Abstract
Successful detection of SARS-COV-2 in wastewater suggests the potential utility of wastewater-based epidemiology (WBE) for COVID-19 community surveillance. This systematic review aims to assess the performance of wastewater surveillance as early warning system of COVID-19 community transmission. A systematic search was conducted in PubMed, Medline, Embase and the WBE Consortium Registry according to PRISMA guidelines for relevant articles published until 31st July 2021. Relevant data were extracted and summarized. Quality of each paper was assessed using an assessment tool adapted from Bilotta et al.'s tool for environmental science. Of 763 studies identified, 92 studies distributed across 34 countries were shortlisted for qualitative synthesis. A total of 26,197 samples were collected between January 2020 and May 2021 from various locations serving population ranging from 321 to 11,400,000 inhabitants. Overall sample positivity was moderate at 29.2% in all examined settings with the spike (S) gene having maximum rate of positive detections and nucleocapsid (N) gene being the most targeted. Wastewater signals preceded confirmed cases by up to 63 days, with 13 studies reporting sample positivity before the first cases were detected in the community. At least 50 studies reported an association of viral load with community cases. While wastewater surveillance cannot replace large-scale diagnostic testing, it can complement clinical surveillance by providing early signs of potential transmission for more active public health responses. However, more studies using standardized and validated methods are required along with risk analysis and modelling to understand the dynamics of viral outbreaks.
Keywords: COVID-19, Wastewater, Epidemiological factors, Systematic review
Graphical abstract
1. Introduction
Wastewater surveillance, commonly used to monitor the epidemiology of poliovirus and noroviruses, is a potential approach for community surveillance of COVID-19 (Kitajima et al., 2020). Established evidence on fecal shedding of SARS-COV-2 by patients and successful virus genomic detection in wastewater studies suggest the applicability of this surveillance technique (Wang et al., 2020). Monitoring of sewage for detecting pathogens has been ongoing for over 40 years especially for poliovirus (Sinclair et al., 2008). Past studies have established that small outbreaks and epidemics of enterovirus and adenovirus disease within a community can be predicted by monitoring a community's sewage (Sinclair et al., 2008). Virus during a Coxsackievirus B5 outbreak was detected 10 days before clinical cases were detected while the Public Health Laboratories of Israel have been conducting an environmental surveillance of sewage to assess the spread of wild type poliovirus, on a monthly basis since 1989 (Nelson et al., 1967; Manor et al., 1999). This helped in the detection of the most recent outbreak of wild poliovirus type 1 in 2013–14 in Rahat, Israel (Brouwer et al., 2018). Sensitivity of sewage surveillance has also been validated in Mumbai, India wherein the wild type poliovirus was detected three months before any clinical cases were observed (Deshpande et al., 2003). Viruses causing gastroenteritis can also be detected in wastewater with only a few infected people. Hellmer et al., concluded that detection of pathogenic viruses in sewage provided early warnings of Hepatitis A virus and Norovirus outbreaks even before the causative pathogens were recognized in health care (Hellmér et al., 2014; La Rosa et al., 2014). Wastewater surveillance can also help predict the prevalence of certain types of disease in the community better than clinical data obtained from hospitalizations, like non-polio EV infections wherein EV-A and EV-C tend to produce subclinical infections that do not require hospitalization (Hellmér et al., 2014; Bisseux et al., 2020). These studies highlighted that sustained wastewater surveillance could be used to assess the introduction of a new infectious agent in the community.
The ongoing COVID-19 pandemic is caused by newly diagnosed SARS-CoV-2 virus, which has been detected in feces and urine of infected patients (Agrawal et al., 2021a). One of the public health challenges in this pandemic has been to implement high coverage and timely COVID-19 testing on populations, and the mainstay has been to conduct laboratory-based diagnostic testing on individuals, whether be it with Polymerase Chain Reaction (PCR) or serological tests. The use of wastewater surveillance as an early warning system to monitor the appearance and resurgence of COVID-19 is commonly suggested along with its use is in tailoring containment and mitigation measures and determining target populations for testing (Aguiar-Oliveira et al., 2020). Wastewater surveillance has a potential advantage of being able to predict the overall status of a given catchment area and being able to include asymptomatic individuals with much less effort compared to clinical surveillance. Given the median duration of virus persistence in feces exceeds that in respiratory samples (Wang et al., 2020), the probability of viral RNA detection in wastewater may be greater than clinical testing. Wastewater surveillance can complement the surveillance pyramid by providing mass monitoring via a low-cost, efficient and non-invasive approach. It can also shed light on prevalence rates ‘hidden’ by asymptomatic infections, poor health-seeking behaviour as well as in settings with low diagnostic capacity (Lodder and de Roda Husman, 2020).
There is still limited systematic synthesis of the progress made in applying wastewater surveillance to monitor COVID-19 trends exclusively, but rather reviews on the different methodologies used by various research groups and application of wastewater surveillance for SARS-CoV-2 along with other waterborne pathogens (Zahedi et al., 2021; Bivins et al., 2020). While we came across 22 reviews in our search, only one review by Li. et al. was a systematic review conducted until Jan 2021 (Hamouda et al., 2021). This systematic review can complement Li et al.'s work and assess the performance of wastewater surveillance in conducting qualitative or quantitative risk assessment of COVID-19 community cases until July 2021, to help understand the potential of wastewater surveillance in driving public health decisions.
2. Material and methods
2.1. Search strategy
This systematic review was conducted in line with Cochrane's Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic search was conducted in Pubmed, Medline and Embase for published literature. Additionally, grey literature on wastewater surveillance of COVID-19 were taken from the Wastewater-based epidemiology Consortium Registry which listed resources from COVID-19 dashboards/government websites. (https://www.covid19wbec.org/covidpoops19). Keywords such as “wastewater”, “COVID-19”, “SARS-CoV-2”, “fecal/urine” and “surveillance/detection” were used in the search to identify peer-reviewed articles on wastewater surveillance published between 1st January 2020 and 4th November 2020. An updated search for only published literature was conducted from 4th November 2020 until 31st July 2021. The publications were imported and managed in EndnoteX9 during the screening process. Publications identified were screened for their title & abstracts, and subsequently their full text according to eligibility criteria drawn out with the PICOS tool. The inclusion criteria are as follows:
-
●
Population: Any population-based community in a geographical region or groups within public/private facilities (including hospitals and cruise ships) that have a sewage system
-
●
Intervention: Wastewater epidemiology with any RT-PCR based COVID assay applied to wastewater (fecal &/or urine) samples, regardless of sampling and laboratory methodology. Excluding studies that do not exclusively look at wastewater surveillance sampling
-
●
Comparator: Epidemiological case counts or trends within the defined community.
-
●
Outcome: Any form of comparison (including graphical) or statistical reporting (e.g. Correlation or association/sensitivity & specificity) of wastewater surveillance results with epidemiological trends.
-
●
Study Design: Cross-sectional or prospective, longitudinal (observational) studies, including technical/government reports and unpublished (non-peer-reviewed i.e. preprints) data, excluding newspaper reports.
Studies that conducted any form of wastewater surveillance and sampling were included if they fulfilled all of the aforementioned criteria. The following study types: newspaper reports, reviews, and all non-English language sources were excluded. All screening was done in duplicate by five authors (SS, JK, JN, NL and SXWG). Discrepancies, if any, were resolved by a third author and JP.
2.2. Data extraction
Data was extracted according to five main themes - study details, sample collection process, sample processing and testing, and various outcomes - by five authors (SS, JK, JN, NL, SXWG) independently in an excel sheet template. The following data fields were condensed and presented in this manuscript: data collection period, country, region, population served, sampling site, sample type, sample collection approach, PCR assays used, kit names, gene targets, viral load recovered, number of positive samples, quantitative/qualitative association, temporal trends. To determine sample positivity, the overall number of samples was extracted as the denominator as much as possible. Amount of SARS-CoV-2 recovered was extracted in terms of gene copies as well as Ct values. For studies that presented sample positivity in terms of sites or days, each site or day was considered a positive sample. Outcome measures including samples positive and viral load are also stratified by gene target subject to data availability. Detailed information on the aforementioned fields, sample processing, sequencing and modelling can be found in the supplementary material (Appendix A: Supplementary Tables 1–3). This review defined grab sampling as sample collected at a single time point, and composite sampling as a sample merged from sampling over multiple time points, regardless of usage of autosampler or manual sampler. Settings other than WWTP and sewage systems are considered as settings outside of the conventional sewage system in this review.
2.3. Quality assessment
Given the novelty of wastewater surveillance as an epidemiological tool, there was no precedent in risk assessment for this study's reference. In order to assess the methodological quality of included studies, this review piloted a risk assessment tool adapted from Bilotta et al.'s work on quality assessment tools for environmental science and CDC guidelines on wastewater surveillance (Bilotta et al., 2014; CDC, 2021). Themes from Bilotta et al.'s work were adopted to form the framework of this assessment: 1) study design (selection and performance bias), 2) measurement of outcome (assessment bias), and 3) bias linked to clarity and publication bias. The tool comprises of nine signaling questions, modified according to CDC guidelines on sample collection, processing and testing. There are four questions in the study design domain, and three questions in the measurement of outcome domain and two questions for the clarity domain. Studies were assigned as of “high” or “low” risk of bias in response to each signaling question. Due to the lack of technical expertise to assign numerical scores in accordance to the importance of each aspect in the methodology, the review authors did not establish an approach to derive the overall quality of each study nor deemed fit to confer a study a high risk of bias based on a single question. Quality of the studies was assessed on a domain basis, with any domain rendered at high risk of bias if at least one question in the domain is answered “high”.
Data extraction and risk assessment for each study was conducted by a single author, and further verified by another. Any disagreement was resolved by a third author and JP.
2.4. Role of the funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
3. Results
3.1. Literature search results & selected study characteristics
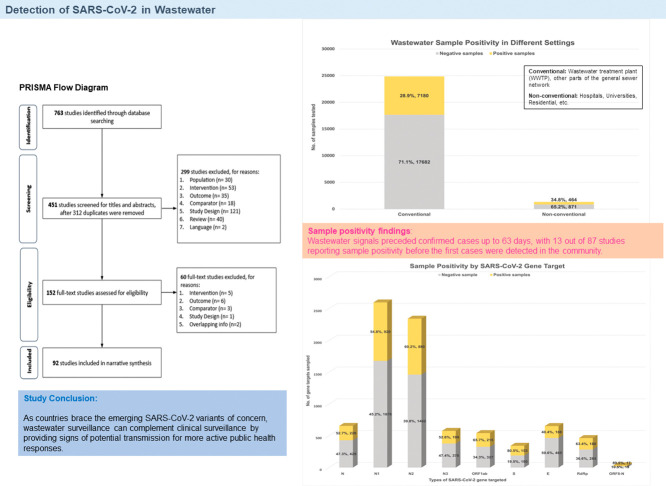
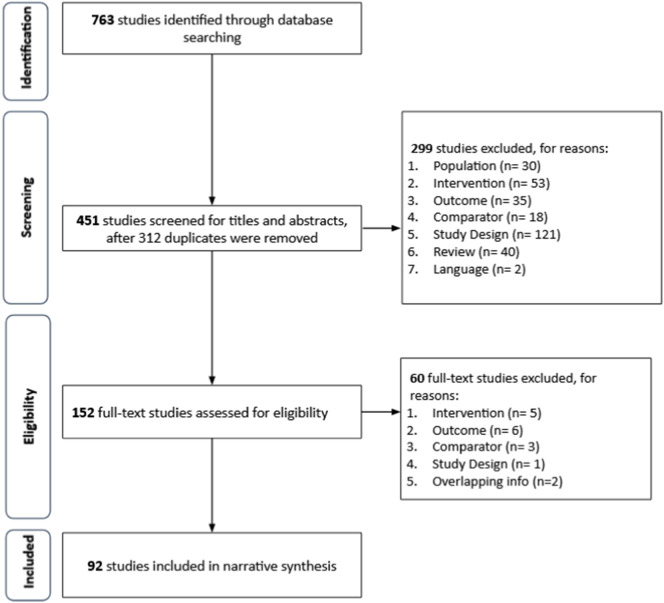
A total of 763 studies were identified from the database search. Following removal of 312 duplicates, 299 studies, including two non-English articles were excluded during the primary screening of title and abstract. Of the 152 studies selected for full-text review, 92 studies were shortlisted for qualitative synthesis (Fig. 1 ).
Fig. 1.
PRISMA flow diagram.
An overview of study characteristics is presented in Table 1 . All 92 included studies reported testing on wastewater samples for SARS-CoV-2 – 87 were research studies on wastewater surveillance (Acosta et al., 2021a; Agrawal et al., 2021b; Ahmed et al., 2021a; Bar-Or et al., 2021a; Bertrand et al., 2021a; Betancourt et al., 2021a; Bhattarai et al., 2021a; Black et al., 2021a; Cao and Francis, 2021a; Chakraborty et al., 2021a; Chavarria-Miró et al., 2021a; Colosi et al., 2021a; D'Aoust et al., 2021a; Ciesielski et al., 2021a; Fernandez-Cassi et al., 2021a; Fongaro et al., 2021a; Gerrity et al., 2021a; Gibas et al., 2021a; Giraud-Billoud et al., 2021a; Graham et al., 2021a; Hillary et al., 2021a; Hokajärvi et al., 2021a; Hong et al., 2021a; Johnson et al., 2021a; Karthikeyan et al., 2021a; Kitamura et al., 2021a; Koureas et al., 2021a; Kumar et al., 2021a; Kumar et al., 2021b; Li et al., 2021a; Melvin et al., 2021a; Mondal et al., 2021a; Mota et al., 2021a; Pillay et al., 2021a; Prado et al., 2021a; Rafiee et al., 2021a; Róka et al., 2021b; Rusiñol et al., 2021a; Saguti et al., 2021a; Saththasivam et al., 2021a; Scott et al., 2021a; Sharma et al., 2021a; Spurbeck et al., 2021a; Tanhaei et al., 2021a; Tomasino et al., 2021a; Wannigama et al., 2021a; Weidhaas et al., 2021a; Wilder et al., 2021a; Wilton et al., 2021a; Wong et al., 2021a; Wu et al., 2021a; Wurtz et al., 2021a; Wurtzer et al., 2020a; Xiao et al., 2021a; Xu et al., 2021a; Yaniv et al., 2021a; Ahmed et al., 2020a; Ahmed et al., 2020b; Albastaki et al., 2021a; D'Aoust et al., 2021b; Fongaro et al., 2021b; Gonçalves et al., 2021a; Gonzalez et al., 2020a; Guerrero-Latorre et al., 2020a; Hasan et al., 2021a; Hata et al., 2021a; Jørgensen et al., 2020a; Kumar et al., 2020a; Kuryntseva et al., 2020a; La Rosa et al., 2020a; La Rosa et al., 2021a; Martin et al., 2020a; Medema et al., 2020a; Nemudryi et al., 2020b; Peccia et al., 2020a; Prado et al., 2020a; Randazzo et al., 2020a; Randazzo et al., 2020b; Rimoldi et al., 2020a; Sharif et al., 2020a; Sherchan et al., 2020a; Trottier et al., 2020a; Vallejo et al., 2020a; Westhaus et al., 2021a; Wu et al., 2020a; Arora et al., 2020a; Haramoto et al., 2020a) while five were reports on ongoing wastewater surveillance programs (Gambier Wastewater SARS-CoV-2 Virus Report 2021, n.d.; Government NSW, 2020a; Government NSW, 2020b; Government NSW, 2020c; Government NSW, 2020d) undertaken by New South Wales, Australia and Kenyon college, USA. Details on the five reports can be found in the supplementary file and tables, and would not be further discussed in this review. Six of the 87 research studies were pre-prints that have yet to be peer reviewed. The 87 research studies were geographically distributed across at least 34 countries – United States (23) (Betancourt et al., 2021a; Bhattarai et al., 2021a; Cao and Francis, 2021a; Ciesielski et al., 2021a; Colosi et al., 2021a; Gerrity et al., 2021a; Gibas et al., 2021a; Graham et al., 2021a; Karthikeyan et al., 2021a; Li et al., 2021a; Melvin et al., 2021a; Mondal et al., 2021a; Scott et al., 2021a; Spurbeck et al., 2021a; Weidhaas et al., 2021a; Wilder et al., 2021a; Wu et al., 2021a; Xiao et al., 2021a; Gonzalez et al., 2020a; Nemudryi et al., 2020b; Peccia et al., 2020a; Sherchan et al., 2020a; Wu et al., 2020a), India (6 studies) (Chakraborty et al., 2021a; Kumar et al., 2021a; Kumar et al., 2021b; Sharma et al., 2021a; Kumar et al., 2020a; Arora et al., 2020a), Brazil (5 studies) (Fongaro et al., 2021a; Mota et al., 2021a; Prado et al., 2021a; Fongaro et al., 2021b; Prado et al., 2020a), Spain (5 studies) (Chavarria-Miró et al., 2021a; Rusiñol et al., 2021a; Randazzo et al., 2020a; Randazzo et al., 2020b; Vallejo et al., 2020a), Australia (4 studies) (Ahmed et al., 2021a; Black et al., 2021a; Ahmed et al., 2020a; Ahmed et al., 2020b), France (4 studies) (Bertrand et al., 2021a; Wurtz et al., 2021a; Wurtzer et al., 2020a; Trottier et al., 2020a), Canada (3 studies) (Acosta et al., 2021a; D'Aoust et al., 2021a; D'Aoust et al., 2021b), Italy (3 studies) (La Rosa et al., 2020a; La Rosa et al., 2021a; Rimoldi et al., 2020a), Japan (3 studies) (Kitamura et al., 2021a; Hata et al., 2021a; Haramoto et al., 2020a), United Kingdom (3 studies) (Hillary et al., 2021a; Wilton et al., 2021a; Martin et al., 2020a), Germany (2 studies) (Agrawal et al., 2021b; Westhaus et al., 2021a), Iran (2 studies) (Rafiee et al., 2021a; Tanhaei et al., 2021a), Israel (2 studies) (Bar-Or et al., 2021a; Yaniv et al., 2021a), South Africa (2 studies) (Johnson et al., 2021a; Pillay et al., 2021a; Albastaki et al., 2021a; Hasan et al., 2021a), United Arab Emirates (2 studies), and Argentina, Ecuador (Guerrero-Latorre et al., 2020a), Finland (Hokajärvi et al., 2021a), Greece (Koureas et al., 2021a), Hong Kong (Xu et al., 2021a), Hungary (Róka et al., 2021b), Netherlands (Medema et al., 2020a), Pakistan (Sharif et al., 2020a), Portugal (Tomasino et al., 2021a), Qatar (Saththasivam et al., 2021a), Russia (Kuryntseva et al., 2020a), Saudi Arabia (Hong et al., 2021a), Singapore (Wong et al., 2021a), Slovenia (Gonçalves et al., 2021a), Sweden (Saguti et al., 2021a), Switzerland (Fernandez-Cassi et al., 2021a), and Thailand (Wannigama et al., 2021a) (one study each) and a mixed-country study involving Belgium, Denmark and France (Jørgensen et al., 2020a). Majority of studies were conducted in the Americas and Europe.
Table 1.
Summary of study details, sample collection and testing methodology of included studies.
