Abstract
Background
The indirect impact of the COVID-19 pandemic on cancer care and timely diagnosis is of increasing concern. We investigated the impact of the COVID-19 pandemic on incidence, time of diagnosis and delivery of healthcare among paediatric oncology patients in Germany in 2020.
Methods
We analysed incident paediatric cancer cases diagnosed in 0- to 17-year olds in Germany in 2020 using data of the German Childhood Cancer Registry. Absolute numbers and age-standardised incidence rates (ASR) in 2020 were compared to the previous five years (2015–2019). Moreover, we conducted a survey with open-ended questions, gathering perceptions of the diagnostic process and healthcare delivery for paediatric oncology patients during the COVID-19 pandemic.
Findings
More or similar numbers of paediatric cancer patients were newly diagnosed each month throughout 2020 in comparison to the previous five years. The estimated ASRs showed markedly higher incidence rates, overall and across diagnostic groups, in 2020 compared to 2015-2019. Results from the qualitative survey indicated that diagnostic processes, timeliness of diagnosis, and delivery of treatment were hardly affected during the COVID-19 pandemic. However, psychosocial supportive care and non-urgent appointments were considerably reduced during the lockdown periods.
Interpretation
We found no indications of severe adverse effects of the COVID-19 pandemic on diagnosis and delivery of healthcare among children with cancer in Germany. The underlying reasons of the increase in incidence rates remain speculative. Continued close monitoring of incidence patterns may shed light on the underlying reasons of the present increase and contribute to understanding disease aetiology.
Funding
None
Key words: Childhood cancer, Incidence, Healthcare delivery, Diagnosis, COVID-19 pandemic, Germany, German Childhood Cancer Registry
Research in Context to the article.
Evidence before this study
An increasing number of reports from different European countries, the US, and elsewhere demonstrated severe detrimental effects of the COVID-19 pandemic on timely diagnosis and cancer care, including a substantial reduction in new cancer diagnoses. Most of the literature focused however on cancer in adults. To identify studies reporting on the impact of the COVID-19 pandemic on incidence, time of diagnosis and delivery of health care among children with cancer, we searched PubMed (15 March 2021), using the following search strategies: (“childhood” OR “paediatric”) AND (“cancer” OR “malignancy”) AND (“COVID-19” OR “coronavirus” OR “SARS-CoV-2”) as well as (“childhood” OR “paediatric”) AND (“cancer” OR “malignancy”) AND (“COVID-19” OR “coronavirus” OR “SARS-CoV-2”) AND (“delay”). We also searched Google Scholar by using the same search terms. We did not apply any language or publication date restrictions.
The evidence for paediatric oncology is limited, mostly of single institutional or regional nature, and capturing only the period of the first pandemic wave. Current literature suggests geographical or institutional differences in the effects of the COVID-19 pandemic on childhood cancer diagnosis and care. While no reduction in the number of children diagnosed with cancer was seen in Greece, substantially fewer paediatric cancer diagnoses were observed in Milan, Italy. A remarkable decrease in leukaemia diagnoses but stable numbers of solid tumours were observed in south-east Norway and in Massachusetts, US, whereas a decrease in solid tumour cases but similar number of children diagnosed with leukaemia were seen in the Bronx, New York, US. Two recent cross-sectional surveys, evaluating the impact of the COVID-19 pandemic on paediatric cancer care in parts of Africa and Asia and worldwide, found significant delays in presentation and cancer care during the first pandemic wave in numerous institutions worldwide. However, those results reflect only the situation of participating institutions and capture only the first wave of the pandemic.
Added value of this study
The present study is the first comprehensive assessment of the impact of the COVID-19 pandemic on paediatric oncology diagnoses and provision of healthcare covering an entire country, namely Germany, with its population of 13.5 million below the age of 18 years. The assessment is based on nationwide high-quality cancer registry data capturing the whole of the year 2020. The assessment is complemented by a qualitative survey exploring perceptions of paediatric oncologists about the diagnostic process and delivery of healthcare during the pandemic. Contrary to our apprehension, we found no decrease in newly diagnosed childhood cancer cases in Germany during the COVID-19 pandemic that would suggest missed or delayed diagnoses. Rather, we found an unexpected remarkable increase in incidence rates of childhood cancer, across all diagnostic groups and age strata. Findings from the qualitative survey indicated that diagnostic processes, timeliness of diagnoses, and delivery of treatment among children with cancer was hardly affected during the pandemic despite the intermittent strained situation in the German healthcare system and the arduous pandemic precautionary measures imposed in Germany during most of 2020. However, psychosocial supportive care and non-urgent appointments such as long-term follow-up care of survivors were markedly reduced during the lockdown periods.
Implications of all available evidence
It is reassuring that we found no indications of missed or delayed childhood cancer diagnoses in Germany throughout 2020. Neither did we find indications of severe adverse effects on the diagnostic process and delivery of treatment among children with cancer. Our observations from Germany are however not generalizable to the situation in other countries and continued global effort to support timely paediatric cancer diagnoses and care is warranted. Furthermore, it should be monitored whether the COVID-19 pandemic-related social isolating periods during early childhood may in the long run increase the incidence of childhood acute lymphoblastic leukaemia.
