Abstract
The main objective of this study was to examine the phenolic compounds and the antibacterial, antioxidant, anti-α-glucosidase and anti-α-amylase activities of the different extracts (methanol, ethanol and hexane) of Musa cavendishii collected from the Anamur district in Turkey. LC–MS/MS was used to identify phenolic compounds. Quinic acid, acotinic acid, hesperidin and amentoflavone were identified in methanol extract. These phenolic compounds, excluding hesperidin, were also identified in the ethanol extract. Methanolic extract appeared the most active in all enzyme inhibition, antibacterial and antioxidative activity assays which is mainly due to its rich phenolic content. The methanol extract of banana showed the highest anti-α-glucosidase and anti-α-amylase activities with IC50 values of 5.45 ± 0.39 mg/mL, 9.70 ± 0.29 mg/mL, respectively. This study showed that methanol and ethanol extract, especially the methanol extract, have potential for use in the development of functional foods for reducing the diabetes and bacterial risks.
Keywords: Musa cavendishii, Antidiabetic potential, Antioxidant activity, Antibacterial effect, LC–MS/MS
Introduction
Banana, as a horticultural crop, is next to cereal crops like wheat, maize and rice in world market rankings as an important food crop (Aurore et al., 2009). The countries producing the largest banana crops in the world are China, India, the Philippines and Ecuador. Turkey used an area of nearly 700 ha for banana production in 1961 with 5200 tons of production. A dramatic increase occurred afterwards and covered 5350 ha of land and produced 251,994 tons of bananas in 2014 (Faostat, 2017).
The banana is an important fruit—among a number of other fruit plants—exhibiting medicinal properties and contributes 16% of the total fruit production worldwide (Lehmann et al., 2002). Kalita et al. (2016) suggested that the banana can be used in the treatment of different diseases.
The banana is considered as an important food in the human diet. It is rich in vitamins, phenolics, minerals, organic acids, dietary fibers and different sugars. These compounds are associated with reduced risks of chronic degenerative diseases such as diabetic, diarrhea, scabies and inflammation (Singh et al., 2016).
Many researchers have conducted a variety of studies on banana, its chemical composition and its biological activities in researching the traditional uses of the fruit. These studies indentified various compounds in banana, although further studies were needed to determine the structure of phytochemicals that might impart important biological properties (Linde, 2009). Banana contains different important bioactive compounds like other fruits of importance. The bioactive compounds in green and ripened banana, which are worthy of special attention, are caretonoids, flavonoids, biogenic amines and phenolics. Banana pulp also contains a small amount of phytosterols. Banana shows higher antioxidant activities in comparison to other herbs, vegetables and berries due to their bioactive compounds. The antioxidant activities of banana are greater in their mature state (Singh et al., 2016).
Special emphasis has been paid in the previous decade to the potential of the extracts obtained from the banana in assisting the treatment of diabetes mellitus type I and type II. Diabetes mellitus is a metabolic disorder and the occurrence of the disease is rising significantly in the West (Silva et al., 2016). Different underlying mechanisms of diabetes have been investigated because of their multifactorial etiology. Researchers have frequently reported the potential of phytochemicals present in banana that affect the adsorption of glucose, and they also have antioxidant activity and an inhibitory effect on carbohydrate-digesting enzymes (α-glucosidase and α-amylase) (Jaber et al., 2013). Further research has been recommended for the development of phytomedicines and edible compounds that have functional properties (Ayoola et al., 2017). Many studies have been carried out on the chemical and biological properties of banana although no such research has previously been performed on the Anamur banana. The Anamur is a dwarf banana variety that is cultivated in Turkey.
This study was designed to show the antidiabetic activity of different extracts of the Anamur banana through its anti-glucosidase and anti-amylase activities in addition to its antioxidant and antibacterial potential. Incidentally, this is the first study to evaluate the phenolic profiles of the Anamur banana by LC–MS/MS to show its anti-glucosidase, anti-amylase activities. The goal of the study was to provide new findings to help develop new functional foods reducing the diabetes and bacterial risks using the Anamur banana.
Materials and methods
Plant material
Ripe Anamur bananas (20 kg) were obtained from their natural habitats in Anamur, a district in the southern Turkish province of Mersin located next to the Mediterranean Sea in 2018.
Reagents
All of the chemicals used in the LC–MS/MS analysis as standards were acquired from Sigma-Aldrich (Steinheim, Germany). Chloroform, hexane, methanol, ammonium formate, ethanol, formic acid and acetonitrile (HPLC grade) were bought from Merck KGaA (Darmstadt, Germany) in addition to Folin–Ciocalteau, sodium carbonate, disodium hydrogen phosphate and sodium hydrogen phosphate. Gallic acid was obtained from Fluka (USA). Sigma-Aldrich (Australia) supplied 3,5-Dinitrosalicylic acid, p-nitrophenyl-α-D-glucopyranoside, p-nitrophenol (pNPG), porcine pancreatic-α-amylase, potassium sodium tartrate tetrahydrate, and α-glucosidase. All analytical grade chemicals were used in this study.
The preparation of extracts for bioactivity assays and their analysis by LC–MS/MS
Extractions were carried out using methanol, ethanol and hexane as the solvent. The freeze-dried and peeled bananas (15 g) were homogenized in 150 mL of solvent for one minute using a Waring blender and then sonicated at 30 °C for 45 min. The slurry was centrifuged at 3500 g for 10 min. After that, the supernatant was discarded and 30 mL of solvent was added to the solid portion and this method was duplicated. A rotary evaporator was used to dry all extracts at a temperature below 45 °C at low pressure. The solvent crude extracts were preserved at − 20 °C after lyophilization (Kam et al., 2013).
Instrumentation and analytical conditions
LC–MS/MS analysis
LC–MS/MS equipment and chromatographic conditions
The quantitative analysis of 53 phytochemicals was analyzed by following a previously validated method for LC–MS/MS (Yilmaz, 2020). Analysis was carried out using an ultra high performance liquid chromatograph (UHPLC), the Shimadzu-Nexera model. A Shimadzu LCMS-8040 model tandem mass spectrometer coupled with an electrospray ionization (ESI) source was used for the mass spectrometric detection.
Total phenolic content
The total phenolic content of the extracts was analyzed spectrophotometrically using the Folin-Ciocalteu colorimetric method (Ough and Amerine, 1988). An aliquot (1 ml) of extract was poured into a 100 mL conical flask and 5 mL of Folin-Ciocalteu reagent, 60 mL of deionized water and 15 mL of sodium carbonate (20%) were added. The content was mixed thoroughly afterwards. The absorption was measured at a wavelength of 765 nm using a Shimadzu 300 UV–vis spectrophotometer (Shimadzu UV-1700, Kyoto, Japan). The gallic acid equivalent (GAE) in g was used to express the total phenolic content of the extract.
Inhibitory assay on α-glucosidase
The inhibitory activity of the extracts on α-glucosidase was performed by following the method mentioned in Liu et al. (2013), with slight modifications. Briefly, 50 μL of α-glucosidase at a concentration of 2U/mL was mixed with 1.15 mL of phosphate buffer (pH6.8) and 50 μL of Anamur banana extract to produce an inhibitor. The pre-incubation was done for 10 min at 37 °C, and 50 μL of 5 mM p-nitrophenyl-α-D-glucopyranoside (pNPG) was added into the same buffer. Afterwards, the mixture was incubated for 30 min at 37 °C. Subsequently, 2.0 mL of 0.2 M sodium carbonate and 4.7 mL of distilled water were added to terminate the reaction. After that the absorbance of the liquid was measured at a wavelength of 405 nm using a Shimadzu 300 UV–vis spectrophotometer (Shimadzu UV-1700, Kyoto, Japan). Acarbose was used for the positive control.
Inhibitory assay on α-amylase
For an assay to determine inhibition of α-amylase, 40 μL of 6U/mL α-amylase was added to 560 μL of 0.1 M sodium phosphate buffer (pH 6.9, containing 0.006 M NaCl) and 100 μL of Anamur banana extract to form the inhibitor. After 20 min of incubation at 37 °C, 300 μL of a starch solution (1%) in 0.1 M sodium phosphate buffer (pH6.9, with 0.006 M NaCl) was added. The mixture was incubated again for 20 min, followed by the addition of 750 μL of dinitrosalicylic acid. The contents were mixed thoroughly and kept for 5 min in a bath of boiling water. The reaction mixture was then diluted with 6 mL of distilled water. After that the absorbance was measured at a wavelength of 540 nm. Acarbose was used for the positive control.
% inhibition of all enzymes was estimated using the following equation:
where Ao is the enzyme activity without inhibitor and Ai is the enzyme with inhibitor.
A logarithmic regression analysis was used to determine IC50 (Liu et al., 2013).
The determination of antioxidant activity
The antioxidant activities of the extracts were determined according to the free radical scavenging 1 1-diphenyl-2-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) methods (Thaipong et al., 2006).
The DPPH (1 1-diphenyl-2-picrylhydrazyl) method
Determination of the DPPH value was carried out as follows: for stock solution preparation, 24 mg of DPPH was dissolved in 100 mL of methanol and kept at 20 °C until the time of use. The working solution was prepared by mixing 10 mL of stock solution with 45 mL of methanol. The extracts (150 μL) were kept in the dark for 24 h and then reacted with 2850 mL of DPPH. The absorbance values of the extract were determined at a wavelength of 515 nm. The standard curve was linear between 25 and 800 mM Trolox. The results are expressed in mM of Trolox equivalent (TE)/g of extract.
The FRAP (ferric reducing antioxidant power) method
The stock solutions contained 20 mM FeCl3.6H2O solution, 30 mM acetate buffer (3.1 g of C2H3NaO2.3H2O and 16 mL of C2H4O2), at a pH of 3.6, with 10 mM TPTZ (2, 4, 6-tripyridyl-s-triazine) solution in 40 mM HCl. The fresh working solution was obtained by adding 2.5 mL of TPTZ solution and 2.5 mL of FeCl3.6H2O solution in 25 mL of acetate buffer. The temperature of the solution was set to 37 °C in a water bath before use. The extracts (150 μL) were kept in the dark for 24 h and then reacted with 2850 mL of DPPH. The absorbance value of the colored product [ferrous tripyridyltriazine complex] was measured at a wavelength of 593 nm. The standard curve was linear between 25 and 800 mM Trolox. The results are expressed in mM TE/g of extract.
Antibacterial activity with the disk diffusion method
The antibacterial effect of the extracts was determined using the filter paper disk agar method. Antibacterial activity was studied in vitro for ethanol, hexane and methanol extracts obtained from Anamur banana pulp. Colonies from the agar plates were inoculated into a sterile nutrient broth to form a turbidity of 0.5 McFarland. Bacterial suspensions with an approximately one times CFU/ml suspension were streaked onto nutrient agar plates using a sterile swab stick. The test disc was prepared by incorporating 20 μL of each extract (500 mg/ml) to 5 mm disc of sterilized filter paper to obtain a final concentration of 10 mg/disc. The discs were dried overnight in a biosafety cabinet. Then, the discs were placed in their respective locations. Ampicillin (30 μg/disc) was utilized as a positive control to inhibit the growth of Escherichia coli and Staphylococcus aureus. The culture plates were incubated at 37 °C for 24 h. Microbial growth was determined by measuring the diameter of the zone of inhibition in mm (Fernandez-Agullo et al., 2013).
Statistical analyses
The outcomes of the enzyme inhibition, antioxidant properties, antibacterial assays and total phenols were expressed as a mean ± SD of three parallel measurements. The student t test was used for the statistical significance. The significant value was considered to be at p < 0.05. Means difference was compared using the Duncan’s multiple range test.
Results and discussion
LC–MS/MS analysis
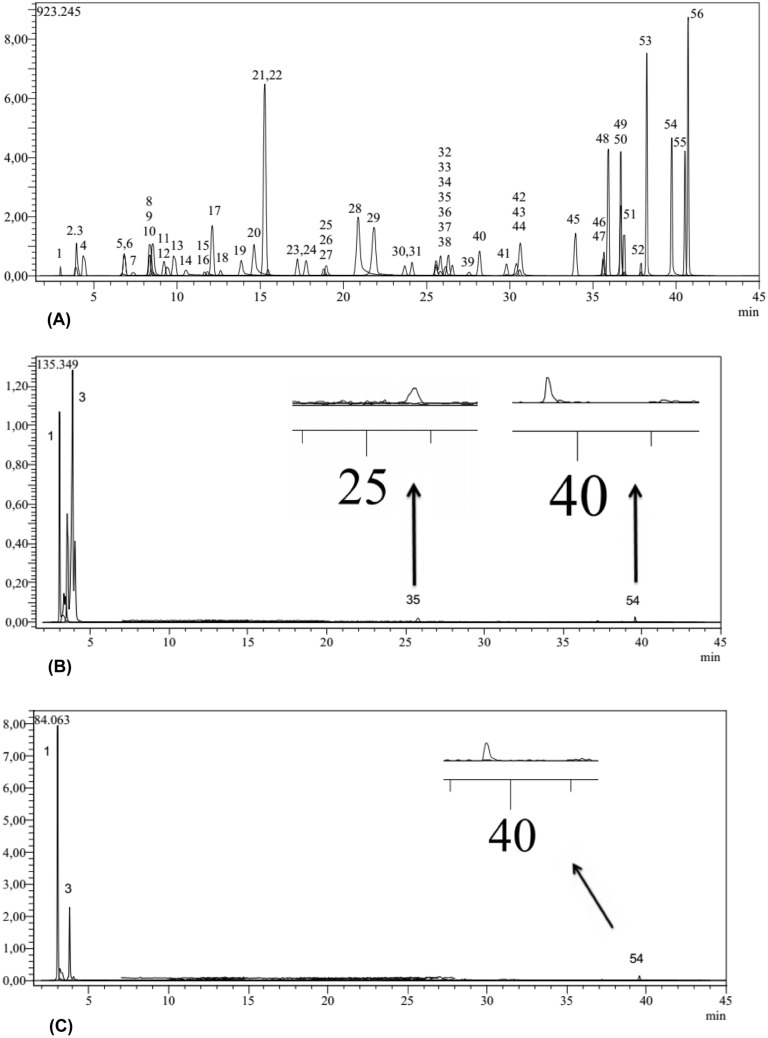
LC–MS/MS was used to examine the phenolic compositions of the methanol and ethanol extract (Figs. 1A, B and C). The extracts were obtained from Anamur banana collected from the Anamur district of Mersin province in Turkey. The hexane extract was not analyzed by LC–MS/MS due to its very low total phenolic content. According to the results, 4 metabolites: hesperidin, quinic acid, aconitic acid and amentoflavone were identified in the methanol extract, and 3 metabolites: quinic acid, aconitic acid and amentoflavone were identified in the ethanol extract (Table 1). A previously validated method was followed for the LC–MS/MS analyses of the compounds in question (Yilmaz, 2020). It was found that the methanol extract contained the highest total phenolic content among all the extracts. Quinic acid is the most common phenolic acid present in the examined methanol and ethanol extracts. The methanol extract was found to be richer (6.51 ± 0.002 mg/g) in phenols compared to the ethanol extract (4.54 ± 0.001 mg/g). The aconitic acid content of the methanol extract (0.23 ± 0.000 mg/g) was found to be much higher than the ethanol extract (0.008 ± 0.000 mg/g). The amentoflavone content in the methanol extract was found to be relatively richer (0.006 ± 0.000 mg/g) in comparison to the ethanol extract (0.004 ± 0.000 mg/g). The methanol extract also contained hesperidin (0.015 ± 0.000 mg/g) and this compound was not found in the ethanol extract.
Fig. 1.
A TIC (Total Ion Chromatogram) chromatogram of standard phenolic compounds (1: Quinic acid, 2: Fumaric acid, 3: Aconitic acid, 4: Gallic acid, 5: Epigallocatechin, 6: Protocatechuic acid, 7: Catechin, 8: Gentisic acid, 9: Chlorogenic acid, 10: Protocatechuicaldehyde, 11: Tannic acid, 12: Epigallocatechin gallate, 13: 1,5-dicaffeoylquinic acid, 14: 4 OH Benzoic acid, 15: Epicatechin, 16: Vanilic acid, 17: Caffeic acid, 18: Syringic acid, 19: Vanillin, 20: Syringic aldehyde, 21: Daidzin, 22: Epicatechin gallate, 23: Piceid, 24: p-Coumaric acid, 25: Ferulic acid D3, 26: Ferulic acid, 27: Sinapic acid, 28: Coumarin, 29: Salicylic acid, 30: Cynaroside, 31: Miquelianin, 32: Rutin D3, 33: Rutin, 34: isoquercitrin, 35: Hesperidin, 36: o-Coumaric acid, 37: Genistin, 38: Rosmarinic acid, 39: Ellagic acid, 40: Cosmosiin, 41: Quercitrin, 42: Astragalin, 43: Nicotiflorin, 44: Fisetin, 45: Daidzein, 46: Quercetin D3, 47: Quercetin, 48: Naringenin, 49: Hesperetin, 50: Luteolin, 51: Genistein, 52: Kaempferol, 53: Apigenin, 54: Amentoflavone, 55: Chrysin, 56: Acacetin) analysed by the developed LC–MS/MS method. (B), TIC chromatogram of Anamur banana methanol extract analyzed by the LC–MS/MS. (C), TIC chromatogram of Anamur banana ethanol extract analyzed by the LC–MS/MS
Table 1.
Quantitative determination (mg/g) of 53 phytochemicals in methanol and ethanol extracts of Anamur banana
| No | Compounds | RTa | Mother Ion (m/z) | Fragment Ions | Ion Mode | Amounts (mg/g) | |
|---|---|---|---|---|---|---|---|
| Methanol | Ethanol | ||||||
| 1 | Quinic acid | 3.0 | 190.8 | 93.0 | Neg | 6.51 ± 0.002 | 4.54 ± 0.001 |
| 2 | Fumaric aid | 3.9 | 115.2 | 40.9 | Neg | – | – |
| 3 | Aconitic acid | 4.0 | 172.8 | 129.0 | Neg | 0.23 ± 0.000 | 0.008 ± 0.000 |
| 4 | Gallic acid | 4.4 | 168.8 | 79.0 | Neg | – | – |
| 5 | Epigallocatechin | 6.7 | 304.8 | 219.0 | Neg | – | – |
| 6 | Protocatechuic acid | 6.8 | 152.8 | 108.0 | Neg | – | – |
| 7 | Catechin | 7.4 | 288.8 | 203.1 | Neg | – | – |
| 8 | Gentisic acid | 8.3 | 152.8 | 109.0 | Neg | – | – |
| 9 | Chlorogenic acid | 8.4 | 353.0 | 85.0 | Neg | – | – |
| 10 | Protocatechuic aldehyde | 8.5 | 137.2 | 92.0 | Neg | – | – |
