Abstract
Purpose:
To determine if metformin is associated with non-infectious uveitis (NIU).
Methods:
Patients in an insurance claims database who initiated metformin (n=359,139) or other oral anti-diabetic medications (n=162,847) were followed for NIU development. Both cohort and case-control analyses were performed to assess differing exposure lengths using Cox and conditional logistic regression, respectively.
Results:
The hazard ratio (HR) for incident NIU was not significantly different between the metformin and non-metformin cohorts [HR=1.19, 95% Confidence Interval (CI): 0.92-1.54, P=0.19]. The case-control analysis similarly showed no association between any metformin use 2 years before the outcome date and NIU [Odds ratio (OR)=0.64, 95% CI: 0.39-1.04, P=0.07]. However, there was a protective association between cumulative metformin duration [445-729 days adjusted OR (aOR)=0.49, 95% CI: 0.27-0.90, P=0.02) and dosage (>390,000 mg aOR=0.44, 95% CI: 0.25-0.78, P=0.001] compared with no metformin use.
Conclusions:
Our results suggest metformin use for longer durations may be protective of NIU onset.
Uveitis is a significant cause of permanent vision loss1-3 accounting for up to 10% of blindness.3-5 Etiologically, uveitis is categorized as infectious or non-infectious (immune-mediated), with most cases in developed countries being non-infectious.6 As uveitis often affects younger people of working age, the disease is responsible for longer durations of blindness and a higher per case cost to society compared to other age-associated ocular diseases.7 Though the human and economic costs correlated with the disease are severe, because of the challenges of conducting studies for less common diseases, few population-based studies currently exist that identify factors that affect uveitis incidence.8-11
Non-infectious uveitis (NIU) is typically treated with corticosteroids and/or systemic immunomodulatory agents.12 These are effective therapies but can also be associated with serious adverse effects, although such side effects are uncommon when treatment occurs according to expert panel recommendations.13,14 A notable proportion of NIU cases are recurrent or chronic, requiring effective secondary prevention strategies to prevent vision loss.9,15
Metformin, an oral drug commonly prescribed for treating type 2 diabetes mellitus (T2DM), is known to have anti-inflammatory effects regardless of one’s diabetes status.16 In addition, previous studies have demonstrated that a metformin analog can prevent uveitis onset in two animal models.17,18 These preliminary findings suggest metformin could modify uveitis risk. To date, no human studies of metformin and uveitis have been performed. As a relatively safe and inexpensive medication, metformin could be a good candidate as adjunctive therapy to reduce incidence of uveitis, to induce remission, or to prevent relapse.19 The purpose of this study is to utilize a large dataset assembled from a United States (US) medical claims database to determine whether use of metformin is associated with a lower risk of NIU incidence.
Methods
Study Population
Optum’s Clinformatics™ Data Mart Database contains the de-identified medical claims of all beneficiaries in a large US insurer’s commercial and Medicare Advantage administrative database and was used for this study. Included within the database are all outpatient medical claims (office visits, associated diagnoses, and in-office procedures), all pharmaceutical prescriptions filled, all laboratory testing claims (including selected results) and demographic data for each beneficiary during their enrollment in the insurance plan. The subset of data available for this study included all claims from January 1, 2000 to June 30, 2019. The Massachusetts Eye and Ear Infirmary’s and University of Pennsylvania’s Institutional Review Boards deemed this study exempt from review due to the de-identified nature of the data.
Study Design
We planned a priori two parallel analyses which were performed to examine the risk of NIU with regards to metformin use. First, we conducted a cohort study using similar methodology to a previous study evaluating the risk of uveitis associated with female hormonal therapy.20 The first analysis assessed a new user cohort study design with exposure status determined at baseline to most closely emulate a clinical trial with per protocol analysis using observational data. However, this study design can be problematic due to low statistical power if patients are only adherent to their baseline exposure for a short period of time, particularly for less common outcomes like uveitis and in situations where prolonged exposure is required to elicit a benefit. Therefore, as an additional analysis, we also performed a case-control study (described in detail below) using the same population identified for the cohort study.
Cohort Study
A new user active comparator cohort design was used to create cohorts from patients newly exposed to metformin (metformin cohort) or other oral anti-diabetic medications (non-metformin cohort). Patients who started a combination drug that included metformin were considered metformin users. All patients 18 years old or over who filled a prescription for an oral anti-diabetic medication were eligible for inclusion. The index date was defined as the date the first prescription for an oral anti-diabetic medication was filled. Patients with a prior diagnosis of infectious uveitis or NIU were also excluded. Please see eTable 1 in the Supplement for a full list of the International Classifications of Diseases (ICD), Ninth and Tenth Revisions (ICD-9 and ICD-10) codes used. Patients with less than one-year enrollment in the insurance plan (to ensure new user status) or who did not have a visit to an eye care provider prior to the index date (to reduce the possibility of undiagnosed subclinical uveitis) were excluded. In addition, to reduce variability in T2DM disease severity, patients were excluded if they had a prescription for insulin prior to the index date.
