Abstract
Background
Cancer patients are at a higher risk of developing severe coronavirus disease 2019 (COVID-19). However, the safety and efficacy of COVID-19 vaccination in cancer patients undergoing treatment remain unclear.
Patients and methods
In this interventional prospective multicohort study, priming and booster doses of the BNT162b2 COVID-19 vaccine were administered 21 days apart to solid tumor patients receiving chemotherapy, immunotherapy, targeted or hormonal therapy, and patients with a hematologic malignancy receiving rituximab or after allogeneic hematopoietic stem cell transplantation. Vaccine safety and efficacy (until 3 months post-booster) were assessed. Anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor-binding domain (RBD) antibody levels were followed over time (until 28 days after the booster) and in vitro SARS-CoV-2 50% neutralization titers (NT50) toward the wild-type Wuhan strain were analyzed 28 days after the booster.
Results
Local and systemic adverse events (AEs) were mostly mild to moderate (only 1%-3% of patients experienced severe AEs). Local, but not systemic, AEs occurred more frequently after the booster dose. Twenty-eight days after the booster vaccination of 197 cancer patients, RBD-binding antibody titers and NT50 were lower in the chemotherapy group {234.05 IU/ml [95% confidence interval (CI) 122.10-448.66] and 24.54 (95% CI 14.50-41.52), respectively} compared with healthy individuals [1844.93 IU/ml (95% CI 1383.57-2460.14) and 122.63 (95% CI 76.85-195.67), respectively], irrespective of timing of vaccination during chemotherapy cycles. Extremely low antibody responses were seen in hematology patients receiving rituximab; only two patients had RBD-binding antibody titers necessary for 50% protection against symptomatic SARS-CoV-2 infection (<200 IU/ml) and only one had NT50 above the limit of detection. During the study period, five cancer patients tested positive for SARS-CoV-2 infection, including a case of severe COVID-19 in a patient receiving rituximab, resulting in a 2-week hospital admission.
Conclusion
The BNT162b2 vaccine is well-tolerated in cancer patients under active treatment. However, the antibody response of immunized cancer patients was delayed and diminished, mainly in patients receiving chemotherapy or rituximab, resulting in breakthrough infections.
Key words: antineoplastic treatment, BNT162b2 COVID-19 vaccination, anti-RBD IgG antibody response, safety, cancer
Highlights
-
•
The BNT162b2 vaccine is well-tolerated in cancer patients, including patients under immunotherapy.
-
•
Full BNT162b2 vaccination results in a blunted humoral immune response in cancer patients under active treatment.
-
•
The humoral immune response after BNT162b2 vaccination varies between different antineoplastic treatments.
-
•
Two doses of BNT162b2 vaccination may insufficiently protect patients receiving chemotherapy or rituximab against SARS-CoV-2.
Introduction
After the initial discovery of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, the coronavirus disease 2019 (COVID-19) pandemic has raged all over the globe, affecting >180 million individuals and leading to >4 million deaths up to now.1 Although the use of dexamethasone and remdesivir may provide patients with a modest benefit, there is currently no effective treatment for severe COVID-19.2,3 The rapid development and approval of several SARS-CoV-2 vaccines have provided a powerful tool to control further spread of COVID-19.4
Recently, it was shown that SARS-CoV-2-exposed patients with hematological malignancies display heterogeneous humoral immune response, an exhausted T-cell phenotype and a high prevalence of prolonged viral shedding.5, 6, 7, 8 Although patients with solid tumors often develop immune response signatures similar to those of noncancer patients,9 they remain at increased risk of severe COVID-19.10, 11, 12, 13 Hence, according to various oncological associations, high priority for vaccination should be given to these patients, regardless of age.
The Pfizer–BioNTech BNT162b2 messenger RNA (mRNA) vaccine was proven 95% effective at preventing PCR-confirmed COVID-19 in people without evidence of prior infection.14 However, ongoing antineoplastic treatment with cytotoxic drugs was an exclusion criterion in the pivotal phase III trial.14 Hence, it remains unclear how antineoplastic treatment affects patient’s ability to mount protective immunity after BNT162b2 vaccination15, 16, 17 and data regarding the safety of BNT162b2 vaccination in these patients are lacking.
To address this knowledge gap, the B-VOICE study prospectively investigated antibody response, vaccine effectiveness and safety of BTN162b2 vaccination in cancer patients receiving different types of antineoplastic treatment.
Methods
Trial design and participants
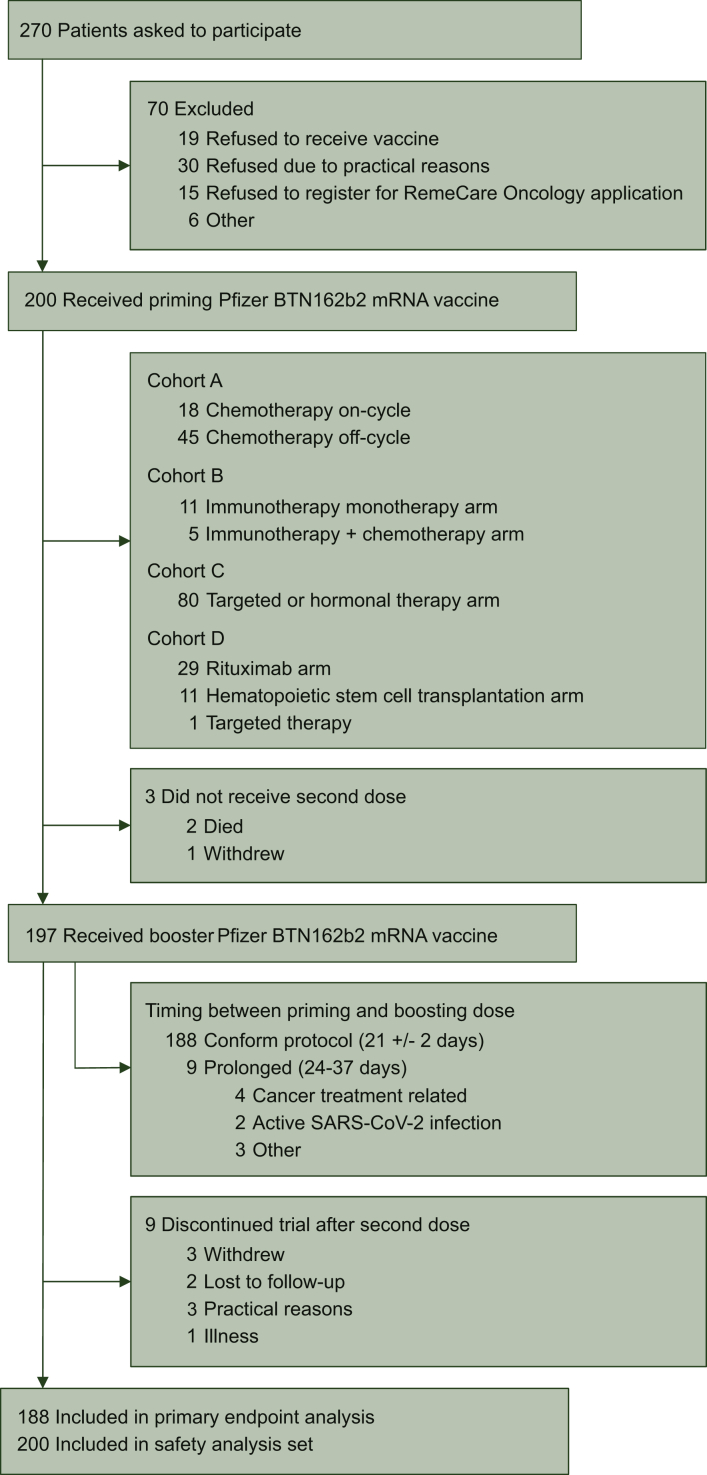
