), and N structural protein (Fig. 2e) compared to PBMCs. The composition of the T-cell compartment after expansion was not greatly affected by exercise but there was an observable shift toward CD8 T-cell dominance against all SARS-CoV-2 antigens. Similar to PBMCs, deep TCR-β sequencing revealed that VSTs expanded after exercise maintain diversity (Fig. 2f) but there was a slight skewing in T-cell clonality toward M glycoprotein and N phosphoprotein in VSTs expanded from PBMCs collected during exercise compared to VSTs expanded before exercise and 1 h post-exercise (Fig. 2 g/h/i).
3.3. SARS-CoV-2 infection was associated with an increased metabolic demand to graded exercise that was restored after COVID-19 vaccination
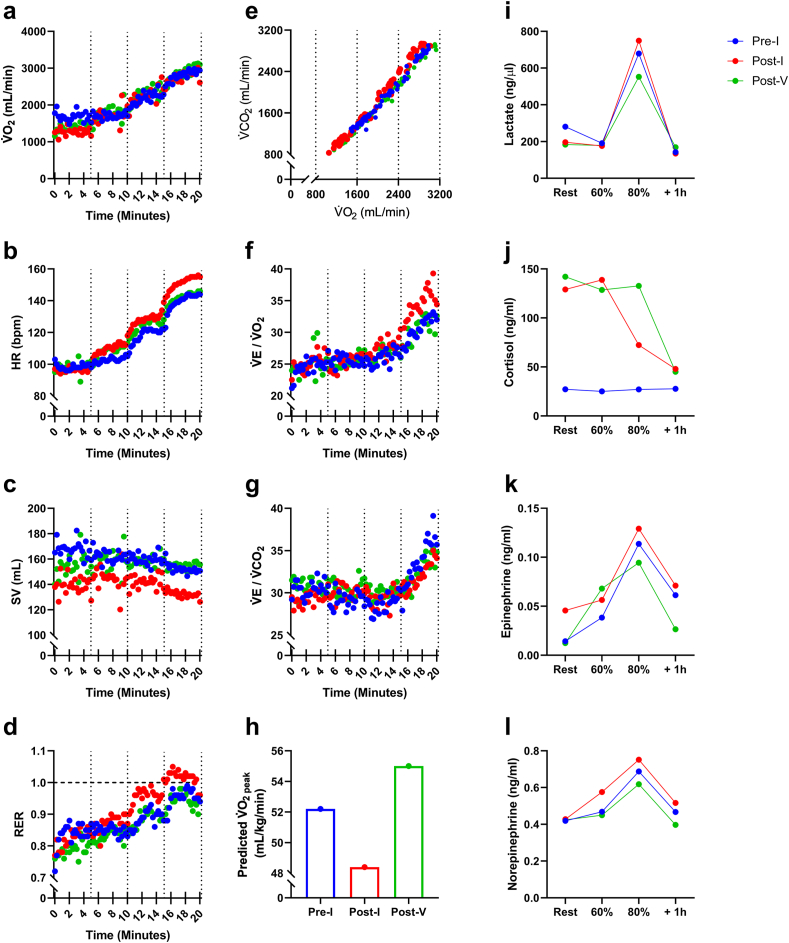
The volume of oxygen (V̇O2) consumed and the ratio of ventilation to carbon dioxide exhaled (V̇E/V̇CO2) did not differ across the three exercise trials (Fig. 3). However, discernible elevations in heart rate (HR), ventilation/oxygen consumption (V̇E/V̇O2), respiratory exchange ratio (RER), V-slope (V̇O2 versus V̇CO2), epinephrine, norepinephrine and blood lactate, with concomitant reductions in stroke volume (SV) and predicted V̇O2peak, were noted in the Post-I trial compared to the both the Pre-I and Post-V trials. The metabolic response to exercise in the Post-V trial was comparable (or slightly improved) to the Pre-I trial. Resting cortisol levels were markedly elevated in the Post-I and Post-V trials compared to the Pre-I trial.
Fig. 3.
Metabolic responses to graded exercise before and after SARS-CoV-2 infection and COVID-19 vaccination. (a) V̇O2, (b) HR, (c) SV, (d) RER, (e) V-slope, (f) V̇E/V̇O2, (g) V̇E/V̇CO2, (h) Predicted V̇O2peak, (i) Blood Lactate, (j) Epinephrine, and (l) Norepinephrine.