Alt-text: Unlabelled box
1. Introduction
Over the past five decades, advances in diagnostics, understanding of tumour biology, pharmacology, treatment combinations and supportive care have led to remarkable advancements in treatment of and cure rates from childhood cancer [1,2]. Nowadays, five-year survival of childhood cancer exceeds 80% in Europe and most high-income countries [2], [3], [4]. However, not all children benefit equally from these improvements and survival varies widely by cancer type, age at clinical onset, stage of disease (in solid tumours), and somatic genetic lesions. Timely diagnosis is essential for good prognosis and preventing advanced disease, which commonly requires more intensive therapy and involves a higher risk of treatment-induced side and late effects [5,6].
Following the outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; which causes coronavirus disease 2019 (COVID-19)) pandemic in early March 2020, wide-ranging public health measures, social distancing policies and societal restrictions were imposed across Europe and globally to mitigate the spread of SARS-CoV-2 and to prevent overburdening the healthcare systems with seriously ill COVID-19 patients. In many countries, healthcare authorities advised hospitals and healthcare facilities to delay medical care for non-acute or not life-threatening conditions and to postpone cancer screenings whilst tackling the pandemic. According to a report by the World Health Organization, healthcare services for noncommunicable diseases have been severely disrupted since the COVID-19 pandemic began [7]. Indeed, an increasing body of institutional but also nationwide and international evidence points towards major detrimental effects of the COVID-19 pandemic on several areas of healthcare including the provision of cancer care [8], [9], [10], [11], [12], [13], [14]. A remarkable decline in new cancer diagnoses has been observed in a number of European countries [8,10,12,13], the US [11] and Latin America [14], and suggestive evidence for delayed diagnoses, fewer acute admissions, and increases in cancer deaths has been noted [8,9,[12], [13], [14]].
For paediatric cancer specifically, the few reports addressing the impact of the COVID-19 pandemic suggest fewer newly diagnosed paediatric cancers than expected, although observations are not fully consistent. They range from an overall stable number of children diagnosed with cancer [15], to substantially fewer diagnoses than expected [16,17], to a decrease in some diagnostic groups or cancer types but not in others [18], [19], [20]. These reports present however only a snapshot of the impact of the COVID-19 pandemic on paediatric oncology, limited by respectively small sample sizes of mostly institutional or regional data and capturing only the period of the first pandemic wave. Results from two cross-national surveys [21,22] among paediatric oncology units in parts of Africa and Asia and worldwide indicate a global effect of the COVID-19 pandemic on paediatric oncology care.
As of 16 March 2020, also in Germany extensive stay-at-home policies, social distancing restrictions as well as the directions to postpone elective surgeries, inpatient healthcare services and hospitalisations in response to the onset of the first wave of the COVID-19 pandemic were imposed, followed by a nationwide lockdown on 23 March. As of 27 April, the restrictions were gradually scaled back. The second pandemic wave, starting from beginning of October [23], involved substantially higher infection rates compared to the first wave. In consequence, a partial lockdown was imposed from 2 November with schools, kindergartens, and shops remaining initially open. Finally, from 16 December due to worryingly high transmission rates, increasing mortality rates and overwhelmed intensive care units a stricter lockdown was imposed.
The indirect impact of the COVID-19 pandemic on cancer care and timely diagnosis is of increasing concern. We therefore examined the impact of the COVID-19 pandemic on the incidence, time of diagnosis and delivery of healthcare among children with cancer in Germany in 2020 as well as leverage points to strengthen healthcare for paediatric cancer patients during the COVID-19 pandemic in Germany.
2. Materials and methods
To quantify the monthly number of diagnoses and estimate incidence rates of childhood cancer during the COVID-19 pandemic, we analysed newly diagnosed cases of childhood cancer reported to the German Childhood Cancer Registry (GCCR) throughout 2020 in comparison to the number of cases in previous years. To investigate the delivery of healthcare services for children with cancer in Germany in 2020, we complemented the quantitative analyses with a qualitative survey exploring the perceptions of paediatric oncologists of diagnostic processes and delivery of treatment and follow-up care for this patient group.
2.1. Study population and quantitative data collection
For the register-based part of this study, we identified incident cases of primary cancers diagnosed in 0- to 17-year olds in Germany in 2020 and in 2015-2019 (for comparison purposes) from the GCCR. The GCCR was established in 1980 and is the nationwide population-based childhood cancer registry of Germany, monitoring incident cases of all malignancies as well as non-malignant central nervous system (CNS) tumours diagnosed in 0- to 17-year olds. On average, approximately 2,250 incident cases are observed annually [4] based on a population of about 13.5 million children below the age of 18 years. The registration process is based on daily reporting by all paediatric haematology-oncology units in Germany (the number of paediatric haematology-oncology units varied from 63 to 59 in 2015-2020) with completeness of registration estimated to exceed 95% [4]. A close and well-functioning collaboration with the German Society of Paediatric Oncology and Haematology, the scientific association of all paediatric oncology professionals in Germany, guarantees the coverage of virtually all cases. Although the GCCR receives information on newly diagnosed cases on a daily basis, on average 13.3% (ranging between 11.1% - 15.0% in the previous five years) of all incident cases of a calendar year are only reported with some delay in the subsequent calendar year. Only exceptionally cases (2 – 2.5%) are reported after the subsequent calendar year. For this study we used the most up-to-date status of the GCCR database including late reports received by 15 March 2021. In 2015-2019 approximately 95% (ranging between 93.7% - 96.6%) of all incident cases of a particular year had already been reported by 15 March of the subsequent year.
We classified cancer diagnoses according to the International Classification of Childhood Cancer Third edition (ICCC-3) [24] which classifies tumours according to the IDC-O-3 nomenclature into 12 major diagnostic groups.