| Study details |
Sample collection |
Testing |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Data collection period | Country | City/region | Total population served | Sampling Site | Sample type | Sample collection approach | PCR assays used | PCR kit name | Gene target |
| Wu, F., et al., (2020b) | January–mid-May 2020 | USA | Middlesex, Norfolk, Suffolk (counties in Massachusetts) & Boston (city in Massachusetts) | 2,254,000–2,289,000 | WWTP, sewage system | Raw sewage | Composite & Grab | RT-PCR, RT-qPCR | ProtoScript II Reverse Transcriptase (RT), TaqMan® Fast Advanced Master Mix, TaqMan™ Fast Virus 1-Step Master Mix | N1, N2 |
| Westhaus, S., et al. (2021b) | 8 April 2020 | Germany | North Rhine-Westphalia (State) | 3,725,633 | WWTP | Raw sewage, effluent (post-tertiary treated & after ozonation/filtration) | Composite | RT-qPCR | Luna Universal Probe One-Step RT-qPCR Kit, LightCycler® Multiplex RNA Virus Master | E, M, N, RdRp |
| Vallejo, J. A., et al. (2020b) | 15 April–4 June 2020 | Spain | A Coruña, Oleiros, Culleredo, Cambre & Arteixo (municipalities in A Coruña) | >369,098 | WWTP, hospital sewer (treating COVID-19 patients) | Wastewater | Composite | RT-qPCR | qCOVID-19 | N |
| Trottier, J., et al. (2020b) | 7 May–20 July 2020 | France | Lattes (commune in Montpellier) | 470,000 | WWTP | Effluent | Composite | RT-qPCR | TaqPath One-Step RT-qPCR, CG Master Mix (ThermoFisher Scientific) | N1, N3 |
| Sherchan, S. P., et al. (2020b) | 13 January–29 April 2020 | USA | Louisiana | 290,321 | WWTP | Raw wastewater, secondary treated, final effluent | Composite & Grab | RT-qPCR | PerfecTa qPCR ToughMix | N1, N2 |
| Sharif, S., et al. (2020b) | 20 March–28 April 2020 | Pakistan | 38 districts | 3,800,000–11,400,000 | Pumping station, Open drain | Raw wastewater | Grab | RT-qPCR, RT-PCR | Real-Time Fluorescent RT-PCR Kit, 2019-nCoV Nucleic Acid Diagnostic Kit (PCR Fluorescence Probing), 2019 Novel Coronavirus RNA (PCR Fluorescence Probing) | ORF 1ab, N, E |
| Rimoldi, S.G., et al. (2020b) | 14 & 22 April 2020 | Italy | Milan & Monza e Brianza (city & province in Lombardy) | 2,780,000 | WWTP, River, Canal | Raw/treated wastewater, Riverwater | Grab | RT-qPCR | 2019-nCoV real-time RT-PCR kit panel | N, ORF1ab, E |
| Randazzo, W., et al. (2020c) | 12 March–14 April 2020 | Spain | Murcia, Cartagena, Molina de Segura, Lorca, Cieza, Totana (cities in Murcia) | 750,132 | WWTP | Influent, secondary, tertiary treated effluent | Grab | RT-qPCR | One Step PrimeScript™ RT-PCR Kit (Perfect Real Time) | N1, N2, N3 |
| Randazzo, W., et al. (2020d) | 12 February–14 April 2020 | Spain | Valencia | ~1,200,000 | WWTP | Raw/treated sewage water | Grab | RT-qPCR | PrimeScript™ One Step RT-PCR Kit | N1, N2 |
| Prado, T., et al. (2020b) | 15 April 2020 | Brazil | Niterói | NR | WWTP, hospital sewer, sewage system | Raw sewage | Composite | RT-qPCR | NR | N2 |
| Peccia, J., et al. (2020b) | 19 March–1 June 2020 | USA | New Haven (city in Connecticut) | ~200,000 | WWTP | Primary sewage sludge | NR | RT-qPCR | Bio-Rad iTaq Universal Probes One-Step Kit | N1, N2 |
| Nemudryi, A., et al. (2020a) | Late March–June 2020 | USA | Bozeman (city in Montana) | 49,831 | WWTP | Raw wastewater | Composite | RT-qPCR | 2019-nCoV CDC EUA Kit | N1, N2 |
| Medema, G., et al. (2020b) | 5 February–25 March 2020 | Netherlands | Amsterdam, Den Haag, Utrecht, Apeldoorn, Amersfoot, Tilburg and Schiphol Airport | 2,802,800 | WWTP, airport | Sewage (influent) | Composite | RT-qPCR | Taqman Fast Virus 1-Step Master Mix | N1, N2, N3, E |
| Martin, J., et al. (2020b) | 14 January–12 May 2020 | UK | South East England | 4,000,000 | WWTP | Raw sewage (influent) | Composite | RT-qPCR, nRT-PCR | qScript XLT qPCR Toughmix system, Invitrogen SuperScript III One-Step RT-PCR System with Platinum Taq High-Fidelity DNA Polymerase | RdRP, E, nsp2-PLPro, ORF8b-N |
| La Rosa, G., et al. (2021b) | 9 October 2019–28 February 2020 | Italy | Milan (City in Lombdardy), Turin (City in Piedmont) and Bologna | 4,998,600 | WWTP | Raw sewage | Composite | nRT-PCR, RT-qPCR | PCR reaction Kit Platinum SuperFi Green PCR Master Mix | RdRp, ORF1ab |
| La Rosa, G., et al. (2020b) | 3 February–2 April 2020 | Italy | Milan, Rome | 3,000,000 | WWTP | Raw sewage | Composite | nRT-PCR, RT-qPCR | Kit Platinum™ SuperFi™ Green PCR Master Mix; SuperScript III Reverse Transcriptase, Dream Taq polymerase and buffer, UltraSense one-step qRT-PCR System | ORF1ab, S, RdRP |
| Kuryntseva, P., et al. (2020b) | 30 March & 30 July 2020 | Russia | Kazan | 1790 | Sewage access points (city, Kazan Federal University campus) | Raw sewage | Composite | RT-qPCR | OM-Screen-2019-nCoV-RT | Orf1ab |
| Kumar, M., et al. (2020b) | 8 & 27 May 2020 | India | Ahmedabad (city in Gujarat) | NR | WWTP (hospital included in catchment) | Raw sewage, final effluents (after UASB & aeration pond) | Composite | RT-PCR | TaqPath Covid-19 RT-PCR Kit | ORF1ab, N, S |
| Jorgensen, A., et al. (2020b) | February–June 2020 | Denmark, France, Belgium | NR | NR | WWTP (Denmark, France), hospitals (Denmark, Belgium) | Raw sewage | Composite | RT-PCR | VIRSeek Screen kit, VIRSeek Ident kit | E, RdRP |
| Hata, A., et al. (2021b) | 5 March–23 April 2020 | Japan | Ishikawa and Toyama | 465,243 | WWTP | Influent wastewater | Grab | RT-qPCR | qTOWER3 | N, N2, N3 |
| Hasan, S. W., et al. (2021b) | May–June 2020 | UAE | NR | NR | WWTP, sewer access points, pumping stations | Influents and treated effluents, raw wastewater | Composite | RT-qPCR | GENESIG COVID-19 kits | RdRP |
| Haramoto E., et al. (2020b) | 17 March–7 May 2020 | Japan | Yamanashi Prefecture | NR | WWTP, river | Influent, secondary-treated wastewater activated sludge process before chlorination, river water | Grab | RT-qPCR, nRT-PCR | NR | N, N1, N2, ORF1a, S |
| Guerrero-Latorre, L., et al. (2020b) | 5 June 2020 | Ecuador | Quito | ~3,000,000 | River | River water impacted by urban wastewater | NR | RT-qPCR | TaqMan™ Fast Virus 1-Step Master Mix | N1, N2 |
| Gonzalez, R., et al. (2020b) | 9 March–2 August 2020 | USA | Virginia | 1,700,000 | WWTP | Raw wastewater | Composite & Grab | RT-ddPCR | One-Step RT-ddPCR Advanced Kit for Probes | N1, N2, N3 |
| Fongaro, G., et al. (2021c) | 30 October 2019–4 March 2020 | Brazil | Florianopolis (city in Santa Catalina) | ~5000 | Sewage system | Raw sewage | NR | RT-qPCR | OneStep qPCR Quantinova kit, Seegene Allplex™ 2019-nCoV commercial kit | N1, S, RdRp |
| D'Aoust, P.M., et al. (2021c) | 1 April–30 June 2020 | Canada | Ottawa (City in Ontario) and Gatineau (City in Quebec) | ~1,300,000 | WWTP | Post-grit chamber influent solids (PGS) & primary clarified sludge (PCS) | Composite & Grab | RT-qPCR, RT-ddPCR | Reliance One-Step Multiplex RT-qPCR Supermix, One-Step RT-ddPCR Advanced Kit for Probes | N1, N2, E |
| Arora, S., et al. (2020b) | 3 May–14 June 2020 | India | Jaipur (City in Rajasthan) | NR | WWTP (jail included in catchment), hospitals | Raw sewage, treated sample, tertiary treated sample | Grab | RT-qPCR | Allplex™ 2019-nCoV Assay kit, TaqPath™ COVID-19 Combo Kit | E, RdRp, N, ORF1ab, S |
| Albastaki, A., et al. (2021b) | 22 April–7 July 2020 | UAE | Dubai | NR | WWTP, pumping stations, aircrafts | Raw sewage, treated wastewater, raw sewage from airplane | Grab | RT-qPCR | TaqPath™ Covid-19 RT-PCR Kit | ORF1ab, N, S |
| Ahmed, W., et al. (2020d) | 23 April–10 May 2020 | Australia | NA | >321 | Cruise ship, aircrafts | Influent, effluent of the membrane bioreactor of the cruise ship, aircraft wastewater samples | Grab | RT-qPCR, RT-ddPCR | iTaq™ Universal Probes One-Step ReactionMix | N1, N2, N, E |
| Ahmed, W., et al. (2020e) | 24 February–1 April 2020 | Australia | Brisbane (City in Queensland) | 736,172 | WWTP, pumping station | Raw sewage | Composite | RT-qPCR | iTaq™ Universal Probes One-Step Reaction Mix | N |
| Gonçalves, J., et al. (2021b) | 1 June–15 June 2020 | Slovenia | Ljubljana | NR | Hospital pumping station | Raw wastewater | Composite | RT-qPCR | Roche LightCycler®Multiplex RNA Virus Master, LightMix® Modular SARS-CoV primers and probes mix | E, RdRp |
| Acosta, N., et al. (2021b) | 5 Aug–17 Dec 2020 | Canada | Calgary, Alberta | 2129 | Manholes (3 tertiary-care hospitals) | Raw waste water | Composite | RT-qPCR | TaqMan™ Fast Virus 1-Step Master Mix (Applied Biosystems) | N1, N2, E |
| Agrawal, S., et al. (2021c) | April–August 2020 | Germany | Frankfurt metropolitan area | ~1,200,000 | WWTP | Raw sewage | Composite | RT-qPCR | TaqPath COVID-19 RT-PCR Kit | N, S, ORF1ab |
| Ahmed, W., et al. (2021b) | 24 Feb–1 May 2020 | Australia | Brisbane | 934,000 | WWTP | Raw wastewater | Composite & Grab | RT-qPCR | iTaq™ Universal Probes One-Step Reaction Mix (Bio-Rad Laboratories, Richmond, CA). | N1, N2, E |
| Bar-Or, I., et al. (2021b) | August 2020 to February 2021 | Israel | El Hamra, Tzfat, Haifa, Natanya, Shafdan, Ashdod, Jerusalem, Rahat, Beer Sheva | 4,493,300 | WWTP | Raw wastewater | Composite | RT-qPCR | SensiFast reaction mix (Bioline) | E |
| Bertrand, I., et al. (2021b) | 2 April–28 May 2020 | France | French Grand Est region. | 250,000 | WWTP | Influent wastewater | NR | RT-PCR, RT-ddPCR | RNA UltraSens™ One-Step Quantitative RT-PCR system | RdRp, E |
| Betancourt, W., et al. (2021c) | 24 August–20 November 2020 | USA | the University of Arizona, Tucson | NR | University (sewer manholes) | Raw sewage | Grab | RT-PCR | RT-PCR assays manufactured at Integrated DNA Technologies (IDT, Coralville, IA) | N1, N2 |
| Bhattarai, B., et al. (2021b) | 18 May–21 July 2020 | USA | Utah | 1,449,860 | Water reclamation facilities (WRF) | Raw influent, primary sludge, return activated sludge, digested sludge | Composite & Grab | RT-qPCR | Quantstudio™ 3 Real-Time PCR machine (Thermofisher Scientific, USA). | N1 |
| Black, J., et al. (2021b) | 25 Aug–27 Oct 2020 | Australia | Metropolitan & regional Melbourne | 4,336,602 | WWTP & main sewer pipes | Raw sewage | Composite & Grab | RT-qPCR | PerkinElmer® SARS-CoV-2 Nucleic Acid Detection Kit (RUO) | N, ORF1ab |
| Cao and Francis (2021b) | 8 April 2020–current | USA | Indiana | ~30,000 | WWTP | Influent | Composite | NR | NR | NR |
| Chakraborty, P., et al. (2021b) | Chennai: 5–11 Sept 2020 Hospital WW: Aug–Sep 2020 |
India | Chennai | 9,675,613 | Sewage treatment plant, sewage pumping station, hospital wastewater | Influent, effluent, sludge | Composite and grab | RT-qPCR | IDT 2019-nCoV CDC-EUA kit | N1, N2 |
| Chavarria-Miró, G., et al. (2021b) | 13 April–7 July 2020 | Spain | Barcelona (Metropolitan area) | ~2,700,000 | WWTP | Raw sewage | Composite | RT-qPCR | NR | RdRp, IP2, IP4, E, N1, N2 |
| Ciesielski, M., et al. (2021b) | 9 March–6 Sept 2020 | USA | 18 cities in Virginia | 1,427,336 | WWTP | Influent | Grab & composite | RT-qPCR, RT-ddPCR | Reliance One-Step Multiplex RT-qPCR Supermix; Bio-Rad's One-Step RT-ddPCR Advanced Kit | N2 |
| Colosi, L., et al. (2021b) | 7 July–2 September 2020 | USA | Virginia | >14,505 | WWTP, hospitals, dormitories | Raw sewage, primary solids | Grab & composite | RT-qPCR | NR | N1, N2 |
| D'Aoust, P., et al. (2021d) | 20 June–4 Aug 2020 | Canada | Ottawa | ~1000,000 | water resource recovery facility (WRRF) | Primary clarified sludge | Composite | RT-qPCR | One-Step Multiplex RT-qPCR Supermix | N1, N2 |
| Fernandez-Cassi, X., et al. (2021b) | 26 February–30 April 2020 | Switzerland | Lugano, Lausanne, Zurich | 815,000 | WWTP | Influent | Composite | RT-qPCR | RNA UltraSense™ One-Step | N1, N2 |
| Fongaro, G., et al. (2021d) | Aug-20 | Brazil | Minas Gerais State region (presidio, worker accommodation, rural community) | 1750 | Sink storage box, river water | Raw sewage, river water upstream and downstream | NR | RT-qPCR | NR | N1, N2 |
| Gerrity, D., et al. (2021b) | Early March–late May 2020 | USA | Southern Nevada | 1,060,000 | WWTP, untreated surface water, finished drinking water | Influent and primary effluent | Composite and grab | RT-qPCR | iScript™Select cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) or the Maxima First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) | N1, N2, E, ORFla |
| Gibas, C., et al. (2021b) | 28 September–23 November 2020 | USA | Charlotte, North Carolina | >2550 | University (plumbing cleanouts & manhole access points) | Raw sewage | Composite | RT-qPCR | iTaq universal probes reaction mix (Bio-Rad laboratories), iScript reverse transcriptase (Bio-Rad laboratories), 2019-nCoV CDC RUO Kit (Integrated DNA Technologies IDT, #10006713) | N1 |
| Giraud-Billoud, M., et al. (2021b) | 22 July 2020–January 2021 | Argentina | Mendoza | ⁓1,191,649 | WWTP | Influent samples | Manual | RT-qPCR | 2019 nCoV CDC EUA Kit (IDT#10006606, L#0000512209) | N1, N2 |
| Graham, K., et al. (2021b) | 16 March–12 July 2020 | USA | Santa Clara County, California | ~1,700,000 | WWTP | Influent, primary solids | Grab & composite | RT-qPCR, ddRT-PCR | TopTaq Master Mix Kit, ddPCR SARS CoV-2 BioRad kit triplex assay | N1, N2 |
| Hillary, L., et al. (2021b) | March–July 2020 | UK | Gwynedd, Cardiff, Liverpool, Manchester, the Wirral, Wrexham | 3,032,000 | WWTP | Influent, treated effluent | Grab & composite | RT-qPCR | RNA Ultrasense Reaction Mix, RNA Ultrasense Enzyme Mix | N1, E |
| Hokajärvi, A., et al. (2021b) | 19–20 April and 24–25 May 2020 | Finland | Helsinki | 860,000 | WWTP | Influent | Composite | RT-qPCR | TaqMan Fast Virus 1-step Master Mix and a QuantStudio 6 Flex real-time PCR system | E, N2 |
| Hong, P., et al. (2021b) | 15 April–9 July 2020 | Saudi Arabia | Jeddah | 42–236 | Hospital (WWTP within hospital) | Raw sewage, partially treated wastewater | Grab | RT-qPCR | NR | N1, N2, N3 |
| Johnson, R., et al. (2021b) | 18-Jun-20 | South Africa | Cape Town and Stellenbosch municipality | 2,375,000 | WWTP | Raw influent | Composite | RT-qPCR | iTaq Universal Probes One-Step Reaction Mix | N1, N2 |
| Karthikeyan, S., et al. (2021b) | 20 July 2020–21 October 2020 | USA | San Diego | >2,300,000 | WWTP | Influent | Composite | RT-qPCR | TaqPath™ 1-Step RT-qPCR Master Mix | N1, N2, E |
| Kitamura, K., et al. (2021b) | 9 June–19 August 2020 | Japan | Metropolitan region | NR | Manhole, WWTP | Influent | Grab | RT-qPCR | QIAamp Viral RNA kit (Qiagen) | N1, N2 |
| Koureas, M., et al. (2021b) | 29 October 2020–14 April 2021 | Greece | Larissa and Volos | 305,000 | WWTP | Influent | Composite | RT-PCR | TaqPath™ COVID-19 CE-IVD RT-PCR Kit (Applied Biosystems™) | ORF1ab, S, N |
| Kumar, M., et al. (2021c) | 7 Aug–30 Sept 2020 | India | Gandhinagar | NR | WWTP (4 wards and an academic institution) | Influent | Grab | RT-PCR | TaqPath™ Covid-19 RT-PCR Kit | ORF1ab, N, S |
| Kumar, M., et al. (2021d) | 3 September–26 November 2020 | India | Ahmedabad | NR | WWTP, pumping station, lakes, river | Influent | Grab | RT-PCR | TaqPath™ 1 Step Multiplex Master Mix | N, ORF 1ab, S |
| Li, B., et al. (2021b) | 27 Aug–4 Oct 2020 | USA | Honolulu (Sand Island and Honouliuli) | 697,680 | WWTP | Influent | Composite | RT-qPCR | ABI 7300 qPCR System (Applied Biosystem; Beverly, MA, USA) | E, N1, N2 |
| Melvin, R., et al. (2021b) | May–August 2020 | USA | Minnesota | 2,362,610 | WWTP | Influent | Composite | RT-qPCR | Go-Taq Probe 1-Step RT-qPCR system 2× master mixes | N1, N2 |
| Mondal, S., et al. (2021b) | Mid-October 2020–early Jan 2021 | USA | Wisconsin (Dane county) | NR | WWTP | Primary influent | 24 h composite | RT-qPCR | SARS-CoV-2 RT-qPCR Detection Kit for Wastewater (Promega Corp.) | N1, N2, E |
| Mota, C., et al. (2021b) | 11 May–7 August 2020 | Brazil | Belo Horizonte | ~2,000,000 | WWTP, sewer network | Influent | Composite | RT-qPCR | iTaq™ Universal probes One Step reaction mix & reverse transcriptase | N1 |
| Pillay, L., et al. (2021b) | 7 July–6 October 2020 | South Africa | KwaZulu-Natal | NR | WWTP | Raw sewage | Grab | One-step RT-ddPCR | One-Step RT-ddPCR Advanced Kit for Probes from Biorad (USA) | N2 |
| Prado, T., et al. (2021b) | 15 April–25 August 2020 | Brazil | Niterói, Rio de Janeiro | 513,584 | WWTP, sewer network | Raw sewage | Composite | RT-qPCR | SuperScript TM III Platinum TM One-Step qRT-PCR Kit (Invitrogen) | N2 |
| Rafiee, M., et al. (2021b) | 12–20 November 2020, 6–14 May 2021 | Iran | Tehran | 8,694,000 | Sewer access points (manholes) | Raw sewage | Grab, composite & Moore swabs | RT-qPCR | Novel coronavirus nucleic acid diagnostic real time PCR kit (Sansure Biotech, China) | N, ORF1ab |
| Róka, E., et al. (2021a) | June–November 2020 | Hungary | Budapest | 1,800,000 | WWTP | Raw sewage | Composite & grab | RT-qPCR | LightCycler Multiplex RNA Virus Master kit (Hoffmann-La Roche) | N1 |
| Rusiñol, M., et al. (2021b) | Mid-March–early November 2020 | Spain | Catalonia | 2,011,300 | WWTP | Influent | Composite | RT-qPCR | TaqMan™ Environmental Master Mix 2.0 | N1, N2 |
| Saguti, F., et al. (2021b) | 10 February–5 July 2020 | Sweden | Gothenburg | 755,940 | WWTP | Influent, treated effluent | Composite & grab | RT-qPCR | Reaction Mix (Invitrogen), 20 U RNaseOUTTM (Invitrogen), 1 μL SuperScript® III/platinum® Taq Mix (Invitrogen) | RdRP |
| Saththasivam, J., et al. (2021b) | 21 June–30 August 2020 | Qatar | greater Doha | 2,503,457 | WWTP | Influent | Composite | RT-qPCR | SARS-CoV-2 (2019-nCoV) CDC qPCR Probe Assay Research Use Only (RUO) kit | N1, N2 |
| Scott, L., et al. (2021b) | 19 August–1 December 2020 | USA | New Orleans (Louisiana) | 14,062 | University (manholes, sewer cleanouts, and municipal manholes) | Raw sewage | Grab | RT-qPCR | qScript one-step RT-qPCR ToughMix (QuantaBio) | N1, N2 |
| Sharma, D., et al. (2021b) | Pre-Covid & 11–22 May 2020 | India | Mumbai | 4,704,883 | Pumping station, open drain | Raw sewage | Grab | RT-PCR | LabGun COVID-19 real-time RT-PCR kit (LabGenomics) | E, RdRP |
| Spurbeck, R., et al. (2021b) | 14, 22 & 29 July 2020 | USA | Toledo (Ohio) | NR | Hospitals, nursing home, residential neighbourhood (manholes) | Raw sewage | Composite & grab | RT-qPCR | TaqMan Fast Viral One-Step Master Mix (ThermoFisher) | N1 |
| Tanhaei, M., et al. (2021b) | 30 June–31 July 2020 | Iran | Tehran | NR | WWTP | Raw influent, treated effluent | Grab | RT-qPCR | Sansure Biotech Inc. (Changsha, China), Shanghai Zhijiang Biotechnology Co. (Shanghai, China) Kits | ORF1ab, N |
| Tomasino, M., et al. (2021b) | 14 May 2020–10 March 2021 | Portugal | Porto | 370,000 | WWTP | Raw sewage | Composite | RT-qPCR | 1) BGI's Real-Time Fluorescent RTPCR kit 2) Water SARS-CoV-2 RT-PCR ready-to use kit (IDEXX Laboratories) |
May–Sep: NR Sep–Mar: N1, N2 |
| Wannigama, D., et al. (2021b) | 7 July–27 December 2020 | Thailand | Bangkok & 26 nearby suburbs | 15,934,500 | Bangkok: shopping centers, condominium complex, office complex, and WWTP, Entertainment venues Suburbs: shopping centers, housing complex, office complex, and food markets |
Primary wastewater samples | NR | RT-qPCR | Bio-Rad iTaq Universal Probes One-Step Kit | N1, N2, N3, E |
| Weidhaas, J., et al. (2021b) | 1 April–28 May 2020 | USA | Utah | 1,260,000 | WWTP | Influent, interceptor sample from sub-sewershed, effluent | Composite & grab | RT-qPCR | TaqPath™1-step RT-qPCR (Thermofisher), qScript XLT Onestep RT-qPCR (Quantabio) | N1, N2 |
| Wilder, M., et al. (2021b) | 28 April–24 June 2020 | USA | New York (upstate) | 1000,898 | Sewage Network Access Points | Influent | Composite | RT-qPCR | Reliance One-Step Multiplex RT-qPCR Super- mix (Bio-Rad®, California, USA) | RdRp |
| Wilton, T., et al. (2021b) | 14 January 2020–26 January 2021 | UK | London | 4,000,000 | WWTP | Inlet samples | Composite | nRT-PCR | NR | RdRP, ORF8b, S |
| Wong, J., et al. (2021b) | 4–20 July 2020 | Singapore | Singapore | NR | Manhole of apartment building | Raw sewage | Composite | RT-qPCR | Luna Universal Probe One-Step RT-qPCR Kit (NEB, USA) | ORF1ab |
| Wu, F., et al. (2021b) | 18 February–2 June 2020 | USA | Nationwide (40 States) | 42,500,000 | WWTP or catchments | Raw wastewater | NR | RT-qPCR | TaqMan® Fast Advanced Master Mix, TaqMan™ Fast Virus 1-Step Master Mix | N1, N2 |
| Wurtz, N., et al. (2021b) | 1 July–15 December 2020 | France | Marseille | 973,746 | Sewer networks | Wastewater | NR | nRT-PCR | BioFire COVID-19 Test (BioFire Defense) | NR |
| Wurtzer, S., et al. (2020b) | 5 March–23 April 2020 | France | Paris | 3,000,000 | WWTP | Influent | Composite | RT-qPCR | Fast virus 1-step Master mix 4× (Lifetechnologies) | E, RdRp |
| Xiao, A., et al. (2021b) | 4 March 2020–13 May 2021 | USA | Boston (Massachusetts) | ~2,300,000 | WWTP | Raw sewage | Composite | RT-qPCR | TaqMan™ Fast Virus 1-Step Master Mix | N1, N2 |
| Xu, X., et al. (2021b) | 8 June–29 September 2020 | Hong Kong | Hong Kong | 4,555,001 | Manholes of isolation ward building (hospital), public housing estate, WWTP, sewer network | Raw sewage | Composite | RT-qPCR | TaqMan Fast Virus 1-Step Master Mix | N |
| Yaniv, K., et al. (2021b) | 17–19, 25 May 2020 | Israel | Ashkelon | 34,100 | Manholes, WWTP | Raw sewage | Composite | RT-qPCR | One Step PrimeScript III RT-qPCR mix (Takara) | N1 |
| NSW Government (2020a) | July–October 2020 | Australia | New South Wales | 147,908–6,430,642 | WWTP | Raw sewage | NR | NR | NR | NR |
| NSW Government (2020b) | 4–10 October 2020 | Australia | New South Wales | 6,472,811 | WWTP, quarantine hotels | Raw sewage | NR | NR | NR | NR |
| NSW Government (2020c) | 11–17 October 2020 | Australia | New South Wales | 6,526,714 | WWTP, quarantine hotels | Raw sewage | NR | NR | NR | NR |
| NSW Government (2020d) | 18–24 October 2020 | Australia | New South Wales | 6,475, 464 | WWTP, quarantine hotels | Raw sewage | NR | NR | NR | NR |
| Kenyon College (Gambier Wastewater SARS-CoV-2 Virus Report, n.d.) | Ongoing | USA | Ohio | 700 | NR | NR | Composite | NR | NR | N1, N2 |
Samples were collected between January 2020 and May 2021 from various locations. Settings in which sewage were sampled from include: Waste Water Treatment Plant (WWTP) (69 studies), parts of the sewage system (21 studies), hospitals (11 studies), university (6 studies), river/lakes (5 studies), residential (2 studies), aircraft (2 studies) and canal, cruise, airport and shopping malls/office/food market/entertainment venues (1 study each) (Table 1). Only 61 studies exclusively sampled a single type of setting: WWTP (47 studies), hospital and university (three studies each), river and residential (one study each), parts of the sewage system (six studies). The population served by each WWTP or sampling site ranged from 321 to 11,400,000 inhabitants, totaling at least 188,907,989 inhabitants (Table 1).