This prospective, longitudinal, interventional, multicohort trial was initiated on 15 February 2021 in the Multidisciplinary Oncology Center Antwerp, Antwerp University Hospital, Belgium, aiming to enroll 200 cancer patients. All participants signed written informed consent. All study patients were aged 18 years or older with a life expectancy of at least 6 months. Pregnant or breastfeeding women and patients with an immune deficiency unrelated to cancer or cancer treatment were ineligible. Eligible patients were cancer patients with a solid tumor under (i) chemotherapy (cohort A); (ii) immunotherapy (cohort B) and (iii) targeted therapy or hormonal therapy (cohort C). Patients receiving a combination of immunotherapy and chemotherapy were included in cohort B. Eligible patients for cohort D were cancer patients with a hematologic malignancy receiving rituximab or having received allogeneic hematopoietic stem cell transplantation (HSCT) at least 1 year before inclusion (Figure 1). In addition, cohort A was split up into a subcohort in which vaccine administration took place during peak chemotherapy-induced cytotoxicity (the ‘on-cycle’ subcohort) and a subcohort in which vaccine administration took place during the chemotherapy recovery period (the ‘off-cycle’ subcohort; see Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2021.100274).
Figure 1.
Trial profile.
Trial oversight
The study was approved by the local ethics committee and was executed in accordance with Good Clinical Practice and the Declaration of Helsinki [ICH GCP E6(R2)]. The regulatory sponsor was the Antwerp University Hospital (EudraCT number 2021-000300-38). This work was supported by the Belgian Government through Sciensano [COVID-19_SC004, COVID-19_SC059, COVID-19_SC061].
Procedures
All study participants received a priming and booster dose of 30 μg BNT162b2 vaccine intramuscularly 21 days apart. Blood samples for assessment of anti-SARS-CoV-2-specific antibodies were collected before the priming dose, on the day of the booster dose prior to vaccine administration and at days 7 and 28 after the booster dose and processed by Biobank Antwerp (BB190007, Antwerp, Belgium; ID: BE 71030031000). Results of antibody responses from a cohort of healthy staff members from a nursing home, participating in the PICOV-VAC trial (EUDRA-CT: 2021-000401-24) following the same sampling and vaccination schedule, were used as healthy controls.
Antibody responses were assessed on serum samples with an enzyme-linked immunosorbent assay (ELISA) for quantitative detection of immunoglobulin G (IgG) antibody levels to SARS-CoV-2 receptor-binding domain (RBD) antigen (see Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2021.100274). Quantitative anti-RBD IgG results were converted to international units per ml (IU/ml). Lower limit of quantification (LLQ) was 5 IU/ml. Using an independent exploratory study population of 268 patients participating in ongoing seroprevalence studies or hospitalized due to COVID-19 at the beginning of the SARS-CoV-2 pandemic, we showed that a threshold for anti-RBD IgG of 200 IU/ml predicts a neutralization response required for 50% protection against symptomatic SARS-CoV-2 infection (99%-100% specificity at a sensitivity of 94.94%; see Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2021.100274). As such, this threshold was used to define high and low responders. Moreover, we performed quantitative detection of IgG antibody levels to the SARS-CoV-2 S1 antigen using the Siemens SARS-CoV-2 spike IgG assay (sCOVG) as described before.18 Individual neutralization capacity at 28 days after the booster was assessed using an in vitro SARS-CoV-2 neutralization test toward the Wuhan (wild type) strain (see Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2021.100274).
Safety and vaccine effectiveness
Patient-reported outcomes (PROs) of local and systemic adverse events (AEs) were assessed via RemeCare Oncology, a web-based electronic platform for home toxicity monitoring.19,20 Patients were educated for and equipped with this PRO application and registered local and systemic reactions during 7 days after each vaccine administration. Local reactogenicity was graded as mild, moderate or severe. Systemic AEs were recorded according to the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE 5.0). To map vaccine effectiveness, PCR-confirmed SARS-CoV-2 infections of all participants were actively assessed at baseline and at day 7 after the booster, with a questionnaire offered via the RemeCare app. In addition, all patients attending the oncology day care unit were screened for SARS-CoV-2 using PCR on mouth and oropharyngeal rinse samples before their treatment and in Belgium testing is required for people returning from travel abroad, persons that were in close contact with an infected person or with suggestive symptoms such as fever or acute respiratory illness. For all patients, these data were monitored up to 3 months after the booster dose.
Outcomes
The primary endpoint was IgG antibody level to SARS-CoV-2 RBD antigen at 28 days after the booster BNT162b2 vaccination. Secondary endpoints included level of neutralizing antibodies 28 days after the booster, evolution of IgG antibodies across time points and cohorts, the efficacy of the immune response based on the incidence of PCR-confirmed SARS-CoV-2 infection and PRO-based local and systemic AEs.