To account for differences between individuals taking metformin vs. other oral anti-diabetic medications, a propensity score for having been prescribed metformin was created using multivariable logistic regression. Covariates included in the propensity score were age at the index date, gender, race, education level, household income, geographic region of the country, index year, history of hypertension, hypercholesterolemia, chronic kidney disease, or systemic disease associated with uveitis, smoking status, the diabetes complications severity index (DCSI), number of health care visits within 1 year of index date, and hemoglobin A1c (HbA1c) level within 1 year of index date (with the closest to the index being used if more than one was available). The DCSI is a diabetes disease severity scale derived from outpatient ICD codes that are divided into six diabetes-related complication categories, each scored 0 – 2.21 While smoking often is not directly coded in administrative datasets, we applied an approach using smoking diagnosis codes, use of antismoking drugs and Current Procedural Terminology (CPT) codes for smoking cessation counseling. This method has previously found smoking rates in administrative databases of 10-11%, similar to the general population.22 To account for differences in these baseline covariates between the metformine and non-metformin cohorts, inverse probability of treatment weighting (IPTW) using the estimated propensity scores was performed in all subsequent analyses.
The primary outcome for the cohort analysis was incident NIU defined as two ICD-9 or ICD-10 codes for NIU within a 120-day period. The date of incidence was set to the date of the second confirming NIU code. In addition, to increase the likelihood of our cases being truly “incident”, we required patients who developed uveitis to have at least 2 years in the dataset prior to the index date without a diagnosis of uveitis for inclusion in the study.23 Sub-analyses focusing on anatomic subtype of uveitis were also performed. The different anatomic subtypes of uveitis are indicated in eTable 1.
A secondary outcome definition in which a prescription for a corticosteroid or a CPT code for an intraocular/periocular corticosteroid injection within 120 days of the uveitis code was used instead of requiring a second uveitis code as in our primary outcome definition. This approach has been used previously,20,24,25 and the rationale for this outcome is to capture a slightly different and partially overlapping set of uveitis patients who were seen once for NIU and may have improved significantly with corticosteroid treatment and were not seen again due to resolution of uveitis. For this group, the date of incidence was set to the date of the corticosteroid treatment.
Case-Control Study
As a sensitivity analysis, we conducted a case-control study within the cohort of patients used for the primary analysis. Cases of uveitis were defined using the primary uveitis outcome definition. The date of the second diagnosis code was defined as the outcome date. Controls were randomly selected using incidence density sampling at a 20:1 ratio of controls to cases, and controls were assigned the outcome date of their matched case.
Statistical Analysis Methods
For the cohort study, data were analyzed using Cox proportional hazards regression modeling with IPTW.26 Cumulative incidence of NIU was summarized using Kaplan-Meier curves with IPTW. In this model, patients were censored at disenrollment from the insurance plan, one year after the index date, any occurrence of a cohort exclusion criterion (including diagnosis of infectious uveitis), use of metformin in the non-metformin cohort or vice versa, or at the end of a 30-day gap in oral diabetic prescription coverage. To reduce potential misclassification of postoperative inflammation as uveitis, all patients who had an intraocular surgery were censored at the time of surgery. All patients were censored at 365 days.
For the case-control study, conditional logistic regression adjusting for demographic and clinical covariates was performed to examine the association between NIU and metformin use within 2 years before outcome date. This time point was chosen to examine putative long term effects of metformin on uveitis. An ordinal categorical model was used to calculate Wald p-values for metformin dose and duration effects. Patients using other classes of diabetic medications within 45 days of outcome date were considered active users of those medications and included in the multivariate analysis.
SAS version 9.4 (SAS Institute Inc., Cary, NC) software was used for all statistical analyses. We defined statistical significance using a two-sided alpha = 0.025, as recommended for exploratory, hypothesis-generating studies like this one.27
Results
Cohort Study
There were 359,139 new users of metformin (metformin cohort) and 162,847 new users of other oral anti-diabetic medications (non-metformin cohort) who met eligibility criteria. eFigure 1 delineates the inclusion and exclusion criteria for the cohorts. Table 1 compares the baseline characteristics of the metformin cohort and the non-metformin cohort both before and after IPTW. As noted in the table, IPTW achieved excellent balance between the cohorts for most variables (less than 0.1 standardized mean difference after weighting). The metformin cohort was followed for a median of 166 days [Interquartile range (IQR): 64, 365)] after the index date compared to a median of 130 days (IQR: 60, 351) in the non-metformin cohort.
Table 1.