Raw sewage, the most commonly collected sample type, was collected singly or in combination with other sample types in 80 studies (Table 1). Nineteen studies collected wastewater effluent treated to secondary, tertiary, and post-tertiary levels and seven studies collected primary sludge samples. Three studies were unclear in reporting the sample type collected (Wurtz et al., 2021a; Medema et al., 2020a; Vallejo et al., 2020a). A total of 8078 studies clearly reported their sampling approach – grab samples only (19 studies), composite samples only (43 studies), both grab and composite sampling (18 studies). One study also conducted Moore sampling (Rafiee et al., 2021a). All but one study conducted polymerase-chain testing to ascertain sample positivity – quantitative real-time PCR (RT-qPCR) (74 studies), qualitative PCR (RT-PCR) (10 studies) and digital droplet PCR (RT-ddPCR) (seven studies). Six studies also carried out nested PCR. A total of 49 studies focused on only one genetic target for positive signals (N gene – 4332 studies, RdRp – 3 studies, Orf1ab – 2 studies and E – 1 study), while all others looked at more than one gene target. The N gene (inclusive of N/N1/N2/N3) was the most commonly targeted, being used in 70 of the included studies. Other gene targets used include envelope (E), ORF1ab, Spike (S), RNA-dependent RNA polymerase (RdRp). While only a couple of studies used primers/probe detecting the membrane (M) gene, nsp2-PLPro and ORF8ab-N (Wilton et al., 2021a; Martin et al., 2020a) (Table 1).
3.2. Positivity in wastewater samples
A total of 7644 positive samples were detected from at least 26,197 samples (29.2%) collected by 66 studies (Table 2 ). Twenty-two papers were unclear in reporting the outcome of sampling (Vallejo et al., 2020a; Wu et al., 2020a). Notably, all papers that detailed testing outcome reported sample positivity. Majority of samples reported were attributed to Peccia et al., who made 3632 positive detections in 17,661 samples (20.6%) of primary sludge (Peccia et al., 2020a). Separately, the sewage surveillance program by the New South Wales government saw the detection of positive signals at least 33 times out of 472 sampling events over three months. Raw sewage influent constituted majority of positive sample, followed by secondary and tertiary treated-effluent, effluent after ozonation and filtration, post-grit chamber influent solids, primary sludge, and river samples in no particular order (Table 2).
Table 2.
Summary of outcomes of included studies.
| Author | Viral load |
Sample positivity |
Association |
Temporal trends |
||||
|---|---|---|---|---|---|---|---|---|
| Gene copies | Ct values | Overall | Gene specific | Case trend (Y/N) |
Correlation, estimated prevalence of infection or comparison with case numbers | Trend | Duration | |
| Wu F., et al. (2020b) | N1: NR N2: NR Overall: >15 copies/mL |
NR | NR | NR | Y | No positive detection before March, correspond to absence of case reported. Low viral titer matched low community cases (2 cases in early March), exponential increase in titers matched peak in ILI (late March), decreased titers matched decline in new clinical cases (Mid-April) | Higher correlations when comparing new clinical cases back-dated by 4–10 days; maximum agreement is a 4-day time lag (consistent with 4–5 days incubation period) | 4–10 days before |
| Westhaus S., et al. (2021b) | Overall: 2.7–37 gene equivalents/mL | M: 32–35 RdRP: 33–37 |
13/13 (Influent, effluent, post-tertiary treatment, effluent after ozonation & filtration) | NR | Y | SARS-CoV-2 load correlated to cumulative and acute prevalence at the WWTP; no conclusive correlation between incidence and SARS-CoV-2 concentration | NA | NA |
| Vallejo, J. A., et al. (2020b) | Overall: <1000–~500,000 gene copies/L | NR | NR | NR | Y | - Viral load in WWTP Bens consistent with number of estimated COVID-19 cases in the metropolitan area of A Coruña, both reached lowest numbers by beginning of June; mean viral load at WWTP Bens decreased with asymptotic fashion, while estimated cases decreased approx. 6 folds in the same period - Strong linear correlation between active cases and logarithm of daily mean viral load at Bens. - Higher viral load at CHUAC (hospital) compared to WWTP Bens in mid-May |
NA | NA |
| Trottier, J., et al. (2020b) | NR | N1: 36.4 N3: 37.6 |
7/7 | NR | Y | Low detection of viral RNA in the second half of May but abnormally high COVID-19 cases (might be due to readjustment), in early June, there was increase in viral RNA detected as daily cases remained relatively low. In mid-late June, there was sharp increase in amount of detected viral RNA matched by slowly increasing COVID-19 daily case. By early July, high viral titers was consistently detected, accompanied by sharp increase in COVID-19 daily case. COVID-19 daily cases peaked in late July | Number of new COVID-19 patients surged roughly 2–3 weeks after the increase of SARS-CoV-2 RNA levels in wastewater | 2–3 weeks before |
| Sherchan, S. P., et al. (2020b) | N1: 7.5 × 10^3 copies/L N2: 3.1 × 10^3–4.3 × 10^3 copies/L Overall: 4.3 × 10^3; 3.1 × 10^3–7.5 × 10^3 copies/L |
NR | 2/15 (Influent) | N1: 1/15 N2: 2/15 |
Y | No detection in wastewater before first COVID-19 case (March 9), samples positive in April when COVID-19 cases surfaced. | NA | NA |
| Sharif, S., et al. (2020b) | NR | 32–38 (only known for 6 samples) | 21/78 | (Only known for 20 samples) OFR1ab: 6/20 N: 6/20 E: 6/20 |
Y | Detection of SARs-COV-2 RNA in wastewater samples collected from areas with recent history of COVID-19 patients | NA | NA |
| Rimoldi, S.G., et al. (2020b) | NR | NR | WWTP: 4/12 (raw) River: 4/6 |
WWTP N: 2/12 ORF1ab: 4/12 E: 3/12 River N: 4/6 ORF1ab: 4/6 E: 0/6 |
Y | Lower viral load on 22 April than 14 April, corresponds to the overall declining case trend whereby cases on 14 April higher than 22 April; study planned during first stage of epidemic decline | NA | NA |
| Randazzo, W., et al. (2020c) | N1: 5.1 ± 0.3 log10 gc/L N2: 5.5 ± 0.2 log10 gc/L N3: 5.5 ± 0.3 log10 gc/L |
N1: 34.68–37.28 N2: 35.1–38.39 N3: 35.2–38.14 Values not inclusive of SD |
37/73 (raw, secondary treated) | N1: 22/73 N2: 26/73 N3: 28/73 |
Y | Amplification signals detected in wastewaters when cases were diagnosed within the municipality. | SARS-CoV-2 detected weeks before the first confirmed case. Positive signals in wastewater found 12–16 days before COVID-19 cases were diagnosed clinically. | 12–16 days before |
| Randazzo, W., et al. (2020d) | N1: 5.55; 5.31–5.75 log10 gc/L N2: 5.73; 5.22–5.99 log10 gc/L Overall: 5.56; 5.22–5.99 log10 gc/L |
N1: 34.79; 33.87–36.02 N2: 36.95; 34.17–37.84 Overall: 35.12; 33.87–37.84 |
13/24 (raw) | N1: 12/24 N2: 13/24 |
Y | First positive sample on 24 February corresponds to incipient community cases (first local case reported on 25 Feb); local community transmission likely undergoing when the first cases were declared in Valencia. By 9 & 11 March, consistent detection in samples correspond to 50 and 76 cases in Valencia (not that high yet) | First positive sample detected 1 day before community case; wastewater viral RNA anticipated the ascent in cases (increased and plateaued faster than declared cases) | at least 1 day before |
| Prado, T., et al. (2020b) | NR | N2: 36.3–39.8 | WWTP/SS: 5/10 (raw) Hospital: 0/2 |
WWTP/SS: N2: 5/10 Hospital: N2: 0/2 |
Y | Majority of positive samples (4/5) correctly reflected neighbourhood with higher cases (n = 70), sole detection at Camboinhas suggests outbreak expansion. | NA | NA |
| Peccia, J., et al. (2020b) | 1.7 × 10^3 mL−1 to 4.6 × 10^5 mL−1 of primary sludge | Average Ct N1: 34.6 N2: 34.5 |
3632/17661 (primary sludge) | NR | N | Given the uncertainties in sludge data and epidemiological data, the study did not attempt to correlate absolute numbers of sludge SARS-CoV-2 RNA concentrations and COVID-19 cases. | Sludge results led tests by date of specimen collection by 0–2 days, hospital admissions by 1–4 days, reported positive tests by 6–8 days. | 0–2 days - before positive test by date of specimen collection 1–4 days - before hospital admissions 6–8 days - before positive tests by report date |
| Nemudryi, A., et al. (2020a) | N1: 43.5–5400.5 copies/L (mean) N2: 22.2–6116.2 copies/L (mean) |
NR | 13/17 | N1: 13/17 N2: 13/17 |
Y | After 1 month (late April - early May) of undetectable levels of SARS-CoV-2, the wastewater began testing positive again in late May, which coincided with an increase in COVID-19 cases in the community | In mid-March, SARS-CoV-2 RNA concentration in the wastewater lagged behind symptom onset data by 8 days, preceded laboratory test results for individuals by 2 days. In May, wastewater detection trailed symptom onset by 5 days and foreshadowed the increase in positive tests by 4 days. | 5–8 days after symptom onset 2–4 days before clinical PCR test results |
| Medema, G., et al. (2020b) | N1: 195; 2.6–790 gc/mL N2: 425; 12–2200 gc/mL N3: 430; 6.6–1800 gc/mL Overall N: 310; 2.6–2200 gc/mL E - NA |
N: NR E - 32.95; 29.9–39.2 |
WWTP: 17/25 (influent) Airport: 3/4 (influent) |
WWTP N1: 15/25 N2: 16/25 N3: 16/25 E: 16/25 Airport N1: 3/4 N2: 2/4 N3: 3/4 E: 2/4 |
Y | No samples were positive 3 weeks before the first cases was detected in Netherland (Feb 27). One week into the epidemic (March 4–5), samples were positive in Utrecht, Den Haag, Schipol and Amersfoort although no cases were detected in Amersfoort on March 5 but first case surfaced 6 days later. As the epidemic spread (march 18), all samples (March 15 and 16) were positive and correlated with increase in cases at different rates in each city (Tilburg > Utrecht > Amsterdam). An increase in the SARS-CoV-2 RNA concentration was observed as the number of reported COVID-19 cases increased. | Virus circulation in Amersfoort 6 days before the first cases were noted. N and E assays started to produce signals in sewage samples when the observed COVID-19 prevalence was around or <1 case in 100,000 people. | 6 days before the first case |
| Martin, J., et al. (2020b) | RdRp: <3.50–5.91 Log10 gc/L E: 4.63–5.84 Log10 gc/L |
NR | 4/5 (raw) | RdRP: 4/5 E: 2/5 nsp2-PLPro: 3/5 ORF8b-N: 4/5 |
Y | Low levels of viral RNA detected was detected in February sample; 11 days after first two COVID-19 cases were confirmed in York, northern England, and 3 days before the first case was reported in the population. The viral RNA levels found in sewage in March were higher than cases detected; most likely due to limitations in testing capacity. Large reduction of viral RNA concentration in April–May samples correlated with observed reduction in confirmed cases as lockdown was introduced from 23rd March. | Low levels of viral RNA detected 3 days before the first case was reported in the population | 3 days before the first case |
| La Rosa, G., et al. (2021b) | 1200; 290–56,000 c.g./L | NR | 15/40 (raw) | NR | Y | Samples were positive before the first autochthonous Italian case was reported (February 21). First positive sewage samples from Milan and Turin (18 Dec 2019) and Bologna (29 Jan 2020) correlated with clinical cases detected in January and February samples, except February sample from Bologna. | SARS-CoV-2 was already circulating in northern Italy at the end of 2019, moreover in different geographic regions simultaneously | 23–63 days before first case |
| La Rosa, G., et al. (2020b) | NR | NR | 6/12 (raw) | ORF1ab: 6/12 Spike: 2/12 RdRP: 0 |
Y | While infections were still limited in Milan and the first autochthonous case was reported earlier on 21 February, positive samples were detected on 24 and 28 February. Positive samples in Rome were detected on 31 March and 2 April correlating with considerable spread of the epidemic. | NR | NR |
| Kuryntseva, P., et al. (2020b) | NR | NR | City: 3/20 (sewage) School: 1/2 (sewage) |
NR | Y | Positive samples correlated to an average of 1.2% of sickness rate in the residences that use the sewage inspection chamber. While level of viral particles in March were not detectable, there were positive samples (4/11) detected in July which correlated to the growth in registered COVID-19 cases in the city. | Kazan Federal University Campus (3 buildings); Presence of viral particles in wastewater but none reported feeling sick or having a fever on the day of investigation. 1 case registered within 5 days after investigation, 3 more cases within 7 days. | 5 days before first case |
| Kumar, M., et al. (2020b) | 5.6 × 10–3.5 × 10^2 copies/L | ORFlab: 32.65–35.52 N: 34.18–35.39 S: 34.83–39.56 |
2/4 (raw) | ORF1ab: 2/4 N: 2/4 S: 2/4 |
Y | Higher detection (ten times increase) of positive samples on 27 May as opposed to 8 May which correlated with the infection numbers in the area (4912 and 10,674 cases on 8 and 27 May respectively). | NR | NR |
| Jorgensen, A., et al. (2020b) | NR | 32 (hospital sample) | WWTP: 18/51 Hospital: 6/9 |
NR | Y | High virus concentrations were detected between mid-late March which correlated with the COVID-19 peak in Denmark. Samples from Solrød were positive on 24 Feb, three days prior to the reporting of “patient zero” from another city in Denmark. Decrease in the virus load correlated with the introduction of preventive measures by the local authorities. | Virus was detected in a sample 3 days before patient zero was identified in the country. | 3 days before first case |
| Hata, A., et al. (2021b) | N: 1 × 10^0–1.3 × 10^4 copies/L N2: 1 × 10^0–1.8 × 10^4 copies/L N3: 1 × 10^0–3.5 × 10^4 copies/L Overall: 1 × 10^0–3.5 × 10^4 copies/L |
N: NIL N2: NIL N3: 31.6; 29.9–36.2 Overall: 31.6; 29.9–36.2 |
20/21 (influent) | N2: 12/21 N3: 16/21 N: 4/21 |
Y | The detection frequency seemed to be higher when the number of total confirmed SARS-CoV-2 cases in 100,000 peoples became above 10 in each prefecture. The SARS-CoV-2 detection frequency was 15% (3 positives out of 20 samples) before the number became >10, whereas it reached 57% (4 positives out of 7 samples) after the number became >10. | NR | NR |
| Hasan, S. W., et al. (2021b) | 1.49 × 10^3; 1.06 × 10^2–2.91 × 10^4 gc/L | NR | 33/45 (influent) | NR | Y | When the viral load was high in May, there were more positive wastewater samples detected. More community prevention measures in June and July, lesser cases (decreased viral load) than in May. | NR | NR |
| Haramoto E., et al. (2020b) | 2.4 × 10^3 copies/L | 39.96 | WWTP: 1/10 (secondary treated) River: 0/3 |
WWTP N: 1/10 N1: 0/10 N2: 0/10 ORF1a: 0/10 S: 0/10 River N: 0/3 N1: 0/3 N2: 0/3 ORF1a: 0/3 S: 0/3 |
Y | Correspondence between RNA detection and highest peak in daily cases in Yamanshi Prefecture; Cumulative COVID-19 cases low (4.4 cumulative cases/100,000 inhabitants) but SARS-CoV-2 RNA detected when weekly reported community cases were high. | NR | NR |
| Guerrero-Latorre, L., et al. (2020b) | N1: 2.91 × 10^6; 2.8410^5–3.19 × 10^6 gc/L N2: 8.55 × 10^5; 2.07 × 10^5–2.23 × 10^6 gc/L Overall: 1.54 × 10^6; 2.07 × 10^5–3.19 × 10^6 gc/L |
NR | 3/3 (river) | River N1: 3/3 N2: 3/3 |
Y | There were high viral loads from the samples taken in M1 (south-center city) where higher active cases were registered. | NR | NR |
| Gonzalez, R., et al. (2020b) | 10^1–10^4 copies/100 mL | NR | 150/198 (raw) | N1: 107/198 N2: 125/198 N3: 113/198 |
Y | Positive detection in at least one WWTP on all sample dates, even during the first week of sampling when there were only two cases in the region. Increased detection in Williamsburg and Nansemond (mid-March & mid-April) due to 2 different outbreaks in those areas. | NR | NR |
| Fongaro, G., et al. (2021c) | 5.74; 5.49–6.68 log10 gc/L (SD excluded) | NR | 4/6 (raw) | NR | Y | Stable viral load in November 2019–March 2020. On 4 March 2020, an increase of approximately 1 log10 of viral gene copies were detected in wastewater when the first COVID-19 case diagnosed in region. | 60 days before first case (continent) >90 days before first case (Brazil) |
60 days before first case (continent) >90 days before first case (Brazil) |
| D'Aoust, P.M., et al. (2021c) | 1.7 × 10^3–3.8 × 10^5 copies/L | NR | 11/NR (PCS, PGS) | NR | Y | Positive detection in PCS from Ottawa and Gatineau between 1 April and 30 June 2020; decreasing and continued low COVID-19 prevalence in two cities (56.7 → 4.8 and 57.3 → 10.2 confirmed cases/100 K inhabitants in Ottawa and Gatineau, respectively). No correlation between sample detection and any of the three epidemiological metrics in Ottawa while significant correlations between sample detection and number of active cases in Gatineau. Strong, significant and positive correlation between PMMoV normalized N1/2 signals and 7-day rolling average percent positive epidemiological unit in Ottawa. | NR | NR |
| Arora, S., et al. (2020b) | NR | 33.63; 16.8–37.52 | WWTP: 5/18 (influent) Hospital: 1/7 (influent) |
WWTP: RdRp: 4/18 E: 6/18 N: 5/18 ORF1ab: 0/1 S: 0/1 Hospital: RdRp: 0/6 E: 2/6 N: 2/7 ORF1ab: 1/1 S: 1/1 |
Y | Areas served by WWTPs with positive detections reported a continuous increase in confirmed positive patients soon after the first sampling. A significant increase in positive tested cases within 6–14 days of first sampling (4 May 2020) was correlated to a surge in cases at site 5 (Central jail) within six days of sampling. At site 6, the 1st sample (15 May 2020) was negative owing to low number of positive cases in area. Positive samples in the subsequent weeks correlated with the sudden surge in COVID-19 cases up to fivefold within next six days of detection | 4–15 May 2020: samples positive 10–14 days preceding a large jump in positive cases in the respective area 16 May–12 June 2020: gap between positive sewage detection and positive cases decreased from 14 days to 6 days |
6–14 days before case surge |
| Albastaki, A., et al. (2021b) | NR | 32–34, 33–36 (aircraft) | Municipal - 847/2470 (raw) Aircraft - 27/198 |
NR | Y | Decrease in SARS-CoV-2 concentration in municipal wastewater samples over study duration, corresponding to decrease in cases in the city. | NR | NR |
| Ahmed, W., et al. (2020d) | N: 49.95; 0.66–156 copies/100 mL N1: 163; 43.9–596 copies/100 mL N2: 13; 1.33–60.9 copies/100 mL E: 138; 53.6–272 copies/100 mL RT-ddPCR N1: 103–387 (83.5–387 copies/100 mL) |
RT-qPCR N: 36.6; 35.1–42.1 N1: 34; 32.1–36.8 N2: 38.2; 35.8–41.3 E: 37.7; 36.3–39.0 RT-ddPCR N1: NR |
Aircraft - 4/10 Cruiseship - 8/11 (influent, effluent) |
Aircraft N1: 0/10 N2: 0/10 N: 1/10 E: 3/10 Cruiseship N1: 8/11 N2: 4/11 N: 5/11 E: 3/11 |
Y | SARS-CoV-2 detected in airplanes but no infected passengers identified within 14 days. SARS-CoV-2 detected in cruise ship corresponded to 24 cases onboard. | NR | NR |
| Ahmed, W., et al. (2020e) | 1.9–12 copies/100 mL | N: 37.5–39 | 2/9 | N: 2/9 | N | Estimated a median number of 1090 infections on 27 March: positive sample; 297 cases in the PHNs in Brisbane and median number of 171 infections on 1 April: positive sample; 404 cases in the PHNs in Brisbane | NA | NA |
| Gonçalves J,. et al. (2021b) | NR | E: 33.61–38.45 RdRp: 29.65–38.46 Average Cq in triplicates |
10/15 | E: 7/15 RdRp: 10/15 |
Y | SARS-CoV-2 RNA was detected in hospital wastewater when only one patient was hospitalized. The sample from the 5th of June was negative for RdRP, which may have been caused by the heavy rainfall recorded on that day, which may have diluted the sample collected, or by a variable shedding rate of the patients. | RdRP gene - 1 day after the first hospitalization of one patient (4th of June). E gene - 5 days after the first patient was hospitalized (8th of June). |
1–5 days after hospitalization |
| Acosta, N., et al. (2021b) | N1: 0.12–23,861.79 copies/5 μL (avg 921.27copies/5 μL) N2: 0.096–26,881 (avg 1014) E: 8.65–20,323.42 (avg 3261.92) |
Overall: Avg (min, max) N1: 33.4 (26.1, 39.8) N2: 36.9 (27.5,44.9) E: 30.7 (25.9, 39.2) N1 median (IQR) H1: 32.5 (30.6–36.4) H2: 31.8–35.9 H3A: 32.8. (30.7–35.6) H3B: 34.6 (33.8–36.5) H3C: 36.4 (34.7–37.1) N2 median (IQR) H1: 35.3 (33.5–37.7) H2: 35.9 (34.5–37.8) H3A:36.2 (33.2–41.2) H3B:39.6 (38.1–41.7) H3C: 41.1 (39.7–41.7) E median (IQR): H1: 35.5 (31.3–37.3) H3A: 30.6(28.5–30.7) H3C:34.9 |
102/165 | N1: 102/165 N2: 79/165 E: 9/96 |
Y | SARS-CoV-2-RNA signal in wastewater increased over time in both the amount detectable and the proportion of samples that were positive, consistent with increasing cases and hospitalizations, coinciding with Calgary's COVID-19 ‘second wave’. | Significant differences in median SARS-CoV-2 N-1,2 between outbreak-free periods vs outbreak periods when measured as copies/mL and normalized for PMMoV was observed at each hospitals | 5–14 days before first hospital acquired case |
| Agrawal, S., et al. (2021c) | 1.29 × 10^12–1.63 × 10^15 copies/day | NR | 44/44 | NR | Y | Continuous increase again in COVID-19 cases in Frankfurt since August, with an average of 28.6 incidences, compared to 28.7 incidences in April. In August, a resurgence in the SARS-CoV-2 RNA load was observed, reaching 3× 10^13 copies/day, which represented similar levels compared to April with approx. 2 × 10^14 copies/day. A corresponding increase in total viral load across all samples was observed when incidence increased. In June and July, the incidences were less than ten COVID-cases per 100.000 persons per week and the load ranged between 1.29 × 10^12 and 1.91 × 10^13 copies/d. Increase in SARS-CoV-2 RNA load clearly preceded the reported cases with the first step-increase in the middle of July 2020. |
Increase in the SARS-CoV-2 RNA load clearly preceded the reported cases with the first step-increase in the middle of July 2020. | NR |
| Ahmed, W., et al. (2021b) | N1: 135–11,992 gc/100 mL E: 113 & 222 gc/100 mL |
N1: 32.8–41.46 N2: 0 E: 36.6 & 37.6 |
21/63 | N1:19/63 N2: 0/63 E: 2/63 |
Y | Wastewater samples were generally positive during the period with highest caseload data. Most SARS-CoV-2 RNA detections were in WWTP C wastewater, and were one month before and during the main wave of the pandemic. The decay of the 7-day average detections in mid-April align with the decrease of active cases; As might be expected, the 7-day average wastewater detections tracked similarly to the 7-day average GC/100 mL; however, the GC/100 mL did not appear to agree well with daily case numbers. | Detections were made in a Southern Brisbane WWTP in late February 2020, up to three weeks before the first clinical case was reported there | 2–3 weeks before the first case |
| Bar-Or, I., et al. (2021b) | NR | 27–35 | 58/58 | E: 58/58 | Y | Mutation analyses of SARS-CoV-2 in wastewater samples successfully identified the penetration of the B.1.1.7 variant into Israel in December 2020 in the Shafdan and Tzfat regions, and its spread into additional regions by February 2021, corresponding with clinical sampling results (first identified B.1.1.7 case in Israel in December 2020). Identification of B.1.1.7 variant in Tzfat in December 2020, a region in which non-sufficient clinical sampling was available, exemplifies that surveillance by wastewater is a robust approach that can cover large areas by few samples. | Penetration of the B.1.1.7 variant into Israel in December 2020 in the Shafdan and Tzfat regions, first identified case in Israel via clinical sampling and sequencing in December 2020. | NR |
| Bertrand, I., et al. (2021b) | ≥3.0 × 10^3 gc/L | NR | 12-Oct | RdRp: 9/10 E: 10/10 |
Y | A parallel decrease in cases in patients and genome concentration in wastewater was observed, confirming the link between the circulation of the virus in the human population and its presence in wastewater. | NR | NR |