4. Discussion
Individuals that regularly exercise have improved clinical outcomes and symptomology following SARS-CoV-2 infection (Cummins et al., 2021; de Souza et al., 2021; Sallis et al., 2021). This has raised awareness on the potential for lifestyle changes, such as exercise, to safeguard against viral disease and has encouraged investigations into the role of exercise in the recovery of the so-called long COVID syndrome (McGregor et al., 2021; Udina et al., 2021). The frequent mobilization and redistribution of SARS-CoV-2 specific T-cells with each bout of exercise, combined with an increased lymphatic transportation of neutralizing antibodies, is one potential mechanism by which exercise can improve immunity against SARS-CoV-2 (Simpson and Katsanis, 2020); but how exercise affects cellular and humoral immunity against this novel coronavirus has not been determined. This case study is the first report of immune responses to exercise following SARS-CoV-2 infection and COVID-19 vaccination in an otherwise healthy individual.
We have previously shown that T-cells specific to adenovirus and common herpesviruses (e.g. CMV, EBV) are mobilized to blood in response to acute exercise, and that the effect is largely influenced by the secretion of catecholamines acting on T-cell β2 adrenergic receptors (Kunz et al., 2018, 2020; Spielmann et al., 2014, 2016). Here we show that exercise also mobilizes SARS-CoV-2 specific T-cells in an individual recently infected with the virus. The IFN-γ response to whole blood SARS-CoV-2 peptide stimulation (using overlapping peptide pools that span the S, M and N structural proteins) was elevated after exercise in an exercise-intensity dependent manner, with the greatest absolute (post – pre) response seen for T-cells recognizing S protein (including the α-variant) followed by M and N structural proteins. Strikingly, the absolute change in IFN-γ secretion to combined SARS-CoV-2 peptide stimulation was higher than the T-cell response to peptide pools derived from 9 other viruses, including known exercise-responsive ADV, CMV and EBV-specific T-cells, as well as T-cells responding to other respiratory viruses such as influenza and RSV. This robust response of SARS-CoV-2 specific T-cells is most likely due to the exercise test being performed shortly after (∼6-weeks) primary infection when increased numbers of peripheral antigen-specific T-cells are present. Exercise transiently increased the circulating levels of SARS-CoV-2 neutralizing antibodies, likely due to increased lymphatic flow and the return of protein rich fluid back to the blood compartment. We purport that increases in lymphatic flow with exercise may facilitate the transportation of neutralizing antibodies to facilitate viral clearance.
The enhanced whole blood IFN-γ response to SARS-CoV-2 peptides during exercise appears largely due to increased numbers of SARS-CoV-2 T-cells in blood, as highly sensitive IFN-γ ELISPOT assays showed that exercise increased total T-cell numbers (adjusted per volume of whole blood) recognizing all four of the SARS-CoV-2 antigen peptide pools used in this study. However, when we expanded SARS-CoV-2 VSTs ex vivo, the relative expansion of VSTs after 10-days of culture was markedly elevated after exercise (particularly for T-cells recognizing S, M protein and the α-variant). The relative response in SARS-CoV-2 VST expansions were greatest for those recognizing the M protein, indicating that exercise may enhance T-cell immunity against SARS-CoV-2 antigens that are not targets of current COVID-19 vaccines. Collectively, these observations indicate that exercise mobilized SARS-CoV-2 specific T-cells are more responsive (i.e. by releasing IFN-γ and proliferating) to recall antigen exposure than equivalent cells present in resting blood. The majority of T-cells responding to SARS-CoV-2 antigens at rest were of the CD4+ subset (56–65%), but exercise was found to increase the proportion of CD8+ T-cells and ‘double negative’ T-cells recognizing all SARS-CoV-2 antigens in both PBMCs and expanded VSTs. Further, the exercise-mobilized cells maintained broad antigen specificity as confirmed by deep TCR sequencing, although there was a notable skewing in T-cell clonality toward M glycoprotein, N phosphoprotein, and ORF6 in both PBMCs and expanded VSTs. As with the majority of T-cell subpopulations, SARS-CoV-2 specific T-cells, specifically ORF7, surface glycoprotein, and N phosphoprotein, egressed the blood compartment upon exercise cessation, falling below the pre-exercise values at 1 h post-exercise (Walsh et al., 2011). It is purported that T-cells leaving the blood compartment during exercise recovery migrate to tissues that require enhanced immune surveillance following a stress response (e.g. the lungs and the intestines) (Kruger et al., 2008).
