Abstract
Background
In this study, we report clinical outcomes in COVID-19 infection in a large cohort of people with cystic fibrosis (pwCF) and compare these outcomes to a propensity score matched cohort of people without CF.
Methods
Analysis of a multicenter research network TriNETX was performed including patients more than 16 years of age diagnosed with COVID-19. Outcomes in COVID-19 positive pwCF were compared with a propensity-matched cohort of people without CF.
Results
A total of 507,810 patients with COVID-19 were included (422 patients, 0.08% with CF; 507,388 patients, 99.92% without CF. Mean age at COVID-19 diagnosis in CF cohort was 46.6 ± 19.3 years, with female predominance (n = 225, 53.32%). Majority of the participants were Caucasian (n = 309, 73.22%). In the crude, unmatched analysis, mortality, hospitalization, critical care need, mechanical ventilation, acute kidney injury and composite (combination of intubation and mortality) outcome at 30 days was higher in the pwCF. Following robust propensity matching, pwCF had higher hospitalization rate (RR 1.56, 95% CI 1.20–2.04), critical care need (RR 1.78, 95% CI 1.13–2.79), and acute renal injury (RR 1.60, 95% CI 1.07–2.39) as compared to patients without CF.
Conclusion
People with CF are at risk of poor outcomes with COVID-19.5.2% of these patients died within one month of COVID-19 diagnosis, and more than one in 10 patients required critical care. Therefore, the relatively young median age of cystic fibrosis patients, and lower prevalence of obesity do not protect these patients from severe disease contrary to prior reports.
Keywords: Coronavirus, COVID 19, Cystic fibrosis
Abbreviations: BMI, body mass index; CF, Cystic Fibrosis; COVID-19, Coronavirus disease 2019; CDC, Centers for Disease Control and Prevention; EHRs, Electronic health record management systems; HCOs, Health Care Organizations; pwCF, people with Cystic Fibrosis; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); SD, Standard deviation; WVCTSI, West Virginia University Clinical and Translational Science Institute
1. Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) related disease, COVID 19, has taken the shape of a pandemic, with more than 4 million deaths reported worldwide. Its impact on groups of patients with pre-existing pulmonary disease needs to be studied in order to better inform patients, families, as well as for formulation of public health policies and preventive strategies.
There is a paucity of data on COVID-19 disease in people with cystic fibrosis (pwCF). COVID-19 infection triggers cytokine dysregulation, and can result in acute respiratory distress syndrome [1]. Worse outcomes in patients with chronic lung diseases have previously been noted, and previously, there was some concern that patient's with cystic fibrosis, due to pre-existing pulmonary disease, as well as CF related hyper inflammation and cytokine dysfunction [[2], [3], [4]], may be at risk of severe disease with COVID-19. However the limited previously available data has noted that outcomes may not be as poor as previously thought, and the course of disease may be milder than the general population [[5], [6], [7]].
Here in, we report the outcomes of COVID-19 disease in a large cohort of patients with pre-existing cystic fibrosis and compare these outcomes to a propensity score matched cohort of patients without cystic fibrosis.
2. Methods
2.1. Study design and data source
This is a retrospective cohort study, conducted on multi-institutional research network TriNETX (Cambridge, MA, USA) platform. TriNETX is a federated research network which includes more than 40 Health Care Organizations (HCOs) in the United States of America. It provides real time access to healthcare records and includes data of more than 40 million patients from different healthcare organizations, in a de-identified fashion. The data is retrieved in real-time from the hospital's electronic health record management systems (EHRs) of each participating organization, which are large academic centers that operate both tertiary care and satellite outpatient office locations.
The server derives clinical variables (referred as facts directly from clinical documents through a built-in Natural Language Processing system. Robust quality assurance is achieved at the time of extraction from EHRs before inclusion in this database. TriNETX only provides aggregate counts and statistical summaries to protect identifiable patient's health information and ensures that the data remain de-identified at all levels of retrieval and dissemination.
TriNetX has received waiver from Western IRB. At our institution West Virginia University Clinical and Translational Science Institute (WVCTSI) manages the TriNetX platform and provides access to the end-users.