| Betancourt, W., et al. (2021c) | From 1/13 dorms N1: 1.00 × 10^4–3.84 × 10^5 gc/L N2: 1.61 × 10^5–1.06 × 10^6 gc/L |
NR | 99/319 | NR | Y | On August 25, wastewater from dorm A tested positive. On August 26, antigen testing identified one positive individual (Person A) despite demonstrating no symptoms, person B with symptoms reported. Person C inconclusive by antigen test. PCR testing on 26 August revealed all three positive. 76% (sensitivity), 79.8% (PPV), 88.6% (NPV) | On August 26, wastewater from dorm A tested positive. On August 26, antigen testing identified one positive individual (Person A) despite demonstrating no symptoms. Person C inconclusive by antigen test on August 25, but tested positive on August 29. Persons A, B, C were positive by PCR testing (tested on August 26, results out several days later). | ≤4 days before first case in building |
| Bhattarai, B., et al. (2021b) | RAS: 6.89E+02 (3.33E+02)–1.59E+04 (1.03E+04) gc/L Influent: 2.38E+04 (1.83E+04)–4.60E+04 (6.97E+04) gc/L |
NR | NR | NR | Y | Overall, while positive COVID-19 cases increased significantly in most sewersheds, the gene copy number for influent and sludge samples remained relatively stable over the sampling period. Of the seven WRFs, only ECWRF exhibited a positive correlation between the influent gene copies per liter and the daily number of COVID-19 cases. | A significant correlation between SARS-CoV-2 gene copy numbers in influent and daily reported cases of COVID-19 was seen in ECWRF, after accounting for a one-week time lag. Digested sludge gene copy numbers significantly predicted the weekly number of cases with a two-week lag for all three WRFs that possessed anaerobic digesters. |
1 week before (sludge) 2 weeks before (digested sludge) |
| Black, J., et al. (2021b) | NR | NR | 71/346 | NR | Y | Probability of detection varied widely with the number of known infected people. It fell with decreasing case numbers, but was still around 10% for the presence of a single known case at any point in the illness course. Although unlikely, it provides some evidence that a single infected person in a catchment on a given day could trigger the detection of virus at the sampling site on the same day. | The odds of detection in sewage were between 5 and 20 times higher where known cases were present, with less effect of distance than time since onset. Each known SARS-CoV-2-infected person was determined on each day from two days before onset to 55 days after | 2 days before - 55 days after onset |
| Cao and Francis (2021b) | Mean (SD): 318.37 (463.98) × 10^6 gc/L Median (IQR): 137.9 (458.72) × 10^6 gc/L |
NR | NR | NR | Y | It is clear that an increase in SARS-CoV-2 concentration has been consistently associated with an increased number of COVID-19 infections (weekly). (Model) An increase in the SARS-CoV-2 concentration in the current week implies a steadily increase in the COVID-19 cases in the following three weeks and with the maximum impact occur in the third week. Then the impact will go down and eventually disappear after 6 weeks in the future. | An increase in the SARS-CoV-2 concentration in the current week implies a steadily increase in the COVID-19 cases in the following three weeks, peaking in the third week. | 3 weeks before case surge |
| Chakraborty, P., et al. (2021b) | N1: STP/SPS: 1.41 × 10^4–1.99 × 10^5 gc/L HWW: >1.19 × 10^4 gc/L |
STP and SPS overall: N1: 29.2–36.87 N2: 30.03–36.08 HWW: N1: 28.68–38.24 N2:25.85–32.07 |
12/17 (incl 0 effluent) | NR | Y | Higher viral loading in HWW samples during partial lock-down (1.10 × 105–1.62 × 105 gc/L) over post-lockdown (1.20 × 104–1.15 × 105 gc/L) can be related with the lower number of active COVID-19 patients admitted in SRM hospital during that period. The wastewater surveillance showed the presence of higher number of infected people in communities with high population density. | NR | NR |
| Chavarria-Miró, G., et al. (2021b) | NR | NR | NR | NR | Y | In WWTP 2, the analysis of archival samples revealed the increasing occurrence of SARS-CoV-2 genomes in samples from 15 January to 4 March employing the IP2, IP4, and E targets. Of note, SARS-CoV-2 was detected in sewage 41 days (15 January) ahead of the declaration of the first COVID-19 case (25 February), clearly evidencing the validity of wastewater surveillance to anticipate cases in the population. Progressive decline in genome copy numbers in both WWTPs paralleled the diminution in the estimated cumulative number of shedders, based on the actual number of reported symptomatic cases and figures for 7-day, 14-day, and 21-day excretion periods before the sampling date; evidences the effect of lockdown measures on the spread of the infection. |
Of note, SARS-CoV-2 was detected in sewage 41 days (15 January) ahead of the declaration of the first COVID-19 case (25 February). | 41 days before first case |
| Ciesielski, M., et al. (2021b) | RT-ddPCR: 0–3.2E+05 copies/L RT-qPCR: 0–1.16E+06 copies/L |
N2: RT-ddPCR: 0–3.2E+05 copies/L RT-qPCR: 0–1.16E+06 copies/L |
NR | N2: 39/60 | N | NR | NR | NR |
| Colosi, L., et al. (2021b) | NR | Wastewater (influent) Hospital N1: 30.6–41.9 N2: 32.5–41.2 WWTP N1: 30.3–34.2 N2: 31.2–35.2 School dormitory N1: 32.6–38.1 N2: 35.1–41.5 Primary solids WWTP N1: 30.8–36.2 N2: 32.4–39.2 |
Wastewater (Influent) Hospital: 11/11 WWTP: 2/3 School dorms: 15/29 Primary solids WWTP: 3/3 Overall: 31/46 |
NR | Y | Wastewater results are highly consistent with known presence or absence of COVID-19 cases detected via clinical testing. Across all hospital and dorm samples collected after completion of the side-by-side method comparison, outcomes were as follows: 25 true positives, 0 false positives, 9 true negatives, and 1 apparent false negative. Corresponding sensitivity was 96.2% and specificity was 100% (94.74% and 100%, respectively, if duplicate samples are excluded). There was a very weak negative correlation between CT values and known hospital case counts by date for both the N1 and N2 amplicons. | Interval between successive clinical and wastewater-based testing was never more than 8 days. Positive wastewater results were obtained 8 days after the first positive clinical results and 5 days after the second set of clinical positive results. | 5–8 days after sample positivity |
| D'Aoust, P., et al. (2021d) | N1 and N2: below 1.00 × 10–4 copies/copies PMMoV An absolute increase of 2.03 × 10–4 and 3.01 × 10–4 copies/copies PMMoV is reported during this period of increase for N1 and N2 gene regions |
NR | NR | NR | Y | Positive correlations were observed between the normalized viral RNA signal (N1 and N2) and both the number of new daily positive COVID-19 cases and clinical testing percent positivity. A weak positive correlation was also seen between N1 and N2 SARS-CoV-2 PMMoV-normalized RNA signal and COVID-19-caused hospitalizations | A times-step correlation analysis suggests that increases in SARS-CoV-2 signal in wastewater precedes increases in new daily positive COVID-19 cases and clinical testing percent positivity by two days. Furthermore, it also suggests that increases in SARS-CoV-2 signal in wastewater precedes increases in the number of hospitalized cases by four days | 2 days before case resurgence 4 days before hospitalization increased rates |
| Fernandez-Cassi, X., et al. (2021b) | NR | NR | NR | NR | Y | RNA (N1) load generally corresponded to confirmed cases in the regions over sampling period. Wastewater-derived incidence (model) outperformed case numbers with respect to the timing and shape of the peak incidence, whereas confirmed case numbers were a better indicator for incidence decline. | NR | NR |
| Fongaro, G., et al. (2021d) | Sewage: average of 1.3 × 10^4–4.3 × 10^4 gc/mL Downstream river water: average 1.1 × 10^2–1.7 × 10^3 gc/mL Upstream river water: ND |
NR | NR | NR | N | NR | NR | NR |
| Gerrity, D., et al. (2021b) | N1: 3.6 10^4 gc/L N2: 1.2 10^5 gc/L Overall: 10^4–10^6 gc/L |
N1: 33.8 ± 1.4 N2: 35.1 ± 1.6 E_Sarbeco: 35.2 ± 1.1 orf1a: 33.3 ± 1.8 |
NR | N1: 30/56 N2: 26/56 E_Sarbeco: 9/56 orf1a: 4/56 |
Y | Consistent wastewater detections of SARS-CoV-2 occurred once the confirmed daily case load and cumulative cases exceeded 0.001% relative incidence and 0.01% relative prevalence, respectively. The strongest observed wastewater signal, as determined by the number of positive assays and replicate reactions, occurred when the confirmed daily case load and cumulative cases reached 0.01% relative incidence and 0.1% relative prevalence, respectively, which corresponds with a model concentration of nearly 105 gc/L. | NR | NR |
| Gibas, C., et al. (2021b) | 394–2,990,271 copies/L | 28.38–45.21 | 40/332 | N1: 40/332 | Y | The number of new daily positive cases in Mecklenburg County as a whole and the number of buildings detected as positive on each sampling event are correlated over this time frame. | NR | NR |
| Giraud-Billoud, M., et al. (2021b) | N1: 3.97 ∗ 10^2–1.05 ∗ 10^5 gc/100 mL (avg 1.03 ∗ 10^4) N2: 0.5 ∗ 10^2–1.45 ∗ 10^5 gc/100 mL (avg 1.49 ∗ 10^4) |
N1: 30.7–38.3 (avg 34.4) N2: 30.8–40 (avg 34.6) |
39/46 | N1: 32/46 N2: 37/46 |
Y | Wastewater samples were not detectable in July when the weekly cases were 356. The samples were positive for SARS-CoV-2 RNA genetic markers on August 3rd and 11th before the biweekly doubling of weekly COVID-19 cases. Between September and the end of October, when the cases were high, wastewater samples were positive for N1 and N2 consecutively. WW samples from EP remained detectable between late November and December, when only 140 and 290 weekly COVID-19 cases were reported. | N2 was the first marker to become positive on July 30, 2020. N1 and N2, showed the highest concentration when the cases were at its peak. Wastewater samples were detectable before the exponential growth phase of weekly COVID-19 cases. As cases diminished from November 9 to November 29, wastewater samples remained positive. In addition, the N2 marker showed a second peak in the week of November 2–8, 2020. | NR |
| Graham, K., et al. (2021b) | NR | NR | NR | N1: 79/96 N2: 68/96 |
Y | Positive association between N1 and N2 in solids and the number of new COVID-19 infections. The concentrations of N1 and N2 tracked the reporting of new cases in the sewershed (based on specimen collection date) when accounting for autocorrelation and technical errors associated with the wastewater measurements. Down sampling wastewater data collection and analysis frequency to twice per week yields significant associations between case counts and wastewater concentrations, not fortnightly or once a week. | Our downsampling analysis suggests that sampling solids twice per week would be frequent enough to identify the global trends in the clinical case data. | NR |
| Hillary, L., et al. (2021b) | <1.2 × 10^3–1.5 × 10^4 gc/100 mL | NR | NR | NR | Y | Drop in wastewater SARS-CoV-2 RNA concentration, new positive clinical tests and COVID-19 related deaths following the imposition of the UK-wide lockdown in late March 2020. WWTPs in Manchester, Liverpool and the Wirral showed strong correlations between SARS-CoV-2 RNA concentration and daily positive tests. Negative correlations were also observed between viral concentrations in all sites and time following the implementation of national lockdown, except Cardiff, indicating these measures lowered the prevalence of the virus in local populations. | If only considering daily clinical testing data, the SARS-CoV-2 wastewater RNA concentration leads testing data by 2–4 days but this can be extended by approximately 1 day by using a rolling sum of positive clinical test cases over a series of days leading up to the clinical testing date being considered. The overall effect of varying these parameters is minimal since correlation coefficients stay between 0.8 and 0.9 over a range of permutations. | 2–4 days before testing |
| Hokajärvi, A., et al. (2021b) | E: mean 2.8–3.2 log10 copies/100 mL N2: mean 3.6–4 log10 copies/100 mL |
NR | 2-Feb | E: 2/2 N2: 2/2 |
Y | The presence of SARS-CoV-2 in wastewater influent samples collected during April and May 2020 were in agreement with confirmed COVID-19 cases in the municipalities of the Viikinmäki WWTP sewerage network area preceding the sampling. | NR | NR |
| Hong, P., et al. (2021b) | Overall: 81.1 ± 11.0–1327.4 ± 176.6 gc/L Untreated wastewater N1: 173.7 ± 32.2 gc/L N2: 772.1 ± 172.5 gc/L N3: 1327.4 ± 176.6 gc/L Partially treated wastewater N1: 81.1 ± 11.0 gc/L N2: 1115.8 ± 173.1 gc/L N3: 411.2 gc/L |
NR | 43/57 | N1: 31/57 N2: 26/57 N3: 23/57 |
Y | Despite presence of infected patients since the first sampling date, SARS-CoV-2 was not detected in untreated wastewaters sampled on 15, 16, 22 and April 24, 2020. A relatively consistent detection of the SARS-CoV-2 N1 gene was only observed after April 27, 2020. N1 and N2 gene exhibited a weak positive correlation, but there was no apparent correlation between abundance of N3 genes and the number of hospitalized patients. An estimated range of 253–409 positive cases out of 10,000 persons is required prior to detecting SARS-CoV-2 RNA in wastewater. |
NR | NR |
| Johnson, R., et al. (2021b) | Overall: 4.6 × 10^3–454 × 10^3 copies/mL N1: ~5 × 10^3 - ~400 × 10^3 copies/mL N2: 4.6 × 10^3–454 × 10^3 copies/mL |
Overall: 29–32 | 5-May | N1: 5/5 N2: 5/5 |
Y | The number of confirmed COVID-19 cases on 18 June 2020 in the Khayelitsha subdistrict (one of the areas served by the Zandvliet WWTP) was higher (n = 80) than in the Stellenbosch municipality (n = 43) which corresponded with the SARS-CoV-2 data from Zandvliet and Stellenbosch WWTP. | NR | NR |
| Karthikeyan, S., et al. (2021b) | 487,224.07–4,303,251.63 gc/L | NR | 94/94 | NR | Y | Peaks in the wastewater data were frequently followed by peaks in the clinically confirmed cases at a later date; suggests a correlation between wastewater and the number of new cases with the caveat of a time delay, where the wastewater data predicts future trends in the new number of cases. SARS-CoV-2 viral gene copies correlated with the daily hospital caseload; suggesting wastewater data could at least be used to identify the peak. | Forecasts successfully captured general trends on the number of new cases (shown here up to 3 weeks in advance), ultimately reinforcing that wastewater analysis can be expository of previously undetected SARS-CoV-2 infections in the population. | 3 weeks before |
| Kitamura, K., et al. (2021b) | Overall: 1.1 × 10^2–1.3 × 10^4 gene copies/L | NR | 18/32 | NIID_N2: 13/32 CDC_N1N2: 17/32 |
Y | Spearman's correlation analysis revealed a significant correlation between the number of COVID-19 cases and viral RNA concentration in WWTP B. No SARS-CoV-2 RNA was detected within the first two weeks of the sample collection period (June 9–17) at any of the three collection sites. The first detection was made within the third week, on June 23, corresponded to a sample from Manhole A. Viral RNAs were continuously detected in samples from Manhole A and WWTP B after the third and sixth weeks, respectively. Notably, WWTP B is downstream of Manhole A and is located in a high COVID-19 prevalence area. Conversely, WWTP C is located in a low COVID 19 prevalence area, and samples from this WWTP rarely showed the presence of SARS-CoV-2 RNA. | NR | NR |
| Koureas, M., et al. (2021b) | 12–8000 copies/mL | NR | NR | NR | Y | Wastewater based predictions seem to capture short term changes in disease incidence and resembled the epidemic curve in both municipalities. Considerable deviations between actual and predicted cases were observed in certain periods (e.g., January), while very high accuracy was observed during the first epidemic wave in both municipalities (from October until December). | Our results indicate that estimating cumulative cases choosing a 7-day window is feasible. | NR |
| Kumar, M., et al. (2021c) | Overall S: average ~1223 copies/L (21–4159 copies/L) N: average ~1022 copies/L (22–6768 copies/L) ORF 1 ab: average ~485 copies/L (34–2441 copies/L) Wards S: 42–4159 copies/L N: 22–6768 copies/L ORF 1 ab: 34–2441 copies/L Academic institute S: 21–2976 copies/L N: 101–2357 copies/L ORF 1 ab: 43–1476 copies/L |
Average values: S: 32.66 N: 33.03 ORF 1 ab: 33.95 |
Overall: 39/43 Wards: 32/33 Academic institute: 7/10 |
Overall: N: 41/43 ORF: 39/43 S: 39/43 Ward: N: 33/33 ORF: 32/33 S: 32/33 Academic institute: N: 8/10 ORF: 7/10 S: 7/10 |
Y | A comparison of monthly variation demonstrated higher SARS-CoV-2 genome concentration in September (~925 copies/L) than August (~898 copies/L) in line with the ~2.2-fold rise in the number of confirmed cases during the study period | Examining the potential of WBE for COVID-19 surveillance as a potential tool showed that the percentage change in genome concentration level on a particular date was in conjunction with the confirmed cases registered 1–2 weeks later on a temporal scale by the regulatory authority based on clinical tests | 1–2 weeks before |
| Kumar, M., et al. (2021d) | Overall: 0.13 × 10^2–1049.2 × 10^2 copies/L N: 0.15 × 10^2–522.7 × 10^2 copies/L ORF1ab: 0.13 × 10^2–1049.2 × 10^2 copies/L S: 0.15 × 10^2–374.5 × 10^2 copies/L |
Average N: 32.50 ORF 1ab: 32.36 S: 33.85 |
111/116 | N: 109/116 ORF1ab: 109/116 S: 109/116 |
Y | Monthly variation depicted a significant decline in gene concentration in October compared to September 2020, followed by a sharp increment in November 2020. The PCR products and effective gene concentration for all three genes were maximum in wastewater samples of November, followed by September and October, in line with a ~1.5-fold rise in the number of confirmed cases during the study period (3rd September 2020 and 26th November 2020). The linear regression between changes in SARS-CoV-2 effective gene concentration and the number of confirmed cases showed a positive but statistically insignificant correlation. |
Percentage change in effective gene concentration level on a particular date was in conjunction with the confirmed cases registered 1–2 weeks later; relationship between percentage changes in effective gene concentration and confirmed cases that can be used as a pre-alarming tool, which gives a lead of ~2 weeks for the upcoming scenario. | 1–2 weeks before |
| Li, B., et al. (2021b) | Liquid fractions N1: 10^3.0–10^5.1 GC/L N2: 10^1.2–10^4.5 GC/L E: 10^2.0–10^4.5 GC/L Solid fractions N1: 10^4.1–10^5.5 GC/g N2: 10^1.5–10^6.0 GC/g E: 10^1.4–10^6.2 GC/g |
NR | NR | NR | Y | Corresponding to the decrease in new clinical cases resulting from the lockdown, the measured SARS-CoV-2 RNA concentration in the wastewater samples also exhibited an overall downward trend and significant concentration fluctuations in both WWTPs. | detected significant daily fluctuation of the wastewater SARS-CoV-2 RNA abundance, even within the same weeks, indicating the fine-scale temporal dynamics of SARS-CoV-2 RNA in wastewater needs to be taken into consideration in designing and implementing wastewater-based surveillance | NR |
| Melvin, R., et al. (2021b) | NR | NR | NR | NR | Y | The clinical data for the state showed that SARS-CoV-2 virus was present in late Spring and then began to decrease until around mid-July, but then it steadily increased through August. Similar trends were observed for indexed wastewater SARS-CoV-2 RNA in each region across Minnesota, with the exceptions of the South East and South West regions. Interestingly, indexed wastewater SARS-CoV-2 RNA data pooled for the entire state had a similar pattern to clinical cases of COVID-19 around the same period, except it appeared to be slightly offset. Levels in wastewater samples (indexed SARS-CoV-2) appeared to increase by mid-June, preceding the apparent increase in new clinical cases. This trend was recapitulated in most of the other regions of Minnesota, expect for the South West and South East regions. Pearson correlation coefficient comparing N1 to new cases was 0.38, that comparing N2 to new cases was 0.38, and that comparing the mean of N1 and N2 to new cases was 0.39. |
Statewide, lag analysis showed that wastewater predicted confirmed new cases by 15 d for N1 and 17 d for N2. The predictive window when Melvin's Indices for N1 and N2 were averaged was 17 d. Lag analysis had less predictive power when regions were considered. | 15–17 days before |
| Mondal, S., et al. (2021b) | NR | NR | NR | N1: 36/36 N2: 36/36 E: 36/36 |
Y | The peak of SARS-CoV-2 genetic signal observed in the wastewater is concurrent with the peak of positive SARS-CoV-2 reported in mid-November of 2020 | Concurrent peak with 7 day moving average | 7 days |
| Mota, C., et al. (2021b) | N1: 56–214,430 gc/L | NR | 202/202 | N1: 202/202 | Y | The four-week cumulative number of individuals seeking health care with COVID-19 symptoms and the load of SARS-CoV-2 RNA in sewage followed similar trends, with sharp increases in weeks 24 to 26, followed by surges and later decreases in weeks 27 to 32. Local restrictions had a strong effect on hospital bed occupation, as well as suspected COVID-19 cases and SARS CoV-2 RNA loads in sewage. | NR | NR |
| Pillay, L., et al. (2021b) | N2: average 0–7.32 × 10^5 copies/100 mL | NR | 39/42 | N2: 39/42 | Y | The increase in clinical cases during the period of 9th–12th August 2020, corroborated with an increase in the SARS-CoV-2 loads in wastewater. This trend continued to rise and reaching its peak on 18th August 2020. | NR | NR |
| Prado, T., et al. (2021b) | 3.07–7.12 log10 gc/100 mL | NR | 188/223 | N2: 188/223 | Y | High titers of SARS-CoV-2 RNA in sewage samples coincided with the period when the city registered the highest numbers of COVID-19 cases and related deaths (end of April to mid-June). | NR | NR |
| Rafiee, M., et al. (2021b) | NR | N: 29.59–39 ORF1ab: 31.06–40 |
34/34 | N: 34/34 ORF1ab: 31/34 |
Y | As expected, the temporal variations on November 2020 and May 2021 did not reach statistical significance (p = 0.074). These results are consistent with the so-called second and fourth epidemic waves in Iran (Iran Ministry of Health and Medical Education, 2020). | NR | NR |
| Róka, E., et al. (2021a) | N1: 8 × 10^11–1.93 × 10^14 gc/day (up to 7.14 × 105 GC/L) Hospital building: 3.27 × 105 GC/L |
NR | NR | NR | Y | First positive signals of SARS-CoV-2 in sewage were detected 2 weeks before case numbers started to rise. From week 34, new cases doubled every week, and the viral titres also increased exponentially. Viral load correlated to the number of new cases on the week of sampling and the active cases on the next week. Highest correlation was seen at +3 and +11 days for new cases and active cases, respectively. The correlation was more pronounced in the uprising phase of infection. No clear association was seen when the number of cases plateaued. Correlation to hospitalisations and deaths were low. | Viral load correlated to the number of new cases on the week of sampling and the active cases on the next week. Highest correlation was seen at +3 and +11 days for new cases and active cases, respectively. | 2 weeks before case surge |
| Rusiñol, M., et al. (2021b) | N1: 2.18 × 10–3.92 × 10^6 gc/L N2: 9.24–2.82 × 10^6 gc/L Overall: 9.24–3.92 × 10^6 gc/L |
NR | N1: 118/184 N2: 102/184 Overall: 128/184 |