Annual population estimates by age were available from The Federal Statistical Office [25]. As at time of analysis population estimates for 2020 had not yet been available, we applied the population estimates of 2019 to 2020 based on a press release by The Federal Statistical Office stating that population size did not increase in 2020.
2.2. Qualitative data collection
Based on experience from previous research projects in similar settings, we anticipated to reach theme saturation after approximately 10-12 qualitative surveys. We therefore invited 16 (27%) of the 60 German paediatric haematology-oncology units to participate in the survey, taking into account potential non-response. To capture diverse perspectives, we purposefully sampled 16 units from different geographical parts of Germany and of different size but with at least 100 paediatric oncology patients treated during 2015 – 2019.
To gather personal perspectives of the heads of paediatric haematology-oncology units, the survey comprised open-ended questions on (i) the diagnostic process and timeliness of diagnosis for children with suspected cancer, (ii) the delivery of healthcare for children with confirmed cancers including the provision of psychosocial supportive care and overall changes in structures and processes of healthcare provision in response to the COVID-19 pandemic, and (iii) potential leverage points to ensure high-quality care during the pandemic. In December 2020, we approached the heads of the selected paediatric haematology-oncology units by email and invited them to participate in our study by completing the digital survey. In case we did not obtain any response, up to two reminders were sent. In total, twelve completed surveys were received by 19 January 2021 (response rate: 75%) which were all included in the qualitative content analysis.
2.3. Statistical and qualitative analyses
Absolute numbers of newly diagnosed childhood cancer cases for each month of the year 2020 were compared to the counts of the previous five years (2015–2019) for the respective month. We used the database of the GCCR as of 15 March 2021 and as of 15 March of the years 2016 to 2020 for the comparison with 2015-2019 (in order to avoid bias). The population size and age distribution was virtually stable for 0- to 17-year olds for the past five years [25]. Separate analyses were conducted for leukaemias (ICCC-3 Group I), lymphoid leukaemias (ICCC-3 Group Ia), lymphomas (ICCC-3 Group II), tumours of the central nervous system (CNS; ICCC-3 Group III), and the combined group of non-CNS solid tumours (ICCC-3 Group IV-XII), as well as by age at diagnosis (groups: <1, 1-4, 5-9, and 10-17 years). We were specifically interested in investigating differential impacts of the COVID-19 pandemic during the first lockdown (16 March – end of April 2020), the inter-lockdown period (beginning of May – 1 November 2020), and the second lockdown (2 November – end of 2020); hence we show data by month and by – roughly – lockdown period (January-February, March-April, May-October, November-December).
Furthermore, we compared the incidence rate of childhood cancer in 2020 to the mean incidence rate in the previous five years; the incidence rates for 2015-2019 included all cases reported in the respective year or the subsequent year. We estimated age-standardised incidence rates (to the Segi 1960 World Standard Population [26] (ASR)) per 1,000,000 children together with 95% confidence intervals. To take into account different hypothetical scenarios in relation to the number of additional cases diagnosed in 2020 but reported only later in 2021, we i) estimated the ASR based on the database of the GCCR as of 15 March 2021 considering no additional cases due to late reporting after 15 March 2021, ii) used the database of the GCCR as of 31 December 2020 and estimated the ASR by adding the minimum proportion of additional cases (by diagnostic group) observed in 2015-2019, and iii) used the database of the GCCR as of 31 December 2020 and estimated the ASR by adding the mean proportion of additional cases (by diagnostic group) observed in 2015-2019. To enable international comparisons, ASRs were calculated for two different age ranges: for childhood cancer diagnosed between ages 0 to 17 years and 0 to 14 years. ASRs were calculated for the same diagnostic groups as specified above. To examine whether changes in incidence rates varied among geographical regions of Germany, we performed additional analyses stratified by region (Northern, Western, Eastern and Southern Germany).
The statistical software SAS, version 9.4, was used for data management and statistical analyses.
We used qualitative content analysis to analyse the responses to the open-ended survey questions. Two researchers (MW: female, cancer epidemiologist with training in public health and epidemiology and ME: male, head of a research group on paediatric health services research and paediatrician with training in qualitative research methods and biostatistics) analysed all responses independently. To ensure that all emerging themes were captured we coded text segments inductively and derived main themes from the data. Discrepancies were discussed by MW and ME and were resolved by consensus. We used Microsoft Excel to organise coded segments.
Reporting in this manuscript is based on the STROBE statement [27] and the COREQ guidelines [28].
2.4. Role of the funding source
There was no funding source for this study.