2.2. Study population, variables and outcomes
A real time search was conducted on the TriNetX platform to identify patients with SARS-CoV-2 infection more than 16 years of age using International Classification of Diseases, Ninth Revision and tenth Revision, Clinical Modification (ICD-10-CM) codes and Logical Observation Identifiers Names and Codes (LOINC) codes for positive laboratory tests, as recommended by Center of Disease Control (CDC) COVID-19 coding. These codes are illustrated in Supplemental Table 1. This search strategy is previously used in published literature from the TriNetX. Following which the cohort was sub-divided into people with cystic fibrosis and people without cystic fibrosis, utilizing ICD-10-CM code: E84 (Supplemental Table 1). This study method has been previous validated [[8], [9], [10]].
Study duration: Participants diagnosed with COVID-19 disease between January 20, 2020, and January 09, 2021 were included in the study cohort. January 20, 2020 was chosen as it was the date of diagnosis of the first case of SARS-CoV-2 infection in the USA. January 09, 2021 was chosen to ensure 30 days of follow up for all included patients, as the primary study endpoint was a composite outcome at 30 days from diagnosis. The search was updated through February 10, 2021.
Study Outcome: Index event was defined as either positive SARS-CoV-2 test or COVID-19 diagnosis. Study outcomes were assessed at 30 days from index event and included the day of index event. Baseline characteristics for patients were considered until the day of COVID-19 diagnosis or first positive SARS-CoV-2 test result. Primary study outcome was a composite event of death or requirement for mechanical ventilation in 30-day period from the index event. Other outcomes included death, hospitalization, and critical care need in the 30-day follow up period.
2.3. Statistical analysis
All analysis was performed on TriNetX platform. Chi square test and T-test were used for univariate analysis. 1:1 propensity score matching was performed with age, race, diabetes, hypertension, chronic lung diseases, chronic kidney disease, nicotine dependence, heart failure, ischemic heart disease, body mass index (BMI), and gender as covariates and a propensity score matched control group of patients without CF was identified. 1:1 matching was performed based on the propensity scores generated by using greedy nearest neighbor algorithms utilizing a caliper width of 0.1 pooled standard deviations (SD0). Balance on covariates was assessed using standardized mean difference, and absolute values > 0.1 were considered positive for residual imbalance. A two-sided alpha of less than 0.05 was defined a priori for statistical significance. Risk ratios with 95% confidence intervals were calculated for all analyses.
Details of propensity score matching: TriNetX platform utilizes input matrices of user identified covariates and conducts logistic regression analysis to obtain propensity scores for individual subjects. 1:1 matching was performed based on the propensity scores generated by using greedy nearest neighbor algorithms utilizing a caliper width of 0.1 pooled standard deviations (SD). TriNetX randomizes the order of rows in order to eliminate bias resulting from nearest neighbor algorithms. This study method has been previous validated [[8], [9], [10]].
3. Results
3.1. Study population
A total of 507,810 patients with COVID-19 were included. Of these identified patients, 422 patients (0.08%) carried a diagnosis of CF, while the rest 507,388 patients (99.92%) were included in the non-CF cohort. Mean age at the time of COVID-19 diagnosis in pwCF was 46.6 ± 19.3 years, with female predominance (n = 225, 53.32%). Majority of the participants were Caucasian (n = 309, 73.22%). Co-morbidities including hypertension, chronic lower respiratory diseases, diabetes mellitus, ischemic heart disease, nicotine dependence, and chronic kidney disease were more common in pwCF when compared to the people without CF (all p values < 0.01). Baseline demographic characteristics and comorbid conditions are described in Table 1 .
Table 1.