NR | Y | The highest concentration of SARS-CoV-2 in WW matched the infection peak for the first wave of the pandemic in Barcelona. While the second wave of the pandemic (July–onwards) showed higher recorded cases but the concentration of N1 in WW was lower than that recorded in the first wave, suggesting under-diagnosis in the first wave. The efficacy of the lockdown measures implemented nationwide were reflected by WW monitoring, with viral load decreasing with daily new cases and prevalence estimates. Besides the incidence level, concentrations of SARS-CoV-2 (N1 and N2) were consistently higher in larger facilities compared with smallest plants. During the first weeks of the second wave, no SARS-CoV-2 RNA was detected in the smallest WW facilities, it was only when the infection rate relative to population was higher than ever, SARS-CoV-2 was detected and quantified in their WW samples. In all 10 WWTPs, WW loads are congruent with estimations of active cases within the sewershed. The lowest incidence of infection which resulted in a quantifiable concentration of SARS-CoV-2 in WW for medium sized WWTPs was 0.11 cases/1000 inh. while for small WWTPs this was 0.82 cases/1000 inh. | Concentrations of SARS-CoV-2 (N1 assay) in WW showed good correlations with the number of cases that would be diagnosed from 0 to 7 days after sampling. | ≤1 week before |
| Saguti, F., et al. (2021b) | Influent RdRp: <3–16 log10 gc/L Treated effluent RdRp: 0.14–1.87 log10 gc/L |
NR | 18/35 | RdRp: 18/35 | Y | The four peaks of SARS-CoV-2 during the sampling period coincided with the peaks of SARS-CoV-2 in treated effluent wastewater with a 4-log10 reduction compared to the influent wastewater. The SARS-CoV-2 peaks in wastewater preceded the peaks of number of hospitalized patients with COVID-19 at Sahlgrenska University Hospital by three to four weeks. | Correlation between the increase in concentrations of viral genomes in wastewater and the number of newly hospitalized patients with COVID-19 with the former preceding the latter by three to four weeks. | 3–4 weeks before hospitalisations |
| Saththasivam, J., et al. (2021b) | N1: 7889–542,056 copies/L N2: 14,980–485,050 copies/L |
N1: 27.5–32.4 N2: 27.9–33.3 Overall: 27.7–33.3 |
43/43 | N1: 43/43 N2: 43/43 |
Y | Daily reported SARS-CoV-2 positive cases decreased by over 66% during the study time period which was mirrored by declining CRNA trends observed in all the WWTPs. A momentary surge in the CRNA was observed during the first two weeks of August 2020 which coincided with the rise in the daily reported cases. | Overall, the estimated infected population dropped approximately 17-fold the numbers of daily positive cases in Qatar fell by 66.4% between the third week of June and the end of August 2020. Results did not indicate that SARS-CoV-2 had appeared earlier in the wastewater than the confirmed virological laboratory testing. | 0 days before |
| Scott, L., et al. (2021b) | N1: 2.25 × 10–5.27–10^3 gc/100 mL (average 1.75 × 10^3 copies/100 mL) N2: 8.16 × 10–3.91 × 10^4 gc/100 mL (average 6.76 × 10^3 copies/100 mL) Overall: 2.25 × 10–3.91 × 10^4 gc/100 mL |
NR | 60/110 | N1: 58/110 N2: 58/110 |
Y | Concentrations of N1 and N2 genes in wastewater were significantly correlated with the number of COVID-19 cases detected in a dormitory while the type of dormitory was significantly associated with the frequency of the detection of N1 and N2 genes; association stronger when both genes were analyzed together; Together, the N1 and N2 genes had a negative predictive power of 61.3% and a positive predictive power of 71.4%. Highest concentration detection in samples from the isolation dormitory for COVID-19 patients. N1 was a more reliable indicator of presence or absence of cases while N2 was best in predicting number of cases in a single dormitory. |
Temporal trends of SARS-CoV-2 in wastewater, particularly, after human fecal marker normalization, mirrored trends in COVID-19 case data. | NR |
| Sharma, D., et al. (2021b) | NR | E: 31.92–43.54 RdRP: 30.46–42.19 |
20-Dec | E: 12/12 RdRp: 12/12 |
Y | None of the sewage samples (from any site) collected before March 20, 2020 showed amplification of E and RdRp genes of SARS-CoV-2 RNA. All sewage samples collected in May 2020 (except one sample from Kurla) were positive for SARS-CoV-2, just as cases exceeded 1000 cases in 5 of 6 regions sampled. | NR | NR |
| Spurbeck, R., et al. (2021b) | N1 Overall: 1–110.6 gc/mL Hospital 1 N1: 1–2.3 gc/mL Hospital 2 N1: 9–110.6 gc/mL Nursing home N1: 0.6 gc/mL Community N1: 2.9–39.1 gc/mL |
NR | 12-Sep | N1: 9/12 Hospital: 5/6 Nursing Home: 1/3 Residential: 3/3 |
Y | For Hospital V and Nursing Home N, and a weak positive correlation was observed between the number of infected and viral load in the wastewater. Data from Hospital P and Community C were not available for correlation. | NR | NR |
| Tanhaei, M., et al. (2021b) | N: 7.18E + 01 to 1.09E + 03 GC/mL | ORF1ab: 29.62–34.45 N: 27.60–30.53 |
11-Sep | ORF1ab - 9/9 N - 9/9 |
Y | Comparison of the average level of SARS-CoV-2 genomes in the last wastewater samples with the earlier wastewater samples confirmed that the increase of viral genome in effluent perfectly monitored the increase in the number of new cases confirmed in the country on the onset of the second peak of COVID-19. | NR | NR |
| Tomasino, M., et al. (2021b) | 2.05–1104.9 ng/μL | Overall: 28.3–43.9 | 16/68 | NR | Y | From week 21 (2020/09/30) onwards, we started detecting SARS-CoV-2 RNA in both WWTPs and on week 23 (2020/10/14) we detected SARS-Cov-2 RNA for the first time in all samples collected for this study. The improved detection in mid-October was concomitant with an increase in the number of COVID-19 cases in the region. Good fit between the SARS-CoV-2 gene copy numbers in the analyzed wastewater and the COVID-19 incidence peaks in the region were observed, especially with the peak observed in mid-November. The highest SARS-CoV-2 copy numbers observed for both WWTPs in the liquid and solid phases were coincide with the mid-November peak, with the exception of a few higher values observed during the mid-January peak in the liquid phase of Sobreiras WWTP. Significant positive relationships between the SARS-CoV-2 copy numbers and the weekly moving average of COVID-19 cases in both phases of both WWTPs. |
NR | NR |
| Wannigama, D., et al. (2021b) | Overall: 22.86–967.926 copies/mL | NR | NR | NR | Y | In December, the number of SARS-CoV-2 RNA copies from the city center and suburbs in wastewater increased, reflecting the increased number of positive SARS-CoV-2 patients (previously 0 in Bangkok and nearby suburbs); higher copy number of the viral genome could correlate with an elevation in infected people in the community. | NA | NA |
| Weidhaas, J., et al. (2021b) | 0.6 (1.7) gc/mL–1038 (1294) × 10^3 gc/mL | NR | 109/242 | NR | Y | Communities with higher confirmed COVID-19 caseloads tended to have higher SARS-CoV-2 MVGC/cap/d in wastewater while the SARS-CoV-2 wastewater loads in ECWRF decreased over the nine-week observation period as the COVID-19 case rates dropped. A sharp increase in viral loads was seen from 2 WWTPs just prior just prior to increases in their weekly COVID-19 case rates. While the overall estimated number of SARS-CoV-2 shedders in each sewershed was found to be linearly correlated with the cumulative diagnosed COVID-19 cases, the daily estimated number of SARSCoV-2 shedding individuals did not correlate with daily COVID-19 cases |
When a one-week lag was applied to the weekly COVID-19 case rates, the ECWRF virus RNA in wastewater did correlate with the COVID-19 case rates, suggesting that the increase in case counts may occur concurrently or precede the increase in SARS-CoV-2 RNA in wastewater, while the decline in SARS-CoV-2 RNA in wastewater may lag the decline in case counts. | NR |
| Wilder, M., et al. (2021b) | Average: 2.16 × 10^4 (2.11 × 10^4) gc/L Maximum: 1.02 × 10^5 (7.96 × 10^3) gc/L |
NR | 111/169 | RdRp: 111/169 | Y | Samples with quantifiable levels of SARS-CoV-2 RNA were associated with higher levels of positive test results the week following sampling. Over the seven days following wastewater sample collection, both the average number of new positive tests per 10,000 persons and the testing positivity rate (among people contributing to a sewershed in 7 days after sampling) were significantly higher in quantifiable samples than in samples classified as BLOD or DNQ for SARS-CoV-2. Significant relationship between SARS-CoV-2 RNA (normalized by crAssphage DNA) and the number of positive tests per 10,000 population. |
Significant relationship between the SARS-CoV-2 (normalized by crAssphage DNA) and the number of positive tests per 10,000 population the week following sampling. | 7 days before cases/10,000 population |
| Wilton, T., et al. (2021b) | NR | NR | 13/14 | RdRp: 12/14 ORF8n-N: 13/14 |
Y | SARS-CoV-2 RNA in sewage samples from London throughout the COVID-19 pandemic, with changes in viral RNA levels shown to be in good agreement with those of confirmed COVID-19 cases reflecting national restrictions. | NA | NA |
| Wong, J., et al. (2021b) | 600–246,700 copies/L | NR | NR | NR | Y | The index case (Case 1) returned from the healthcare facility on 8 July 2020 after clinical recovery. Therefore, wastewater signals detected on 9 July 2020 could be either due to viral shedding from the newly identified case (Case 10) or from the returned index case. SARS-CoV-2 levels declined after the newly identified case was conveyed to a healthcare facility (1100–39,400 to 600–1700 RNA copies/L sewage from 10 to 13 July 2020). SARS-CoV-2 levels increased again during 15–16 July 2020 (3900–28,300 RNA copies/L sewage) and was attributed to two recovered cases who returned on 14 July 2020. SARS-CoV-2 levels were not detected from the evening of 19 July 2020, corroborating the viral shedding pattern of recovered cases and the cessation of active virus transmission in the building. | 3–4 days before case was symptomatic | 3–4 days before case was symptomatic |
| Wu, F., et al. (2021b) | 2.47–16,878.71 copies/mL | NR | 830/1751 | NR | Y | A weak positive correlation was found between the incidence of daily new cases at the county level, and the wastewater viral titers at the catchment. A linear relationship was observed between the total viral load and catchment size-normalized daily new cases. | Mean viral titers increased from early March and became relatively stable between late March to late April, followed by a small downward trend until June 2, mirroring the trends of clinical new cases and deaths at the national level, and precedes clinical data. Wastewater viral titers also reflected, and seemed to precede, the rise and fall of hospitalization and intensive care unit admissions. | NR |
| Wurtz, N., et al. (2021b) | <300–9000 copies/mL | NR | NR | NR | Y | When looking at the evolution between the number of SARS CoV- 2 copies in wastewater and the number of SARS-CoV-2 positive cases, from 1 July to 1 September, the amount of virus in the sewer increases as does the number of positive cases. Then, while the number of positive patients stagnates, the amount of virus in the sewer drops from 1 to 23 September. In this phase, a discrepancy between the number of SARS-CoV-2 positive cases and the amount of virus in the wastewater was observed. From September 24, a perfect correspondence was observed between the number of positive cases and the amount of virus observed in the sewers, with a peak observed on October 22. Then, a decrease in the amount of virus was observed in wastewater, in agreement with the decrease in the number of SARS-CoV-2 positive cases. The cross correlation testing of the curves shows a maximal correlation with a lag = 0. | NR | NR |
| Wurtzer, S., et al. (2020b) | Max: 2.5 × 10^6 genome units/L | NR | NR | NR | Y | The shape of the concentration curve was reminiscent of the disease dynamics at the regional level. Observed delay between epidemiological curves in humans compared to viral RNA quantification in WW. | Viral concentration similar to the number of daily positive cases, with an 8-day temporal shift. | 8 days before |
| Xiao, A., et al. (2021b) | NR | NR | NR | NR | Y | Wastewater viral titers generally mirrored trends in disease incidence: an exponential rise from March to mid-April, a decline through July, a slow increase over the summer, followed by a sharper increase in the fall and second peak in the winter. Trends in wastewater data differed from clinical data after some key events; a short peak in wastewater viral titers at the start of August, which was only slightly reflected in the clinical data; after colleges and universities welcomed students back in late August/early September, we observed another peak in wastewater viral titers, but not in clinical cases; after the start of Phase 3 Step 2 reopening, wastewater viral titers increased steeply, while clinical cases had a shallower slope. WW data more sensitive than clinical data. | The relationship and time lag between wastewater viral titers and positive clinical tests changed over the course of the pandemic. Wastewater was more effective as a leading indicator in the first wave of the pandemic, and this early warning effect diminished drastically in the second wave, perhaps as clinical testing availability increased. Delay between wastewater data and clinical reporting shrank from average of 6.2 days to no significant difference between the wastewater and clinical trend after August 15, 2020. | 6.2 days before in early stage of pandemic (low testing capacity) |
| Xu, X., et al. (2021b) | N: 0.3–1975 copies/mL | NR | 23/107 | Hospital N: 7/12 Individual buildings N: 4/8 WWTP/sewer network N: 12/87 Total N: 23/107 |
Y | Hospital A strikingly high level of SARS-CoV-2 concentration was observed in the sewage directly collected from the manhole of the isolation ward of the COVID-19 hospital. Residential buildings SARS CoV-2 was detected in sewage samples, 2 days before COVID-19 was first diagnosed in two individual buildings of the housing estates located in the Wong Tai Sin district. WWTP Although the association between sewage measurements of SARS CoV-2 and clinically reported COVID-19 cases has been observed in sewage treatment facilities, a similar relationship was not observed in this study. |
SARS CoV-2 was detected in sewage samples 2 days before COVID-19 was first diagnosed in two individual building, community testing for these buildings only began on 27 July, after the positive sewage tests were discovered. | 2 days before first case in building |
| Yaniv, K., et al. (2021b) | N1: 3.32 × 10^6–3.71 × 10^7 copies/L | N1: 33.18–36.66 | 19-Sep | N1: 9/19 | Y | In May, no relationship was observed between wastewater signals and reported COVID-19 cases. The low morbidity in the city reported during May, particularly at the end of the month, might have been an underestimation of COVID-19 cases due to limited testing and untested asymptomatic cases. Despite no new COVID-19 positive cases reported in Ashkelon, there were traces of the SARS-CoV-2 RNA in sewage originating from the different sampling manholes in the city. A positive SARS-CoV-2 RNA signal was detected in the city sewage system about one week prior to July COVID-19 initial outbreak of the second wave of in the city. A resurgence in COVID-19 cases became evident in the city's population in June–July 2020 a month following the wastewater sampling. | A positive SARS-CoV-2 RNA signal was detected in the city sewage system about one week prior to July COVID-19 initial outbreak of the second wave of in the city. | 7 days before second outbreak |
| NSW Government (2020a) | NR | NR | 17/312 | NR | N | Detections from North Richmond and West Camden not associated with previously reported cases (only one case in West Camden WWTP area since 3 October); suggest recently recovered or undiagnosed cases | NA | NA |
| NSW Government (2020b) | NR | NR | 4/52 | NR | N | Sites with positive detections had previously reported cases, i.e. positive detection at West Camden WWTP; recent cases identified due to increased testing | NR | NA |
| NSW Government (2020c) | NR | NR | 6/55 | NR | N | Sites with positive detections had previously reported cases, i.e. positive detection at West Camden WWTP; recent cases identified due to increased testing | NR | NA |
| NSW Government (2020d) | NR | NR | 6/53 | NR | Y | All detections apart from Bathurst were associated with reported cases | Positive sample not linked to reported cases in Bathurst | NA |
| Kenyon College (Gambier Wastewater SARS-CoV-2 Virus Report, n.d.) | NR | NR | NR | NR | NR | NR | Wastewater signal often precede individual clinical tests by 2 weeks; likely that wastewater reveals earlier cases missed by clinical qPCR (false negative rate = 30%) | 2 weeks before |
Positive samples were detected in various settings – WWTP, parts of the sewage system, hospitals, rivers, aircrafts and airport. In settings within the conventional sewage system, 7180 samples were positive (28.9%) of 24,862 samples taken. In settings outside of the conventional sewage system (WTP, sewage access points, drains, pumping stations), samples were positive on 464 out of 1335 occasions (34.8%). Nine of 10 papers that sampled sewage systems serving hospitals reported clear outcomes showing a total of 185/284 (65.1%) positive samples. Three papers that examined samples from aviation settings, specifically two from aircrafts and one from airport, found 34 positive samples from 212 samples (Ahmed et al., 2020b; Albastaki et al., 2021a; Medema et al., 2020a). In the sole paper that sampled cruise ships, Ahmed et al., reported eight positive detections in 11 samples constituting both influent and effluent of the cruise sewage system (Ahmed et al., 2020b). Studies investigating travel-related settings (airport, aircraft & cruise) reported 42/223 (18.8%) samples positive collectively (Ahmed et al., 2020b; Albastaki et al., 2021a; Medema et al., 2020a). Another two of three papers that sampled rivers made seven positive detections in 12 (58.3%) collected samples (Guerrero-Latorre et al., 2020a; Rimoldi et al., 2020a; Haramoto et al., 2020a). Six of seven university-based studies reported clear outcomes with 222 positive samples (27.7%) of 802 samples collected. Two studies involving samples from residential areas reported 7 out of 11 (63.6%) samples positive (Tomasino et al., 2021a; Yaniv et al., 2021a). Separately, a single study collected wastewaters from nursing home (1 of 3 samples positive; 33.3%) (Tanhaei et al., 2021a). Viral load measured in the positive samples ranged from 1 to 4.8 × 109 gene copies per liter of wastewater (Table 2).
In terms of sample positivity, regions within the spike (S) gene had the highest percentage of positive samples: S (153/190; 80.5%), ORF1ab (215/327; 65.8%), RdRp (180/284; 63.4%), N2 (880/1462; 60.2%), N1 (920/1678; 54.8%), N (226/429; 52.7%), N3 (199/378; 52.7%), E (188/465; 40.4%). Although high positivity was recorded when ORF8-N (17/19 samples; 89.5%) was targeted, this should be interpreted with caution as the data was only obtained from two studies (Wilton et al., 2021a; Martin et al., 2020a). N genes remain the most commonly used gene targets in samples taken from settings outside of the conventional sewage system. Maximum sample positivity outside of the conventional sewage system was seen in ORF1ab (12/20; 60%), followed by S (8/14; 57.1%), N (48/99; 48.5%), RdRp (10/21; 47.6%), N2 (185/397; 46.6%), N3 (26/61; 42.6%), N1 (254/707; 35.9%), E (26/148; 17.6%). For river samples, N1 and N2 had positivity rates of 50% each given by 3/6 positive samples, while N and ORF1ab had positivity rates of 44.4% given by 4/9 positive detections each and six samples that were tested with E gene assay returned null detection. In the hospital setting, N1 gene was the most commonly used (138/228; 47.4%), followed by N2 (105/224; 46.9%), E (18/117; 15.4%), N3 (23/57; 40.4%), RdRp (10/21; 47.6%), N (9/19; 47.4%), ORF1ab and S (1/1; 100% each). In the university setting, the highest sample positivity was observed in N gene (8/10; 80%), followed by ORF1ab and S at 70% each (7/10 samples positive), N2 gene (58/110; 52.7%), and N1 gene (98/442; 22.2%). In residential setting, N gene was 50% positive (4/8 samples), and N1 gene was 100% positive (3/3 samples). The N2 gene was used in the only study involving nursing home, which reported 1 out of 3 positive samples (33.3%). Samples taken from travel-related setting were only tested with N and E genes, giving sample positivity as follows – N3 (3/4; 75%), N1 (11/25; 44%), E (8/25; 32%), N (6/21; 28.6%) and N2 (6/25; 24%) (Table 2).
3.3. Association with COVID-19 cases in the community
All but four studies reported potential association between wastewater signals and community COVID-19 cases. Twenty-three studies reported an association between positive detection in wastewater samples and number of community cases. These studies that focused on qualitative detection of SARS-CoV-2 in wastewater consistently reported the following three trends: 1) positive detection in areas with recent history of COVID-19 patients, 2) positive detection in wastewater samples coinciding with presence or increase in community cases, or 3) decreasing number of positive wastewater samples coinciding with decline in community cases. Another 53 studies reported associations between trend in viral load and community cases, with number of community cases directly proportional to the viral load. Six studies reported both qualitative and quantitative associations between WWTP positivity and case trends, while a single study by Bar-Or et al. (2021a) reported the presence of B.1.1.7 variant concurrently with clinical detection. Notably, thirteen studies reported detection of positive signals in wastewater before community cases surfaced in the population. The most prominent finding was that by Fongaro et al., who reported stable viral load in November 2019 through early March 2020, nearly 60 days before the first confirmed case in South America, and more than 90 days before the first confirmed case in Brazil (Fongaro et al., 2021b). La Rosa et al., reported similar findings of SARS-CoV-2 in wastewater samples dated between 18 December 2019 and 29 January 2020, two months before the first autochthonous Italian case was reported on 21 February 2020 (La Rosa et al., 2021a). Likewise, Chavarria-Miro et al. detected SARS-CoV-2 in sewage 41 days before the first COVID-19 cases was declared on 25 February 2020 (Chavarria-Miró et al., 2021a). In Australia, Ahmed et al. Detected SARS-CoV-2 up to up to three weeks before the first clinical case in Brisbane (Ahmed et al., 2021a). Interestingly, Martin et al., and Jorgensen et al., both detected viral RNA in samples dated three days before the first case surfaced in the sampled population in Southeastern England and a city in Denmark respectively (Jørgensen et al., 2020a; Martin et al., 2020a). Randazzo et al., reported detection of positive cases in two studies conducted in Spain, 12–16 days before in cities in Murcia and at least one day before in Valencia (Randazzo et al., 2020a; Randazzo et al., 2020b). Two studies detected SARS-CoV-2 in apartment buildings 2–3 days before the detection of a positive case in the buildings (Wong et al., 2021a; Xu et al., 2021a). Two campus-dormitories studies detected positive signals 4–5 days before a case was registered (Betancourt et al., 2021a; Kuryntseva et al., 2020a). Lastly, Medema et al., reported their earliest positive detection on 24 February 2020 when community case was incipient in Valencia, Spain, further affirming community transmission in affected regions before clinical cases were reported (Medema et al., 2020a).