Vaccination increased total SARS-CoV-2 specific T-cells by 1.48-fold. Expectedly, vaccination induced a greater T-cell response to S and not M or N proteins as S protein is the sole target of all current mass-produced COVID-19 vaccines (Dhama et al., 2020). Vaccination did, however, increase T-cell responses to the α-variant indicating that current COVID-19 vaccines may be effective in enhancing cellular immunity against evolving SARS-CoV-2 escape variants in those previously exposed. The SARS-CoV-2 T-cell response to exercise was also increased after vaccination by 1.25-fold. For instance, T-cell IFN-γ response to S protein increased by 1.68 and 1.61-fold in response to exercise after infection and vaccination, respectively. Simultaneously, since S protein is a major inducer of neutralizing antibodies, it is not surprising vaccination increased neutralizing antibodies by 1.92-fold. Despite overall increases in neutralizing antibodies after vaccination, the exercise effect was smaller with just a 1.18-fold increase in neutralizing antibodies after exercise post vaccine compared to a 2.23-fold increase after natural infection. This could be due to a ceiling effect in neutralizing antibody titers following vaccination.
Fatigue, lethargy and low exercise tolerance are symptoms of long COVID syndrome (Jimeno-Almazán et al., 2021). The individual in this case study was highly active and presented with an age-adjusted maximum aerobic capacity score (52.4 mL/kg/min) considered ‘superior’ by the American College of Sports Medicine (American College of Sports Medicine et al., 2018). At 46-days post infection, the predicted V̇O2peak from the graded exercise test had dropped by ∼9%. This was accompanied by notable elevations in exercising HR, V̇E/V̇O2, RER and blood lactate with a concomitant reduction in SV, all of which indicate that the metabolic demand of the exercise bout was markedly elevated after natural infection. Interestingly, vaccination was associated with a complete recovery in the metabolic response to exercise, as all respiratory gas exchange, blood lactate and catecholamine responses had returned to pre-infection values (or slightly improved) at 21-days post vaccination (114-days post infection). Whether this restoration in exercise performance was facilitated by the vaccine, the exercise habits of the individual, or to more recovery time following infection warrants further investigation. The participant did, however, report that he was still experiencing afternoon tiredness even after receiving the vaccine. It is possible that the increased fatigue/tiredness is associated with the elevated baseline cortisol levels that were observed after both infection and vaccination (Kumari et al., 2009). Cortisol did not increase during exercise, which is typical for individuals of high fitness performing relatively short bouts (<20-min) of exercise (Duclos et al., 1997). There did, however, appear to be a ‘cortisol clearance’ effect of exercise when baseline levels were high following SARS CoV-2 infection and COVID-19 vaccination.
In conclusion, the findings from this case study indicate that a single exercise bout mobilizes large numbers of SARS-CoV-2 specific T-cells with broad TCR diversity against COVID antigens and an enhanced ability to respond to recall antigens. Notwithstanding the obvious limitations of a case study (e.g. limited sample size, lack of statistical sampling, low external validity, limited control conditions), the findings do support the narrative that every exercise bout promotes neutralizing antibody transportation and mobilizes and redistributes highly functional virus-specific T-cells with the potential to increase immune surveillance and facilitate viral clearance. Of particular importance is the potential that an exercise boost in anti-SARS-CoV-2 immunity could reduce the viral reservoir and improve symptomology in those with long COVID syndrome. Indeed, vaccination in people previously infected with SARS-CoV-2 has been associated with improved symptoms, possibly due to the boost in neutralizing antibodies and SARS-CoV-2 specific T-cells contributing to the clearance of residual virus (Reynolds et al., 2021). Future experimental studies with sufficient sample sizes are warranted to determine the role of exercise, and the underpinning immunological mechanisms involved, in mitigating viral propagation, reducing symptoms of viral disease and restoring functional exercise capacity following SARS-CoV-2 and other viral infections.