3. Results
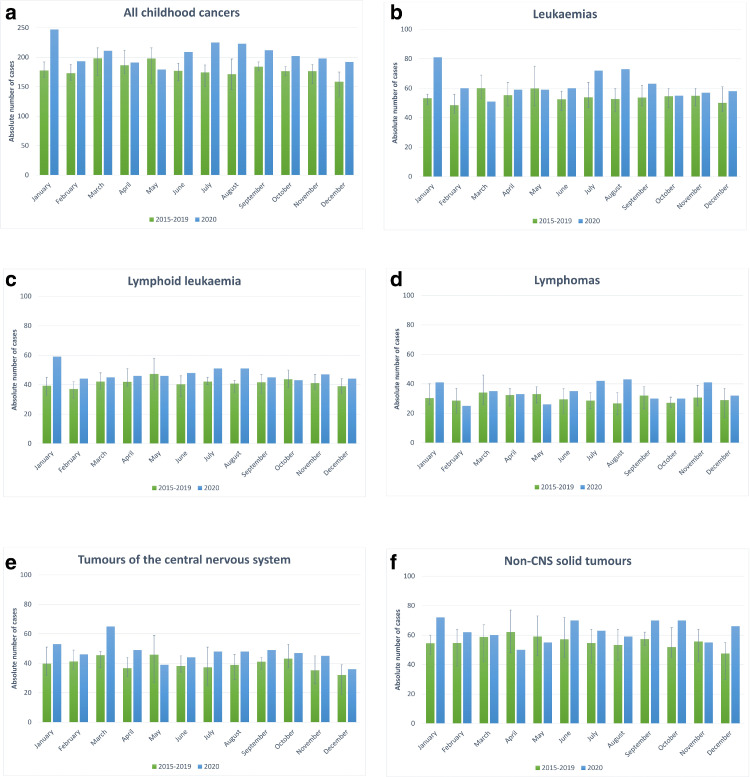
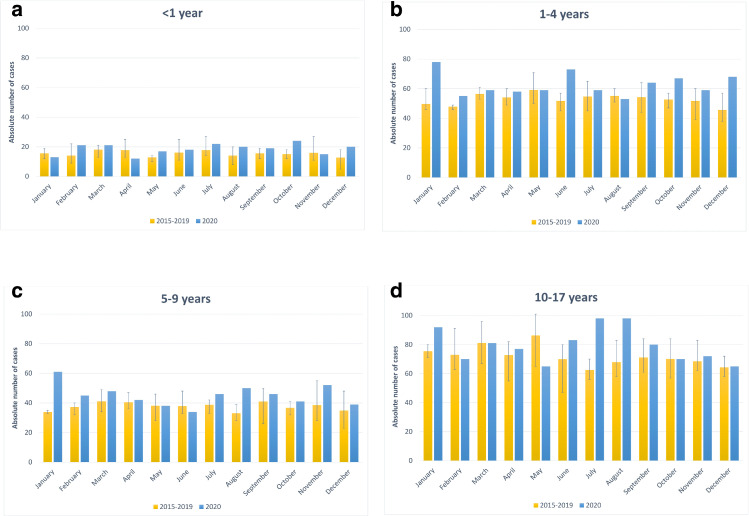
During 2020, 2,482 children and adolescents were newly diagnosed with cancer and reported to the GCCR by 15 March 2021. Fig. 1, Fig. 2 present the number of cases diagnosed in 2020 by calendar month in comparison to the average, minimum and maximum number of childhood cancer cases for the respective month in 2015 – 2019; Supplemental Figures 1 and 2 show the number of cases by the different lockdown periods as described in the Material and Methods section. For all childhood cancers combined in all months but May the 2020 numbers exceeded the respective monthly averages of the previous five years, in nine months (January, February, June to December) the 2020 numbers even exceeded the maximum number of the respective month of the previous five years. This pattern was broadly consistent across diagnostic groups (Fig. 1) and age strata (Fig. 2), albeit with stronger variations due to smaller sample sizes. The particular high number of newly diagnosed cases seen for January, i.e. before the pandemic had arrived in Germany, was preceded by a deficit of newly diagnosed cases in December 2019 (data not shown), possibly related to the early Christmas break in Germany in 2019 and postponed completion of some diagnostic procedures in January 2020.
Fig. 1.
a-f: Absolute numbers of newly diagnosed childhood cancer cases (in 0 – 17 year olds) in 2020 by calendar months versus the average numbers of childhood cancer cases during 2015–2019. The whiskers display the respective minimum and maximum number of cancer cases by calendar month during 2015-2019. The comparison is given for all cancers combined (a), and separately for leukaemias (b), lymphoid leukaemias (c), lymphomas (d), tumours of the central nervous system (e) and solid tumours other than in the central nervous system (non-CNS solid tumours) (f). Diagnostic groups and cancer types were defined according to the International Classification of Childhood Cancer – 3rd version (ICCC-3). The group of non-CNS solid tumours includes ICCC-3 diagnostic groups IV to XII (IV. Neuroblastoma and other peripheral nervous cell tumours, V. Retinoblastomas, VI. Renal tumours, VII. Hepatic tumours, VIII. Malignant bone tumours, IX. Soft tissue and other extraosseous sarcomas, X. Germ cell tumours, trophoblastic tumours, and neoplasm of gonads, XI. Other malignant epithelial neoplasms and melanomas, and XII. Other and unspecified malignant neoplasms).
Fig. 2.
a-d: Absolute numbers of newly diagnosed childhood cancer cases (in 0 – 17 year olds) in 2020 by calendar months and age at diagnosis (<1 year (a), 1-4 years (b), 5-9 years (c), 10-17 years (d)) versus the average numbers of childhood cancer cases during 2015–2019. The whiskers display the respective minimum and maximum number of cancer cases by calendar month during 2015-2019.
The estimated ASRs indicated markedly higher incidence rates of childhood cancer, overall and across individual diagnostic groups, in 2020 compared to the rates in previous years (Table 1). Even when applying the most conservative scenario of no additional cases due to late reporting after 15 March 2021, the ASRs were seven to eleven percent higher than those observed in 2015-2019. When applying the hypothetical scenarios of either adding the minimum proportion of additional cases observed in 2015-2019 or the mean proportion of additional cases in 2015-2019, the estimated ASRs of 2020 were substantially higher than those in 2015-2019. This applied to ASRs of all childhood cancers combined at ages 0 to 14 years and 0 to 17 years as well as across diagnostic groups for both age ranges (Table 1). Additional analyses by geographical region showed largely similarly increased incidence rates of childhood cancer across Germany, with the increase being somewhat less pronounced in Southern Germany (Supplemental Table 1).