Comparison of demographic and clinical characteristics of COVID-19 patients with and without Cystic Fibrosis before and after propensity score matching.
|
Variable |
Before matching |
After matching |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cystic Fibrosis cohort (n = 422) |
Non-CF cohort (n = 507,388) |
Std Diff | Cystic Fibrosis cohort (n = 413) |
Non-CF cohort (n = 413) |
Std Diff | |||||
| Number | Percent/SD | Number | Percent/SD | Number | Percent/SD | Number | Percent/SD | |||
| Demographics | ||||||||||
| Age (Mean) | 46.6 | 19.3 | 46.7 | 19.1 | <0.01 | 47.0 | 19.3 | 47.5 | 19.3 | 0.01 |
| Male | 197 | 46.68 | 223,703 | 44.09 | 0.05 | 191 | 46.25 | 187 | 45.28 | 0.02 |
| Female | 225 | 53.32 | 281,108 | 55.40 | 0.04 | 222 | 53.75 | 226 | 55.42 | 0.02 |
| BMI (30 and above) | 113 | 26.78 | 107,267 | 21.14 | 0.13 | 113 | 27.36 | 130 | 31.48 | 0.09 |
| African American | 58 | 13.74 | 86,406 | 17.03 | 0.09 | 58 | 14.04 | 62 | 15.01 | 0.03 |
| Caucasian | 309 | 73.22 | 310,774 | 61.25 | 0.26 | 301 | 72.88 | 298 | 72.16 | 0.02 |
| Hispanic or Latino | 47 | 11.34 | 65,000 | 12.81 | 0.05 | 47 | 11.38 | 44 | 10.65 | 0.02 |
| Comorbidities | ||||||||||
| Hypertension | 203 | 48.10 | 146,339 | 28.84 | 0.40 | 197 | 47.7 | 211 | 51.09 | 0.08 |
| Chronic kidney disease | 91 | 21.56 | 31,005 | 6.11 | 0.46 | 87 | 21.07 | 63 | 15.25 | 0.15 |
| Chronic lower respiratory diseases | 259 | 61.37 | 84,682 | 16.69 | 1.03 | 250 | 60.52 | 251′ | 60.78 | 0.01 |
| Diabetes mellitus | 197 | 46.68 | 73,411 | 14.47 | 0.75 | 188 | 45.52 | 198 | 47.94 | 0.05 |
| Ischemic heart disease | 95 | 22.51 | 44,337 | 8.74 | 0.39 | 92 | 22.8 | 93 | 22.52 | 0.01 |
| Nicotine dependence | 52 | 12.32 | 39,685 | 7.82 | 0.15 | 52 | 12.59 | 40 | 9.69 | 0.09 |
Abbreviation: CF: Cystic Fibrosis, SD: Standard Deviation, Std Diff: Standard Difference.
A total of 58 patients (14%) had a history of lung transplant. Sixty-eight patients (16.15%) had documented use of CFTR potentiator agent use before COVID-19 infection (Ivacaftor was most common, 33 patients). Regarding anti-pseudomonal agent use, eighty-two and forty patients each were prescribed Tobramycin and Aztreonam respectively in the 6 months preceding COVID-19 infection.
There was a trend towards lower hospitalization rates in patients with CFTR potentiator agent use however it failed to reach statistical significance 13/68 vs 104/353, OR: 0.57 (95% CI: 0.297,1.08).
3.2. Clinical outcomes
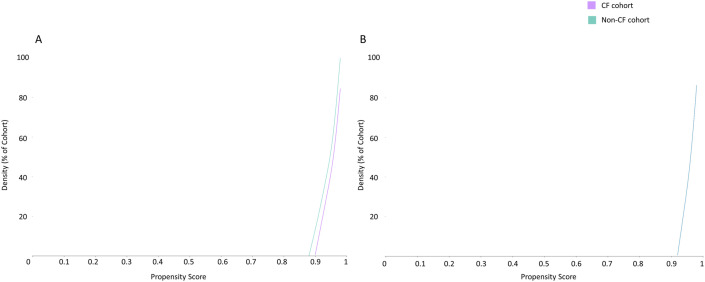
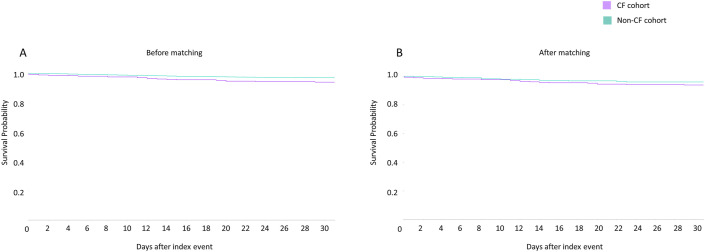
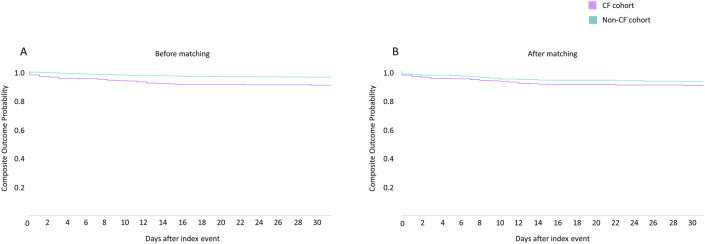
All the covariates used for matching in the two groups were similar after propensity score matching (mean standard difference <0.1) and are illustrated in Fig. 1 and Table 2 . In the 30-day period post COVID-19 diagnosis, 22 (5.21%) deaths and 37 (8.77%) composite outcomes (death or mechanical ventilation) was reported in the CF cohort (Fig. 2, Fig. 3 , Table 2).