Declaration of competing interest
We have no conflicting interests to declare.
Acknowledgment
This work was supported by the following NASA Grants: NASA (JSC) 80NSSC19K1480, NASA (JSC) 80NSSC21K0452.
References
- American College of Sports Medicine, Riebe D., Ehrman J.K., Liguori G., Magal M. Wolters Kluwer; Philadelphia: 2018. ACSM's Guidelines for Exercise Testing and Prescription. [Google Scholar]
- Arnold D.T., Milne A., Samms E., Stadon L., Maskell N.A., Hamilton F.W. Are vaccines safe in patients with Long COVID? A prospective observational study. medRxiv. 2021 doi: 10.1101/2021.03.11.21253225. 2021.03.11.21253225. [DOI] [Google Scholar]
- Cummins L., Ebyarimpa I., Cheetham N., Tzortziou Brown V., Brennan K., Panovska-Griffiths J. 2021. Factors Associated with COVID-19 Related Hospitalisation, Critical Care Admission and Mortality Using Linked Primary and Secondary Care Data. Influenza and Other Respiratory Viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza F.R., Motta-Santos D., Dos Santos Soares D., de Lima J.B., Cardozo G.G., Guimarães L.S.P., Negrão C.E., Dos Santos M.R. Association of physical activity levels and the prevalence of COVID-19-associated hospitalization. J. Sci. Med. Sport. 2021;24(9):913–918. doi: 10.1016/j.jsams.2021.05.011. Epub 2021 May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccines Immunother. 2020;16:1232–1238. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. The Lancet. Infectious diseases. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos M., Corcuff J.B., Rashedi M., Fougère V., Manier G. Trained versus untrained men: different immediate post-exercise responses of pituitary adrenal axis. A preliminary study. Eur. J. Appl. Physiol. Occup. Physiol. 1997;75:343–350. doi: 10.1007/s004210050170. [DOI] [PubMed] [Google Scholar]
- Jimeno-Almazán A., Pallarés J.G., Buendía-Romero Á., Martínez-Cava A., Franco-López F., Sánchez-Alcaraz Martínez B.J., Bernal-Morel E., Courel-Ibáñez J. Post-COVID-19 syndrome and the potential benefits of exercise. Int. J. Environ. Res. Publ. Health. 2021;18:5329. doi: 10.3390/ijerph18105329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M.D., Harris K.M., Jensen-Wachspress M.A., Kankate V.V., Lang H., Lazarski C.A., Durkee-Shock J., Lee P.-H., Chaudhry K., Webber K., Datar A., Terpilowski M., Reynolds E.K., Stevenson E.M., Val S., Shancer Z., Zhang N., Ulrey R., Ekanem U., Stanojevic M., Geiger A., Liang H., Hoq F., Abraham A.A., Hanley P.J., Cruz C.R., Ferrer K., Dropulic L., Gangler K., Burbelo P.D., Jones R.B., Cohen J.I., Bollard C.M. SARS-CoV-2–specific T cells are rapidly expanded for therapeutic use and target conserved regions of the membrane protein. Blood. 2020;136:2905–2917. doi: 10.1182/blood.2020008488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger K., Lechtermann A., Fobker M., Volker K., Mooren F.C. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav. Immun. 2008;22:324–338. doi: 10.1016/j.bbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kumari M., Badrick E., Chandola T., Adam E.K., Stafford M., Marmot M.G., Kirschbaum C., Kivimaki M. Cortisol secretion and fatigue: associations in a community based cohort. Psychoneuroendocrinology. 2009;34:1476–1485. doi: 10.1016/j.psyneuen.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Kunz H.E., Agha N.H., Hussain M., LaVoy E.C., Smith K.A., Mylabathula P., Diak D., Baker F.L., O'Connor D.P., Bond R.A., Katsanis E., Bollard C.M., Simpson R.J. The effects of β(1) and β(1+2) adrenergic receptor blockade on the exercise-induced mobilization and ex vivo expansion of virus-specific T cells: implications for cellular therapy and the anti-viral immune effects of exercise. Cell Stress & Chaperones. 2020;25:993–1012. doi: 10.1007/s12192-020-01136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz H.E., Spielmann G., Agha N.H., O'Connor D.P., Bollard C.M., Simpson R.J. A single exercise bout augments adenovirus-specific T-cell mobilization and function. Physiol. Behav. 2018;194:56–65. doi: 10.1016/j.physbeh.2018.04.035. [DOI] [PubMed] [Google Scholar]