Table 1.
Estimated age-standardised incidence rates of childhood cancer (ages 0 – 14 years and 0-17 years) in Germany in 2020, applying different hypothetical scenarios of additional cases due to late reporting.
| 0-14 years |
0-17 years |
|||||||
|---|---|---|---|---|---|---|---|---|
| ASR1 per 1,000,000 [95% CI] |
ASR1 per 1,000,000 [95% CI] |
|||||||
| 2015-20193 | 2020 (SI)4 | 2020 (SII)5 | 2020 (SIII)6 | 2015-20193 | 2020 (SI)4 | 2020 (SII)5 | 2020 (SIII)6 | |
| All cancers2 | 172.9 [169.4-176.4] |
186.0 [178.0-194.2] |
192.8 [184.6-201.1] |
197.5 [189.2-205.9] |
171.3 [168.2-174.5] |
185.8 [178.5-193.2] |
193.0 [185.6-200.6] |
198.0 [190.5-205.7] |
| Leukaemias | 55.2 [53.2-57.2] |
61.2 [56.7-66.0] |
62.3 [57.7-67.1] |
63.8 [59.1-68.7] |
51.5 [49.8-53.3] |
56.9 [52.9-61.1] |
57.9 [53.8-62.1] |
59.4 [55.3-63.7] |
| Lymphoid leukaemia | 43.1 [41.4-44.9] |
47.9 [43.8-52.1] |
47.7 [43.6-51.9] |
48.9 [44.8-53.1] |
39.2 [37.7-40.8] |
43.4 [39.9-47.1] |
43.3 [39.8-46.9] |
44.3 [40.8-48.0] |
| Lymphomas | 21.2 [20.0-22.4] |
23.4 [20.7-26.3] |
22.9 [20.2-25.7] |
23.4 [20.7-26.3] |
26.1 [24.9-27.3] |
29.1 [26.4-32.0] |
28.8 [26.1-31.7] |
29.7 [27.0-32.7] |
| CNS tumours | 41.3 [39.6-43.0] |
44.3 [40.5-48.3] |
48.0 [44.0-52.2] |
50.2 [46.1-54.4] |
39.3 [37.8-40.9] |
42.5 [39.1-46.1] |
46.5 [42.9-50.2] |
48.4 [44.8-52.2] |
| Non-CNS solid tumours | 55.2 [53.2-57.3] |
57.0 [52.6-61.6] |
58.5 [54.0-63.2] |
60.3 [55.8-65.0] |
54.4 [52.6-56.2] |
57.2 [53.2-61.4] |
58.9 [54.8-63.2] |
60.7 [56.6-65.1] |
ASR: age-standardized incidence rate (using Segi World Standard Population) per 1,000,000 person-years.
Diagnostic groups defined using the International Classification of Childhood Cancer Third edition (ICCC-3). The group of non-CNS solid tumours includes ICCC-3 diagnostic groups IV to XII.
Age-standardized incidence rate per 1,000,000 person-years in 2015-2019. Incidence rates for 2015-2019 included all cases reported in the respective year or the subsequent year, cases reported only after the subsequent calendar year were neglected.
Scenario I: considering no additional cases due to late reporting after 15 March 2021.
Scenario II: considering the minimum proportion of additional cases due to late reporting (by diagnostic group) observed in 2015-2019. The minimum proportion of additional cases due to late reporting for childhood cancer at ages 0-14 years amounted to 12.5% for all cancers combined, 6.1% for leukaemias, 3.3% for lymphoid leukaemias, 10.4% for lymphomas, 20.1% for CNS tumours and 12.7% for non-CNS solid tumours. The minimum proportion of additional cases due to late reporting for childhood cancer at ages 0-17 years amounted to 12.5% for all cancers combined, 6.1% for leukaemias, 3.3% for lymphoid leukaemias, 8.8% for lymphomas, 21.2% for CNS tumours and 13.0% for non-CNS solid tumours.
Scenario III: considering the mean proportion of additional cases due to late reporting (by diagnostic group) observed in 2015-2019. The mean proportion of additional cases due to late reporting for childhood cancer at ages 0-14 years amounts to 15.2% for all cancers combined, 8.7% for leukaemias, 5.9% for lymphoid leukaemias, 13.1% for lymphomas, 25.5% for CNS tumours and 16.1% for non-CNS solid tumours. The mean proportion of additional cases due to late reporting for childhood cancer at ages 0-17 years amounts to 15.4% for all cancers combined, 9.0% for leukaemias, 5.8% for lymphoid leukaemias, 12.2% for lymphomas, 26.2% for CNS tumours and 16.4% for non-CNS solid tumours.
The survey respondents described largely no or no severe delay in presentation, diagnostic procedures and start of cancer treatment but a heterogeneous picture of mild disruptions to the delivery of healthcare services across place and time. While some of the participating haematology-oncology units reported hardly any negative impacts on healthcare delivery, other units described multidimensional disruptions ranging from limited psychosocial support to increased administrative burden. Disruptions seemed to differ between the first and the second lockdown, and the inter-lockdown period. While some units noted more pronounced disruptions during the first lockdown, disruptions were reported to be less severe during the second lockdown and the inter-lockdown period when structures and processes had been adapted after the first lockdown. The main themes identified in the qualitative content analysis are reported in Table 2.
Table 2.