Thirty-nine studies reported temporal trends between wastewater signals and community cases, with wastewater signals found to precede confirmed cases in the community by up to 63 days. Positive signals typically anticipated confirmed cases by 10 days −25 studies reported a minimum duration of up to 10 days, nine studies reported a minimum precedence of 14 days, and three studies detected SARS-CoV-2 minimally 23 days before a clinical case surfaced, and up to a maximum of 63 days. Only one study conducted by Saththasivam et al., concluded that SARS-CoV-2 in wastewaters did not precede clinical cases (Saththasivam et al., 2021a). At the minimum, studies reported that wastewater signals anticipated cases up to five days in advance. Peccia et al., Nemudryi et al. and Saguti et al., further investigated temporal association between wastewater signals and other markers of community cases, including positive tests by date of specimen collection, hospital admission and days after symptom onset (Saguti et al., 2021a; Nemudryi et al., 2020b; Peccia et al., 2020a). Wastewater signals led positive tests by date of specimen collection by a mere 0–2 days as compared to 1–4 days for hospital admissions. Notably, there were three occurrences of wastewater signals lagging cases/hospitalisations – when cases were defined by symptom onset and in a low prevalence setting respectively (Colosi et al., 2021a; Gonçalves et al., 2021a; Nemudryi et al., 2020b) (Table 2).
3.4. Sequencing & modelling studies
Twenty-five out of 87 studies conducted sequencing analysis of their SARS-CoV-2 isolates, fifteen of which reported the resemblance of the strains with those listed in GenBank database. A study conducted in Brisbane between 24 Feb–1 April 2020 reported the presence of genome that originated in Israel (Ahmed et al., 2020a), another study conducted in Japan between March and April 2020 reported genome that was partially identical to the Wuhan strain (Hata et al., 2021a). A study conducted in North Rhine-Westphalia, Germany found a strain in April 2020 that was closely related to the California strain, which was also detected in a study conducted in Bozeman, Montana, USA between late March and June 2020 (Nemudryi et al., 2020b; Westhaus et al., 2021a). Local strains were identified in four studies, namely, two studies conducted in Italy between 3 Feb–2 April 2020 and mid-April, a study conducted in the metropolitan region of Japan between 9 June–19 Aug, 2020 and a study conducted in Dane county, Wisconsin between mid-October 2020–early Jan 2021 (Kitamura et al., 2021a; Mondal et al., 2021a; La Rosa et al., 2020a; Rimoldi et al., 2020a). Notably, Bar-Or et al. and Wilton et al. detected the B.1.1.7 variant in wastewater in Israel from Aug 2020–Feb 2021 and London from Jan 2020 to Jan 2021 (Bar-Or et al., 2021a; Wilton et al., 2021a). Ten studies reported genome coverage of their isolates (85–100%). Among the 17 studies that conducted predictive modelling analysis of COVID-19 cases, three studies adopted the Monte Carlo simulation approach to estimate infection prevalence according to viral load (Prado et al., 2021a; Ahmed et al., 2020a; Hata et al., 2021a), while majority of the studies conducted regression modelling to estimate the number of infected individuals based on viral load. Separately, Wu et al. simulated wastewater titers and detection probability using a Poisson model (Wu et al., 2020a). The results were varied with the probability for detection being reported between 88% and 100% (Supplementary Table 4).
3.5. Risk assessment
Majority of the studies (30 out of 87) had a high risk of bias linked to clarity and publication. A total of 26 out of 31 studies (29.3%) had a high risk of bias for the study design domain and 22 (25.3%) studies had a high risk of bias for assessment of outcome domain. Notably two studies, only four studies, Agrawal et al., Jorgensen et al., Prado et al. and Wurtz et al., showed high bias in all three domains (Agrawal et al., 2021b; Wurtz et al., 2021a; Jørgensen et al., 2020a; Prado et al., 2020a), while 33 (38%) studies had low bias for all three domains.
Within the study design domain, majority of the studies (20 out of 87) showed high bias for clarity of sampling, while only three studies had a high bias for the method of virus extraction (Wurtz et al., 2021a; Yaniv et al., 2021a; Jørgensen et al., 2020a). Presence of viral quantification process showed maximum risk of bias in the assessment of outcome domain while least bias was seen for association with human cases/community transmission with only six studies (Sharif et al., 2020a) showing high risk of bias. Bias linked to clarity on sampling site had eight studies with high bias, while nineteen studies showed high bias for transparency in results (Supplementary Table 5).
4. Discussion
This review sought to provide a systematic synthesis of wastewater surveillance studies for SARS-CoV-2. Studies in this review covered wide geographical areas serving up to 42,500,000 inhabitants as well as targeted areas like university, residential area, cruise, airlines and hospitals. Although the performance of wastewater surveillance in settings outside conventional sewage system was better (34.8% vs. 28.9%) than conventional sewage system, number of samples in the latter were far more that the former (24,862 vs. 1335). On the note of ethical considerations, wastewater surveillance seems better suited for larger catchment areas as focusing on targeted and high risk areas allows population to be easily identified (Zahedi et al., 2021). In case of wastewater surveillance for pharmaceuticals, it is generally accepted that populations over >10,000 can be considered to give anonymity and posing no risk to smaller groups of people (Sims and Kasprzyk-Hordern, 2020). However, targeting confined sub-populations within high-risk facilities may provide clearer indication of infection source as opposed to widespread geographical testing. Key high-risk facilities such as schools, dormitories, nursing homes that entail frequent prolonged close interactions between individuals have been shown to promote COVID-19 outbreaks (Daughton, 2020; Koh, 2020; Thompson et al., 2020). In this study, SARS-CoV-2 was detected in the targeted surveillance of hospitals (65.1%), university settings (27.7%), residential buildings/regions (63.6%) and nursing home (33.3%). Notably, in their study on hospital wastewater, Gonçalves et al. reported positive signals in wastewater in the presence of only one hospitalized COVID-19 case (Gonçalves et al., 2021a). Persistent positive signals were detected in the hospital's wastewater throughout the study period, which saw only a maximum of four hospitalized COVID-19 patients at its peak (Gonçalves et al., 2021a), suggesting high sensitivity of facility based sewage sampling. Similar findings were reported by Betancourt et al. and Xu et al., which detected the presence of SARS-CoV-2 even before the first case surfaced in the facilities (Betancourt et al., 2021a; Xu et al., 2021a). Targeted surveillance of living facilities can also sieve out sub-populations underserved by healthcare due to unavailability of healthcare services or avoidance in seeking healthcare (Daughton, 2020). Given that sewage can be diluted by rain or industrial discharge running into the sewer network (CNA, 2021), infection prevalence deduced from WWTP samples may be underestimated without proper accountancy of these factors during analysis. Nevertheless, it would still be challenging to interpret the wastewater samples tested positive as potential COVID-19 transmission when there are known reported recovered cases who have returned back into these targeted non-healthcare facilities (Wang et al., 2020; Wong et al., 2021a).
Inadequate sewage infrastructure in developing countries can hinder the application of wastewater surveillance, further stunting surveillance efforts in the very countries that ought to reap the most benefit from this cost-effective surveillance approach. Several constraints in sanitation infrastructure of developing countries challenge the representativeness of wastewater surveillance, such as high proportions of households not being connected to the sewage network, underperformance of WWTP, dysfunctional operational facilities, and mismanagement of sewage. Surveillance approaches that typically begin with sampling influent of WWTP is not representative of actual disease prevalence in developing countries (Panchal et al., 2021; Pandey et al., 2021; Street et al., 2020). Despite several studies based in developing countries, these limitations were insufficiently addressed. Only a single paper included in this review focused on SARS-CoV-2 surveillance in low sanitation settings, detecting three positive samples out of three samples collected from rivers receiving untreated sewage in Ecuador. Approaches should be contextualized to consider both areas served and not-served by sewage networks to present an accurate picture of estimated prevalence (Calabria de Araujo et al., 2020). More investigation of wastewater surveillance approach in developing/low-income settings is warranted.
Positive samples were detected as early as two months before the first case was clinically identified (Hata and Honda, 2020). Virus shedding in feces is suggested to occur five days prior to symptom onset and last up to 17.2 days (Cevik et al., 2021; Jones et al., 2020). COVID-19 has a short serial interval of 3–8 days, given that SARS-CoV-2 reportedly has an incubation period of 5.6 days, it suggests that transmission typically occurred before or near symptom onset (Quesada et al., 2021; Cheng et al., 2020; Park et al., 2020; Pung et al., 2020). In this review, Kuryntseva et al. detected SARS-CoV-2 in wastewater discharged from Kazan Federal University Buildings when nobody reported illness, five days before the first COVID-19 case was registered (Kuryntseva et al., 2020a) while dormitories in the USA and residential buildings in Hong Kong reported positive samples 2–4 days before the first case surfaced. Four cases were detected within seven days after investigation, demonstrating fecal shedding of virus in pre-symptomatic individuals. Studies included in this review typically reported anticipation of COVID-19 cases 16 days prior to clinical detection (≤10 days; 24 studies, ≥14 days; 8 studies), which provides adequate time for containment measures to be put in place to prevent an imminent cluster/outbreak given the short serial interval of COVID-19. However, there were studies that did not report any positive detection before cases were clinically reported in the community (Medema et al., 2020a; Sherchan et al., 2020a; Wu et al., 2020a), or detection was only observed when there were higher incidence rate reported (Hata et al., 2021a; Nemudryi et al., 2020b; Prado et al., 2020a; Haramoto et al., 2020a). This phenomenon may be due to the fact that only about 50% of the infected cases would shed viruses in the excreta (Gupta et al., 2020). Reports of SARS-CoV-2 signals in wastewater at the beginning of the COVID-19 pandemic should be interpreted with caution given the initial delay in establishing targeted assays and laboratory surveillance for a novel virus.
All papers reported SARS-CoV-2 detection with viral load ranging from 1 to 4.8 × 109 gene copies per liter of wastewater, overall sample positivity was relatively low at 29.2%. Detection of SARS-CoV-2 signals in wastewater depends on viral load present and sensitivity of detection assays. Hata et al. reported that SARS-CoV-2 was detectable in WWT if one in 100,000 persons sheds 109 copies/g-feces in 200 g of feces, and the success of wastewater surveillance is dependent on the viral load in feces and the detection limit of the method of analysis, estimated as 2 copies/mL in wastewater using the RT-qPCR assay (Hata and Honda, 2020). Evidence from two meta-analyses demonstrated pooled virus detection in stool of 40.5% and 48.1% of patients respectively (Cheung et al., 2020; Parasa et al., 2020). Higher detection rates were also reported in females, those with gastrointestinal symptoms and more severe disease (Wong et al., 2020). Such differential viral shedding profiles in feces further challenge the representation of disease prevalence deduced by wastewater surveillance. On this note, targeting endogenous biomarkers instead of utilizing clinical diagnostic testing platforms can potentially mitigate the aforementioned limitations (Daughton, 2020). The accuracy of disease prevalence captured can be potentially improved with the identification of infection markers that are more likely to be universally excreted in either urine or feces. An example of its potential is provided by a preprint published on medRxiv, which reported heighted sensitivity by assaying viral proteins using Multiplex Paired-antibody Amplified Detection (Neault et al., 2020). Since significant inflammation is typically induced in severe patients, the identification of appropriate endogenous markers warrant deeper biomarker evaluation (Panchal et al., 2021), especially for universal indicators applicable to mild cases. Apart from cost reduction from lowered reliance on SARS-CoV-2 immuno- and molecular assays (Daughton, 2020), false negativity caused by low viral load falling below assay detection and analytical limits can be mitigated. Other plausible benefits include earlier alerts supplemented by infection indicators and simplified sampling and processing workflow from using biomarkers present in urine (Daughton, 2020).
Process efficiency is a major challenge as little is known of the recovery efficiency of SARS-CoV-2 (Ahmed et al., 2020c). Beyond proving SARS-CoV-2 detection in wastewater, several studies were driven by the need to propose an adequate workflow to optimise recovery efficiency, comparing the use of different concentration methods adapted from surveillance of other pathogenic viruses, primers and probes for detection (Arora et al., 2020a; Haramoto et al., 2020a). Nineteen studies included in this review trialed more than one virus concentration protocol to evaluate performance differences. The most commonly used approaches were ultrafiltration (34 studies) and polyethylene glycol (PEG) precipitation (31 studies). This was further corroborated by Hjelmsø et al., who concluded PEG as a powerful extraction method in their comparison of viral concentration and RNA isolation methods (Hjelmsø et al., 2017). Some also reported detection of SARS-CoV-2 in different sample types, prompting questions on the optimal wastewater type to sample. While suspended solids – primary sewage sludge, post-grit chamber influent solids and primary clarified sludge – were less frequently collected (7 studies) as compared to raw sewage into WWTP (80 studies), Bhattarai et al. concluded that sludge samples had greater predictability than influent samples, especially for monitoring purposes that exhibits a time lag (Bhattarai et al., 2021a). Since suspended solids confer protective effect by allowing virus adsorption (Gundy et al., 2008), Balboa et al. confirmed their hypothesis that enveloped virus had higher affinity for biosolids, by identifying higher virus concentrations in thickened and primary sludge than WWTP influent (Balboa et al., 2021). The sparsity of studies utilizing solid samples hitherto, on top of promising results reported by Peccia et al. prompt further investigation into the sample type optimal for SARS-CoV-2 detection (Peccia et al., 2020a). Further evaluation of processing methodologies was not undertaken in this review as it was beyond the expertise of the review authors. In-depth assessment of the optimal methodology is warranted in future studies to guide the adoption of wastewater surveillance. A more recently conducted study on a college campus in Arizona, USA reported a sensitivity of 76.0% and a positive predictive value of 79.8% when comparing wastewater surveillance results with clinical testing (Betancourt et al., 2021b). In Colosi et al.'s analysis of hospital and dormitories, wastewater surveillance was reported to have a sensitivity of 96.2% and specificity of 100% (Colosi et al., 2021a). This suggests that wastewater surveillance can potentially serve as an “early warning system” to monitor trends of SARS-CoV-2 transmission; however, more papers need to look at sensitivity and positive predictive value to allow a meta-analysis providing more robust evidence.
Finally, this review piloted the first tool to assess the risk of bias in wastewater surveillance studies across the domains of study design, outcome measurement and clarity/publication bias. Our risk of bias assessment highlighted the urgent need for more well-established guidance in the aspects of study design, execution and interpretation of wastewater surveillance studies for use globally and to allow robust meta-review. The importance of such an assessment lies in its potential to explain heterogeneity in findings and the ability to grade the strength of evidence presented by studies, which can influence the interpretation of results synthesized (Viswanathan et al., 2017). This assessment tool can 1) provide a platform to guide the consistency of future research design and presentation, which can help strengthen the conclusions made by future systematic reviews; 2) can act as a foundation for the refinement of future risk of bias assessments.
5. Strengths & limitations
This review is limited by the usage of solely qualitative synthesis due to the largely descriptive nature of existing literature. Units in which the viral load in various papers were presented were inconsistent and did not allow for meta-analysis. It is recommended to present it in terms of the daily loads per capita (mg/day/1000 inhabitants) to allow comparisons between cities in different geographic locations, and to be able to normalize the findings (Hata and Honda, 2020). Furthermore, there is also no consistent guidance on the definition of a positive wastewater result, either based on a significantly high viral load cut-off value and/or a consecutive number of days with wastewater sampling tested positive. There is a lack of systematic methods in existing literature to assess association between COVID-19 cases and wastewater signals as well as the accuracy of this wastewater testing over a fixed period of time. Ideally, the association between COVID-19 incidence and wastewater signals should be based on daily regular surveillance of the wastewater and the weekly PCR nasopharyngeal swab test of the susceptible (naïve) population of interest. Though funnel plot analysis for publication bias assessment was not feasible in this review, it would be prudent to assume that most, if not all, studies with negative findings on their wastewater testing and its association with COVID-19 incidences, would be challenging to publish when making interpretation from this review.
Sample positivity was moderately high with respect to the nucleocapsid gene amidst low rates of sample positivity seen among studies overall. While wastewater surveillance omits the factors of time consumption, cost, exposure risk, and avoids the biases of epidemiological indicators arising from limitations in the healthcare system, such as diagnostic testing capacity, symptomatic testing and hospitalization lags, it does not provide a true quantitative estimation of the number of people who are infected in the population. More studies using accurate and validated methods are required along with risk analysis and modelling to understand the dynamics of viral outbreaks. Future studies need to present their findings in a more consistent manner – provide clarity of sampling approach and sampling sites, virus detection and quantification process, use of laboratory controls and genetic targets in addition to mere reporting of sample positivity so as to guide and enable valid comparisons of study design. Guidance should also be provided on analysis methods to enable standardized, statistically robust association of findings with community/clinical data.
6. Conclusion
As countries embrace the emerging SARS-CoV-2 variant of concerns, wastewater surveillance can complement clinical surveillance by providing signs of potential transmission for more active public health responses.
CRediT authorship contribution statement
Junxiong Pang: Conceptualization, Supervision, Writing - Reviewing and Editing. Shimoni Shah: Data curation, Methodology, Writing - Original draft preparation, Writing - Reviewing and Editing. Sylvia Xiao Wei Gwee: Data curation, Methodology, Writing - Original draft preparation, Writing - Reviewing and Editing. Jamie Qiao Xin Ng: Data curation, Writing - Reviewing and Editing. Nicholas Lau: Data curation. Jiayun Koh: Data curation.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data sharing
All data generated in this study are included in this article and its supplementary files.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor: Jay Gan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.150060.