Fig. 1.
Propensity score density graph in the unmatched (A) and matched (B) cystic fibrosis (purple) and non-cystic fibrosis (green) cohorts, depicting similar propensity score density in the two cohorts in the matched groups (B). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Outcomes in the two cohorts of COVID-19 patients with and without cystic fibrosis before and after propensity score matching.
| Outcome | Cystic Fibrosis cohort (n = 422) | Percentage | Non-CF cohort (n = 507,388) | Percentage | Risk Ratio | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|---|
| Before propensity score matching | |||||||
| 30-day Mortality | 22 | 5.21 | 8,705 | 1.72 | 3.74 | 2.02 | 4.57 |
| Inpatient services | 117 | 27.73 | 39,471 | 7.78 | 3.56 | 3.05 | 4.16 |
| Critical Care | 49 | 11.61 | 12,953 | 2.55 | 4.55 | 3.49 | 5.92 |
| Mechanical ventilation | 26 | 6.16 | 7,842 | 1.55 | 3.99 | 2.75 | 5.79 |
| 30-day composite outcome | 37 | 8.77 | 13,288 | 2.62 | 3.35 | 2.46 | 4.56 |
| Acute renal injury | 60 | 14.22 | 19,646 | 3.87 | 3.67 | 2.90 | 4.64 |
| Outcome | Cystic Fibrosis cohort (n=413) | Percentage | Non-CF cohort (n=413) | Percentage | Risk Ratio | 95% CI Lower | 95% CI Upper |
| After propensity score matching | |||||||
| 30-day Mortality | 22 | 5.33 | 12 | 2.91 | 1.83 | 0.92 | 3.66 |
| Inpatient services | 111 | 26.88 | 71 | 17.19 | 1.56 | 1.20 | 2.04 |
| Critical Care | 48 | 11.62 | 27 | 6.54 | 1.78 | 1.13 | 2.79 |
| Mechanical ventilation | 26 | 6.30 | 17 | 4.12 | 1.53 | 0.84 | 2.78 |
| 30-day composite outcome | 37 | 8.96 | 23 | 5.57 | 1.61 | 0.97 | 2.66 |
| Acute renal injury | 56 | 13.56 | 35 | 8.48 | 1.60 | 1.07 | 2.39 |
Abbreviation: CF: Cystic Fibrosis, CI: Confidence Interval.
Fig. 2.
Kaplan Meier (KM) plots of 30-day mortality in SARS-CoV-2 infected patients with cystic fibrosis (purple) and without cystic fibrosis (green), before (A) and after propensity matching (B). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Kaplan Meier (KM) plots of composite endpoint (mortality and mechanical ventilation combined) in SARS-CoV-2 infected patients with cystic fibrosis (purple) and without cystic fibrosis (green), before (A) and after propensity matching (B). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In the crude, unmatched analysis, mortality, inpatient services, critical care need, mechanical ventilation, acute kidney injury and composite endpoint at 30 days was higher in the CF group (Table 2). Following robust propensity matching, no differences were noted between the two cohorts in terms of 30-day mortality, mechanical ventilation, 30-day composite outcome. However, following propensity score matching, pwCF had higher hospitalization rate (RR 1.56, 95% CI 1.20–2.04), critical care need (RR 1.78, 95%CI 1.13–2.79), and acute renal injury (RR 1.60, 95% CI 1.07–2.39) as compared to people without CF (Fig. 2, Fig. 3, Table 2).