Comparison of baseline characteristics between metformin and non-metformin cohorts before and after inverse probability of treatment weighting (IPTW)
| Non-metformin cohort |
Metformin cohort | Standardize d Mean difference |
Weighted non- metformin users |
Weighted metformin users |
Standardized Mean difference |
|
|---|---|---|---|---|---|---|
| Age [Mean years (SD)] | 64.3 (13.3) | 61.2 (13.9) | −0.226 | 61.5 (14.7) | 62.1 (13.7) | 0.042 |
| Gender (Female) | 86928 (53%) | 201210 (56%) | 0.054 | 92850 (57%) | 198987 (55%) | 0.023 |
| Race | 0.103 | 0.005 | ||||
| White | 105010 (64%) | 226967 (63%) | 103902 (63%) | 227596 (63%) | ||
| Black | 21436 (13%) | 42131 (12%) | 19835 (12%) | 43630 (12%) | ||
| Hispanic | 12940 (8%) | 37608 (10%) | 16083 (10%) | 35011 (10%) | ||
| Asian | 5294 (3%) | 14349 (4%) | 6257 (4%) | 13556 (4%) | ||
| Unknown | 18167 (11%) | 38084 (11%) | 17972 (11%) | 38891 (11%) | ||
| Education level | 0.084 | 0.002 | ||||
| ≤ High School Diploma | 52513 (32%) | 104044 (29%) | 49095 (30%) | 107502 (30%) | ||
| <Bachelor Degree | 77196 (47%) | 177759 (49%) | 80029 (49%) | 175072 (49%) | ||
| ≥Bachelor Degree | 18593 (11%) | 47068 (13%) | 20595 (13%) | 45003 (13%) | ||
| Unknown | 14545 (9%) | 30268 (8%) | 14331 (9%) | 31106 (9%) | ||
| Household income | 0.244 | 0.004 | ||||
| <$40K | 32530 (20%) | 69134 (19%) | 31960 (20%) | 69928 (20%) | ||
| $40K - $49K | 9317 (6%) | 21388 (6%) | 9652 (6%) | 21126 (6%) | ||
| $50K - $59K | 9668 (6%) | 23658 (7%) | 10412 (6%) | 22945 (6%) | ||
| $60K - $74K | 12862 (8%) | 32714 (9%) | 14321 (9%) | 31436 (9%) | ||
| $75K - $99K | 16988 (10%) | 46178 (13%) | 19727 (12%) | 43375 (12%) | ||
| $100K+ | 26342 (16%) | 79362 (22%) | 33401 (20%) | 72767 (20%) | ||
| Unknown | 55140 (34%) | 86705 (24%) | 44576 (27%) | 97106 (27%) | ||
| Geographic location | 0.165 | 0.009 | ||||
| Upper Midwest | 40283 (25%) | 87351 (24%) | 39811 (24%) | 86989 (24%) | ||
| Southern Midwest | 29571 (18%) | 68354 (19%) | 31409 (19%) | 67819 (19%) | ||
| Northeast | 16332 (10%) | 36690 (10%) | 16475 (10%) | 36473 (10%) | ||
| Mountain | 8525 (5%) | 28627 (8%) | 11600 (7%) | 25511 (7%) | ||
| Pacific | 11992 (7%) | 34150 (10%) | 14692 (9%) | 31649 (10%) | ||
| South Atlantic | 55648 (34%) | 102824 (29%) | 49561 (30%) | 109124 (30%) | ||
| Unknown | 496 (0%) | 1143 (0%) | 502 (0%) | 1119 (0%) | ||
| Index year | 0.816 | 0.010 | ||||
| 2001 | 4249 (3%) | 3803 (1%) | 2522 (2%) | 5591 (2%) | ||
| 2002 | 5703 (4%) | 3872 (1%) | 2996 (2%) | 6647 (2%) | ||
| 2003 | 6348 (4%) | 3415 (1%) | 3035 (2%) | 6585 (2%) | ||
| 2004 | 8793 (5%) | 1670 (0%) | 3259 (2%) | 6979 (2%) | ||
| 2005 | 14049 (9%) | 7259 (2%) | 6616 (4%) | 14591 (4%) | ||
| 2006 | 13323 (8%) | 13365 (4%) | 8274 (5%) | 18219 (5%) | ||
| 2007 | 14650 (9%) | 16468 (5%) | 9771 (6%) | 21455 (6%) | ||
| 2008 | 15553 (10%) | 15746 (4%) | 9794 (6%) | 21574 (6%) | ||