Appendix A. Supplementary data
Supplementary tables
References
- Acosta N., Bautista M.A., Hollman J., McCalder J., Beaudet A.B., Man L., et al. A multicenter study investigating SARS-CoV-2 in tertiary-care hospital wastewater. viral burden correlates with increasing hospitalized cases as well as hospital-associated transmissions and outbreaks. Water Res. 2021;201 doi: 10.1016/j.watres.2021.117369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta N., et al. A multicenter study investigating SARS-CoV-2 in tertiary-care hospital wastewater. viral burden correlates with increasing hospitalized cases as well as hospital-associated transmissions and outbreaks. Water Res. 2021;201:117369. doi: 10.1016/j.watres.2021.117369. in eng. Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S., Orschler L., Lackner S. Long-term monitoring of SARS-CoV-2 RNA in wastewater of the Frankfurt metropolitan area in Southern Germany. Sci. Rep. 2021;11(1):5372. doi: 10.1038/s41598-021-84914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S., Orschler L., Lackner S. Long-term monitoring of SARS-CoV-2 RNA in wastewater of the Frankfurt metropolitan area in Southern Germany. Sci. Rep. 2021;11(1):5372. doi: 10.1038/s41598-021-84914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S., Orschler L., Lackner S. Long-term monitoring of SARS-CoV-2 RNA in wastewater of the Frankfurt metropolitan area in Southern Germany. Sci. Rep. 2021;11(1):5372. doi: 10.1038/s41598-021-84914-2. in eng. Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar-Oliveira MdL, Campos A., R Matos A., Rigotto C., Sotero-Martins A., PFP Teixeira, et al. Wastewater-based epidemiology (WBE) and viral detection in polluted surface water: a valuable tool for COVID-19 surveillance-a brief review. Int. J. Environ. Res. Publ. Health. 2020;17(24):9251. doi: 10.3390/ijerph17249251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Angel N., Bibby K., Bivins A., Dierens L., et al. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travellers. J. Travel Med. 2020;27(5) doi: 10.1093/jtm/taaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., et al. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739:139960. doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., et al. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travellers. J. Travel Med. 2020;27(5):taaa116. doi: 10.1093/jtm/taaa116. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Tscharke B., Bertsch P.M., Bibby K., Bivins A., Choi P., et al. SARS-CoV-2 RNA monitoring in wastewater as a potential early warning system for COVID-19 transmission in the community: a temporal case study. Sci. Total Environ. 2021;761:144216. doi: 10.1016/j.scitotenv.2020.144216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., et al. SARS-CoV-2 RNA monitoring in wastewater as a potential early warning system for COVID-19 transmission in the community: a temporal case study. Sci. Total Environ. 2021;761:144216. doi: 10.1016/j.scitotenv.2020.144216. in eng. Mar 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albastaki A., Naji M., Lootah R., Almeheiri R., Almulla H., Almarri I., et al. First confirmed detection of SARS-COV-2 in untreated municipal and aircraft wastewater in Dubai, UAE: the use of wastewater based epidemiology as an early warning tool to monitor the prevalence of COVID-19. Sci. Total Environ. 2021;760:143350. doi: 10.1016/j.scitotenv.2020.143350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albastaki, et al. First confirmed detection of SARS-COV-2 in untreated municipal and aircraft wastewater in Dubai, UAE: The use of wastewater based epidemiology as an early warning tool to monitor the prevalence of COVID-19. Sci. Total Environ. 2021;760:143350. doi: 10.1016/j.scitotenv.2020.143350. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S., Nag A., Sethi J., Rajvanshi J., Saxena S., Shrivastava S.K., et al. Sewage surveillance for the presence of SARS-CoV-2 genome as a useful wastewater based epidemiology (WBE) tracking tool in India. Water Sci. Technol. 2020;82(12):2823–2836. doi: 10.2166/wst.2020.540. [DOI] [PubMed] [Google Scholar]
- Arora S., et al. Sewage surveillance for the presence of SARS-CoV-2 genome as a useful wastewater based epidemiology (WBE) tracking tool in India. Water Sci. Technol. 2020;82(12):2823–2836. doi: 10.2166/wst.2020.540. [184] [DOI] [PubMed] [Google Scholar]
- Balboa S., Mauricio-Iglesias M., Rodriguez S., Martínez-Lamas L., Vasallo F.J., Regueiro B., et al. The fate of SARS-COV-2 in WWTPS points out the sludge line as a suitable spot for detection of COVID-19. Sci. Total Environ. 2021;772:145268. doi: 10.1016/j.scitotenv.2021.145268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or I., Weil M., Indenbaum V., Bucris E., Bar-Ilan D., Elul M., et al. Detection of SARS-CoV-2 variants by genomic analysis of wastewater samples in Israel. Sci. Total Environ. 2021;789:148002. doi: 10.1016/j.scitotenv.2021.148002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or, et al. Detection of SARS-CoV-2 variants by genomic analysis of wastewater samples in Israel. Sci. Total Environ. 2021;789:148002. doi: 10.1016/j.scitotenv.2021.148002. in eng. May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand I., Challant J., Jeulin H., Hartard C., Mathieu L., Lopez S., et al. Epidemiological surveillance of SARS-CoV-2 by genome quantification in wastewater applied to a city in the northeast of France: comparison of ultrafiltration- and protein precipitation-based methods. Int. J. Hyg. Environ. Health. 2021;233:113692. doi: 10.1016/j.ijheh.2021.113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand, et al. Epidemiological surveillance of SARS-CoV-2 by genome quantification in wastewater applied to a city in the northeast of France: Comparison of ultrafiltration- and protein precipitation-based methods. Int. J. Hyg. Environ. Health. 2021;233:113692. doi: 10.1016/j.ijheh.2021.113692. in eng. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt W.Q., Schmitz B.W., Innes G.K., Prasek S.M., Pogreba Brown K.M., Stark E.R., et al. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci. Total Environ. 2021;779:146408. doi: 10.1016/j.scitotenv.2021.146408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt W.Q., Schmitz B.W., Innes G.K., Prasek S.M., Pogreba Brown K.M., Stark E.R., et al. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci. Total Environ. 2021;779:146408. doi: 10.1016/j.scitotenv.2021.146408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt W.Q., et al. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci. Total Environ. 2021;779:146408. doi: 10.1016/j.scitotenv.2021.146408. in eng. Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai B., Sahulka S.Q., Podder A., Hong S., Li H., Gilcrease E., et al. Prevalence of SARS-CoV-2 genes in water reclamation facilities: from influent to anaerobic digester. Sci. Total Environ. 2021;796:148905. doi: 10.1016/j.scitotenv.2021.148905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai, et al. Prevalence of SARS-CoV-2 genes in water reclamation facilities: from influent to anaerobic digester. Sci. Total Environ. 2021;796:148905. doi: 10.1016/j.scitotenv.2021.148905. in eng. Jul 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilotta G.S., Milner A.M., Boyd I.L. Quality assessment tools for evidence from environmental science. Environ. Evid. 2014;3(1):14. [Google Scholar]
- Bisseux M., Debroas D., Mirand A., Archimbaud C., Peigue-Lafeuille H., Bailly J.-L., et al. Monitoring of enterovirus diversity in wastewater by ultra-deep sequencing: an effective complementary tool for clinical enterovirus surveillance. Water Res. 2020;169:115246. doi: 10.1016/j.watres.2019.115246. [DOI] [PubMed] [Google Scholar]
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., et al. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. Environ. Sci. Technol. 2020;54(13):7754–7757. doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- Black J., Aung P., Nolan M., Roney E., Poon R., Hennessy D., et al. Epidemiological evaluation of sewage surveillance as a tool to detect the presence of COVID-19 cases in a low case load setting. Sci. Total Environ. 2021;786 [Google Scholar]
- Black, et al. Epidemiological evaluation of sewage surveillance as a tool to detect the presence of COVID-19 cases in a low case load setting. Sci. Total Environ. 2021;786 doi: 10.1016/j.scitotenv.2021.147469. in English. [DOI] [Google Scholar]
- Brouwer A.F., Eisenberg J.N.S., Pomeroy C.D., Shulman L.M., Hindiyeh M., Manor Y., et al. Epidemiology of the silent polio outbreak in Rahat, Israel, based on modeling of environmental surveillance data. Proc. Natl. Acad. Sci. U. S. A. 2018;115(45) doi: 10.1073/pnas.1808798115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabria de Araujo J., Gavazza S., Leao T.L., Florencio L., da Silva H.P., Albuquerque JdO, et al. SARS-CoV-2 sewage surveillance in low-income countries: potential and challenges. J. Water Health. 2020;19(1):1–19. [Google Scholar]
- Cao Y., Francis R. On forecasting the community-level COVID-19 cases from the concentration of SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;786:147451. doi: 10.1016/j.scitotenv.2021.147451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Francis R. On forecasting the community-level COVID-19 cases from the concentration of SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;786:147451. doi: 10.1016/j.scitotenv.2021.147451. in eng. Sep 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Wastewaster Surveillance: CDC. 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/wastewater-surveillance.html Available from:
- Cevik M., Tate M., Lloyd O., Maraolo A.E., Schafers J., Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P., Pasupuleti M., Jai Shankar M.R., Bharat G.K., Krishnasamy S., Dasgupta S.C., et al. First surveillance of SARS-CoV-2 and organic tracers in community wastewater during post lockdown in Chennai, South India: methods, occurrence and concurrence. Sci. Total Environ. 2021;778:146252. doi: 10.1016/j.scitotenv.2021.146252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P., et al. First surveillance of SARS-CoV-2 and organic tracers in community wastewater during post lockdown in Chennai, South India: methods, occurrence and concurrence. Sci. Total Environ. 2021;778:146252. doi: 10.1016/j.scitotenv.2021.146252. in eng. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Miró G., Anfruns-Estrada E., Martínez-Velázquez A., Vázquez-Portero M., Guix S., Paraira M., et al. Time evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in wastewater during the first pandemic wave of COVID-19 in the metropolitan area of Barcelona, Spain. Appl. Environ. Microbiol. 2021;87(7) doi: 10.1128/AEM.02750-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Miró G., et al. Time evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in wastewater during the first pandemic wave of COVID-19 in the metropolitan area of Barcelona, Spain. Appl. Environ. Microbiol. 2021;87(7) doi: 10.1128/aem.02750-20. in eng. Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.-Y., Jian S.-W., Liu D.-P., Ng T.-C., Huang W.-T., Lin H.-H., et al. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern. Med. 2020;180(9):1156–1163. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski M., Blackwood D., Clerkin T., Gonzalez R., Thompson H., Larson A., et al. Assessing sensitivity and reproducibility of RT-ddPCR and RT-qPCR for the quantification of SARS-CoV-2 in wastewater. J. Virol. Methods. 2021;114230 doi: 10.1016/j.jviromet.2021.114230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski M., et al. Assessing sensitivity and reproducibility of RT-ddPCR and RT-qPCR for the quantification of SARS-CoV-2 in wastewater. J. Virol. Methods. 2021:114230. doi: 10.1016/j.jviromet.2021.114230. in eng. Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CNA Channel News Asia; 2021. Sewage testing could protect schools, hospitals from COVID-19 outbreaks Channel News Asia. https://www.channelnewsasia.com/news/world/covid-19-sewage-testing-could-protect-schools-hospitals-outbreak-13537284 Available from:
- Colosi L.M., Barry K.E., Kotay S.M., Porter M.D., Poulter M.D., Ratliff C., et al. Development of wastewater pooled surveillance of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from congregate living settings. Appl. Environ. Microbiol. 2021;87(13) doi: 10.1128/AEM.00433-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosi M., et al. Development of wastewater pooled surveillance of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from congregate living settings. Appl. Environ. Microbiol. 2021;87(13):e0043321. doi: 10.1128/aem.00433-21. in eng. Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aoust P.M., Graber T.E., Mercier E., Montpetit D., Alexandrov I., Neault N., et al. Catching a resurgence: increase in SARS-CoV-2 viral RNA identified in wastewater 48 h before COVID-19 clinical tests and 96 h before hospitalizations. Sci. Total Environ. 2021;770:145319. doi: 10.1016/j.scitotenv.2021.145319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aoust P.M., Mercier E., Montpetit D., Jia J.-J., Alexandrov I., Neault N., et al. Quantitative analysis of SARS-CoV-2 RNA from wastewater solids in communities with low COVID-19 incidence and prevalence. Water Res. 2021;188:116560. doi: 10.1016/j.watres.2020.116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aoust P.M., et al. Quantitative analysis of SARS-CoV-2 RNA from wastewater solids in communities with low COVID-19 incidence and prevalence. Water Res. 2021;188:116560. doi: 10.1016/j.watres.2020.116560. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aoust P.M., et al. Catching a resurgence: Increase in SARS-CoV-2 viral RNA identified in wastewater 48 h before COVID-19 clinical tests and 96 h before hospitalizations. Sci. Total Environ. 2021;770:145319. doi: 10.1016/j.scitotenv.2021.145319. in eng. May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020;736:139631. doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande J.M., Shetty S.J., Siddiqui Z.A. Environmental surveillance system to track wild poliovirus transmission. Appl. Environ. Microbiol. 2003;69(5):2919–2927. doi: 10.1128/AEM.69.5.2919-2927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cassi X., Scheidegger A., Bänziger C., Cariti F., Tuñas Corzon A., Ganesanandamoorthy P., et al. Wastewater monitoring outperforms case numbers as a tool to track COVID-19 incidence dynamics when test positivity rates are high. Water Res. 2021;200:117252. doi: 10.1016/j.watres.2021.117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cassi X., et al. Wastewater monitoring outperforms case numbers as a tool to track COVID-19 incidence dynamics when test positivity rates are high. Water Res. 2021;200:117252. doi: 10.1016/j.watres.2021.117252. in eng. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fongaro G., Rogovski P., Savi B.P., Cadamuro R.D., Pereira J.V.F., Anna I.H.S., et al. SARS-CoV-2 in human sewage and river water from a remote and vulnerable area as a surveillance tool in Brazil. Food Environ. Virol. 2021:1–4. doi: 10.1007/s12560-021-09487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fongaro G., Stoco P.H., Souza D.S.M., Grisard E.C., Magri M.E., Rogovski P., et al. The presence of SARS-CoV-2 RNA in human sewage in Santa Catarina, Brazil, November 2019. Sci. Total Environ. 2021;778:146198. doi: 10.1016/j.scitotenv.2021.146198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fongaro G., et al. The presence of SARS-CoV-2 RNA in human sewage in Santa Catarina, Brazil, November 2019. Sci. Total Environ. 2021;778:146198. doi: 10.1016/j.scitotenv.2021.146198. 2021/07/15/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fongaro G., et al. SARS-CoV-2 in human sewage and river water from a remote and vulnerable area as a surveillance tool in Brazil. Food Environ. Virol. 2021:1–4. doi: 10.1007/s12560-021-09487-9. in eng. Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambier Wastewater SARS-CoV-2 Virus Report. https://biology.kenyon.edu/slonc/covid/Gambier_Wastewater_Report.html
- Gambier Wastewater SARS-CoV-2 Virus Report 2021. https://biology.kenyon.edu/slonc/covid/Gambier_Wastewater_Report.html Available from:
- Gerrity D., Papp K., Stoker M., Sims A., Frehner W. Early-pandemic wastewater surveillance of SARS-CoV-2 in Southern Nevada: methodology, occurrence, and incidence/prevalence considerations. Water Res. X. 2021;10:100086. doi: 10.1016/j.wroa.2020.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity K., Papp M., Stoker A. Sims, Frehner W. Early-pandemic wastewater surveillance of SARS-CoV-2 in Southern Nevada: methodology, occurrence, and incidence/prevalence considerations. Water Res. X. 2021;10:100086. doi: 10.1016/j.wroa.2020.100086. in eng. Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibas C., Lambirth K., Mittal N., Juel M.A.I., Barua V.B., Roppolo Brazell L., et al. Implementing building-level SARS-CoV-2 wastewater surveillance on a university campus. Sci. Total Environ. 2021;782 doi: 10.1016/j.scitotenv.2021.146749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibas, et al. Implementing building-level SARS-CoV-2 wastewater surveillance on a university campus. Sci. Total Environ. 2021;782:146749. doi: 10.1016/j.scitotenv.2021.146749. in eng. Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud-Billoud M., Cuervo P., Altamirano J.C., Pizarro M., Aranibar J.N., Catapano A., et al. Monitoring of SARS-CoV-2 RNA in wastewater as an epidemiological surveillance tool in Mendoza, Argentina. Sci. Total Environ. 2021;796:148887. doi: 10.1016/j.scitotenv.2021.148887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud-Billoud, et al. Monitoring of SARS-CoV-2 RNA in wastewater as an epidemiological surveillance tool in Mendoza, Argentina. Sci. Total Environ. 2021;796:148887. doi: 10.1016/j.scitotenv.2021.148887. in eng. Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves J., Koritnik T., Mioč V., Trkov M., Bolješič M., Berginc N., et al. Detection of SARS-CoV-2 RNA in hospital wastewater from a low COVID-19 disease prevalence area. Sci. Total Environ. 2021;755:143226. doi: 10.1016/j.scitotenv.2020.143226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves J., et al. Detection of SARS-CoV-2 RNA in hospital wastewater from a low COVID-19 disease prevalence area. Sci. Total Environ. 2021;755:143226. doi: 10.1016/j.scitotenv.2020.143226. 2021/02/10/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R., Curtis K., Bivins A., Bibby K., Weir M.H., Yetka K., et al. COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Res. 2020;186:116296. doi: 10.1016/j.watres.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R., et al. COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Res. 2020;186:116296. doi: 10.1016/j.watres.2020.116296. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government NSW . New South Wales Government; 2020. COVID-19 Weekly Surveillance in NSW - Epidemiological Week 40, Ending 3 October 2020. [Google Scholar]
- Government NSW . New South Wales Government; 2020. COVID-19 Weekly Surveillance in NSW - Epidemiological Week 41, Ending 10 October 2020. [Google Scholar]
- Government NSW . New South Wales Government; 2020. COVID-19 Weekly Surveillance in NSW - Epidemiological Week 42, Ending 17 October 2020. [Google Scholar]
- Government NSW . New South Wales Government; 2020. COVID-19 Weekly Surveillance in NSW - Epidemiological Week 43, Ending 24 October 2020. [Google Scholar]
- Graham E., et al. SARS-CoV-2 RNA in wastewater settled solids is associated with COVID-19 cases in a large urban sewershed. Environ. Sci. Technol. 2021;55(1):488–498. doi: 10.1021/acs.est.0c06191. in eng. Jan 5. [DOI] [PubMed] [Google Scholar]
- Graham K.E., Loeb S.K., Wolfe M.K., Catoe D., Sinnott-Armstrong N., Kim S., et al. SARS-CoV-2 RNA in wastewater settled solids is associated with COVID-19 cases in a large urban sewershed. Environ. Sci. Technol. 2021;55(1):488–498. doi: 10.1021/acs.est.0c06191. [DOI] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: implications in low sanitation countries. Sci. Total Environ. 2020;743:140832. doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: Implications in low sanitation countries. Sci. Total Environ. 2020;743:140832. doi: 10.1016/j.scitotenv.2020.140832. 2020/11/15/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2008;1(1):10. [Google Scholar]
- Gupta S., Parker J., Smits S., Underwood J., Dolwani S. Persistent viral shedding of SARS-CoV-2 in faeces - a rapid review. Color. Dis. 2020;22(6):611–620. doi: 10.1111/codi.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamouda M., Mustafa F., Maraqa M., Rizvi T., Aly Hassan A. Wastewater surveillance for SARS-CoV-2: lessons learnt from recent studies to define future applications. Sci. Total Environ. 2021;759:143493. doi: 10.1016/j.scitotenv.2020.143493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. 2020/10/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.W., Ibrahim Y., Daou M., Kannout H., Jan N., Lopes A., et al. Detection and quantification of SARS-CoV-2 RNA in wastewater and treated effluents: surveillance of COVID-19 epidemic in the United Arab Emirates. Sci. Total Environ. 2021;764:142929. doi: 10.1016/j.scitotenv.2020.142929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.W., et al. Detection and quantification of SARS-CoV-2 RNA in wastewater and treated effluents: surveillance of COVID-19 epidemic in the United Arab Emirates. Sci. Total Environ. 2021;764:142929. doi: 10.1016/j.scitotenv.2020.142929. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Honda R. Potential sensitivity of wastewater monitoring for SARS-CoV-2: comparison with norovirus cases. Environ. Sci. Technol. 2020;54(11):6451–6452. doi: 10.1021/acs.est.0c02271. [DOI] [PubMed] [Google Scholar]
- Hata A., Hara-Yamamura H., Meuchi Y., Imai S., Honda R. Detection of SARS-CoV-2 in wastewater in Japan during a COVID-19 outbreak. Sci. Total Environ. 2021;758:143578. doi: 10.1016/j.scitotenv.2020.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata H., Hara-Yamamura Y., Meuchi S. Imai, Honda R. Detection of SARS-CoV-2 in wastewater in Japan during a COVID-19 outbreak. Sci. Total Environ. 2021;758:143578. doi: 10.1016/j.scitotenv.2020.143578. 2021/03/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., et al. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80(21):6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary L.S., Farkas K., Maher K.H., Lucaci A., Thorpe J., Distaso M.A., et al. Monitoring SARS-CoV-2 in municipal wastewater to evaluate the success of lockdown measures for controlling COVID-19 in the UK. Water Res. 2021;200:117214. doi: 10.1016/j.watres.2021.117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary S., et al. Monitoring SARS-CoV-2 in municipal wastewater to evaluate the success of lockdown measures for controlling COVID-19 in the UK. Water Res. 2021;200:117214. doi: 10.1016/j.watres.2021.117214. in eng. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmsø M.H., Hellmér M., Fernandez-Cassi X., Timoneda N., Lukjancenko O., Seidel M., et al. Evaluation of methods for the concentration and extraction of viruses from sewage in the context of metagenomic sequencing. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokajärvi A.M., Rytkönen A., Tiwari A., Kauppinen A., Oikarinen S., Lehto K.M., et al. The detection and stability of the SARS-CoV-2 RNA biomarkers in wastewater influent in Helsinki, Finland. Sci. Total Environ. 2021;770:145274. doi: 10.1016/j.scitotenv.2021.145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokajärvi M., et al. The detection and stability of the SARS-CoV-2 RNA biomarkers in wastewater influent in Helsinki, Finland. Sci. Total Environ. 2021;770:145274. doi: 10.1016/j.scitotenv.2021.145274. in eng. May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong P.Y., Rachmadi A.T., Mantilla-Calderon D., Alkahtani M., Bashawri Y.M., Al Qarni H., et al. Estimating the minimum number of SARS-CoV-2 infected cases needed to detect viral RNA in wastewater: to what extent of the outbreak can surveillance of wastewater tell us? Environ. Res. 2021;195:110748. doi: 10.1016/j.envres.2021.110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong P.Y., et al. Estimating the minimum number of SARS-CoV-2 infected cases needed to detect viral RNA in wastewater: to what extent of the outbreak can surveillance of wastewater tell us? Environ. Res. 2021;195:110748. doi: 10.1016/j.envres.2021.110748. in eng. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Muller C.J.F., Ghoor S., Louw J., Archer E., Surujlal-Naicker S., et al. Qualitative and quantitative detection of SARS-CoV-2 RNA in untreated wastewater in Western Cape Province, South Africa. S. Afr. Med. J. 2021;111(3):198–202. doi: 10.7196/SAMJ.2021.v111i3.15154. [DOI] [PubMed] [Google Scholar]
- Johnson R., et al. Qualitative and quantitative detection of SARS-CoV-2 RNA in untreated wastewater in Western Cape Province, South Africa. S. Afr. Med. J. 2021;111(3):198–202. doi: 10.7196/SAMJ.2021.v111i3.15154. in eng. Jan 28. [DOI] [PubMed] [Google Scholar]
- Jones D.L., Baluja M.Q., Graham D.W., Corbishley A., McDonald J.E., Malham S.K., et al. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci. Total Environ. 2020;749:141364. doi: 10.1016/j.scitotenv.2020.141364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen A.C.U., Gamst J., Hansen L.V., Knudsen I.I.H., Jensen S.K.S. Eurofins Covid-19 SentinelTM wastewater test provide early warning of a potential COVID-19 outbreak. medRxiv. 2020 (2020.07.10.20150573) [Google Scholar]
- Jørgensen C.U., Gamst J., Hansen L.V., Knudsen I.I.H., Jensen S.K.S. Eurofins Covid-19 SentinelTM wastewater test provide early warning of a potential COVID-19 outbreak. medRxiv. 2020 doi: 10.1101/2020.07.10.20150573. p. 2020.07.10.20150573. [DOI] [Google Scholar]
- Karthikeyan S., Ronquillo N., Belda-Ferre P., Alvarado D., Javidi T., Longhurst C.A., et al. High-throughput wastewater SARS-CoV-2 detection enables forecasting of community infection dynamics in San Diego County. mSystems. 2021;6(2) doi: 10.1128/mSystems.00045-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan S., et al. High-throughput wastewater SARS-CoV-2 detection enables forecasting of community infection dynamics in San Diego County. mSystems. 2021;6(2) doi: 10.1128/mSystems.00045-21. in eng. Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., et al. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Sadamasu K., Muramatsu M., Yoshida H. Efficient detection of SARS-CoV-2 RNA in the solid fraction of wastewater. Sci. Total Environ. 2021;763:144587. doi: 10.1016/j.scitotenv.2020.144587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Sadamasu M. Muramatsu, Yoshida H. Efficient detection of SARS-CoV-2 RNA in the solid fraction of wastewater. Sci. Total Environ. 2021;763:144587. doi: 10.1016/j.scitotenv.2020.144587. in eng. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D. Migrant workers and COVID-19. Occup. Environ. Med. 2020;77(9):634–636. doi: 10.1136/oemed-2020-106626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koureas M., Amoutzias G.D., Vontas A., Kyritsi M., Pinaka O., Papakonstantinou A., et al. Wastewater monitoring as a supplementary surveillance tool for capturing SARS-COV-2 community spread. A case study in two Greek municipalities. Environ Res. 2021;200:111749. doi: 10.1016/j.envres.2021.111749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koureas, et al. Wastewater monitoring as a supplementary surveillance tool for capturing SARS-COV-2 community spread. A case study in two Greek municipalities. Environ. Res. 2021;200:111749. doi: 10.1016/j.envres.2021.111749. in eng. Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., et al. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., et al. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Joshi M., Patel A.K., Joshi C.G. Unravelling the early warning capability of wastewater surveillance for COVID-19: a temporal study on SARS-CoV-2 RNA detection and need for the escalation. Environ. Res. 2021;196:110946. doi: 10.1016/j.envres.2021.110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Joshi M., Shah A.V., Srivastava V., Dave S. Wastewater surveillance-based city zonation for effective COVID-19 pandemic preparedness powered by early warning: a perspectives of temporal variations in SARS-CoV-2-RNA in Ahmedabad, India. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, Joshi M., Patel A.K., Joshi C.G. Unravelling the early warning capability of wastewater surveillance for COVID-19: a temporal study on SARS-CoV-2 RNA detection and need for the escalation. Environ. Res. 2021;196:110946. doi: 10.1016/j.envres.2021.110946. in eng. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, Joshi M., Shah A.V., Srivastava V., Dave S. Wastewater surveillance-based city zonation for effective COVID-19 pandemic preparedness powered by early warning: a perspectives of temporal variations in SARS-CoV-2-RNA in Ahmedabad, India. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148367. in English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryntseva P.A., Karamova K.O., Fomin V.P., Selivanovskaya S.Y., Galitskaya P.Y. A simplified approach to monitoring the COVID-19 epidemiologic situation using waste water analysis and its application in Russia. medRxiv. 2020 (2020.09.21.20197244) [Google Scholar]
