Dear Editor,
Inflammatory pain, such as arthritic pain, is a common health problem worldwide. Inflammatory pain is generally treated by opioids and non-steroidal anti-inflammatory drugs including cyclooxygenase-2 (COX-2) inhibitors. However, side-effects limit the analgesic efficiency of current treatments and there is an urgent need to develop novel strategies for treatment.
Mounting evidence suggests that acute inflammation includes two distinct phases, an early inflammatory phase and a late resolution phase [1]. During the inflammatory phase, immune cells, especially neutrophils, infiltrate into the injured tissue [2], producing proinflammatory cytokines to induce inflammatory pain by activating nociceptors throughout skin and tissues [3]. Pro-resolution lipid mediators, such as resolvins and protectins, are actively involved in the resolution phase, resulting in the resolution of inflammation and return to homeostasis, partly by boosting the phagocytic activity of macrophages (MΦ) [4, 5]. We have shown that exogenous resolvins produce potent antinociceptive effects in animal models of inflammatory pain at doses lower than morphine [6]. However, the mechanisms underlying the resolution of inflammatory pain are not fully understood.
Chemerin is an endogenous peptide ligand for ChemR23 (Chemerin Receptor 23), a G protein-coupled receptor (GPCR). Although ChemR23 is involved in the analgesic effects of resolvins [6], the role of the endogenous chemerin-ChemR23 signal in inflammatory pain has yet to be revealed. Given the critical role of chemerin and ChemR23 in the resolution of inflammation and their abundant distribution in the epidermis [7], we presumed that endogenous chemerin-ChemR23 might play a role in the resolution of inflammatory pain. Here, we examined the role of endogenous chemerin in an inflammatory pain model induced by intraplantar injection of carrageenan in mice. We further investigated the mechanism underlying the resolution of inflammatory pain by chemerin-ChemR23.
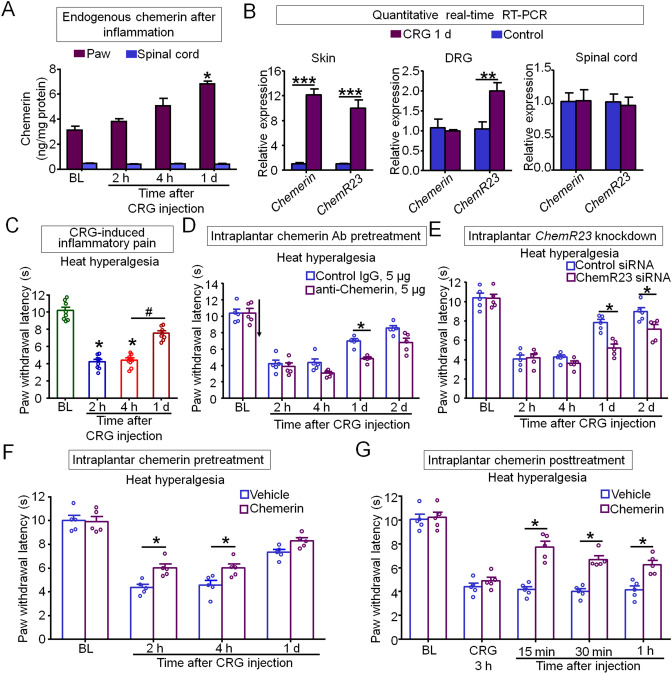
First, we measured the expression of endogenous chemerin in mouse hindpaw skin and spinal cord tissues by enzyme-linked immunosorbent assays. Interestingly, chemerin was highly expressed and significantly up-regulated in paw skin at 1 day after carrageenan (CRG) injection, but not in dorsal root ganglia (DRGs) and spinal cord (Fig. 1A, B). Moreover, ChemR23 mRNA was dramatically boosted in paw skin and DRGs, but not in spinal cord at 1 day after CRG treatment (Fig. 1B). Given this expression pattern, we hypothesized that the endogenous chemerin-ChemR23 signal might act on skin cells or local nerve fibers in skin. Intraplantar CRG induced a quick (<2 h) heat hyperalgesia, a cardinal feature of inflammatory pain, as revealed by a reduction in paw withdrawal latency to radiant heat stimulation. The CRG-induced heat hyperalgesia, which peaked at 2 and 4 h, was significantly resolved at 1 day after injection (Fig. 1C). Based on the consistent time-course of chemerin upregulation and pain resolution, we hypothesized that up-regulated chemerin in the inflamed hindpaw may contribute to the resolution of inflammatory pain.
Fig. 1.
Chemerin and its receptor ChemR23 contribute to the resolution of inflammatory pain. A Endogenous chemerin is up-regulated in the paw but not the spinal cord [*P < 0.05 vs baseline (BL), one-way ANOVA; n = 3–6 mice/group]. B Chemerin and ChemR23 mRNAs are boosted in hindpaw skin, but not in the spinal cord (***P < 0.001, **P < 0.01 vs control, Student’s t-test; n = 5 mice/group). C Time-course of the initiation and resolution of inflammatory pain induced by intraplantar injection of carrageenan [CRG, 20 μL; *P < 0.05 vs BL; #P < 0.05, 4 h vs 1 day (d), one-way ANOVA; n = 8 mice/group]. D, E Neutralizing endogenous chemerin by intraplantar injection of chemerin neutralizing antibody (chemerin Ab, 5 μg in 20 μL; D) or knocking down ChemR23 by intraplantar siRNA pretreatment (E) delays the resolution of inflammatory pain. ChemR23 siRNA (4 μg in 20 μL) or control siRNA was intraplantarly injected at 48 h, 24 h, and 3 h before CRG injection (*P < 0.05 vs control, two-way ANOVA; n = 5 mice/group). F Pretreatment with exogenous chemerin partially reduces CRG-induced heat hyperalgesia. Chemerin (0.9 μg in 20 μL) was intraplantarly injected before CRG injection. G Posttreatment with exogenous chemerin reverses CRG-induced heat hyperalgesia. Chemerin (0.45 μg in 20 μL) was intraplantarly injected 3 h after CRG injection [*P < 0.05 vs vehicle (saline), two-way ANOVA, n = 5 mice/group]. Data are the mean ± SEM.
To test our hypothesis, we investigated whether CRG-induced heat hyperalgesia would be altered after neutralizing endogenous chemerin by intraplantar injection of its antibody. Indeed, the resolution of heat hyperalgesia was significantly delayed by pretreatment with anti-chemerin neutralizing antibody (Fig. 1D). Furthermore, knockdown of ChemR23, the GPCR receptor for chemerin, with a specific siRNA, also largely blocked the resolution of heat hyperalgesia induced by CRG (Fig. 1E). Therefore, these data suggest that endogenous chemerin and its receptor ChemR23 contribute to the resolution of inflammatory pain. To further clarify the role of chemerin in promoting inflammatory pain resolution, we tested the effect of exogenous chemerin in CRG-induced heat hyperalgesia. As expected, intraplantar pretreatment with chemerin partially reduced the CRG-induced heat hyperalgesia (Fig. 1F). Similarly, intraplantar posttreatment with chemerin reversed the established heat hyperalgesia induced by CRG (Fig. 1G). Thus, chemerin is necessary and sufficient to promote inflammatory pain resolution.
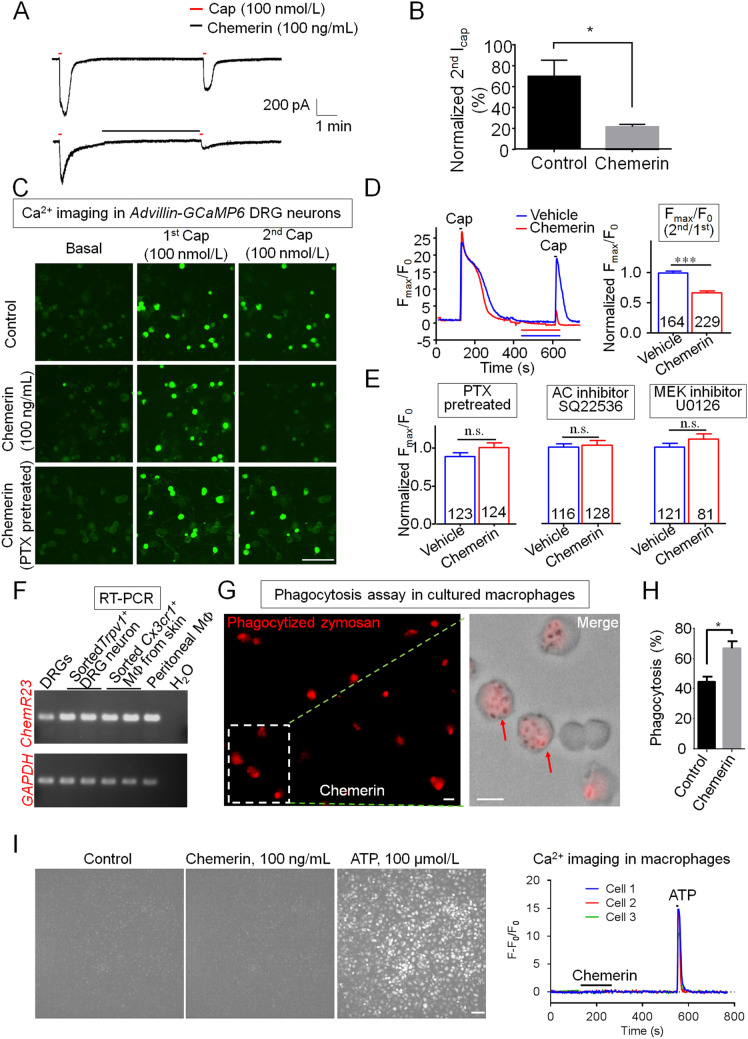
Transient receptor potential vanilloid subtype 1 (TRPV1) is one of the most important ion channels expressed in nociceptors and plays a critical role in inflammatory pain and heat hyperalgesia [8]. We investigated whether chemerin would inhibit TRPV1 activity in small (diameter < 25 μm) DRG neurons. Capsaicin (Cap) is a TRPV1 agonist and specifically activates TRPV1 to induce inward current and intracellular calcium (iCa2+) elevation. Patch-clamp recordings showed that the Cap (100 nmol/L)-induced inward current was largely inhibited by chemerin (100 ng/mL) in small DRG neurons (Fig. 2A, B). To further study the Ca2+ response in DRG neurons, we generated AdvCre; GCamp6fl/− (Advillin-GCaMP6) mice by crossing GCamp6fl/fl mice with sensory-neuron-specific Cre line AdvillinCre mice. We performed Ca2+ imaging in dissociated DRG neurons from Advillin-GCaMP6 mice to test whether chemerin would inhibit the TRPV1-mediated intracellular Ca2+ elevation. Ca2+ imaging showed that Cap (100 nmol/L) induced a robust iCa2+ elevation in small DRG neurons (Fig. 2C, D). Interestingly, chemerin (100 ng/mL) treatment inhibited the iCa2+ elevation induced by Cap (Fig. 2C, D). The peak amplitude of iCa2+ elevation mediated by a second Cap bath was significantly decreased by chemerin pretreatment in Cap-responsive neurons (Fig. 2D). These data indicate that chemerin directly inhibits TRPV1 activation (currents and iCa2+ signals) in nociceptors. Since the distribution of ChemR23 was in Trpv1+ DRG neurons (Fig. 2F), we next tested whether chemerin would inhibit TRPV1 activity via a ChemR23-associated signaling pathway. Given that ChemR23 is Gαi-coupled GPCR [9], pertussis toxin (PTX) was applied to block ChemR23-associated signaling pathways. We found that the inhibitory effect of chemerin on TRPV1 activity was absolutely relieved in DRG neurons pretreated with PTX (0.5 μg/mL) for 18 h (Fig. 2C, E). Adenylyl cyclase (AC) and extracellular signal-regulated kinase (ERK) are important downstream effectors of the GPCR signal and involve in the regulation of TRPV1 activity [6, 10]. Perfusion of an AC inhibitor (SQ22536) and an ERK kinase MEK inhibitor (U0126) inhibited Cap-induced TRPV1 activity in primary cultured DRG neurons [10]. Furthermore, if we adequately blocked AC and MEK via SQ22536 and U0126 (5 μmol/L, 5 min) pretreatment, chemerin did not further suppress the Cap-induced iCa2+ elevation in DRG neurons (Fig. 2E). Thus, chemerin negatively modulates TRPV1 activity via the Gαi-mediated inhibition of AC and ERK signaling in DRG neurons.
Fig. 2.
Chemerin inhibits TRPV1 activation in nociceptive DRG neurons and enhances phagocytic activity of macrophages. A Patch-clamp recording showing the inhibition of capsaicin-induced inward current in small DRG neurons after chemerin treatment (100 ng/mL, 5 min). B Normalized amplitude of 2nd capsaicin currents [(the 2nd current/the 1st current) × 100%] in small DRG neurons with vehicle (control) or chemerin treatment (*P < 0.05 vs Control; unpaired Student’s t-test; n = 5 neurons/group). C–E Ca2+ imaging in DRG neurons from AdvCre; GCamp6fl/− mice. C Representative images showing Ca2+ elevation in small DRG neurons after capsaicin (Cap, 100 nmol/L) treatment. Note chemerin (100 ng/mL, 3 min) treatment reduces the peak amplitude of iCa2+ elevation induced by the second capsaicin bath, which was relieved by pretreatment with pertussis toxin (PTX, 0.5 μg/mL, 18 h) (scale bar, 100 μm). D Left: representative traces showing increases in iCa2+ induced by capsaicin (Cap, 100 nmol/L) in small DRG neurons in the absence and presence of chemerin (100 ng/mL). Right: normalized Fmax/F0 amplitude of 2nd Cap-induced iCa2+ elevation in small DRG neurons with or without chemerin treatment. Normalized 2nd Fmax/F0 = (2nd Fmax/1st Fmax)/(average of 2nd Fmax/1st Fmax in Vehicle group). ***P < 0.001, vs Vehicle group; unpaired Student’s t-test. n = 164–229 neurons were analyzed for each group. E Effects of PTX (0.5 μg/mL, pretreatment for 18 h), the adenylyl cyclase inhibitor SQ22536 (5 μmol/L, bath application for 5 min), and the MEK inhibitor U0126 (5 μmol/L, bath application for 5 min) on chemerin-induced inhibition of TRPV1 activity (n.s., not significant. P = 0.13 for PTX pretreatment, P = 0.76 for SQ22536 treatment, P = 0.21 for U0126 treatment vs Vehicle; unpaired Student’s t-test; n = 81–128 neurons were analyzed for each group). F RT-PCR showing the expression of ChemR23 in DRG tissue, sorted Trpv1+ DRG neurons, Cx3cr1+ macrophages (MΦ) from skin, and peritoneal MΦ. Gapdh was used as internal control. H2O was used as negative control. G, H Chemerin treatment enhances phagocytosis in cultured MΦ. G Representative images of phagocytosis in MΦ with chemerin (100 ng/mL) treatment. Note that only intracellular zymosan particles (pH-sensitive and dye-conjugated) display red fluorescence. Red arrows indicate the phagocytized zymosan particles in MΦ. Scale bar, 10 μm. H Chemerin treatment enhances phagocytic activity as revealed by the percentage of cells with phagocytosis (*P < 0.05 vs Control, unpaired Student’s t-test, n = 897–1116 cells were analyzed for each group). I Ca2+ imaging in MΦ from EIIaCre; GCamp6fl/− mice. Left, representative images showing iCa2+elevation in MΦ after ATP (100 μmol/L) treatment but not chemerin (100 ng/mL) treatment (scale bar, 50 μm). Right, representative traces showing increases in iCa2+ induced by ATP but not chemerin in MΦ. Data are presented as the mean ± SEM.
Phagocytosis is a key function of MΦ and critical for the resolution of inflammation and inflammatory pain [11]. To determine whether chemerin is involved in the phagocytosis of MΦ, we first examined the expression of its receptor ChemR23 in MΦ. RT-PCR showed that ChemR23 was expressed in both chemokine (C-X3-C) motif receptor 1-positive (Cx3cr1+) skin MΦ and peritoneal MΦ (Fig. 2F). Interestingly, CRG treatment induced the striking upregulation (15–25-fold change) of Chemerin and ChemR23 mRNA in sorted Cx3cr1+ skin MΦ (Supplementary Fig. 1B), which was higher than their elevated level in paw skin tissue (Fig. 1B). Zymosan is a well-studied pathogen and triggers marked inflammation and inflammatory pain after intraplantar injection [11]. We further investigated whether chemerin enhances phagocytosis in MΦ using pHrodo® Red dye-conjugated zymosan particles, which show red fluorescence after phagocytosis due to the lower pH values in intracellular compartments such as phagosomes [11]. Chemerin treatment (100 ng/mL for 30 min) caused an increase of phagocytic activity in cultured MΦ, and more phagocytized zymosan particles were found after chemerin treatment (Fig. 2G, H). The percentage of MΦ with phagocytosis was significantly increased from 45% in the control group to 67% in the chemerin-treated group (Fig. 2H). Intracellular Ca2+ plays a distinct role in GPCR-mediated phagocytosis in macrophages [11, 12]. We took advantage of EIIaCre;GCamp6fl/− (EIIa-GCaMP6) mice, in which GCaMP6 was expressed globally including MΦ, to test whether chemerin can induce iCa2+ elevation in MΦ. Ca2+ imaging in MΦ showed that chemerin treatment failed to induce iCa2+ elevation in MΦ, but positive control ATP treatment did induce robust iCa2+ elevation (Fig. 2I). It appears that chemerin induces phagocytosis of MΦ in an iCa2+-independent manner. Thus, these findings suggest an important role of chemerin in macrophage phagocytosis and the resolution of inflammatory pain.
Here, we found that endogenous chemerin and ChemR23 contribute to the resolution of inflammatory pain. Pro-resolution lipid mediators, derived from EPA and DHA, are well known for their potent anti-inflammatory and pro-resolving actions in acute inflammation [1]. Resolvin E1 suppresses inflammatory pain via inhibiting neutrophil infiltration and proinflammatory cytokines, partially in a ChemR23-dependent manner [6]. In this study, we revealed that chemerin, the natural endogenous ligand for ChemR23, had an up-regulated expression pattern in the inflamed paw, which coincided with the resolution of inflammatory pain. Furthermore, endogenous chemerin efficiently accelerated inflammatory pain resolution but did not influence the initiation (Fig. 1D, E). Considering the vital role of the proinflammatory response for the removal of pathogenic microorganisms, chemerin seems to have a distinct role in promoting the resolution of inflammatory pain without blocking the proinflammatory response. Mechanistically, chemerin exerted its analgesic effect via blocking TRPV1 activity in nociceptor sensory neurons which are important for generating heat hyperalgesia [8]. Moreover, ChemR23 was expressed by MΦ and chemerin treatment enhanced the phagocytic activity of MΦ in an iCa2+-independent manner. The mimic peptides of chemerin also possess prophagocytic activity in a ChemR23-dependent manner, which involves dynamic change in F-actin and phagosome formation regulated by tyrosine kinase Syk [13]. Phagocytosis by MΦ is crucial for effectively eliminating apoptotic cells and inflammatory leukocytes during the resolution phase of acute inflammation [1]. Thus, the enhancement effect of chemerin/ChemR23 on phagocytic activity of MΦ could be critical to the repair of inflamed tissues and the reestablishment of tissue homeostasis, which is an important prerequisite for the resolution of inflammatory pain [14]. Taken together, our studies demonstrate an important role of endogenous chemerin and ChemR23 in the resolution of inflammatory pain. Recent studies have also shown the anti-inflammatory roles and pharmacological benefits of ChemR23 ligands [15]. Given the well-known side-effects of opioids and COX-2 inhibitors, the development of chemerin analogs or ChemR23 agonists may lead to new interventions for inflammation-related painful diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Ru-Rong Ji (Duke University) for reading and discussing the manuscript, Dr. Fan Wang (Duke University) for providing AdvillinCre/+ mice, and Dr. Shumin Duan (Zhejiang University) for providing Cx3cr1-GFP mice. This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LZ18C090002) and the National Natural Science Foundation of China (31771162).
Conflict of interest
All authors claim that there is no conflict of interests.
References
- 1.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghasemlou N, Chiu IM, Julien JP, Woolf CJ. CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci USA. 2015;112:E6808–E6817. doi: 10.1073/pnas.1501372112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang Y, Zhu J, Duan W, Xie Y, Ma C. Inhibition of muscular nociceptive afferents via the activation of cutaneous nociceptors in a rat model of inflammatory muscle pain. Neurosci Bull. 2020;36:1–10. doi: 10.1007/s12264-019-00406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gisondi P, Lora V, Bonauguri C, Russo A, Lippi G, Girolomoni G. Serum chemerin is increased in patients with chronic plaque psoriasis and normalizes following treatment with infliximab. Br J Dermatol. 2013;168:749–755. doi: 10.1111/bjd.12118. [DOI] [PubMed] [Google Scholar]
- 8.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 9.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park CK, Lü N, Xu ZZ, Liu T, Serhan CN, Ji RR. Resolving TRPV1- and TNF-α-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci. 2011;31:15072–15085. doi: 10.1523/JNEUROSCI.2443-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji RR. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest. 2018;128:3568–3582. doi: 10.1172/JCI99888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang NN, Becker S, Boularan C, Kamenyeva O, Vural A, Hwang IY, et al. Canonical and noncanonical g-protein signaling helps coordinate actin dynamics to promote macrophage phagocytosis of zymosan. Mol Cell Biol. 2014;34:4186–4199. doi: 10.1128/MCB.00325-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cash JL, Christian AR, Greaves DR. Chemerin peptides promote phagocytosis in a ChemR23- and Syk-dependent manner. J Immunol. 2010;184:5315–5324. doi: 10.4049/jimmunol.0903378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen O, Donnelly CR, Ji RR. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol. 2020;62:17–25. doi: 10.1016/j.conb.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle JR, Krishnaji ST, Zhu G, Xu ZZ, Heller D, Ji RR, et al. Development of a membrane-anchored chemerin receptor agonist as a novel modulator of allergic airway inflammation and neuropathic pain. J Biol Chem. 2014;289:13385–13396. doi: 10.1074/jbc.M113.522680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.