Statistical analysis
All analyses were performed with the use of an intention-to-treat principle. As prespecified in the statistical analysis plan, geometric means of the SARS-CoV-2 RBD-IgG titer at 28 days after the booster vaccination was compared using an analysis of variance between treatment cohorts with pairwise comparison using Tukey's honestly significant difference (HSD) post hoc test. Robustness of results was confirmed using nonparametric testing with Kruskal-Wallis test between treatment cohorts with pairwise comparison using Dunn's post hoc test. As an exploratory analysis, a linear regression mode for outcome at day 28 after the booster vaccination and a linear mixed-effects model over time were fitted to log-transformed IgG measurements. Proportions of high-responding and low-responding patients at day 28 after the booster were compared by Fisher’s exact test. In addition, high-responding and low-responding cancer patients were compared using a logistic model for outcome at day 28. Exploratory analysis in treatment subcohorts was performed using similar statistical techniques. A two-sided P value of <0.05 after Holm-Bonferroni correction for multiple testing was considered statistically significant (for further details, see Supplementary Appendix and protocol, available at https://doi.org/10.1016/j.esmoop.2021.100274). Spearman's correlation was calculated between IgG antibody level to SARS-CoV-2 RBD and S1 antigen per timepoint for all cancer patients and between IgG antibody level to SARS-CoV-2 RBD at 28 days after the booster and 50% neutralization titers (NT50) titers at this timepoint.
Results
Patient characteristics
Between 15 February and 2 March 2021, 159 patients with a solid tumor and 41 patients with hematological malignancy were enrolled in this study (demographic details for patients and healthy controls presented in Table 1 and Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2021.100274). While all the healthy controls were SARS-CoV-2 naïve, seven cancer patients (4%) had a PCR-confirmed SARS-CoV-2 infection at least 14 days before the start of the study.
Table 1.
Demographics.
| Demographics | Target/hormone therapy (N = 80) | Immunotherapy (N = 16) | Chemotherapy (N = 63) | Hematology (N = 41) | Overall (N = 200) |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Female | 71 (88.8) | 4 (25.0) | 43 (68.3) | 17 (41.5) | 135 (67.5) |
| Male | 9 (11.2) | 12 (75.0) | 20 (31.7) | 24 (58.5) | 65 (32.5) |
| Age, years | |||||
| Mean (SD) | 59.8 (12.2) | 68.3 (8.09) | 60.0 (13.2) | 61.2 (11.5) | 60.8 (12.3) |
| Median (range) | 60.0 (31.0-86.0) | 69.5 (56.0-84.0) | 61.0 (26.0-88.0) | 63.0 (25.0-79.0) | 62.0 (25.0-88.0) |
| BMI | |||||
| Mean (SD) | 25.7 (4.72) | 27.0 (4.13) | 25.5 (5.19) | 25.2 (3.88) | 25.6 (4.66) |
| Median (range) | 25.3 (17.8-40.0) | 26.9 (19.7-34.5) | 24 (18.9-44.8) | 24.4 (17.1-35.5) | 25.0 (17.1-44.8) |
| Missing, n (%) | 0 (0) | 0 (0) | 3 (4.8) | 2 (4.9) | 5 (2) |
| ECOG score, n (%) | |||||
| 0 | 74 (92.5) | 11 (68.8) | 48 (76.2) | 38 (92.7) | 171 (85.5) |
| 1 | 6 (7.5) | 5 (31.2) | 13 (20.6) | 3 (7.3) | 27 (13.5) |
| 2 | 0 (0) | 0 (0) | 1 (1.6) | 0 (0) | 1 (0.5) |
| Missing | 0 (0) | 0 (0) | 1 (1.6) | 0 (0) | 1 (0.5) |
| Autoimmune disease, n (%) | 4 (5.0) | 0 (0) | 1 (1.6) | 3 (7.3) | 8 (4.0) |
| Kidney disease, n (%) | 2 (2.5) | 1 (6.2) | 5 (7.9) | 1 (2.4) | 9 (4.5) |
| Hypertension, n (%) | 20 (25.0) | 4 (25.0) | 22 (34.9) | 8 (19.5) | 54 (27.0) |
| Diabetes, n (%) | 3 (3.8) | 2 (12.5) | 10 (15.9) | 5 (12.2) | 20 (10.0) |
| Coronary disease, n (%) | 5 (6.2) | 2 (12.5) | 10 (15.9) | 7 (17.1) | 24 (12.0) |
| Smoking status, n (%) | |||||
| Current smoker (all tobacco) | 5 (6.2) | 1 (6.2) | 5 (7.9) | 2 (4.9) | 13 (6.5) |
| Former smoker (all tobacco) | 21 (26.2) | 11 (68.8) | 21 (33.3) | 18 (43.9) | 71 (35.5) |
| Nonsmoker | 51 (63.8) | 3 (18.8) | 29 (46.0) | 21 (51.2) | 104 (52.0) |
| Missing | 3 (3.8) | 1 (6.2) | 8 (12.7) | 0 (0) | 12 (6.0) |
| Stage, n (%) | |||||
| I | 20 (25.0) | 0 (0) | 6 (9.5) | NA | 26 (16.4)a |
| II | 17 (21.2) | 2 (12.5) | 6 (9.5) | NA | 25 (15.7)a |
| II | 2 (2.5) | 0 (0) | 0 (0) | NA | 2 (1.2)a |
| III | 6 (7.5) | 2 (12.5) | 6 (9.5) | NA | 14 (8.8)a |
| IV | 33 (41.2) | 12 (75.0) | 42 (66.7) | NA | 87 (54.7)a |
| Missing | 2 (2.5) | 0 (0) | 3 (4.8) | NA | 46 (28.9)a |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; NA, not applicable; SD, standard deviation.
Percentage of total of patient with solid tumor.
All 200 patients received a priming dose of the BTN162b2 vaccine, and 197 (98.5%) received a booster dose. A total of 188 patients (95.4%) received the booster dose after 21 (±2) days; nine patients (4.6%) received a delayed booster dose due to active infection or cancer treatment-related complications (Figure 1). All patients in the healthy control cohort received both priming and booster doses exactly 21 days apart.