3.3. Laboratory values
Supplemental Table 2 describes mean values of C-reactive protein (mg/L), Lactate dehydrogenase (U/L), Erythrocyte sedimentation rate (mm/hr), Alanine aminotransferase (U/L), Aspartate aminotransferase (U/L), Serum Bilirubin (mg/dL) and Serum Ferritin (ng/ml). No statistically significant difference was observed in both cohort following propensity matching.
4. Discussion
Theoretical mechanisms for both possible accentuation and mitigation of COVID 19 viral replication and disease impact in pwCF have been proposed in literature [4]. Data currently available regarding COVID-19 disease outcomes in pwCF are limited to two overlapping registries of cystic fibrosis patients with COVID-19.
McClenaghan et al. in their report for ‘Cystic Fibrosis Registry Global Harmonization Group’, reported COVID-19 disease for 181 pwCF [5]. Their report includes 40 patients previously reported from this registry by Cosgriff et al. [6]. Of their total cohort (n = 181), 51.2% required hospitalization, 11 patients were admitted to the intensive care unit, and a total of 7 deaths were recorded. The authors concluded that pwCF may have better outcomes compared to other chronic diseases due to younger age and lower prevalence of obesity. The European Cystic Fibrosis Society Patient Registry (ECFSPR) reported 414 confirmed cases of COVID-19 in pwCF from 37 countries across Europe in their continually updated registry till January 20, 2021 [7]. Many of these patients are included in the ‘Cystic Fibrosis Registry Global Harmonization Group’ study noted above. A total of 7 deaths have been noted in patients with cystic fibrosis included in that registry. Notably, 150/414 patients had unknown severity of COVID-19 disease. Of the patients that required hospitalization, 17 needed ICU care (15%).
In our analysis of a large a cohort of patients from the United States, we found high rates of death (5.2%) with COVID-19 disease in patients with cystic fibrosis, noting that outcomes in this population are not as good as previously hypothesized. Rates of critical care need and acute kidney injury were also higher in the cystic fibrosis group compared to the general population, and this risk remained high even after propensity score matching.
The above-mentioned available data on COVID-19 disease in CF could not be compared to a control group of subjects, and therefore it could not be inferred whether these patients fared better or worse compared to the general population. We have found that the crude mortality of COVID-19 disease in pwCF is 3.5 times worse compared to the general population. Even after robust control for confounders, there was a signal towards worse mortality in these patients which failed to achieve statistical significance. Rates of critical care need and acute kidney injury remained higher after matching. Interestingly, there was a trend towards lower hospitalization rates in patients with CFTR agent use, however it could not reach statistical significance.
Several factors may have resulted in the different outcomes that we have noted in our population. Firstly, many patients in the reports discussed above, did not have follow-up available, and many of them were reported as not yet recovered from COVID-19, with some still admitted to the intensive care at the time of publication. All of our patients had 30-day follow-up available, during which time their disease course should have truly manifested itself, and therefore this analysis results in a more ‘real world’ and accurate picture of the course of COVID-19 disease in this population. Interestingly, the rate of hospitalization for COVID-19 in pwCF reported by both registries noted above (51.2% and 27.54%) were even higher than the rates of hospitalization that we have noted. Their analysis lacked a control group; however, it should be safe to assume that such high levels of hospitalization with COVID-19 would not be expected in the general population. Secondly, due to the availability of a control group, and propensity scores matching performed in our analysis, conclusions can be drawn regarding the risk of severe disease compared to the general population. As our data is directly pulled from electronic health records, and not reported by providers manually, it should also suffer less from selection bias.
Limitations of our study include its retrospective nature and the biases inherent to studies conducted on electronic health record, including mis-documentation. We could not analyze patient with and without lung transplant separately and compare their outcomes, owing to the nature of the data. 58 patients (14%) of the CF cohort were lung transplant recipients. This may introduce some bias in the result, given the significant degree of immunosuppression that these people receive and any possible differences in response to SARS-COV2 vaccination in transplant patients. Regardless, this first report outcomes from the United States of America adds important comparative data to the existing scientific pool and owing to robust follow-up data as well as a match control allows for drawing of important conclusions.