- Logue J.K., Franko N.M., McCulloch D.J., McDonald D., Magedson A., Wolf C.R., Chu H.Y. Sequelae in adults at 6 Months after COVID-19 infection. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0830. e210830-e210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor G., Sandhu H., Bruce J., Sheehan B., McWilliams D., Yeung J., Jones C., Lara B., Smith J., Ji C., Fairbrother E., Ennis S., Heine P., Alleyne S., Guck J., Padfield E., Potter R., Mason J., Lall R., Seers K., Underwood M. Rehabilitation Exercise and psycholoGical support after covid-19 InfectioN' (REGAIN): a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22:8. doi: 10.1186/s13063-020-04978-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlsen-Cannarella S.L., Nieman D.C., Jessen J., Chang L., Gusewitch G., Blix G.G., Ashley E. The effects of acute moderate exercise on lymphocyte function and serum immunoglobulin levels. Int. J. Sports Med. 1991;12:391–398. doi: 10.1055/s-2007-1024700. [DOI] [PubMed] [Google Scholar]
- Reynolds C.J., Pade C., Gibbons J.M., Butler D.K., Otter A.D., Menacho K., Fontana M., Smit A., Sackville-West J.E., Cutino-Moguel T., Maini M.K., Chain B., Noursadeghi M., Brooks T., Semper A., Manisty C., Treibel T.A., Moon J.C., Valdes A.M., McKnight Á., Altmann D.M., Boyton R. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372:1418. doi: 10.1126/science.abh1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis R., Young D.R., Tartof S.Y., Sallis J.F., Sall J., Li Q., Smith G.N., Cohen D.A. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br. J. Sports Med. 2021;55(19):1099–1105. doi: 10.1136/bjsports-2021-104080. [DOI] [PubMed] [Google Scholar]
- Simpson R.J., Katsanis E. The immunological case for staying active during the COVID-19 pandemic. Brain Behav. Immun. 2020;87:6–7. doi: 10.1016/j.bbi.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann G., Bollard C.M., Bigley A.B., Hanley P.J., Blaney J.W., LaVoy E.C., Pircher H., Simpson R.J. The effects of age and latent cytomegalovirus infection on the redeployment of CD8+ T cell subsets in response to acute exercise in humans. Brain Behav. Immun. 2014;39:142–151. doi: 10.1016/j.bbi.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Spielmann G., Bollard C.M., Kunz H., Hanley P.J., Simpson R.J. A single exercise bout enhances the manufacture of viral-specific T-cells from healthy donors: implications for allogeneic adoptive transfer immunotherapy. Sci. Rep. 2016;6:25852. doi: 10.1038/srep25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., Pujol J.C., Klaser K., Antonelli M., Canas L.S., Molteni E., Modat M., Jorge Cardoso M., May A., Ganesh S., Davies R., Nguyen L.H., Drew D.A., Astley C.M., Joshi A.D., Merino J., Tsereteli N., Fall T., Gomez M.F., Duncan E.L., Menni C., Williams F.M.K., Franks P.W., Chan A.T., Wolf J., Ourselin S., Spector T., Steves C.J. Attributes and predictors of long COVID. Nat. Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udina C., Ars J., Morandi A., Vilaró J., Cáceres C., Inzitari M. Rehabilitation in adult post-COVID-19 patients in post-acute care with Therapeutic Exercise. The Journal of frailty & aging. 2021;10:297–300. doi: 10.14283/jfa.2021.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh N.P., Gleeson M., Pyne D.B., Nieman D.C., Dhabhar F.S., Shephard R.J., Oliver S.J., Bermon S., Kajeniene A. Position statement. Part two: maintaining immune health. Exerc. Immunol. Rev. 2011;17:64–103. [PubMed] [Google Scholar]
- Yong S.J. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infectious diseases. 2021:1–18. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]