Safety and tolerability
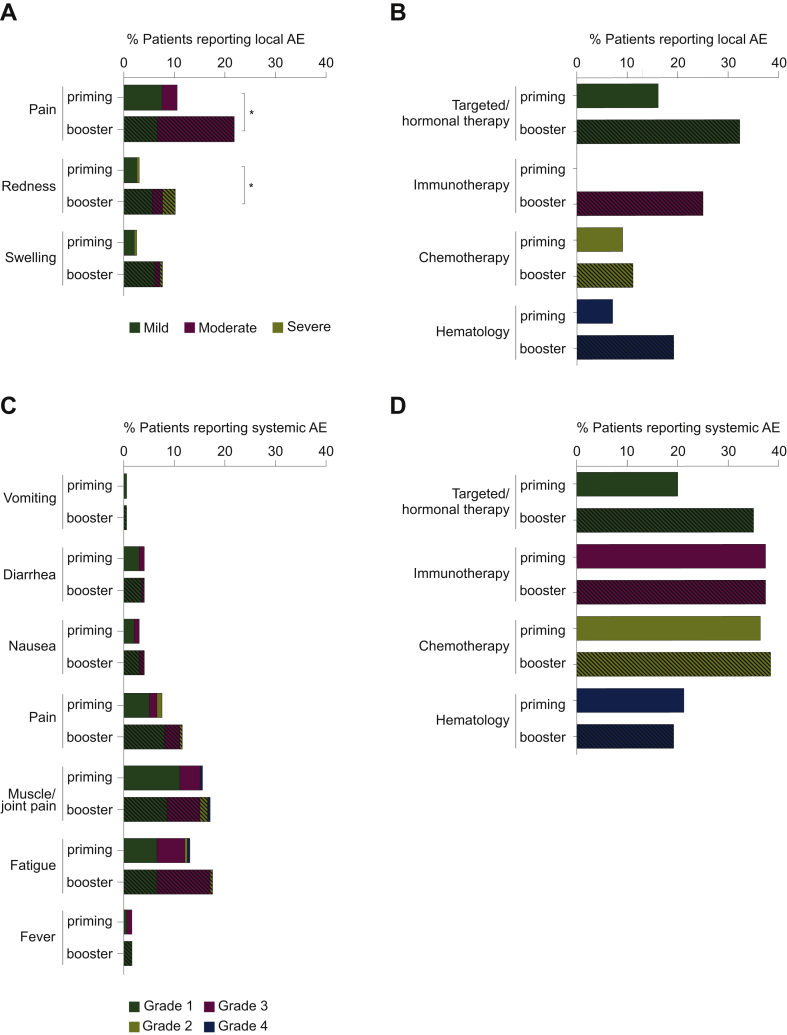
The most frequently reported local AE was mild-to-moderate pain at the injection site. Severe reactogenicity occurred in 1% of the patients following the priming dose and in 3% of the patients following the booster dose (Figure 2A). The percentage of patients reporting localized pain and redness was higher following the booster dose (21.5% versus 9%; 10.2% versus 3% respectively; Figure 2A). For patients receiving targeted/hormonal therapy, the proportion reporting a local AEs was higher after the booster than after the priming dose (32.5% versus 516.3%, Figure 2B), while no significant differences were observed in other cohorts. All local AEs resolved within 3-5 days.
Figure 2.
Local and systemic adverse events (AEs) reported within 7 days after priming and booster BNT162b2 vaccination.
(A) Local and (C) systemic AEs have been pooled from all study cohorts and are represented as a percentage of the total study population (n = 200 for priming vaccination, n = 197 for booster vaccination). To show differences in the occurrence of AEs between study cohorts, the proportion of patients reporting AEs are represented as a percentage of the number of patients in that cohort (first vaccine dose: hematology cohort, n = 41; targeted/hormonal therapy cohort, n = 80; chemotherapy cohort, n = 63; immunotherapy cohort, n = 16; second vaccine dose: hematology cohort, n = 41; targeted/hormonal therapy cohort, n = 80; chemotherapy cohort, n = 60; immunotherapy cohort, n = 16) (B and D). Open bars represent the AEs reported after the first vaccine dose while the dashed bars represent the ones reported after the second vaccine dose. Comparisons between both vaccine administrations were performed using a McNemar test with Bonferroni-Holm correction for the number of cohorts (n = 4) and the number of different local AEs (n = 3) and systemic AEs (n = 7) are reported: ∗P < 0.05.
The reported systemic AEs were similar after each dose, with fatigue (13% after the priming dose, 17.5% after the booster dose) and muscle/joint pain (15.5% after the priming dose and 17% after the booster dose) being the most common (Figure 2C). Severe systemic AEs were reported in <2% after either dose (fatigue in 0.5% and muscle/joint pain in 1%; Figure 2C). The proportion of patients suffering from systemic AEs after either of the vaccine administrations was similar across all study cohorts (Figure 2D).
Seven serious AEs were reported. One patient with stage IV breast cancer under oral capecitabine treatment had a nonfatal pulmonary embolism. As it is well-known that the risk of thromboembolic events is higher among cancer patients,21 this was considered as cancer related and not vaccine related by the investigators. Two patients had transient mild neurological complaints (5-6 days after vaccine administration) which disappeared within 48 h, with normal brain magnetic resonance imaging and no evidence for thrombotic or immunologic etiology. Four patients died during the study period. Three cancer-related deaths were considered unrelated to the BNT162b2 vaccine. One patient died due to myocardial infarction. This patient had a history of atrial fibrillation (under treatment with bisoprolol) which in combination with the presence of an infection provoked the myocardial infarction. However, we cannot complete rule out that this event was vaccine related.