Overall, from our large cohort of pwCF with COVID-19 matched on the basis of propensity scores to COVID-19 patients without CF, it can be inferred that pwCF are at risk of poor outcomes with COVID-19. Continued preventative measures will be important in this cohort, and this finding may hold implications for measures such as prioritization for vaccination. 5.2% of these patients died within one month of COVID-19 diagnosis, and more than 1 in 10 patients required critical care. Therefore, the relatively young median age of pwCF, and lower prevalence of obesity do not protect these patients from severe disease contrary to prior reports.
Funding source
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 5U54GM104942-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Guarantor
ARS.
CRediT authorship contribution statement
Yousaf B. Hadi: Data curation, Funding acquisition, Acquisition of the data, Formal analysis, Analysis of the data, Interpretation of the data, Writing – original draft, Drafting the work or revising it critically for important intellectual content, Final approval of the version to be published. Dhairya A. Lakhani: Data curation, Funding acquisition, Acquisition of the data, Formal analysis, Analysis of the data, Interpretation of the data, Writing – original draft, Drafting the work or revising it critically for important intellectual content, Final approval of the version to be published. Syeda F. Naqvi: Data curation, Funding acquisition, Acquisition of the data, Formal analysis, Analysis of the data, Interpretation of the data, Final approval of the version to be published. Nida Ul Fatima: Data curation, Funding acquisition, Acquisition of the data, Formal analysis, Analysis of the data, Interpretation of the data, Final approval of the version to be published. Arif R. Sarwari: Data curation, Interpretation of the data, Final approval of the version to be published.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2021.106606.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ye Q., Wang B., Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarosz-Griffiths H.H., Scambler T., Wong C.H., Lara-Reyna S., Holbrook J., Martinon F., Savic S., Whitaker P., Etherington C., Spoletini G. Different CFTR modulator combinations downregulate inflammation differently in cystic fibrosis. Elife. 2020;9 doi: 10.7554/eLife.54556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manti S., Parisi G.F., Papale M., Mulè E., Aloisio D., Rotolo N., Leonardi S. Elsevier; 2021. Looking beyond Pulmonary Disease in COVID-19: A Lesson from Patients with Cystic Fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korkmaz H., Sahin F., Ipekci S.H., Temel T., Kebapcilar L. Increased pulse wave velocity and relationship with inflammation, insulin, and insulin resistance in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2014;26(7):725–732. doi: 10.1097/MEG.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 5.McClenaghan E., Cosgriff R., Brownlee K., Ahern S., Burgel P.-R., Byrnes C.A., Colombo C., Corvol H., Cheng S.Y., Daneau G. The global impact of SARS-CoV-2 in 181 people with cystic fibrosis. J. Cyst. Fibros. 2020;19(6):868–871. doi: 10.1016/j.jcf.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosgriff R., Ahern S., Bell S.C., Brownlee K., Burgel P.-R., Byrnes C., Corvol H., Cheng S.Y., Elbert A., Faro A. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J. Cyst. Fibros. 2020;19(3):355–358. doi: 10.1016/j.jcf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COVID-CF project in Europe. https://www.ecfs.eu/covid-cf-project-europe
- 8.Hadi Y.B., Naqvi S.F.Z., Kupec J.T., Sarwari A.R. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS. 2020 Nov 1;34(13):F3–F8. doi: 10.1097/QAD.0000000000002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadi Y.B., Naqvi S.F.Z., Kupec J.T., Sofka S., Sarwari A. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021 Jun 1;105(6):1365–1371. doi: 10.1097/TP.0000000000003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadi Y.B., Lakhani D.A., Naqvi S.F.Z., Singh S., Kupec J.T. Outcomes of SARS-CoV-2 infection in patients with pulmonary sarcoidosis: a multicenter retrospective research network study. Respir. Med. 2021 Jul 22;187:106538. doi: 10.1016/j.rmed.2021.106538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.