| 2009 | 10210 (6%) | 27743 (8%) | 12034 (7%) | 26157 (7%) | ||
| 2010 | 11335 (7%) | 23504 (7%) | 10881 (7%) | 23974 (7%) | ||
| 2011 | 9236 (6%) | 27331 (8%) | 11525 (7%) | 25170 (7%) | ||
| 2012 | 8170 (5%) | 21537 (6%) | 9235 (6%) | 20378 (6%) | ||
| 2013 | 8273 (5%) | 26150 (7%) | 10835 (7%) | 23655 (7%) | ||
| 2014 | 6008 (4%) | 23801 (7%) | 9518 (6%) | 20509 (6%) | ||
| 2015 | 6733 (4%) | 31343 (9%) | 11879 (7%) | 26156 (7%) | ||
| 2016 | 5441 (3%) | 30317 (8%) | 11440 (7%) | 24584 (7%) | ||
| 2017 | 6247 (4%) | 32645 (9%) | 12433 (8%) | 26774 (7%) | ||
| 2018 | 5380 (3%) | 31100 (9%) | 11453 (7%) | 25100 (7%) | ||
| 2019 | 3146 (2%) | 18070 (5%) | 6551 (4%) | 14586 (4%) | ||
| Hypertension | 132323 (81%) | 276663 (77%) | −0.104 | 126867 (77%) | 280673 (78%) | 0.022 |
| High cholesterol | 133671 (82%) | 284980 (79%) | −0.069 | 130186 (79%) | 287690 (80%) | 0.021 |
| Uveitis-associated diseases * | 20445 (13%) | 40028 (11%) | −0.044 | 19268 (12%) | 41903 (12%) | −0.002 |
| Smokers | 29484 (18%) | 78669 (22%) | 0.095 | 33911 (21%) | 74307 (21%) | 0.001 |
| Chronic kidney disease | 0.183 | 0.002 | ||||
| No disease | 123113 (76%) | 295642 (82%) | 131614 (80%) | 287553 (80%) | ||
| Chronic kidney disease | 35060 (22%) | 59273 (17%) | 29678 (18%) | 65135 (18%) | ||
| End-stage renal disease | 4674 (3%) | 4224 (1%) | 2758 (2%) | 5996 (2%) | ||
| Diabetes Complication | 1.8 (2.1) | 1.3 (1.7) | −0.285 | 1.4 (1.8) | 1.5 (1.8) | 0.009 |
| Severity Index [Mean (SD)] | ||||||
| N of health care visits | 9.1 (6.9) | 7.7 (6.2) | −0.202 | 8.2 (6.4) | 8.2 (6.7) | −0.009 |
| within 1 year before | ||||||
| index date [Mean (SD)] | ||||||
| HbA1c within 1 year | 7.3 (1.6) | 7.0 (1.4) | −0.203 | 7.1 (1.8) | 7.1 (1.4) | 0.018 |
| before index date [Mean (SD)] † |
A list of uveitis-associated diseases can be found in eTable 1.
HbA1c was available on 30% of patients in the metformin cohort and 19% of patients in the non-metformin cohort.
K = thousand
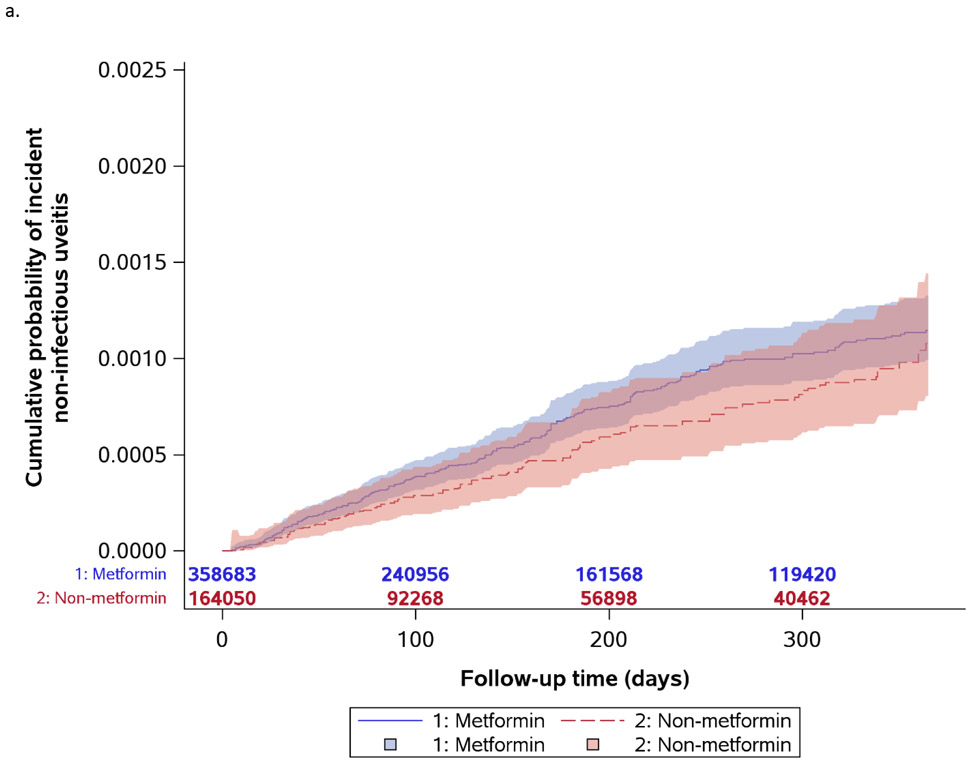
Primary Outcome
Figure 1a shows the Kaplan-Meier curves for incident uveitis diagnosis. For the primary outcome, the metformin cohort had 230 events over 195,300 years (unweighted incidence rate=0.12 per 100 person-years) and the non-metformin cohort had 104 events over 80,660 years (unweighted incidence rate=0.13 per 100 person-years). Table 2 provides the results for the Cox regression analyses for the primary outcome. NIU incidence was not significantly different between the metformin and non-metformin cohorts [Hazard Ratio (HR) = 1.19, 95% Confidence Interval (CI): 0.92-1.54, P=0.19]. In the sub-analyses based on uveitis anatomic subtype, no statistically significant difference in the rate of incident anterior uveitis (HR=1.33, 95% CI: 0.97-1.83, P=0.08), intermediate uveitis (HR=1.71, 95% CI: 0.52-5.60, P=0.38) or posterior/panuveitis (HR=0.63, 95% CI: 0.10-4.07, P=0.63) was found.
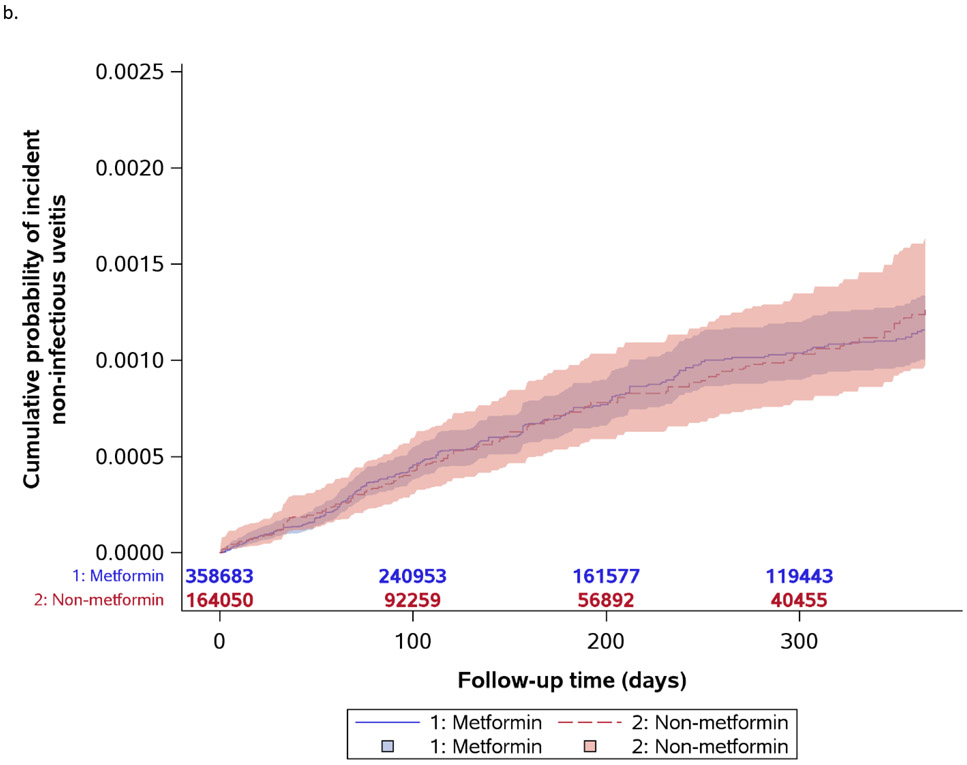
Figure 1.
Kaplan-Meier curves for incident non-infectious uveitis by metformin exposure status
Table 2.
Cox regression results of primary outcome weighted by IPTW
| Unweighted summary statistics |
Propensity score-weighted results | |||||
|---|---|---|---|---|---|---|
| Cohort | N | Person- years |
N of events |
KM-estimated cumulative incidence at 1 year |
Hazard Ratio (95% CI) |
P value |
| Any Uveitis | 0.19 | |||||
| Non-metformin | 162847 | 80660 | 104 | 0.0011 | REF | |
| Metformin | 359139 | 195300 | 230 | 0.0011 | 1.19 (0.92, 1.54) |
|
| Anterior Uveitis | 0.08 | |||||
| Non-metformin | 162847 | 80670 | 66 | 0.0007 | REF | |
| Metformin | 359139 | 195320 | 164 | 0.0008 | 1.33 (0.97, 1.83) |
|
| Intermediate Uveitis | 0.38 | |||||
| Non-metformin | 162847 | 80670 | 4 | 0.00004 | REF | |
| Metformin | 359139 | 195320 | 11 | 0.00005 | 1.71 (0.52, 5.60) |
|
| Posterior/Panuveitis | 0.63 | |||||
| Non-metformin | 162847 | 80670 | 2 | 0.00002 | REF | |
| Metformin | 359139 | 195320 | 3 | 0.00001 | 0.63 (0.10, 4.07) |
|
KM = Kaplan Meier, IPTW = inverse probability of treatment weighting
Secondary Outcome
Figure 1b provides the Kaplan-Meier curves for the pre-specified secondary outcome analysis where the outcome definition included a NIU diagnosis code and corticosteroid prescription. In this analysis, the metformin cohort had 237 events over 195,309 years (unweighted incidence rate=0.12 per 100 person-years) and the non-metformin cohort had 131 events over 80,651 years (unweighted incidence rate=0.16 per 100 person-years). Table 3 shows the results using this outcome. The hazard for NIU was not significantly different between the metformin and non-metformin cohorts (HR=0.97, 95% CI: 0.75-1.24, P=0.80). No significant differences were found based on uveitis subtype either (anterior uveitis - HR=1.12, 95% CI: 0.83-1.52, P=0.46; intermediate uveitis - HR=0.48, 95% CI: 0.19-1.23, P=0.12; posterior/panuveitis - HR=0.96, 95% CI: 0.20-4.64, P=0.95).
Table 3.
Cox regression results of secondary outcome weighted by IPTW
| Unweighted summary statistics |
Propensity-score weighted results | |||||
|---|---|---|---|---|---|---|
| Cohort | N | Person- years |
N of events |
KM-estimated cumulative incidence at 1 year |
Hazard Ratio (95% CI) |
P value |
| Any Uveitis | 0.80 | |||||
| Non-metformin | 162847 | 80651 | 131 | 0.0013 | REF | |
| Metformin | 359139 | 195309 | 237 | 0.0012 | 0.97 (0.75, 1.24) |
|
| Anterior Uveitis | 0.46 | |||||
| Non-metformin | 162847 | 80662 | 77 | 0.0007 | REF | |
| Metformin | 359139 | 195326 | 158 | 0.0008 | 1.12 (0.83, 1.52) |
|
| Intermediate Uveitis | 0.12 | |||||
| Non-metformin | 162847 | 80662 | 12 | 0.00012 | REF | |
| Metformin | 359139 | 195326 | 9 | 0.00004 | 0.48 (0.19, 1.23) |
|
| Posterior/Panuveitis | 0.95 | |||||
| Non-metformin | 162847 | 80662 | 3 | 0.00004 | REF | |
| Metformin | 359139 | 195326 | 7 | 0.00004 | 0.96 (0.20, 4.64) |
|
KM = Kaplan Meier, IPTW = inverse probability of treatment weighting
Case-Control Analysis
For the primary outcome definition of NIU, we identified 502 NIU cases and selected 10,040 controls. eTable 2 presents the characteristics of these cases and controls. Table 4 presents the results of the conditional logistic regression analysis comparing the use of metformin within 2 years prior to the index date between cases and controls. NIU cases had a non-statistically significant lower odds of having any metformin use within the 2 years before the index date [adjusted odds ratio (aOR) = 0.64, 95% CI: 0.39–1.04, P=0.07]. However, when assessing duration of metformin use, a linear trend analysis found lower odds of uveitis with longer use when compared to the reference group of no metformin use (445-729 days of use - aOR=0.49; 95% CI: 0.27-0.90, trend P=0.02). Assessment of cumulative dose found a similar trend with higher metformin doses being associated with a lower odds of uveitis when compared to the reference group of no metformin use (165,001 – 390,000 mg - aOR=0.57; 95% CI: 0.34-0.98; >390,000 mg - aOR=0.44; 95% CI: 0.25-0.78, P=0.001).
Table 4.
Conditional logistic regression analysis for association between metformin use 2 years before outcome date and incident non-infectious uveitis
| Use of metformin within 2 years before index date |
Case N=502 n (%) |
Control N=10,040 n (%) |
Adjusted Odds Ratio (95% CI)* |
Adjusted P value*¶ |
|---|---|---|---|---|
| No | 167 (33%) | 2871 (29%) | REF | 0.07 |
| Yes | 335 (67%) | 7169 (71%) | 0.64 (0.39, 1.04) | |
| Cumulative duration (days)£ | ||||
| 0 | 167 (33%) | 2871 (29%) | REF | 0.02 |
| 4-68 | 90 (18%) | 1795 (18%) | 0.76 (0.43, 1.34) | |
| 69-176 | 86 (17%) | 1796 (18%) | 0.69 (0.40, 1.20) | |
| 177-444 | 81 (16%) | 1780 (18%) | 0.60 (0.34, 1.06) | |
| 445-729 | 78 (16%) | 1797 (18%) | 0.49 (0.27, 0.90) | |
| Cumulative dosage (mg) £ | ||||
| 0 | 167 (33%) | 2871 (29%) | REF | 0.001 |
| 2,000 – 60,000 | 95 (19%) | 1843 (18%) | 0.81 (0.46, 1.41) | |
| 60,001 – 165,000 | 89 (18%) | 1725 (17%) | 0.76 (0.45, 1.31) | |
| 165,001 – 390,000 | 82 (16%) | 1799 (18%) | 0.57 (0.34, 0.98) | |
| 390,001 – 1,777,350 | 69 (14%) | 1801 (18%) | 0.44 (0.25, 0.78) |
Adjusted for age, gender, race, education, income, geographic location, history of hypertension, hypercholesterolemia, chronic kidney disease, uveitis-associated diseases, smoking, diabetes complications severity index, use of insulin, use of other diabetic medication within 45 days before the index date (sulfonylurea, thiazolidinedione, alpha glucosidase inhibitor, meglitinide, dipeptidyl peptidase 4 inhibitor, bile sequestrant), HbA1c level and number of health care visits within 1 year before index date.
Categories based on quartiles.
Linear trend p value
REF= reference group
Discussion
We performed two main types of analyses to assess the association between metformin and NIU. The first was a cohort analysis which compared new users of metformin to new users of other oral anti-diabetic medications. Our primary outcome definition of uveitis that used multiple diagnosis codes within 120 days found no difference in the hazard (HR=1.19) based on exposure. Analyses using our secondary outcome definition of diagnosis plus use of a corticosteroid was also not statistically significant. Of note, the median follow up time in the metformin cohort was only 166 days and all patients were censored after 365 days.
Recognizing the possible limitation of our method to detect differences for longer metformin exposure, we also performed a case-control analysis that allowed for two years of assessment. When examining the association with any metformin use within two years prior to the outcome date, there was no statistically significant difference between uveitis cases and controls. However, a statistically significantly decreased odds of uveitis was found in assessing (1) longer duration of metformin use and (2) higher metformin cumulative doses. The odds of uveitis decreased incrementally with each quartile of higher cumulative metformin duration and dose (duration P=0.02 and dose P=0.001). Onset of NIU is an uncommon event and the triggers of uveitis onset, which are not well understood, may result from haphazardly occurring trigger events over time. These circumstances may explain why the effects of a putatively protective medication like metformin may only be detectable over longer periods of exposure, which would allow a large number of these trigger events to occur and be suppressed by metformin to detect differences between groups.
Animal studies have suggested that a related agent, aminoimidazole carboxamide ribofuranoside (AICAR), can attenuate uveitis in animal models through reducing expression of pro-inflammatory cytokines and inhibiting T-cell proliferation.17,18 Both metformin and AICAR activate AMP-activated protein kinase (AMPK) and have been shown to exert anti-inflammatory activity in many experimental models by reducing oxidative stress and interleukin (IL)-1 beta-induced pro-inflammatory cytokines (IL-6 and IL-8) production in macrophages and endothelial cells.28 It is possible the protective association between longer-term use of metformin and lower uveitis incidence are due to a similar physiologic pathway.
An additional question is whether the hypoglycemic effects of metformin may contribute to its impact on NIU risk. Randomized controlled trials have shown that metformin achieves lower HbA1c levels than glyburide, a common sulfonylurea, but higher HbA1c levels than rosiglitazone, a common thiazolidinedione.29 Sulfonylureas and thiazolidinediones made up nearly 70% of the medications in non-metformin cohort. Given its intermediate effects on blood glucose, it is less likely that the hypoglycemic effects of metformin account for the difference in NIU risk.
Some limitations to our study should be noted. First, although we made efforts to reduce differences in disease severity through controlling for the DCSI, HbA1c level, and insulin use after the index date, it is still possible that the common practice of using metformin as a first line therapy for T2DM treatment allowed for unmeasured confounding. We did not have a complete dataset for HbA1c; we had HbA1c on approximately 30% and 19% of patients in the metformin and non-metformin cohorts, respectively, and therefore could not completely account for differences in hyperglycemia severity in propensity scores. Also, we cannot be certain that patients who were prescribed metformin, actually took the metformin. This, however, would cause a misclassification bias which biases to a null (no difference) finding if non-adherence is non-differential with respect to other patient characteristics. This could in fact explain why only prolonged metformin use was associated with a protective effect with respect to uveitis.
The database does not allow independent verification of the accuracy of the ICD codes with medical chart level data. However, ICD codes have been shown to correlate highly with the corresponding clinical diagnosis and are widely used in health research, including in the ophthalmic literature.22,30-33 While the dataset has the strength of being a national, multiethnic data set, the insurance-based nature may limit the generalizability to patients not covered by this specific insurance company. The majority of patients in this analysis had T2DM (apart from a small subset that might have received metformin for a non-T2DM indication such as polycystic ovarian syndrome), whereas the majority of patients with uveitis do not have T2DM. This may limit the generalizability of the findings. Despite this dataset being the largest of its kind to be used for risk factor studies in uveitis, the rare nature of uveitis is still evident in the small number of patients in the analyses of the rarer anatomic subtypes of uveitis, intermediate and posterior/panuveitis.
In summary, our analysis suggested that metformin may be protective after prolonged periods of exposure for incident uveitis. While not all analyses were significant, the duration of exposure and cumulative dose results suggest that metformin as a protective agent warrants further investigation, particularly because it is a generally safe agent.
Supplementary Material
Acknowledgments
Financial Support: National Institutes of Health R21 EY029851 and University of Pennsylvania Core Grant for Vision Research P30 EY001583. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding was provided by Research to Prevent Blindness and Sight for Souls. None of the funding organizations had any role in the design or conduct of the study.
Footnotes
Conflicts of Interest: Dr. Kempen has served as a consultant for Gilead (Data and Safety Monitoring Committee Chair) Dr. Hubbard has received financial support from Pfizer and Humana. The remaining authors have no conflicts of interest to disclose.
References
- 1.ten Doesschate J Causes of blindness in The Netherlands. Doc Ophthalmol. 1982;52(3-4):279–285. [DOI] [PubMed] [Google Scholar]
- 2.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80(9):844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subcommittee NAECPP. Vision Research - A National Plan: 1983-1987: Volume 1: The 1983 Report of the National Advisory Eye Council. National Institutes of Health, Public Health Service, US Department of Health and Human Services, Bethesda, MD. 1983:13. [Google Scholar]
- 4.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14(5-6):303–308. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein H The reported demography and causes of blindness throughout the world. Adv Ophthalmol. 1980;40:1–99. [PubMed] [Google Scholar]
- 6.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004;218(4):223–236. [DOI] [PubMed] [Google Scholar]
- 8.Darrell RW, Wagener HP, Kurland LT. Epidemiology of uveitis. Incidence and prevalence in a small urban community. Arch Ophthalmol. 1962;68:502–514. [DOI] [PubMed] [Google Scholar]
- 9.Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol. 2013;131(11):1405–1412. [DOI] [PubMed] [Google Scholar]
- 10.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500. [DOI] [PubMed] [Google Scholar]
- 11.Suhler EB, Lloyd MJ, Choi D, Rosenbaum JT, Austin DF. Incidence and prevalence of uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. Am J Ophthalmol. 2008;146(6):890–896 e898. [DOI] [PubMed] [Google Scholar]
- 12.Bro T, Tallstedt L. Epidemiology of uveitis in a region of southern Sweden. Acta Ophthalmol. 2020;98(1):32–35. [DOI] [PubMed] [Google Scholar]
- 13.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492–513. [DOI] [PubMed] [Google Scholar]
- 14.Writing Committee for the Multicenter Uveitis Steroid Treatment T, Follow-up Study Research G, Kempen JH, et al. Association Between Long-Lasting Intravitreous Fluocinolone Acetonide Implant vs Systemic Anti-inflammatory Therapy and Visual Acuity at 7 Years Among Patients With Intermediate, Posterior, or Panuveitis. JAMA. 2017;317(19):1993–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80(4):332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron AR, Morrison VL, Levin D, et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ Res. 2016;119(5):652–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki J, Manola A, Murakami Y, et al. Inhibitory effect of aminoimidazole carboxamide ribonucleotide (AICAR) on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2011;52(9):6565–6571. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki J, Yoshimura T, Simeonova M, et al. Aminoimidazole carboxamide ribonucleotide ameliorates experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012;53(7):4158–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasri H, Rafieian-Kopaei M. Metformin: Current knowledge. J Res Med Sci. 2014;19(7):658–664. [PMC free article] [PubMed] [Google Scholar]
- 20.Sobrin L, Yu Y, Susarla G, et al. Risk of Non-infectious Uveitis with Female Hormonal Therapy in a Large Healthcare Claims Database. Ophthalmology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14(1):15–23. [PMC free article] [PubMed] [Google Scholar]
- 22.Desai RJ, Eddings W, Liao KP, Solomon DH, Kim SC. Disease-modifying antirheumatic drug use and the risk of incident hyperlipidemia in patients with early rheumatoid arthritis: a retrospective cohort study. Arthritis Care Res (Hoboken). 2015;67(4):457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein JD, Blachley TS, Musch DC. Identification of persons with incident ocular diseases using health care claims databases. Am J Ophthalmol. 2013;156(6):1169–1175 e1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobrin L, Stanwyck LK, Pan W, Hubbard RA, Kempen JH, VanderBeek BL. Association of Hypovitaminosis D With Increased Risk of Uveitis in a Large Health Care Claims Database. JAMA Ophthalmol. 2018;136(5):548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forooghian F, Maberley D, Albiani DA, Kirker AW, Merkur AB, Etminan M. Uveitis risk following oral fluoroquinolone therapy: a nested case-control Study. Ocul Immunol Inflamm. 2013;21(5):390–393. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Y, Heagerty PJ. Partly conditional survival models for longitudinal data. Biometrics. 2005;61(2):379–391. [DOI] [PubMed] [Google Scholar]
- 27.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine--reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–2194. [DOI] [PubMed] [Google Scholar]
- 28.Gejjalagere Honnappa C, Mazhuvancherry Kesavan U. A concise review on advances in development of small molecule anti-inflammatory therapeutics emphasising AMPK: An emerging target. Int J Immunopathol Pharmacol. 2016;29(4):562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. [DOI] [PubMed] [Google Scholar]
- 30.Bearelly S, Mruthyunjaya P, Tzeng JP, et al. Identification of patients with diabetic macular edema from claims data: a validation study. Arch Ophthalmol. 2008;126(7):986–989. [DOI] [PubMed] [Google Scholar]
- 31.Muir KW, Gupta C, Gill P, Stein JD. Accuracy of international classification of diseases, ninth revision, clinical modification billing codes for common ophthalmic conditions. JAMA Ophthalmol. 2013;131(1):119–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto M, Ohl ME, Schweizer ML, Perencevich EN. Accuracy of administrative code data for the surveillance of healthcare-associated infections: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(5):688–696. [DOI] [PubMed] [Google Scholar]
- 33.Santos CA, Brennan DC, Olsen MA. Accuracy of Inpatient International Classification of Diseases, Ninth Revision, Clinical Modification Coding for Cytomegalovirus After Kidney Transplantation. Transplant Proc. 2015;47(6):1772–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.