Vaccine-induced antibody response and efficacy of the immune response
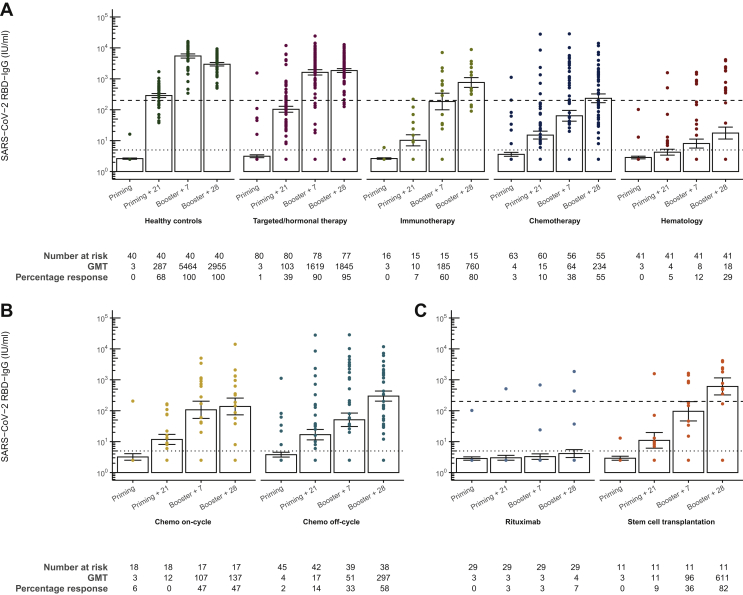
The geometric mean titer (GMT) of SARS-CoV-2 RBD-IgG antibodies 28 days after the booster in patients with solid tumors receiving chemotherapy was significantly lower than the healthy controls {GMT 2955.04 IU/ml [95% confidence interval (CI) 2280.17-3829.65] and 234.05 IU/ml (95% CI 122.10-448.66), respectively}. Hematologic cancer patients mount significantly lower SARS-CoV-2 RBD-IgG titers [GMT 17.61 IU/ml (95% CI 7.17-43.24)] than healthy controls and also than all other cancer cohorts (Figure 3A, Supplementary Figures S1 and S2, available at https://doi.org/10.1016/j.esmoop.2021.100274). All differences remained after correction for age at inclusion, sex, body mass index and PCR-confirmed COVID-19 infection before or during the study period (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100274). No difference could be demonstrated in the SARS-CoV-2 RBD-IgG antibody levels 28 days after the booster between vaccinated cancer patients with solid tumors receiving targeted/hormonal therapy [GMT 1844.93 IU/ml (95% CI 1383.57-2460.14)] and healthy controls, and both cohorts could be categorized as high responding, when considering the predefined cut-off of 200 IU/ml (100% control cohort; 95% targeted/hormonal therapy cohort). The proportion of high responders among the immunotherapy cohort was 80%, similar to healthy controls. Moreover, no difference could be seen between patients receiving immunotherapy with or without chemotherapy. However, the percentage of high responders in the chemotherapy and hematology cohort (respectively 55% and 29%) was significantly lower compared with the high-responders in the healthy control group (Figure 3A, Supplementary Figures S1 and S2, available at https://doi.org/10.1016/j.esmoop.2021.100274). Within the chemotherapy cohort, no difference between the ‘off-cycle’ subcohort and the ‘on-cycle’ subcohort was seen (Figure 3B). Among the hematology cohort, SARS-CoV-2 RBD-IgG levels in the rituximab cohort [GMT 4.12 IU/ml (95% CI 2.25-7.52)] were lower compared with patients with prior allogeneic HSCT [GMT 610.67 IU/ml (95% CI 148.77-2506.63)]. In addition, significantly less high responders were seen in patients receiving the CD20 antibody rituximab (7%) compared with patients with prior allogeneic HSCT (82%; Figure 3B).
Figure 3.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) anti-RBD antibody response generated after BNT162b2 vaccine administration.
(A) SARS-CoV-2 anti-RBD immunoglobulin G (IgG) levels before the priming dose on day 0, 21 days after the priming dose, 7 and 28 days after the booster dose in the different study cohorts. (B and C) Differences between the subcohorts of the hematology and chemotherapy cohorts, respectively. All samples were analyzed using an ELISA for the quantitative detection of IgG class antibodies to RBD. Geometric mean titers (GMTs) of the anti-RBD antibody titers are presented with the dotted line indicating lower limit of quantification (LLQ). Values below the LLQ are imputed to half the LLQ. I bars indicate standard errors. RBD, receptor-binding domain.
Exploratory analysis of log-transformed IgG titers over time showed that the evolution over time of the treatment cohorts was significantly different from healthy controls, mainly between baseline and 21 days after the priming dose, suggesting a delayed antibody response in cancer patients undergoing treatment (see Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2021.100274).
Fifteen cancer patients (7.5%) and six healthy controls (15%) already had SARS-CoV-2 RBD-IgG titers above LLQ before priming doses, indicating a previous SARS-CoV-2 infection, but only four cancer patients and none of the healthy controls reported PCR-confirmed COVID-19 before the start of the study. Excluding patients with baseline IgG titers above LLQ yielded comparable results for primary outcome (see Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2021.100274).
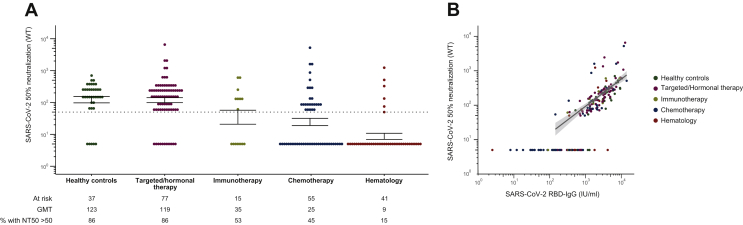
The vaccine-induced humoral immune response was assessed more in-depth by analyzing the anti-S1 antibody response and the in vitro NT50 toward the wild-type Wuhan SARS-CoV-2 strain. Results on the anti-S1 antibody response highly correlated with the results on the RBD-specific response (see Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2021.100274). Moreover, although we could identify a subgroup of individuals showing high SARS-CoV-2 RBD-IgG titers and low NT50, in general the SARS-CoV-2 RBD-IgG also correlated with NT50 28 days after the booster (Figure 4B). Hence, the significant differences in SARS-CoV-2 RBD-IgG titers that could be detected between the different cohorts persist when looking at the NT50 values (Figure 4A). The NT50 of healthy controls [GMT 122.63 (95% CI 76.85-195.67)] and patients receiving targeted/hormonal therapy [GMT 188.69 (95% CI 83.22-169.30)] were comparable, while the NT50 of patients receiving immunotherapy, chemotherapy as well as hematologic cancer patients was significantly lower [GMT: 34.55 (95% CI 11.67-102.23), 24.54 (95% CI 14.50-41.52) and 8.68 (95% CI 5.55-13.57) respectively] (Figure 4A). Moreover, neutralization capacity in the rituximab cohort was extremely low with only one of these patients having a NT50 above the limit of detection.
Figure 4.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibody response generated after BNT162b2 vaccine administration.
Virus neutralizing test with neutralizing titer 50% neutralization titers (NT50) defined as the sample dilution (reciprocal titer) conveying 50% neutralization in SARS-CoV-2 (strain 2019-nCoV-Italy-INMI1, reference 008V-03893, passage 5) infected wells. (A) In vitro SARS-CoV-2 NT50 toward the wild-type Wuhan strain 28 days after the booster dose in the different study groups. (B) Correlation between SARS-CoV-2 anti-RBD immunoglobulin G (IgG) and NT50 with regression line for all values above the lower limit of quantification (LLQ). Geometric mean titers (GMTs) of the NT50 are presented, with the dotted line indicating LLQ. Values below the LLQ are imputed to 1/10 LLQ for NT50. I bars indicate standard errors.
Of all patients that received a second dose, 52% underwent PCR testing for SARS-CoV-2 infection at least once between the priming and booster dose, among which only two patients (both receiving targeted/hormonal therapy) tested positive. One patient was asymptomatic and one showed moderate COVID-19. In the 3 months following the booster dose, patients were actively screened for SARS-CoV-2 infection upon symptoms or hospital admission for treatment, resulting in 62% of patients receiving at least one SARS-CoV-2 PCR test over the entire 3-month follow-up period. Three cancer patients (two receiving targeted/hormonal therapy and one suffering from a hematological malignancy) tested positive for SARS-CoV-2 after the booster dose (17, 38 and 39 days after). The patient receiving hormonal therapy showed a proper immune response 28 days after the booster (anti-RBD IgG titer 1825 IU/ml), explaining the asymptomatic disease. The patient under poly (ADP-ribose) polymerase inhibitor, which is known to have immunosuppressive capacities, was categorized as low responder 28 days after the booster (anti-RBD IgG titer: 151 IU/ml) and experienced mild symptoms. The patient under rituximab treatment did not show any sign of a humoral immune response 28 days after the booster, explaining the severe form of COVID-19 with a 2-week hospital admission.
Discussion
Cancer patients under antineoplastic treatment have an increased risk for severe COVID-19.10, 11, 12, 13 In addition, low or even absent antibody response after SARS-CoV-2 infection has been reported, particularly in patients with advanced cancer and B-cell hematological malignancies.7,8 Emerging evidence shows that SARS-CoV-2 vaccination efficacy might be lower in elderly and immunocompromised.22,23 Our prospective interventional trial demonstrated an acceptable safety profile of the BNT162b2 mRNA COVID-19 vaccine with a reduced and delayed antibody response in both solid and hematological cancer patients receiving antineoplastic treatment.
The lowest antibody response after complete BNT162b2 vaccination could be observed in the hematology cohort. This observation is in line with previous studies showing blunted antibody responses after BNT162b2 vaccination in patients with hematological malignicies,24 an effect that is more pronounced compared with patients with a solid tumor.25,26 Among all hematology patients, those that are actively treated at the time of vaccination show a lower antibody response compared with untreated patients or stem cell transplantation patients (both autologous and allogenic),24 which is fully in line with our observations. In addition, our study focuses on patients under rituximab, a CD20-antibody inducing B-cell aplasia, which compromises the antibody formation.25 Unsurprisingly, almost all the hematological high-responders are patients with prior allogeneic HSCT, with only two receiving rituximab. One of these rituximab high responders had detectable SARS-CoV-2 RBD-IgG titers before priming, possibly leading to increased vaccine efficacy. A recent study showed decreased IgG titers 5 weeks after full BNT162b2 vaccination in multiple myeloma patients and myeloproliferative malignancy patients on active antineoplastic treatment, supporting our findings.27 Moreover, higher antibody response rates were seen in chronic lymphatic leukemia patients in clinical remission or in treatment-naïve patients.28 In addition, patients with multiple myeloma show a lower antibody response after the first dose of BNT162b2 compared with postallogeneic HSCT patients.29
Solid tumor patients, who were vaccinated while undergoing targeted/hormonal therapy, had antibody responses close to healthy controls, which is in line with the known better response after influenza vaccination in comparison to patients under cytotoxic treatment.30 Among this cancer cohort, the majority of the patients received hormonal therapy having little myelosuppressive effect,31 so an adequate immune response could be expected.
By contrast, solid tumor patients who were vaccinated while undergoing chemotherapy had decreased anti-RBD antibody levels as well as decreased neutralizing capacity 28 days after the booster. This corresponds to the lower antibody response in patients receiving cytotoxic chemotherapy in a study which made the comparison with cancer patients on clinical surveillance, 3-4 weeks after being fully vaccinated with either BNT162b2 or mRNA-1273.26 As seen in the latter study, in our study some of these patients mount no or very low levels (e.g. below the lower limit of detection) of (neutralizing) antibodies. In our study, adapting the timing of vaccination relative to the chemotherapy administration did not affect the vaccine-induced immune response. Although in contradiction with recommendations for vaccine administration between chemotherapy cycles,32 this is in line with non-mRNA vaccines such as influenza33 and pneumococcal vaccines.34 As a beneficial effect of booster vaccination against influenza has been demonstrated,30 this supports further studies to investigate the role of additional booster vaccination (BNT162b2) in improving vaccine efficacy.
Indeed, two of our patients got infected between priming and booster dose administration, and three [one receiving hormonal therapy, one poly (ADP-ribose) polymerase inhibitor therapy, one rituximab] patients still got infected with SARS-CoV-2 after being fully vaccinated, with two of them categorized as low responder. While poly (ADP-ribose) polymerase inhibitor therapy and rituximab are known to be immunosuppressive,35,36 the immune system of the patients under hormonal therapy was most probably not fully recovered after four cycles of doxorubicin and cyclophosphamide, which has highly immunodepressing activities including suppression of B-cell functions.37,38 Despite these breakthrough infections, only one of these patients developed severe symptoms and needed hospital admission, in line with the idea that vaccination is more effective against COVID-19 compared with asymptomatic infection.39 It has indeed been demonstrated that while the effectiveness toward hospitalization after the priming dose is almost as high as after the booster dose (74% and 87%), for the protection against symptomatic disease, the effectiveness increases steeply after the booster dose (57% compared with 94%).40 It should be noted that the patient showing severe symptoms did not show seroconversion 28 days after the booster dose, most probably due to the rituximab treatment at the moment of vaccination, which is known to affect antibody production.
While patients under immunotherapy showed a clearly delayed anti-RBD antibody response, the 28-day postbooster response rate was similar to the control cohort, in line with previous literature on influenza vaccination.41 However, a recent meta-analysis indicates an increased rate of seroconversion after vaccination for patients under immunotherapy, together with an increased occurrence of immune-related AEs such as fever.42 As such, concerns were raised regarding enhanced immune-related side-effects after vaccination of patients under immunotherapy. In our study population, the vaccine was well-tolerated. Local side-effects were more frequent after the booster vaccination, confirming the phase III trial in healthy volunteers.14 Systemic side-effects occurred in patients of all cohorts and were similar after the first and second vaccination, in line with the general population.14 Our results, together with the findings of a recent study showing the safe use of the BNT162b2 vaccine in cancer patients treated with immune checkpoint inhibitors,43 demonstrate the safety of the BNT162b2 vaccine in cancer patients, including those under immunotherapy.
In this study, the immune response after a COVID-19 vaccine in a cancer population under treatment is monitored. In contrast to preliminary findings in cancer patients, where the main difference between healthy controls and patients was the failure to produce any response, rather than the magnitude of the response,25 a substantial proportion of our patients has seroconversion (e.g. an IgG level above the LLQ) without reaching levels necessary for 50% protection against symptomatic SARS-CoV-2 infection (<200 IU/ml). Moreover, even though it is widely accepted that SARS-CoV-2 RBD-IgG antibodies correlate with neutralizing antibody capacity14 and this is indeed the case for the overall study population, we here identified a subgroup of participants showing seroconversion without neutralization capacity against the wild-type strain. This phenomenon was seen in patients from all subgroups, including the healthy controls, and already described in a small cohort of donors of convalescent plasma, where donors with similar NT50 values of 240 had different IgG ELISA titers of 5400 and 16200.44 Speculation on this subject points toward the presence of epitopes on RBD that do not result in in vitro receptor binding or the presence of the ‘original antigenic sin’ phenomenon in which previous exposure to common coronaviruses might lead to an early and high-titer immune response after SARS-CoV-2 infection—or in this case vaccination—that uses memory, instead of naïve B cells, as such producing IgG that are typical for common coronaviruses and thus may not be neutralizing against SARS-CoV-2.45 However, as we see here, this phenomenon occurs more frequently in cancer patients, including over half of the patients receiving chemotherapy or immunotherapy and those with a hematological malignancy (24/49, 7/15 and 8/14 respectively); therefore, it is tempting to speculate that antineoplastic treatment during vaccination might lead to the formation of dysfunctional antibodies. As this is fully speculative at the moment, further research in this context is needed.
The strength of this study is that we specifically investigate RBD antibodies rather than spike protein antibodies, as cross-reactivity with other coronavirus subtypes has been reported.46 Moreover, in contrast to the previous studies in this context, we combine both anti-RBD IgG levels with in vitro neutralizing capacity to have a more in-depth representation of the humoral immune response. Furthermore, we report a long-term follow-up of the antibody response up to 28 days (4 weeks) after the booster dose. Despite all this, we acknowledge the lack of data on cellular immunity for these patients, which is needed to be able to fully study the vaccine induced immune response.
This study demonstrates that although the BNT162b2 COVID-19 vaccine is well-tolerated in cancer patients under active treatment, including patients under immunotherapy, some cancer patients lack the capacity to mount an efficient immune response, especially those receiving chemotherapy or rituximab treatment and irrespective of the timing of vaccine administration. As such, group immunity and self-protection are of paramount importance in these cases and caregivers, household members and/or close contacts of these patients (adults regardless of age) should be vaccinated as early as possible.47 In addition, this study raises the question for the third dose vaccination to boost immunity in cancer patients, as this has recently been shown to significantly improve the immunogenicity of the BNT162b2 vaccine.48
Conclusions
In conclusion, BNT162b2 vaccine administration is safe in cancer patients under antineoplastic treatment, but the reduced SARS-CoV-2 RBD-IgG antibody levels indicate a reduced efficacy, having important public health implications given the increased risk of severe COVID-19 in cancer patients.
Acknowledgements
We kindly thank the B-voice patients as well as the participants of the PICOV-VAC study, the nursing staff members at the Day Care Unit of the Antwerp University Hospital, the staff members of the Biobank Antwerpen and all recruiting physicians: Altintas Sevilay, Berneman Zwi, De Bondt Charlotte, Janssens Annelies, Papadimitriou Konstantinos, Prenen Hans, Raskin Jo, Rasschaert Marika, Kirsten Saevels, Specenier Pol, Tjalma Wiebren, Trinh Xuan Bich, Van Berckelaer Christophe, Van den Brande Jan, Van de Velde Ann and Verlinden Anke. We also thank Caroline Rodeghiero, Fabienne Jurion, Elfriede Heerwegh, Antoine Francotte and Stéphane De Craeye for their technical and logistical support in the laboratory and acknowledge Marie-Noëlle Schmickler MD, PhD and Mathieu Verbrugghe PhD from Mensura Occupational Health Service Belgium to enable institutional support for this study. We are grateful to Antoine Francotte, Caroline Rodeghiero and Fabienne Jurion for performing serological analyses, to Leo Heyndrickx, Johan Michiels and Betty Willems for neutralising antibody testing and to Laure Mortgat and Elza Duysburgh for the coordination of the HCW study that sampled part of the exploratory samples.
Funding
This work was supported by the Belgian Government through Sciensano [grant numbers COVID-19_SC004, COVID-19_SC059, COVID-19_SC061].
Disclosure
MP declares to have an advisory role within Remedus. All other authors have declared no conflicts of interest.
Data sharing
Data are available upon reasonable request.
Supplementary data
References
- 1.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ Available at.
- 2.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RECOVERY Collaborative Group. Horby P., Lim W.S. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aschwanden C. The false promise of herd immunity for COVID-19. Nature. 2020;587:26–28. doi: 10.1038/d41586-020-02948-4. [DOI] [PubMed] [Google Scholar]
- 5.Marra A., Generali D., Zagami P. Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2. Ann Oncol. 2021;32:113–119. doi: 10.1016/j.annonc.2020.10.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdul-Jawad S., Bau L., Alaguthurai T. Acute immune signatures and their legacies in severe acute respiratory syndrome coronavirus-2 infected cancer patients. Cancer Cell. 2021;39:257–275 e6. doi: 10.1016/j.ccell.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dam P.A., Huizing M., Papadimitriou K., Prenen H., Peeters M. High mortality of cancer patients in times of SARS-CoV-2: do not generalize! Eur J Cancer. 2020;138:225–227. doi: 10.1016/j.ejca.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dam P., Huizing M., Roelant E. Immunoglobin G/total antibody testing for SARS-CoV-2: a prospective cohort study of ambulatory patients and health care workers in two Belgian oncology units comparing three commercial tests. Eur J Cancer. 2021;148:328–339. doi: 10.1016/j.ejca.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johannesen T.B., Smeland S., Aaserud S. COVID-19 in cancer patients, risk factors for disease and adverse outcome, a population-based study from Norway. Front Oncol. 2021;11:652535. doi: 10.3389/fonc.2021.652535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson E.J., Walker A.J., Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lievre A., Turpin A., Ray-Coquard I. Risk factors for coronavirus disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19) Eur J Cancer. 2020;141:62–81. doi: 10.1016/j.ejca.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q., Berger N.A., Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7:220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dam P.A., Huizing M., Mestach G. SARS-CoV-2 and cancer: are they really partners in crime? Cancer Treat Rev. 2020;89:102068. doi: 10.1016/j.ctrv.2020.102068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotton C.N., Poznansky M.C. Vaccination of oncology patients: an effective tool and an opportunity not to be missed. Oncologist. 2012;17:1–2. doi: 10.1634/theoncologist.2011-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rieger C.T., Liss B., Mellinghoff S. Anti-infective vaccination strategies in patients with hematologic malignancies or solid tumors-Guideline of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO) Ann Oncol. 2018;29:1354–1365. doi: 10.1093/annonc/mdy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsigrelis C., Ljungman P. Vaccinations in patients with hematological malignancies. Blood Rev. 2016;30:139–147. doi: 10.1016/j.blre.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Irsara C., Egger A.E., Prokop W. Clinical validation of the Siemens quantitative SARS-CoV-2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. Clin Chem Lab Med. 2021;59:1453–1462. doi: 10.1515/cclm-2021-0214. [DOI] [PubMed] [Google Scholar]
- 19.Rasschaert M., Vanclooster P., Mertens T. The tele-transition of toxicity management in routine oncology care during the severe acute respiratory syndrome (SARS-CoV-2) pandemic. Br J Cancer. 2021;124:1366–1372. doi: 10.1038/s41416-020-01235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peeters M., van Dam P., Rasschaert M.A. Prescreening for COVID-19 in patients receiving cancer treatment using a patient-reported outcome platform. ESMO Open. 2020;5:e000817. doi: 10.1136/esmoopen-2020-000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Razeq H., Mansour A., Abdulelah H. Thromboembolic events in cancer patients on active treatment with cisplatin-based chemotherapy: another look! Thromb J. 2018;16:2. doi: 10.1186/s12959-018-0161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazza J.J., Yale S.H., Arrowood J.R. Efficacy of the influenza vaccine in patients with malignant lymphoma. Clin Med Res. 2005;3:214–220. doi: 10.3121/cmr.3.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massarweh A., Eliakim-Raz N., Stemmer A. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maneikis K., Šablauskas K., Ringelevičiūtė U. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8:e583–e592. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monin L., Laing A.G., Munoz-Ruiz M. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Addeo A., Shah P.K., Bordry N. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39:1091–1098.e2. doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pimpinelli F., Marchesi F., Piaggio G. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14:81. doi: 10.1186/s13045-021-01090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herishanu Y., Avivi I., Aharon A. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bird S., Panopoulou A., Shea R.L. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8:e389–e392. doi: 10.1016/S2352-3026(21)00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rousseau B., Loulergue P., Mir O. Immunogenicity and safety of the influenza A H1N1v 2009 vaccine in cancer patients treated with cytotoxic chemotherapy and/or targeted therapy: the VACANCE study. Ann Oncol. 2012;23:450–457. doi: 10.1093/annonc/mdr141. [DOI] [PubMed] [Google Scholar]
- 31.Amir E., Seruga B., Niraula S., Carlsson L., Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103:1299–1309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 32.Dulek D.E., Halasa N.B. Timing isn't everything: influenza vaccination in cancer patients. Cancer. 2017;123:731–733. doi: 10.1002/cncr.30467. [DOI] [PubMed] [Google Scholar]
- 33.Keam B., Kim M.K., Choi Y. Optimal timing of influenza vaccination during 3-week cytotoxic chemotherapy cycles. Cancer. 2017;123:841–848. doi: 10.1002/cncr.30468. [DOI] [PubMed] [Google Scholar]
- 34.Choi W., Kim J.G., Beom S.H. Immunogenicity and optimal timing of 13-valent pneumococcal conjugate vaccination during adjuvant chemotherapy in gastric and colorectal cancer: a randomized controlled trial. Cancer Res Treat. 2020;52:246–253. doi: 10.4143/crt.2019.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee E.K., Konstantinopoulos P.A. PARP inhibition and immune modulation: scientific rationale and perspectives for the treatment of gynecologic cancers. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920944116. 1758835920944116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pescovitz M.D., Torgerson T.R., Ochs H.D. Effect of rituximab on human in vivo antibody immune responses. J Allergy Clin Immunol. 2011;128:1295–1302.e5. doi: 10.1016/j.jaci.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu L.P., Cupps T.R., Whalen G., Fauci A.S. Selective effects of cyclophosphamide therapy on activation, proliferation, and differentiation of human B cells. J Clin Invest. 1987;79:1082–1090. doi: 10.1172/JCI112922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huyan X.H., Lin Y.P., Gao T., Chen R.Y., Fan Y.M. Immunosuppressive effect of cyclophosphamide on white blood cells and lymphocyte subpopulations from peripheral blood of Balb/c mice. Int Immunopharmacol. 2011;11:1293–1297. doi: 10.1016/j.intimp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Haas E.J., Angulo F.J., McLaughlin J.M. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet (London, England) 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dagan N., Barda N., Kepten E. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Läubli H., Balmelli C., Kaufmann L. Influenza vaccination of cancer patients during PD-1 blockade induces serological protection but may raise the risk for immune-related adverse events. J Immunother Cancer. 2018;6:40. doi: 10.1186/s40425-018-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desage A.L., Bouleftour W., Rivoirard R. Vaccination and immune checkpoint inhibitors: does vaccination increase the risk of immune-related adverse events? A systematic review of literature. Am J Clin Oncol. 2021;44:109–113. doi: 10.1097/COC.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 43.Waissengrin B., Agbarya A., Safadi E., Padova H., Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadkhoda K. COVID-19: are neutralizing antibodies neutralizing enough? Transfusion (Paris) 2020;60:1602–1603. doi: 10.1111/trf.15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan S., Nakajima R., Jain A. Analysis of serologic cross-reactivity between common human coronaviruses and SARS-CoV-2 using coronavirus antigen microarray. bioRxiv. 2020 doi: 10.1101/2020.03.24.006544. [Preprint]. 2020.03.24.006544. [DOI] [Google Scholar]
- 47.Desai A., Gainor J.F., Hegde A. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021;18:313–319. doi: 10.1038/s41571-021-00487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.