Main themes of the qualitative content analysis: diagnostic process, timeliness of diagnosis and delivery of healthcare among paediatric cancer patients as well as leverage points to strengthen services during the COVID-19 pandemic.
| i |
Diagnostic process/ timeliness of diagnosis
|
| ii |
Delivery of healthcare for children with cancer
|
| iii |
Leverage points to ensure high-quality health care for children with cancer during the pandemic
|
Overall, respondents perceived stable numbers of children with suspected or confirmed cancer diagnosis. Minor delays in diagnostic work-ups were only reported for few haematology-oncology units (Table 2). Respondents suggested that these were partly caused by delayed appointments with primary care paediatricians, limited capacity for diagnostic work-ups at tertiary care facilities, and precautionary measures such as mandatory SARS-CoV-2 testing prior to diagnostic procedures. While the responses suggested timely administration of cancer treatment, psychosocial supportive care like music therapy and non-urgent appointments such as long-term follow-up of survivors were markedly reduced in some units during the lockdowns. Several other changes in healthcare delivery related to the COVID-19 pandemic, such as restricted access for individuals accompanying patients, increased administrative and logistic workload for clinical staff or the expansion of digital services like telemedicine are summarised in Table 2.
Several leverage points to ensure high-quality care for children with cancer during the COVID-19 pandemic were identified (Table 2). These included structural changes such as larger facilities to implement isolation measures as well as changes in processes like the consistent implementation of hygiene measures at the hospital level.
4. Discussion
Contrary to our apprehension, we found no decrease in newly diagnosed childhood cancer cases in Germany during the COVID-19 pandemic that would suggest missed or delayed diagnoses, subsequently leading to more advanced stage cancers requiring more intensive treatment which often involves a higher risk of adverse side and late effects. Rather, we found an unexpected considerable increase in incidence rates of childhood cancer, across all diagnostic groups, age strata and geographical regions. This held even true in the most conservative scenario of no additional cases due to late reporting after 15 March 2021. Findings from the qualitative survey indicated that diagnostic processes, timeliness of diagnosis, and delivery of treatment among children with cancer was hardly affected during the pandemic despite the intermittent strained situation in the German healthcare system and the arduous pandemic precautionary measures imposed in Germany during most of 2020. The qualitative data suggested however that psychosocial supportive care and non-urgent appointments such as long-term follow-up care of survivors were markedly reduced during the lockdown periods.
For adults, an increasing number of reports demonstrated severe detrimental effects from the COVID-19 pandemic on cancer diagnosis and care, including a substantial reduction in cancer diagnoses [[8], [9], [10], [11], [12], [13], [14],[16], [17], [18]]. The evidence for paediatric oncology is limited and less consistent, mostly based on single-centre studies, and covering only the period of the first pandemic wave. While no reduction in the number of children diagnosed with cancer was seen in Greece [15], substantially fewer paediatric solid tumours diagnoses were observed in Milan [17], Italy. A remarkable decrease in leukaemia diagnoses but stable numbers of solid tumours were observed in south-east Norway [19] and in Massachusetts, US [16], whereas a decrease in solid tumour cases but a similar number of children diagnosed with leukaemia were seen in the Bronx, New York, US. Two recent cross-sectional surveys [21,22], evaluating the impact of the COVID-19 pandemic on paediatric cancer care in parts of Africa and Asia and worldwide, found significant delays in presentation and cancer care during the first wave in numerous institutions worldwide, pointing towards a global effect of the COVID-19 pandemic on paediatric oncology care. From this international perspective, Germany came overall well through the first wave;[23] albeit we noted somewhat lower numbers of newly diagnosed paediatric cancer cases during spring in comparison to the rest of the year. However, even during those months numbers did never fall below the minimum of diagnoses of the previous five years. Early imposition of pandemic precautionary measures and subsequently lower SARS-CoV-2 transmission rates in Germany in comparison to some other countries may have contributed to ensuring prompt access to paediatric oncological care also during the pandemic waves. Moreover, in Germany, a country with a well-functioning public healthcare system, all citizens have universal and equal access to essential healthcare services including cancer care, irrespective of a families’ economic situation. In contrast to other countries, the German healthcare system does not involve a gatekeeper function to secondary care through general practitioners, but most children receive primary healthcare from paediatricians. Healthcare services provided by paediatricians may have been less affected by the COVID-19 pandemic than the services by general practitioners, thus potentially explaining the mild disruptions in diagnosis and healthcare delivery for children with suspected cancers as also suggested by some survey respondents. Remarkable are however the particularly high numbers of leukaemia and lymphoma diagnoses in July and August, which may on the other hand indeed speak for a small effect of catching up with diagnoses that had been somewhat delayed due the lockdown period in March and April.
To our surprise, contrary to our a priori concerns of a decrease, childhood cancer incidence and numbers were markedly increased in 2020 compared to the five preceding years. The underlying reasons remain speculative at this point but are unlikely to reflect an immediate increase in the actual occurrence of paediatric cancers. Although the increased number of new cases seen for January is likely an effect of slightly delayed diagnoses due to the early Christmas break in Germany in December 2019 (with postponement of some final diagnostic procedures to January 2020), it is highly unlikely that delayed diagnoses from 2019 or under-ascertainment of cases in previous years explain the overall marked increase in incidence rates in 2020. The GCCR has been registering childhood cancer cases for more than 40 years with virtually complete coverage. Moreover, estimated incidence rates of the previous years from Germany were at a similar level, if not even among the highest [4], compared to other European countries, which provides evidence against under-ascertainment of cases in Germany in previous years. We postulate that the increase in incidence rates across diagnostic groups in Germany may reflect enhanced parental attention to early disease symptoms in their child as well as to enhanced attention of paediatricians due to the fear of an infection with SARS-CoV-2, leading to earlier presentation, a shorter diagnostic process and finally earlier diagnoses. Monitoring the incidence of 2021 and subsequent years to assess any potential rebound effect in incidence rates will confirm or refute this hypothesis. If indeed earlier presentation and earlier diagnoses of children with cancer fully explains the increase in incidence rates for 2020, incidence rates of 2021 may potentially drop up to the same extent as they have increased in 2020 compared to the five preceding years.