- Kuryntseva P.A., Karamova K.O., Fomin V.P., Selivanovskaya S.Y., Galitskaya P.Y. A simplified approach to monitoring the COVID-19 epidemiologic situation using waste water analysis and its application in Russia. medRxiv. 2020 doi: 10.1101/2020.09.21.20197244. p. 2020.09.21.20197244. [DOI] [Google Scholar]
- La Rosa G., Della Libera S., Iaconelli M., Ciccaglione A.R., Bruni R., Taffon S., et al. Surveillance of hepatitis A virus in urban sewages and comparison with cases notified in the course of an outbreak, Italy 2013. BMC Infect. Dis. 2014;14(1):419. doi: 10.1186/1471-2334-14-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., et al. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., et al. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Mancini P., Bonanno Ferraro G., Veneri C., Iaconelli M., Bonadonna L., et al. SARS-CoV-2 has been circulating in northern Italy since December 2019: evidence from environmental monitoring. Sci. Total Environ. 2021;750:141711. doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., et al. SARS-CoV-2 has been circulating in northern Italy since December 2019: evidence from environmental monitoring. Sci. Total Environ. 2021;750:141711. doi: 10.1016/j.scitotenv.2020.141711. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Di D.Y.W., Saingam P., Jeon M.K., Yan T. Fine-scale temporal dynamics of SARS-CoV-2 RNA abundance in wastewater during a COVID-19 lockdown. Water Res. 2021;197:117093. doi: 10.1016/j.watres.2021.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Di D.Y.W., Saingam P., Jeon M.K., Yan T. Fine-scale temporal dynamics of SARS-CoV-2 RNA abundance in wastewater during A COVID-19 lockdown. Water Res. 2021;197:117093. doi: 10.1016/j.watres.2021.117093. in eng. Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor Y., Handsher R., Halmut T., Neuman M., Bobrov A., Rudich H., et al. Detection of poliovirus circulation by environmental surveillance in the absence of clinical cases in Israel and the Palestinian authority. J. Clin. Microbiol. 1999;37(6):1670–1675. doi: 10.1128/jcm.37.6.1670-1675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Klapsa D., Wilton T., Zambon M., Bentley E., Bujaki E., et al. Tracking SARS-CoV-2 in sewage: evidence of changes in virus variant predominance during COVID-19 pandemic. Viruses. 2020;12(10):1144. doi: 10.3390/v12101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., et al. Tracking SARS-CoV-2 in sewage: evidence of changes in virus variant predominance during COVID-19 pandemic. Viruses. 2020;12(10):1144. doi: 10.3390/v12101144. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in Sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. 2020/07/14. [DOI] [PubMed] [Google Scholar]
- Melvin R.G., Chaudhry N., Georgewill O., Freese R., Simmons G.E. Predictive power of SARS-CoV-2 wastewater surveillance for diverse populations across a large geographical range. medRxiv. 2021 [Google Scholar]
- Melvin R.G., Chaudhry N., Georgewill O., Freese R., Simmons G.E. Predictive power of SARS-CoV-2 wastewater surveillance for diverse populations across a large geographical range. medRxiv. 2021 doi: 10.1101/2021.01.23.21250376. in eng. Jan 30. [DOI] [Google Scholar]
- Mondal S., Feirer N., Brockman M., Preston M.A., Teter S.J., Ma D., et al. A direct capture method for purification and detection of viral nucleic acid enables epidemiological surveillance of SARS-CoV-2. Sci. Total Environ. 2021;795:148834. doi: 10.1016/j.scitotenv.2021.148834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S., et al. A direct capture method for purification and detection of viral nucleic acid enables epidemiological surveillance of SARS-CoV-2. Sci. Total Environ. 2021;795:148834. doi: 10.1016/j.scitotenv.2021.148834. in eng. Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota C.R., Bressani-Ribeiro T., Araújo J.C., Leal C.D., Leroy-Freitas D., Machado E.C., et al. Assessing spatial distribution of COVID-19 prevalence in Brazil using decentralised sewage monitoring. Water Res. 2021;202:117388. doi: 10.1016/j.watres.2021.117388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota C.R., et al. Assessing spatial distribution of COVID-19 prevalence in Brazil using decentralised sewage monitoring. Water Res. 2021;202:117388. doi: 10.1016/j.watres.2021.117388. in eng. Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N. S. W. Government . New South Wales Government; 2020. COVID-19 Weekly Surveillance in Nsw - Epidemiological Week 40, Ending 3 October 2020.https://www.health.nsw.gov.au/Infectious/covid-19/Documents/covid-19-surveillance-report-20201009.pdf [Online]. Available: [Google Scholar]
- N. S. W. Government . New South Wales Government; 2020. COVID-19 Weekly Surveillance in Nsw - Epidemiological Week 41, Ending 10 October 2020.https://www.health.nsw.gov.au/Infectious/covid-19/Documents/covid-19-surveillance-report-20201010.pdf [Online]. Available: [Google Scholar]
- N. S. W. Government . New South Wales Government; 2020. COVID-19 Weekly Surveillance in NSW - Epidemiological Week 42, Ending 17 October 2020.https://www.health.nsw.gov.au/Infectious/covid-19/Documents/covid-19-surveillance-report-20201017.pdf [Online]. Available: [Google Scholar]
- N. S. W. Government . New South Wales Government; 2020. COVID-19 Weekly Surveillance in NSW - Epidemiological Week 43, Ending 24 October 2020.https://www.health.nsw.gov.au/Infectious/covid-19/Documents/covid-19-surveillance-report-20201024.pdf [Online]. Available: [Google Scholar]
- Neault N., Baig A.T., Graber T.E., D’Aoust P.M., Mercier E., Alexandrov I., et al. SARS-CoV-2 protein in wastewater mirrors COVID-19 prevalence. medRxiv. 2020 (2020.09.01.20185280) [Google Scholar]
- Nelson D.B., Circo R., Evans A.S. Strategic viral surveillance of sewage during and following an oral poliovirus vaccine campaign. Am. J. Epidemiol. 1967;86(3):641–652. doi: 10.1093/oxfordjournals.aje.a120773. [DOI] [PubMed] [Google Scholar]
- Nemudryi, et al. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep. Med. 2020;1(6) doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., et al. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep. Med. 2020;1(6) doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal D., Prakash O., Bobde P., Pal S. SARS-CoV-2: sewage surveillance as an early warning system and challenges in developing countries. Environ. Sci. Pollut. Res. 2021;28(18):22221–22240. doi: 10.1007/s11356-021-13170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey D., Verma S., Verma P., Mahanty B., Dutta K., Daverey A., et al. SARS-CoV-2 in wastewater: challenges for developing countries. Int. J. Hyg. Environ. Health. 2021;231:113634. doi: 10.1016/j.ijheh.2020.113634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasa S., Desai M., Thoguluva Chandrasekar V., Patel H.K., Kennedy K.F., Roesch T., et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Network Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Cook A.R., Lim J.T., Sun Y., Dickens B.L. A systematic review of COVID-19 epidemiology based on current evidence. J. Clin. Med. 2020;9(4):967. doi: 10.3390/jcm9040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., et al. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38(10):1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., et al. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38(10):1164–1167. doi: 10.1038/s41587-020-0684-z. 2020/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay L., Amoah I.D., Deepnarain N., Pillay K., Awolusi O.O., Kumari S., et al. Monitoring changes in COVID-19 infection using wastewater-based epidemiology: a South African perspective. Sci. Total Environ. 2021;786:147273. doi: 10.1016/j.scitotenv.2021.147273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay L., et al. Monitoring changes in COVID-19 infection using wastewater-based epidemiology: a South African perspective. Sci. Total Environ. 2021;786:147273. doi: 10.1016/j.scitotenv.2021.147273. in eng. Sep 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., Fumian T.M., Mannarino C.F., Maranhão A.G., Siqueira M.M., Miagostovich M.P. Preliminary results of SARS-CoV-2 detection in sewerage system in Niterói municipality, Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2020;115 doi: 10.1590/0074-02760200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., Fumian T.M., Mannarino C.F., Maranhão A.G., Siqueira M.M., Miagostovich M.P. Preliminary results of SARS-CoV-2 detection in sewerage system in Niterói municipality, Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2020;115:e200196. doi: 10.1590/0074-02760200196. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., Fumian T.M., Mannarino C.F., Resende P.C., Motta F.C., Eppinghaus A.L.F., et al. Wastewater-based epidemiology as a useful tool to track SARS-CoV-2 and support public health policies at municipal level in Brazil. Water Res. 2021;191:116810. doi: 10.1016/j.watres.2021.116810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., et al. Wastewater-based epidemiology as a useful tool to track SARS-CoV-2 and support public health policies at municipal level in Brazil. Water Res. 2021;191:116810. doi: 10.1016/j.watres.2021.116810. in eng. Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pung R., Chiew C.J., Young B.E., Chin S., Chen M.I.C., Clapham H.E., et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395(10229):1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada J.A., López-Pineda A., Gil-Guillén V.F., Arriero-Marín J.M., Gutiérrez F., Carratala-Munuera C. Incubation period of COVID-19: a systematic review and meta-analysis. Rev. Clin. Esp. (Barc.) 2021;221(2):109–117. doi: 10.1016/j.rceng.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee M., Isazadeh S., Mohseni-Bandpei A., Mohebbi S.R., Jahangiri-Rad M., Eslami A., et al. Moore swab performs equal to composite and outperforms grab sampling for SARS-CoV-2 monitoring in wastewater. Sci. Total Environ. 2021;790:148205. doi: 10.1016/j.scitotenv.2021.148205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee, et al. Moore swab performs equal to composite and outperforms grab sampling for SARS-CoV-2 monitoring in wastewater. Sci. Total Environ. 2021;790:148205. doi: 10.1016/j.scitotenv.2021.148205. in eng. Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Cuevas-Ferrando E., Sanjuán R., Domingo-Calap P., Sánchez G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Int. J. Hyg. Environ. Health. 2020;230:113621. doi: 10.1016/j.ijheh.2020.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Cuevas-Ferrando E., Sanjuán R., Domingo-Calap P., Sánchez G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Int. J. Hyg. Environ. Health. 2020;230:113621. doi: 10.1016/j.ijheh.2020.113621. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Róka, et al. Ahead of the second wave: Early warning for COVID-19 by wastewater surveillance in Hungary. Sci. Total Environ. 2021;786:147398. doi: 10.1016/j.scitotenv.2021.147398. in eng. Sep 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Róka E., Khayer B., Kis Z., Kovács L.B., Schuler E., Magyar N., et al. Ahead of the second wave: early warning for COVID-19 by wastewater surveillance in Hungary. Sci. Total Environ. 2021;786:147398. doi: 10.1016/j.scitotenv.2021.147398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiñol M., Zammit I., Itarte M., Forés E., Martínez-Puchol S., Girones R., et al. Monitoring waves of the COVID-19 pandemic: inferences from WWTPs of different sizes. Sci. Total Environ. 2021;787:147463. doi: 10.1016/j.scitotenv.2021.147463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiñol M., et al. Monitoring waves of the COVID-19 pandemic: Inferences from WWTPs of different sizes. Sci. Total Environ. 2021;787:147463. doi: 10.1016/j.scitotenv.2021.147463. in eng. Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saguti F., Magnil E., Enache L., Churqui M.P., Johansson A., Lumley D., et al. Surveillance of wastewater revealed peaks of SARS-CoV-2 preceding those of hospitalized patients with COVID-19. Water Res. 2021;189:116620. doi: 10.1016/j.watres.2020.116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saguti, et al. Surveillance of wastewater revealed peaks of SARS-CoV-2 preceding those of hospitalized patients with COVID-19. Water Res. 2021;189:116620. doi: 10.1016/j.watres.2020.116620. in eng. Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saththasivam J., El-Malah S.S., Gomez T.A., Jabbar K.A., Remanan R., Krishnankutty A.K., et al. COVID-19 (SARS-CoV-2) outbreak monitoring using wastewater-based epidemiology in Qatar. Sci. Total Environ. 2021;774:145608. doi: 10.1016/j.scitotenv.2021.145608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saththasivam J., et al. COVID-19 (SARS-CoV-2) outbreak monitoring using wastewater-based epidemiology in Qatar. Sci. Total Environ. 2021;774:145608. doi: 10.1016/j.scitotenv.2021.145608. in eng. Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L.C., Aubee A., Babahaji L., Vigil K., Tims S., Aw T.G. Targeted wastewater surveillance of SARS-CoV-2 on a university campus for COVID-19 outbreak detection and mitigation. Environ. Res. 2021;200 doi: 10.1016/j.envres.2021.111374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L.C., Aubee A., Babahaji L., Vigil K., Tims S., Aw T.G. Targeted wastewater surveillance of SARS-CoV-2 on a university campus for COVID-19 outbreak detection and mitigation. Environ. Res. 2021;200:111374. doi: 10.1016/j.envres.2021.111374. in eng. May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif S., Ikram A., Khurshid A., Salman M., Mehmood N., Arshad Y., et al. Detection of SARs-CoV-2 in wastewater, using the existing environmental surveillance network: an epidemiological gateway to an early warning for COVID-19 in communities. medRxiv. 2020 (2020.06.03.20121426) [Google Scholar]
- Sharif S., et al. Detection of SARs-CoV-2 in wastewater, using the existing environmental surveillance network: An epidemiological gateway to an early warning for COVID-19 in communities. medRxiv. 2020 doi: 10.1101/2020.06.03.20121426. p. 2020.06.03.20121426. [DOI] [Google Scholar]
- Sharma D.K., Nalavade U.P., Kalgutkar K., Gupta N., Deshpande J.M. SARS-CoV-2 detection in sewage samples: standardization of method & preliminary observations. Indian J. Med. Res. 2021;153(1 & 2):159–165. doi: 10.4103/ijmr.IJMR_3541_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D.K., Nalavade U.P., Kalgutkar K., Gupta N., Deshpande J.M. SARS-CoV-2 detection in sewage samples: Standardization of method & preliminary observations. Indian J. Med. Res. 2021;153(1 & 2):159–165. doi: 10.4103/ijmr.IJMR_3541_20. in eng. Jan & Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., et al. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., et al. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. in eng. Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims N., Kasprzyk-Hordern B. Future perspectives of wastewater-based epidemiology: monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020;139:105689. doi: 10.1016/j.envint.2020.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair R.G., Choi C.Y., Riley M.R., Gerba C.P. Pathogen surveillance through monitoring of sewer systems. Adv. Appl. Microbiol. 2008;65:249–269. doi: 10.1016/S0065-2164(08)00609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurbeck R.R., Minard-Smith A., Catlin L. Feasibility of neighborhood and building scale wastewater-based genomic epidemiology for pathogen surveillance. Sci. Total Environ. 2021;789:147829. doi: 10.1016/j.scitotenv.2021.147829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurbeck R.R., Minard-Smith A., Catlin L. Feasibility of neighborhood and building scale wastewater-based genomic epidemiology for pathogen surveillance. Sci. Total Environ. 2021;789:147829. doi: 10.1016/j.scitotenv.2021.147829. in eng. Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street R., Malema S., Mahlangeni N., Mathee A. Wastewater surveillance for Covid-19: an African perspective. Sci. Total Environ. 2020;743:140719. doi: 10.1016/j.scitotenv.2020.140719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanhaei M., Mohebbi S.R., Hosseini S.M., Rafieepoor M., Kazemian S., Ghaemi A., et al. The first detection of SARS-CoV-2 RNA in the wastewater of Tehran, Iran. Environ. Sci. Pollut. Res. Int. 2021;28(29):38629–38636. doi: 10.1007/s11356-021-13393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanhaei M., et al. The first detection of SARS-CoV-2 RNA in the wastewater of Tehran, Iran. Environ. Sci. Pollut. Res. Int. 2021;28(29):38629–38636. doi: 10.1007/s11356-021-13393-9. in eng. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.-C., Barbu M.-G., Beiu C., Popa L.G., Mihai M.M., Berteanu M., et al. The impact of COVID-19 pandemic on long-term care facilities worldwide: an overview on international issues. BioMed Res. Int. 2020;2020:8870249. doi: 10.1155/2020/8870249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasino M.P., Semedo M., Vieira e Moreira P., Ferraz E., Rocha A., Carvalho M.F., et al. SARS-CoV-2 RNA detected in urban wastewater from Porto, Portugal: method optimization and continuous 25-week monitoring. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasino M.P., et al. SARS-CoV-2 RNA detected in urban wastewater from Porto, Portugal: method optimization and continuous 25-week monitoring. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148467. in English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier J., Darques R., Ait Mouheb N., Partiot E., Bakhache W., Deffieu M.S., et al. Post-lockdown detection of SARS-CoV-2 RNA in the wastewater of Montpellier, France. One Health. 2020;10:100157. doi: 10.1016/j.onehlt.2020.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier J., et al. Post-lockdown detection of SARS-CoV-2 RNA in the wastewater of Montpellier, France. One Health. 2020;10:100157. doi: 10.1016/j.onehlt.2020.100157. in eng. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo J.A., Rumbo-Feal S., Conde-Pérez K., López-Oriona Á., Tarrío-Saavedra J., Reif R., et al. Predicting the number of people infected with SARS-COV-2 in a population using statistical models based on wastewater viral load. medRxiv. 2020 (2020.07.02.20144865) [Google Scholar]
- Vallejo J.A., et al. Predicting the number of people infected with SARS-COV-2 in a population using statistical models based on wastewater viral load. medRxiv. 2020 doi: 10.1101/2020.07.02.20144865. p. 2020.07.02.20144865. [DOI] [Google Scholar]
- Viswanathan M., Patnode C.D., ND B. Agency for Healthcare Research and Quality (US); Rockville (MD): 2017. Assessing the Risk of Bias in Systematic Reviews of Health Care Interventions Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]https://www.ncbi.nlm.nih.gov/books/NBK519366/ Available from: [PubMed] [Google Scholar]
- Wang X., Zheng J., Guo L., Yao H., Wang L., Xia X., et al. Fecal viral shedding in COVID-19 patients: Clinical significance, viral load dynamics and survival analysis. Virus Res. 2020;289:198147. doi: 10.1016/j.virusres.2020.198147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannigama D.L., Amarasiri M., Hurst C., Phattharapornjaroen P., Abe S., Hongsing P., et al. Tracking COVID-19 with wastewater to understand asymptomatic transmission. Int. J. Infect. Dis. 2021;108:296–299. doi: 10.1016/j.ijid.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannigama D.L., et al. Tracking COVID-19 with wastewater to understand asymptomatic transmission. Int. J. Infect. Dis. 2021;108:296–299. doi: 10.1016/j.ijid.2021.05.005. in eng. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J., Aanderud Z.T., Roper D.K., VanDerslice J., Gaddis E.B., Ostermiller J., et al. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021;775:145790. doi: 10.1016/j.scitotenv.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J., et al. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021;775:145790. doi: 10.1016/j.scitotenv.2021.145790. in eng. Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhaus S., Weber F.-A., Schiwy S., Linnemann V., Brinkmann M., Widera M., et al. Detection of SARS-CoV-2 in raw and treated wastewater in Germany - suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751:141750. doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhaus S., et al. Detection of SARS-CoV-2 in raw and treated wastewater in Germany - suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751:141750. doi: 10.1016/j.scitotenv.2020.141750. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder M.L., Middleton F., Larsen D.A., Du Q., Fenty A., Zeng T., et al. Co-quantification of crAssphage increases confidence in wastewater-based epidemiology for SARS-CoV-2 in low prevalence areas. Water Res. X. 2021;11:100100. doi: 10.1016/j.wroa.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder M.L., et al. Co-quantification of crAssphage increases confidence in wastewater-based epidemiology for SARS-CoV-2 in low prevalence areas. Water Res. X. 2021;11:100100. doi: 10.1016/j.wroa.2021.100100. in eng. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton T., Bujaki E., Klapsa D., Majumdar M., Zambon M., Fritzsche M., et al. Rapid increase of SARS-CoV-2 variant B.1.1.7 detected in sewage samples from England between October 2020 and January 2021. mSystems. 2021;6(3) doi: 10.1128/mSystems.00353-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton T., et al. Rapid increase of SARS-CoV-2 variant B.1.1.7 detected in sewage samples from England between October 2020 and January 2021. mSystems. 2021;6(3):e0035321. doi: 10.1128/mSystems.00353-21. in eng. Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.C.C., Tan J., Lim Y.X., Arivalan S., Hapuarachchi H.C., Mailepessov D., et al. Non-intrusive wastewater surveillance for monitoring of a residential building for COVID-19 cases. Sci. Total Environ. 2021;786:147419. doi: 10.1016/j.scitotenv.2021.147419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.C.C., et al. Non-intrusive wastewater surveillance for monitoring of a residential building for COVID-19 cases. Sci. Total Environ. 2021;786:147419. doi: 10.1016/j.scitotenv.2021.147419. in eng. Sep 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M.C., Huang J., Lai C., Ng R., Chan F.K.L., Chan P.K.S. Detection of SARS-CoV-2 RNA in fecal specimens of patients with confirmed COVID-19: a meta-analysis. J. Inf. Secur. 2020;81(2) doi: 10.1016/j.jinf.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., et al. SARS-CoV-2 titers in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. medRxiv. 2020 doi: 10.1016/j.scitotenv.2021.150121. (2020.06.15.20117747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., et al. SARS-CoV-2 titers in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. medRxiv. 2020 doi: 10.1101/2020.06.15.20117747. p. 2020.06.15.20117747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., et al. Wastewater surveillance of SARS-CoV-2 across 40 U.S. states from February to June 2020. Water Res. 2021;202 doi: 10.1016/j.watres.2021.117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, et al. Wastewater surveillance of SARS-CoV-2 across 40 U.S. states from February to June 2020. Water Res. 2021;202:117400. doi: 10.1016/j.watres.2021.117400. in eng. Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz N., Lacoste A., Jardot P., Delache A., Fontaine X., Verlande M., et al. Viral RNA in city wastewater as a key indicator of COVID-19 recrudescence and containment measures effectiveness. Front. Microbiol. 2021;12:664477. doi: 10.3389/fmicb.2021.664477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz, et al. Viral RNA in city wastewater as a key indicator of COVID-19 recrudescence and containment measures effectiveness. Front. Microbiol. 2021;12:664477. doi: 10.3389/fmicb.2021.664477. in eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., et al. Evaluation of lockdown effect on SARS-CoV-2 dynamics through viral genome quantification in waste water, Greater Paris, France, 5 March to 23 April 2020. Euro Surveill. 2020;25(50) doi: 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., et al. Evaluation of lockdown effect on SARS-CoV-2 dynamics through viral genome quantification in waste water, Greater Paris, France, 5 March to 23 April 2020. Euro Surveill. 2020;25(50) doi: 10.2807/1560-7917.Es.2020.25.50.2000776. in eng. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao A., Wu F., Bushman M., Zhang J., Imakaev M., Chai P.R., et al. Metrics to relate COVID-19 wastewater data to clinical testing dynamics. medRxiv. 2021 doi: 10.1016/j.watres.2022.118070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, et al. Metrics to relate COVID-19 wastewater data to clinical testing dynamics. medRxiv. 2021 doi: 10.1101/2021.06.10.21258580. in eng. Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zheng X., Li S., Lam N.S., Wang Y., Chu D.K.W., et al. The first case study of wastewater-based epidemiology of COVID-19 in Hong Kong. Sci. Total Environ. 2021;790:148000. doi: 10.1016/j.scitotenv.2021.148000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., et al. The first case study of wastewater-based epidemiology of COVID-19 in Hong Kong. Sci. Total Environ. 2021;790:148000. doi: 10.1016/j.scitotenv.2021.148000. in eng. May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv K., Shagan M., Lewis Y.E., Kramarsky-Winter E., Weil M., Indenbaum V., et al. City-level SARS-CoV-2 sewage surveillance. Chemosphere. 2021;283 doi: 10.1016/j.chemosphere.2021.131194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv K., et al. City-level SARS-CoV-2 sewage surveillance. 2021;283 doi: 10.1016/j.chemosphere.2021.131194. in English. Chemosphere. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi A., Monis P., Deere D., Ryan U. Wastewater-based epidemiology-surveillance and early detection of waterborne pathogens with a focus on SARS-CoV-2, Cryptosporidium and Giardia. Parasitol. Res. 2021:1–22. doi: 10.1007/s00436-020-07023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables