Abstract
This study evaluated the anti-inflammatory effect of ginger-cinnamon mixture using an animal model of dextran sulfate sodium (DSS)-induced intestinal inflammation. The mice were administered either distilled water or ginger extract (GE), cinnamon subcritical water extract (CSWE), low GE + CSWE (GCL), and high GE + CSWE (GCH) for 21 days and drinking water containing 5% DSS for the final 7 days to induce intestinal inflammation. We assessed the change of body weight, disease activity index (DAI), histopathological scores, myeloperoxidase (MPO) activity, and mRNA levels. Compared with the DSS group, the GCH group showed increased body weight, inhibited intestinal shortening, and decreased DAI and histopathological score of intestinal inflammation, which was similar to that for the control group. It inhibited MPO activity as well as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α mRNA levels. Therefore, the ginger–cinnamon complex helps to improve intestine inflammation, which is beneficial for gut health.
Keywords: Ginger, Cinnamon, Extract mixtures, Intestine, Anti-inflammatory
Introduction
Inflammatory bowel disease (IBD) is an immune-mediated inflammatory disease of the intestine associated with serious symptoms, such as diarrhea, and it clinically includes Crohn's disease and ulcerative colitis (Carter et al., 2004; Hanauer, 2006). An immune response that is not normally regulated in the gut microbiota can lead to disease due to a defect in intestinal barrier function (Hanauer, 2006). Cinnamon (Cinnamomum schaeff), a natural product with few side effects and low immunomodulatory activity, has various biological functions such as antibacterial, antioxidant, anti-inflammatory, anti-diabetic, anti-tumor properties and used to treat diseases for people with weakened physical strength and digestive diseases, such as diarrhea and gastrointestinal diseases (Baker et al., 2008; Cha et al., 2019; Hanauer, 2006; Khan et al., 2003; Koppikar et al., 2010; Kwon et al., 2010; Roussel et al., 2009). Cinnamon inhibits the production of nitric oxide (NO), cyclooxygenase-2 (COX-2), and prostaglandin E2 (PGE2), thereby suppressing inflammation, invasion, and tissue damage, as well as various anti-inflammatory factors such as β-hexosaminidase and cysteinyl leukotriene (Hagenlocher et al., 2013; Kanuri et al., 2009; Kwon et al., 2011; Tung et al., 2008). Ginger (Zingiber officinale), a natural product that has been widely used against various diseases for over 2500 years is effective against various conditions, etc. digestive diseases, arthritis, and diabetes. Pharmacologically, it is also known for its hypoglycemic, antibacterial, hypocholesterolemic, cytotoxic, anti-inflammatory, and antipyretic properties, and it is recognized as a substance that acts as a pain reliever (Mascolo et al., 1989). In particular, gingerol, the spicy ingredient of ginger, has been recognized as a valuable substance for centuries because of its strong antifungal, anti-inflammatory, and antioxidant properties, and it has been used in past medical formulations to treat abdominal pain, diarrhea, toothache, gingivitis, arthritis, asthma, and respiratory diseases. Furthermore, gingerol interacts with T helper (Th) cells, which are involved in immunity and inflammation through cytokine production. Lymphocytes produce Th1 and Th2 cells (Bhandari et al., 1998; Kim et al., 2017; Mascolo et al., 1989; Thomson et al., 2002). Th1 cells produce increased levels of proinflammatory cytokines that activate cytotoxic and inflammatory functions, and Th2 cells produce strong antibodies that activate allergic reactions with immunoglobulin E (IgE) and enhance humoral immunity. In general, Th1 lymphocytes produce interleukin (IL)-2, IL-6, tumor necrosis factor-α (TNF-α), and interferon-gamma (IFN-γ), and Th2 lymphocytes produce IL-4 and IL-10 (Brotas et al., 2012; Mosmann and Sad, 1996). Structural compounds of the ginger plant, such as gingerol, shogaols, and paradol, are major factors in its anti-inflammatory properties that inhibit the expression of proinflammatory cytokines produced by Th1 lymphocytes (Grzanna et al., 2005; Shen et al., 2005). In a previous study, a combination of ginger extract and cinnamon extract proved the synergistic effect of inhibiting intestinal inflammation (Kim et al., 2017; Park et al., 2020). In this study, the anti-inflammatory effect of a mixture of ginger extract and cinnamon extract were evaluated in an in vivo intestinal inflammation model: BALB/c mice with dextran sulfate sodium (DSS)-induced colitis.
Materials and methods
Materials
DSS (molecular weight: 36,000–50,000) was obtained from MP Biomedicals, LLC (Santa Ana, CA, USA). Formalin (10%) solution in neutral buffer and myeloperoxidase (MPO) activity assay kits were purchased from Sigma-Aldrich (St. Louis, MO, USA). TRIzol was obtained from Life Technologies (Rockville, MD, USA). The BCA protein assay kit was purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA).
Preparation of ginger extract (GE) and cinnamon subcritical water extract (CSWE)
Ginger ethanol extract and CSWE were provided by the Korea Food Research Institute (Gyeonggi, Korea). Briefly, ginger ethanol extracts were prepared add to 50% ethanol at 50 °C for 35 min. A certain amount of CSWE was added to distilled water at 1:10 (w/v), and extraction was performed using a subcritical water extraction device (TFS-3000, Innoway Co., Anyang, Korea) at 110 °C for 40 min and a pressure of 50 bar. The extract was centrifuged at 4 °C (11,000 g, 5 min), filtered (Whatman No.4, Maidstone, England), and lyophilized.
Animals
A total of 48 5-week-old female BALB/c mice were purchased from the Shizuoka Laboratory Center (Shizuoka, Japan). The animals were fed a standard rodent diet with ad libitum access to water and were housed in rooms maintained at 22 ± 1 °C with a 12 h light/dark cycle. The protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Ewha Womans University (Seoul, Korea) (IACUC No. 17-037). All institutional and national guidelines for the care and use of laboratory animals were followed.
Colitis model and administration of ginger and cinnamon mixture
The animals were divided randomly into six groups (n = 8 mice/group): control, DSS (only), DSS + GE (100 mg/kg) (GE), DSS + CSWE (500 mg/kg) (CSWE), DSS + 50 mg/kg GE + 250 mg/kg CSWE (GCL), and DSS + 100 mg/kg GE + 500 mg/kg CSWE (GCH). The control and DSS (only) groups were administered distilled water by oral gavage. The GE, CSWE, GCL, and GCH groups were administered ginger, cinnamon, or a mixture of extracts orally, once a day, at the same time, from day 1 to day 21. Body weight and dietary intake were recorded once every three days until day 21. Colitis was induced via the intake of 5% (w/v) DSS-containing drinking water ad libitum for 7 consecutive days.
Disease activity index (DAI)
DAI values were calculated based on body weight loss, stool consistency, and fecal bleeding (Cooper et al., 1993), as shown in Table 1. The DAI [DAI = (weight loss) + (stool consistency) + (fecal bleeding)] was used to assess the severity of colon inflammation.
Table 1.
Criteria for disease activity indexa
| Score | Weight loss (%) | Stool consistency | Fecal bleeding |
|---|---|---|---|
| 0 | < 0 | Normal | Normal |
| 1 | 1–5 | – | |
| 2 | 6–10 | Soft | Slightly bloody |
| 3 | 11–15 | Loose | Bloody |
| 4 | > 15 | Diarrhea | Severely bloody |
aDAI [= (weight loss) + (stool consistency) + (fecal bleeding)] was used to assess the severity of colonic inflammation
Histological analysis
A 1-cm-thick section from the distal end of the colon was fixed in neutral-buffered 10% formalin solution (Sigma-Aldrich, St. Louis, MO, USA), embedded in paraffin, sliced into 4-µm-thick sections, and stained with hematoxylin and eosin (H&E). Colonic histopathological variations were estimated from all colon sections under light microscopy at 100 × and 200 × magnification. The grading system was as follows (Rees, 1998): (a) inflammation severity (0 = none; 1 = slight; 2 = moderate; 3 = severe); (b) lesion depth (0 = none; 1 = mucosal layer; 2 = submucosal layer; 3 = muscle layer; 4 = transmural); (c) crypt damage (0 = none; 1 = basal 1/3 damaged; 2 = basal 2/3 damaged; 3 = only surface epithelium intact; 4 = entire crypt and epithelium lost); (d) lesion range (1 = 1–25%; 2 = 26–50%; 3 = 51–75%; 4 = 76–100%). The number of goblet cells in the crypts of the three areas was counted on each H&E slide and expressed per three villus-crypt units (Rees, 1998). For objective evaluation, two different researchers each analyzed and compared them.
Myeloperoxidase (MPO) activity assay
MPO is a prominent indicator that is highly expressed in neutrophils in the mouse proximal colon. The proteins in the colon tissues were evaluated for MPO activity using commercial MPO activity kits (Sigma-Aldrich, St. Louis, MO, USA). The excised colon was weighed, rapidly homogenized in MPO analysis buffer, and centrifuged at 13,000 × g for 10 min. The MPO levels in the supernatants were measured at 412 nm and expressed as U/mg of tissue. One unit of MPO activity is defined as the quantity of enzyme that hydrolyzes the substrate and generates taurine chloramine by consuming 1 µmol of the chromophore 5-thio-2-nitrobenzoic acid per minute at 25 °C.
RNA extraction and qRT-PCR
RNA was isolated from colon tissue using TRIzol (Life Technologies, Rockville, MD, USA) following the manufacturer’s protocol. cDNA was synthesized using reverse transcription using a high capacity RNA-to-cDNA kit and used for RT-qPCR using the Universal ProbeLibrary probe method in a Step-One-Plus RT-PCR system (Hoffmann La Roche, Basel, Switzerland). Relative mRNA levels were calculated using the comparative 2−ΔΔCT method and normalized to GAPDH levels. The primers were as follows: IL-1β forward 5′- agttgacggaccccaaaag-3′reverse 5′-agctggatgctctcatcagg-3; TNF-α forward 5′-tcttctcattcctgcttgtgg-3′ reverse 5′-ggtctgggccatagaactga-3′; IL-6 forward 5′-gctaccaaactggatataatcagga-3′ reverse 5’-ccaggtagctatggtactccagaa-3′.
Statistical analysis
Results are displayed as mean ± standard deviation. Data were analyzed using Statistical Analysis System version 9.4 (SAS Institute, Cary, NC). Differences between the groups were analyzed using one-way analysis of variance along with Duncan’s post-hoc test. Statistical significance was set at P < 0.05.
Results and discussion
Improvement in clinical symptoms of DSS-induced colitis
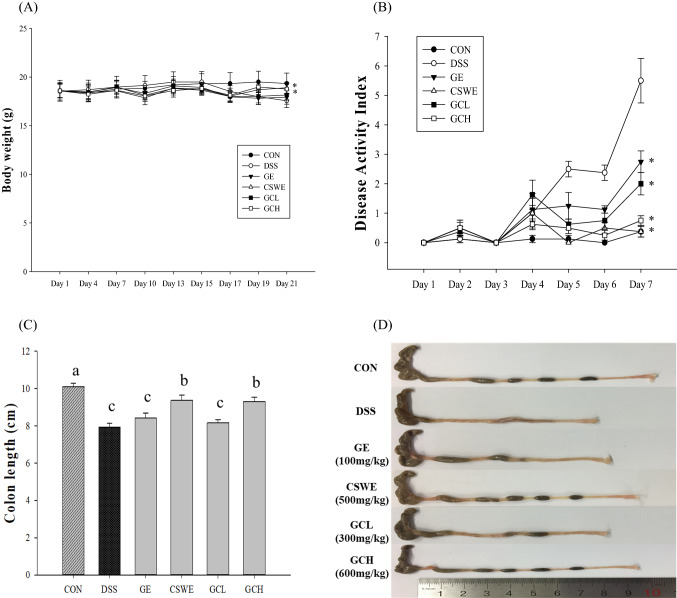
To induce colitis model, mice was provided 5% DSS-containing drinking water for 7 days. During the 7 days (days 15–21) of DSS administration, severe diarrhea, blood loss in feces, and body weight loss were considered as presenting a noticeably high DAI. Although body weight decreased sharply due to DSS intake, weight loss was significantly improved in mice administered ginger, cinnamon, or complex extracts. The CSWE and GCH groups obtained the maximum effect on weight recovery [Fig. 1(A)]. At 21 days, DSS group showed the highest DAI. Whereas all sample groups significantly alleviated DAI relative to the DSS group [Fig. 1(B)]. In particular, the scores were the lowest in CSWE and GCH group. Moreover, colon length in the DSS group was generally shorter than that in the control group. The CSWE and GCH group significantly inhibited reduction of colon length. In case of GE and GCL group, although decrease of colon length slightly prevented, there was no significant difference [Fig. 1(C), (D)]. These results could be attribute CSWE, rather than GE. The cinnamon has few side effects and is well known for its efficacy in treating diarrhea and other digestive disorders (Cha et al., 2019; Mascolo et al., 1989). Previous studies reported that administration of cinnamon extract showed a significantly lower score for symptoms of colitis and DAI (Hansberry et al., 2017). In addition, ginger is known to treat digestive problems such as abdominal pain and diarrhea (Bhandari et al., 1998; Mascolo et al., 1989; Thomson et al., 2002). According to reported studies, the ginger–cinnamon complex was observed to improve DAI and symptoms such as diarrhea, bleeding, and weight loss (Cha et al., 2019; Koppikar et al., 2010). It was considered that the ginger–cinnamon complex has potential to mitigate weight loss, stool consistency, and fecal bleeding in DSS-induced intestinal inflamed mice. These findings supported the reported experimental evidence.
Fig. 1.
Effect of ginger–cinnamon complex on body weight, DAI, and bowel length in DSS-induced colitis. (A) Body weight was recorded at given time on the test day. (B) DAI (C) DSS-induced bowel shortening was improved by ginger–cinnamon extract complex. (D) Representative colons of each group (*p < 0.05 compared with the DSS group)
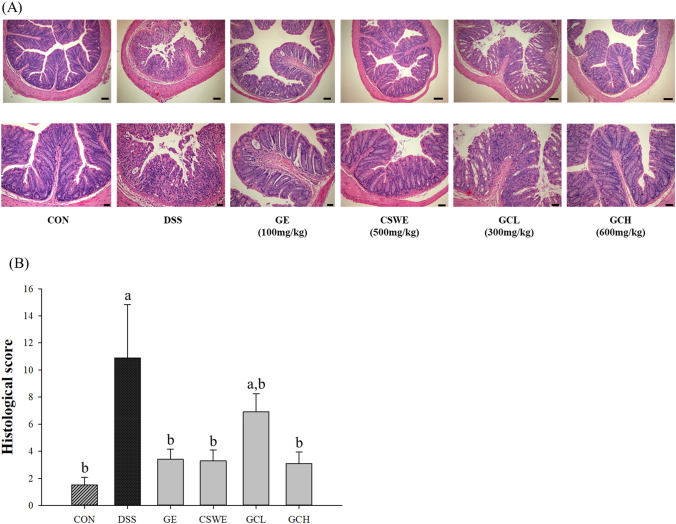
Inhibition of DSS-induced colon histopathological changes
Diarrhea and bloody excrement from daily DSS administration in mice are accompanied by enteritis symptoms and barrier damage. In the histological sections of DSS-administered mice (Kim et al., 2015), crypt destruction and inflammatory cell invasion, symptoms of ulcerative colitis and IBD, were observed. The number of goblet cells decreased in the DSS group as compared with that in the control, whereas CSWE increased these cells and were most similar to those of the control group. The GE, CSWE, and GCH group reduced the histological features of colitis [Fig. 2(A)]. The histological score increased about 7.27-fold in DSS group compared to the control group. Contrastively, all sample groups except for GCL group substantially decreased, similar to that in the control group [Fig. 2(B)]. These results indicated that administration of both ginger and cinnamon improved the symptoms of intestinal inflammation as well as the histopathological symptoms. Cinnamon was revealed to suppress tissue damage by inhibiting the production of NO, COX-2, and PGE2 (Hagenlocher et al., 2013; Kanuri et al., 2009; Kwon et al., 2011; Tung et al., 2008). According to reported study, its administration showed significant improvement in tissue invasion and damage (Yvonne Hagenlocher et al., 2016), and administration of the ginger extract reduced the histopathological characteristics of colitis (Kim and Kim, 2018). In previous study, the number of cells secreting mucus increased in a dose-dependent manner with GE administration (Kim and Kim, 2018). Likewise, this study confirmed that the number of cells secreting mucus increased in microscopic morphology, which proved that administration of both ginger or/and cinnamon suppressed the symptoms of tissue damage and increased the number of cells secreting mucus.
Fig. 2.
Hematoxylin and Eosin staining. The sections were from 5% DSS-induced colitis in mice. Magnification × 100 (bar = 100 mm), X200 (bar = 200 mm). Control, DSS (5% DSS), ginger (ginger extract 100 mg/kg), cinnamon (cinnamon extract 500 mg/kg), GCL (ginger:cinnamon = 1:5, 300 mg/kg), GCH (ginger:cinnamon = 1:5, 600 mg/kg)
Reduction in MPO activity
MPO activity is associated to inflammatory cell infiltration in the proximal colon. MPO is clinically useful as a biomarker of IBD and is known to be overexpressed in a number of inflammatory diseases, including IBD (Hansberry et al., 2017). MPO activity in the DSS group was 1.4 times higher than that in the control group. The CSWE and GCH group significantly inhibited MPO activity induced by DSS, similar to those of the control group [Fig. 3]. Cinnamon is known to exhibit strong anti-inflammatory activity due to its anti-inflammatory properties (Hagenlocher et al., 2013; Kanuri et al., 2009; Tung et al., 2008), and ginger is also recognized as an anti-inflammatory substance. In this study, it was demonstrated that DSS-induced MPO activity was decreased according to CSWE rather than GE, and that the inflammatory symptoms were improved.
Fig. 3.
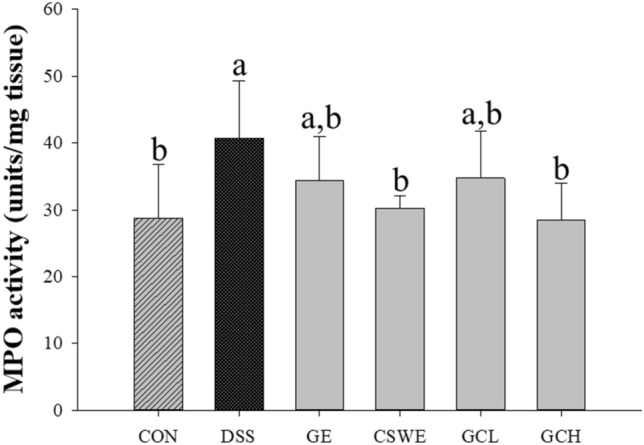
Effect of ginger–cinnamon complex on myeloperoxidase activity in colon of DSS-induced BALB/c mice
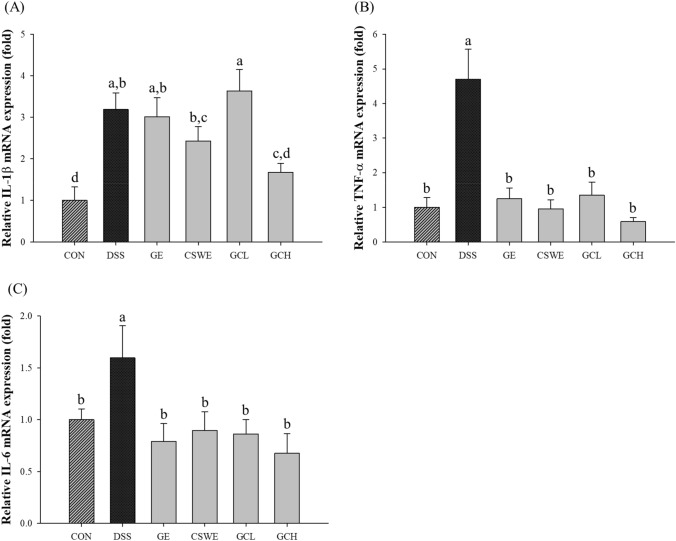
Proinflammatory cytokine mRNA levels in the colon
It was confirmed that IL-1β was increased at the mRNA level in the DSS group by 3.19 times compared with that in control group. Whereas GCH group significantly inhibited mRNA expression of IL-1β compared to DSS group [Fig. 4(A)]. Also, CSWE group showed slight reduction of IL-1β mRNA expression. There was no significant difference in the GE and GCL group. The mRNA expression of TNF-α in the DSS group increased by 4.71 times compared with that in control group [Fig. 4(B)]. Although all groups showed significantly decreased mRNA expression of TNF-α, in particular, GCH group suppressed by 1.7 times compared with that in the DSS group. In the case of IL-6, the mRNA expression in the DSS group increased 1.6 times compared with that in control group and decreased in all sample groups [Fig. 4(C)]. Among the sample groups, GCH group exhibited the highest inhibition on the expression of the proinflammatory cytokines IL-1β, TNF-α, and IL-6. These results indicated that ginger affects the production of proinflammatory cytokines synthesized and secreted at the site of inflammation (Grzanna et al., 2005). The spicy ingredients of ginger, gingerol, shogaol, and paradol inhibit proinflammatory cytokines produced by Th1 lymphocytes. Ginger has been shown to exert anti-inflammatory effects by inhibiting inflammation (Grzanna et al., 2005; Shen et al., 2005). Therefore, it was proved that administration of a ginger–cinnamon complex inhibited the expression of inflammatory cytokines IL-1β, TNF-α, and IL-6 induced by DSS.
Fig. 4.
Effect of ginger–cinnamon complex on inflammatory cytokine mRNA levels in colon. Relative mRNA profiles of IL-1β, TNF-α, and IL-6
In the present study, the combination of a ginger ethanol extract and a cinnamon subcritical water extract showed anti-inflammatory effects in a DSS-induced colitis mouse model. In the case of ginger ethanol extract, the effect of regulating intestinal cell inflammation had been confirmed in a previous study (Kim et al., 2017). The cinnamon supercritical fluid extract had been studied for the selective proliferation activity of beneficial bacteria (Kim, 2016; Kim and Kim, 2016). There was a slight synergistic effect when the complex was administered in the cell line model (Kim and Kim, 2018). In addition, the synergistic or additive effects were observed in the present animal study. When the ginger-cinnamon complex was administered, the anti-inflammatory effect was dose-dependent; as well as, the effect was similar to that of the CSWE-treated group. Along with synergistic effect, when considering flavor and taste, the combination of ginger ethanol extract and cinnamon subcritical water extract might expect development as a functional ingredient for gut health food supplements. Because CSWE contributed to anti-inflammatory activity of the ginger-cinnamon complex in all outcomes, in future studies, detailed mechanisms of action for CSWE and clinical trials should be conducted. This study, however, had several limitations. First, it is necessary to investigate further how there was synergistic effect in the composite sample group. Second, the biomarkers we identified related to inflammation are related to inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and nuclear factor kappa-light-chain-enhancer of activate B cell (NF-kB) signaling pathway (Schottelius and Baldwin Jr, 1999). Therefore, future studies should identify these signaling pathways.
Acknowledgements
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the High Value-added Food Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Project No. 116012-3). We express our sincere appreciation for this support.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jin A Im, Email: zzina.im0116@gmail.com.
Min Seo Kim, Email: muckms@naver.com.
Oran Kwon, Email: orank@ewha.ac.kr.
Jae-Ho shin, Email: shinjh@eulji.ac.kr.
Ji Yeon Kim, Email: jiyeonk@seoultech.ac.kr.
References
- Baker WL, Gutierrez-Williams G, White CM, Kluger J, Coleman CI. Effect of cinnamon on glucose control and lipid parameters. Diabetes Care. 31: 41-43 (2008) [DOI] [PubMed]
- Bhandari U, Sharma J, Zafar R. The protective action of ethanolic ginger (Zingiber officinale) extract in cholesterol fed rabbits. Journal of Ethnopharmacology. 61(2): 167-171 (1998) [DOI] [PubMed]
- Brotas AM, Cunha JMT, Lago EHJ, Machado CCN, Carneiro SCdS. Tumor necrosis factor-alpha and the cytokine network in psoriasis. Anais brasileiros de dermatologia. 87: 673-683 (2012) [DOI] [PubMed]
- Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 53: v1-v16 (2004) [DOI] [PMC free article] [PubMed]
- Cha J, Kim C-T, Kim T-E, Cho Y-J. Optimization of subcritical extraction process for cinnamon (Cinnamomum Cassia Blume) using response surface methodology. Food Science and Biotechnology. 28(6): 1703-1711 (2019) [DOI] [PMC free article] [PubMed]
- Cooper HS, Murthy S, Shah R, Sedergran D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Laboratory Investigation; A Journal of Technical Methods and Pathology. 69: 238-249 (1993) [PubMed]
- Grzanna R, Lindmark L, Frondoza CG. Ginger—an herbal medicinal product with broad anti-inflammatory actions. Journal of Medicinal Food. 8(2): 125-132 (2005) [DOI] [PubMed]
- Hagenlocher Y, Bergheim I, Zacheja S, Schäffer M, Bischoff S, Lorentz A. Cinnamon extract inhibits degranulation and de novo synthesis of inflammatory mediators in mast cells. Allergy. 68: 490-497 (2013) [DOI] [PubMed]
- Hagenlocher Y, Hösel A, Bischoff SC, Lorentz A. Cinnamon extract reduces symptoms, inflammatory mediators and mast cell markers in murine IL-10−/− colitis. The Journal of Nutritional Biochemistry. 30: 85-92 (2016) [DOI] [PubMed]
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflammatory Bowel Diseases. 12: S3-S9 (2006) [DOI] [PubMed]
- Hansberry DR, Shah K, Agarwal P, Agarwal N. Fecal myeloperoxidase as a biomarker for inflammatory bowel disease. Cureus. 9(1): e1004 (2017) [DOI] [PMC free article] [PubMed]
- Kanuri G, Weber S, Volynets V, Spruss A, Bischoff SC, Bergheim I. Cinnamon extract protects against acute alcohol-induced liver steatosis in mice. The Journal of Nutrition. 139: 482-487 (2009) [DOI] [PubMed]
- Khan A, Safdar M, Khan MMA, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 26: 3215-3218 (2003) [DOI] [PubMed]
- Kim CY. Inhibition of interleukin-1α-induced intestinal epithelial tight junction permeability by curcumin treatment in Caco-2 Cells in Caco-2 cells. Journal of Life Science. 26(9): 1082-1087 (2016)
- Kim J, Kim D. Nutritional composition containing ginger extract for enhancing tight junctions in intestinal cells and prepeparing method thereof. Korea Patent 10-2016 (2016)
- Kim K-M, Kim Y-S, Lim JY, Min SJ, Ko H-C, Kim S-J, Kim Y. Intestinal anti-inflammatory activity of Sasa quelpaertensis leaf extract by suppressing lipopolysaccharide-stimulated inflammatory mediators in intestinal epithelial Caco-2 cells co-cultured with RAW 264.7 macrophage cells. Nutrition Research and Practice. 9: 3 (2015) [DOI] [PMC free article] [PubMed]
- Kim MJ, Kim MS, Kang ST, Kim JY. Effect of ginger and cinnamon extract mixtures on the growth of intestinal bacteria and intestinal inflammation. Journal of Applied Biological Chemistry. 60: 321-326 (2017)
- Kim MS, Kim JY. Ginger attenuates inflammation in a mouse model of dextran sulfate sodium-induced colitis. Food Science and Biotechnology. 27(5): 1493-1501 (2018) [DOI] [PMC free article] [PubMed]
- Koppikar SJ, Choudhari AS, Suryavanshi SA, Kumari S, Chattopadhyay S, Kaul-Ghanekar R. Aqueous cinnamon extract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer. 10(1): 1-12 (2010) [DOI] [PMC free article] [PubMed]
- Kwon H-K, Hwang J-S, Lee C-G, So JS, Sahoo A, Im C-R, Jeon WK, Ko BS, Lee SH, Park ZY, Im SH. Cinnamon extract suppresses experimental colitis through modulation of antigen-presenting cells. World Journal of Gastroenterology: WJG. 17: 976 (2011) [DOI] [PMC free article] [PubMed]
- Kwon H-K, Hwang J-S, So J-S, Lee C-G, Sahoo A, Ryu J-H, Jeon WK, Ko BS, Im C-R, Lee S-H, Park ZY, Im S-H. Cinnamon extract induces tumor cell death through inhibition of NFκB and AP1. BMC Cancer. 10(1): 1-10 (2010) [DOI] [PMC free article] [PubMed]
- Mascolo N, Jain R, Jain S, Capasso F. Ethnopharmacologic investigation of ginger (Zingiber officinale). Journal of Ethnopharmacology. 27(1-2): 129-140 (1989) [DOI] [PubMed]
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunology Today. 17(3): 138-146 (1996) [DOI] [PubMed]
- Park S-H, Jung S-J, Choi E-K, Ha K-C, Baek H-I, Park Y-K, Han K-H, Jeong S-Y, Oh J-H, Cha Y-S, Park B-H, Chae S-W. The effects of steamed ginger ethanolic extract on weight and body fat loss: a randomized, double-blind, placebo-controlled clinical trial. Food Science and Biotechnology. 29: 265-273 (2020) [DOI] [PMC free article] [PubMed]
- Rees V. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clinical & Experimental Immunology. 114: 385-391 (1998) [DOI] [PMC free article] [PubMed]
- Roussel A-M, Hininger I, Benaraba R, Ziegenfuss TN, Anderson RA. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. Journal of the American College of Nutrition. 28(1): 16-21 (2009) [DOI] [PubMed]
- Schottelius A, Baldwin Jr A. A role for transcription factor NF-kB in intestinal inflammation. International Journal of Colorectal Disease. 14(1): 18-28 (1999) [DOI] [PubMed]
- Shen C-L, Hong K-J, Kim SW. Comparative effects of ginger root (Zingiber officinale Rosc.) on the production of inflammatory mediators in normal and osteoarthrotic sow chondrocytes. Journal of Medicinal Food. 8(2): 149-153 (2005) [DOI] [PubMed]
- Thomson M, Al-Qattan K, Al-Sawan S, Alnaqeeb M, Khan I, Ali M. The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins, Leukotrienes and Essential Fatty Acids. 67: 475-478 (2002) [DOI] [PubMed]
- Tung Y-T, Chua M-T, Wang S-Y, Chang S-T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresource Technology. 99: 3908-3913 (2008) [DOI] [PubMed]