In the long run, the current COVID-19 pandemic with its stay-at-home policy and social distancing measures may indeed have implications for the incidence of childhood acute lymphoblastic leukaemia (ALL), the most common malignancy in children. The role of the immune system and exposures to infections in ALL development is not fully understood but it has been hypothesised that a lack of immune system stimulation in early life may increase the risk of B-cell precursor ALL, resulting from an abnormal reaction to common infections by an immature and unchallenged immune system later in childhood [29]. The closure of nurseries, kindergartens, and schools, as well as other social distancing measures, have certainly removed young children at these critical ages from training of the immune system to common infections [30]. This scenario is at present incompatible with our observation of an increase in incidence rates that is neither diagnosis- nor age-specific. Such an effect would however not emerge overnight but rather impact future ALL incidence rates.
4.1. Strengths and limitations
A significant strength of this study is its design, combining quantitative and qualitative methods. The study is the first assessment of the impact of the COVID-19 pandemic on incidence rates of childhood cancer based on nationwide high-quality cancer registry data capturing the entire year 2020. Complementing the quantitative results, the qualitative survey enabled a comprehensive exploration of the multidimensional impacts of the COVID-19 pandemic on diagnostic processes and delivery of treatment and follow-up care. Another strength relates to Germany as the study setting; this includes the universal access to healthcare in Germany as described above, the GCCR as one of the oldest and most complete population-based childhood cancer registries worldwide, and the sizeable childhood population of 13.5 million, enabling meaningful analyses despite the rare occurrence of cancer in children. A limitation concerns the uncertain number of additional cases diagnosed in 2020 but only reported to the GCCR after 15 March 2021. We addressed this limitation however by exploring several different scenarios of extra cases due to late reporting when estimating the ASRs. Another limitation is the lack of information about disease stage at diagnosis. Analyses by stage could give important indications whether, for example, the observed increase in cancer diagnoses was limited to diagnoses of early stages.
5. Conclusions
While social distancing, stay-at-home policies, and other pandemic precautionary measures were implemented, cancer occurrence did not pause. It is reassuring that we found no indications of missed or delayed childhood cancer diagnoses in Germany throughout 2020. Neither did we find indications of severe adverse effects on diagnostic processes, timeliness of diagnosis, and delivery of treatment among children with cancer. Instead we observed a marked increase in incidence; the underlying reasons are speculative but may involve greater parental attention to early disease symptoms in their child during the COVID-19 pandemic and hence more timely healthcare consultations. Monitoring the incidence beyond 2020 to examine any potential rebound effect in incidence rates or other temporal patterns is therefore crucial. Moreover, it should be monitored whether the COVID-19 pandemic-related social isolating periods may increase the incidence of childhood ALL, a possible consequence not detectable as early as in 2020.
Additional information
Ethical approval and consent to participate
No ethics approval and consent was required for this study. This research was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent for publication
Not applicable, since no individual person's data was used.
Data sharing
Data on childhood cancer diagnoses used in the present study are available from the German Childhood Cancer Registry. Access to de-identified data and the SAS program used for the quantitative analysis as well as de-identified responses to the open-ended survey questions may be made available upon reasonable request. All data access requests should be directed to the corresponding author.
Declaration of interests
The authors declare that the research was conducted in the absence of any commercial, personal or financial relationships with other people or organisations that could be construed as a potential conflict of interest.
Acknowledgments
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The work of the authors was supported by core funds of their respective institutions, namely the Institute of Medical Biostatistics, Epidemiology and Informatics at the University Medical Center of the Johannes Gutenberg University Mainz, Germany, the International Agency for Research on Cancer and the Department of Pediatrics and Adolescent Medicine, Christian-Albrechts-University and University Medical Center Schleswig-Holstein, Germany. The German Childhood Cancer Registry is part of the Division of Childhood Cancer Epidemiology at the Institute of Medical Biostatistics, Epidemiology and Informatics and is funded by the Federal Ministry of Health and the Health Ministries of the 16 federal states of Germany. The funding sources were not involved in the conceptualisation, design, content or preparation of the manuscript, and decision to submit for publication.
The authors are grateful to the German Society for Paediatric Oncology and Haematology (GPOH) and the paediatric haematology-oncology units for their data contribution to the German Childhood Cancer Registry. Special thanks are due to the individual paediatric oncologists who participated in the qualitative survey.
Authors’ contributions
Conceptualisation, FE, MW, DG, ME; methodology, FE, MW, JS, CS, DG, ME; formal analysis, FE, CT, ME, MW; data curation, FE, MW, CT, CS, DG, ME; writing – original draft preparation, FE, MW, ME; writing – review and editing, FE, JS, MW, CT, CS, MS, DG, ME; visualization, MW, CT, FE, JS; supervision, FE; project administration, FE.
FE and MW had full access to all data and verified the data reported in the study. All authors approved the final manuscript as submitted, agreed to be accountable for all aspects of the work and had the final responsibility for the decision to submit for publication.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/ World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/ World Health Organization.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2021.100188.
Appendix. Supplementary materials
References
- 1.Pritchard-Jones K, Pieters R, Reaman GH. Sustaining innovation and improvement in the treatment of childhood cancer: lessons from high-income countries. Lancet Oncol. 2013;14(3):e95–e103. doi: 10.1016/S1470-2045(13)70010-X. [DOI] [PubMed] [Google Scholar]
- 2.Gatta G, Botta L, Rossi S. Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5—a population-based study. Lancet Oncol. 2014;15(1):35–47. doi: 10.1016/S1470-2045(13)70548-5. [DOI] [PubMed] [Google Scholar]
- 3.Allemani C, Matsuda T, Di Carlo V. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdmann F, Kaatsch P, Grabow D, Spix C. Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University; Mainz: 2020. German Childhood Cancer Registry - Annual Report 2019 (1980-2018) [Google Scholar]
- 5.Erdmann F, Frederiksen LE, Bonaventure A. Childhood cancer: Survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2020 doi: 10.1016/j.canep.2020.101733. [DOI] [PubMed] [Google Scholar]
- 6.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization WH. 2020. COVID-19 significantly impacts health services for noncommunicable diseases.https://www.who.int/news/item/01-06-2020-covid-19-significantly-impacts-health-services-for-noncommunicable-diseases (accessed 30.01.2021) [Google Scholar]
- 8.Skovlund CW, Friis S, Dehlendorff C, Nilbert MC, Mørch LS. Hidden morbidities: drop in cancer diagnoses during the COVID-19 pandemic in Denmark. Acta Oncol (Madr) 2020:1–4. doi: 10.1080/0284186X.2020.1858235. [DOI] [PubMed] [Google Scholar]
- 9.Maringe C, Spicer J, Morris M. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinmohamed AG, Visser O, Verhoeven RHA. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the Number of US Patients With Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Registry NIC . Northern Ireland Cancer Registry; 2020. Recent trends in the number of pathology samples indicating cancer in Northern Ireland. [Google Scholar]
- 13.Maluchnik M, Podwojcic K, Wieckowska B. Decreasing access to cancer diagnosis and treatment during the COVID-19 pandemic in Poland. Acta Oncol. 2021;60(1):28–31. doi: 10.1080/0284186X.2020.1837392. [DOI] [PubMed] [Google Scholar]
- 14.Marques NP, Silveira DMM, Marques NCT, Martelli DRB, Oliveira EA. Martelli-Junior H. Cancer diagnosis in Brazil in the COVID-19 era. Semin Oncol. 2021 doi: 10.1053/j.seminoncol.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kourti M, Markozannes G, Bouka P, Bouka E, Ntzani E, Petridou ET. Pediatric cancer registration fluctuation in Greece due to COVID-19 pandemic and changes in health care delivery. Pediatr Blood Cancer. 2020:e28777. doi: 10.1002/pbc.28777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Neill AF, Wall CB, Roy-Bornstein C, Diller L. Timely pediatric cancer diagnoses: An unexpected casualty of the COVID-19 surge. Pediatr Blood Cancer. 2020;67(12):e28729. doi: 10.1002/pbc.28729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiaravalli S, Ferrari A, Sironi G. A collateral effect of the COVID-19 pandemic: Delayed diagnosis in pediatric solid tumors. Pediatr Blood Cancer. 2020:e28640. doi: 10.1002/pbc.28640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Offenbacher R, Knoll MA, Loeb DM. Delayed presentations of pediatric solid tumors at a tertiary care hospital in the Bronx due to COVID-19. Pediatr Blood Cancer. 2020:e28615. doi: 10.1002/pbc.28615. n/a(n/a) [DOI] [PubMed] [Google Scholar]
- 19.Jarvis KB, Lind A, LeBlanc M, Ruud E. Observed reduction in the diagnosis of acute lymphoblastic leukaemia in children during the COVID-19 pandemic. Acta Paediatr. 2021;110(2):596–597. doi: 10.1111/apa.15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding YY, Ramakrishna S, Long AH. Delayed cancer diagnoses and high mortality in children during the COVID-19 pandemic. Pediatr Blood Cancer. 2020;67(9):e28427. doi: 10.1002/pbc.28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saab R, Obeid A, Gachi F. Impact of the coronavirus disease 2019 (COVID-19) pandemic on pediatric oncology care in the Middle East, North Africa, and West Asia region: a report from the Pediatric Oncology East and Mediterranean (POEM) group. Cancer. 2020;126(18):4235–4245. doi: 10.1002/cncr.33075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graetz D, Agulnik A, Ranadive R. Global effect of the COVID-19 pandemic on paediatric cancer care: a cross-sectional study. The Lancet Child & Adolescent Health. 2021 doi: 10.1016/S2352-4642(21)00031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch-Institute R. 2020. COVID-19-Dashboard. [Google Scholar]
- 24.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer2005; 103(7): 1457-67. [DOI] [PubMed]
- 25.Statistisches Bundesamt (Destatis) 2020. Genesis-Online. [Google Scholar]
- 26.Segi M. Japan Tohoku University of medicine; Sendai: 1960. Cancer mortality for selected sites in 24 countries (1950-57) [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet North Am Ed. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 28.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ) - a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 29.Greaves M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat Rev Cancer. 2018;18(8):471–484. doi: 10.1038/s41568-018-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greaves M. COVID-19 and childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2020;67(12):e28481. doi: 10.1002/pbc.28481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.