| 11 | Tannic acid | 9.2 | 182.8 | 78.0 | Neg | – | – |
| 12 | Epigallocatechingallate | 9.4 | 457.0 | 305.1 | Neg | – | – |
| 13 | 1,5–dicaffeoylquinic acid | 9.8 | 515.0 | 191.0 | Neg | – | – |
| 14 | 4–OH Benzoic acid | 10.5 | 137.2 | 65.0 | Neg | – | – |
| 15 | Epicatechin | 11.6 | 289.0 | 203.0 | Neg | – | – |
| 16 | Vanilic acid | 11.8 | 166.8 | 108.0 | Neg | – | – |
| 17 | Caffeic acid | 12.1 | 179.0 | 134.0 | Neg | – | – |
| 18 | Syringic acid | 12.6 | 196.8 | 166.9 | Neg | – | – |
| 19 | Vanillin | 13.9 | 153.1 | 125.0 | Poz | – | – |
| 20 | Syringic aldehyde | 14.6 | 181.0 | 151.1 | Neg | – | – |
| 21 | Daidzin | 15.2 | 417.1 | 199.0 | Poz | – | – |
| 22 | Epicatechingallate | 15.5 | 441.0 | 289.0 | Neg | – | – |
| 23 | Piceid | 17.2 | 391.0 | 135/106.9 | Poz | – | – |
| 24 | p–Coumaric acid | 17.8 | 163.0 | 93.0 | Neg | – | – |
| 25 | Ferulic acid–D3–ISh | 18.8 | 196.2 | 152.1 | Neg | – | – |
| 26 | Ferulic acid | 18.8 | 192.8 | 149.0 | Neg | – | – |
| 27 | Sinapic acid | 18.9 | 222.8 | 193.0 | Neg | – | – |
| 28 | Coumarin | 20.9 | 146.9 | 103.1 | Poz | – | – |
| 29 | Salicylic acid | 21.8 | 137.2 | 65.0 | Neg | – | – |
| 30 | Cynaroside | 23.7 | 447.0 | 284.0 | Neg | – | – |
| 31 | Miquelianin | 24.1 | 477.0 | 150.9 | Neg | – | – |
| 32 | Rutin-D3-ISh | 25.5 | 612.2 | 304.1 | Neg | – | – |
| 33 | Rutin | 25.6 | 608.9 | 301.0 | Neg | – | – |
| 34 | isoquercitrin | 25.6 | 463.0 | 271.0 | Neg | – | – |
| 35 | Hesperidin | 25.8 | 611.2 | 449.0 | Poz | 0.015 ± 0.000 | – |
| 36 | o-Coumaric acid | 26.1 | 162.8 | 93.0 | Neg | – | – |
| 37 | Genistin | 26.3 | 431.0 | 239.0 | Neg | – | – |
| 38 | Rosmarinic acid | 26.6 | 359.0 | 197.0 | Neg | – | – |
| 39 | Ellagic acid | 27.6 | 301.0 | 284.0 | Neg | – | – |
| 40 | Cosmosiin | 28.2 | 431.0 | 269.0 | Neg | – | – |
| 41 | Quercitrin | 29.8 | 447.0 | 301.0 | Neg | – | – |
| 42 | Astragalin | 30.4 | 447.0 | 255.0 | Neg | – | – |
| 43 | Nicotiflorin | 30.6 | 592.9 | 255.0/284.0 | Neg | – | – |
| 44 | Fisetin | 30.6 | 285.0 | 163.0 | Neg | – | – |
| 45 | Daidzein | 34.0 | 253.0 | 223.0 | Neg | – | – |
| 46 | Quercetin-D3-ISh | 35.6 | 304.0 | 275.9 | Neg | – | – |
| 47 | Quercetin | 35.7 | 301.0 | 272.9 | Neg | – | – |
| 48 | Naringenin | 35.9 | 270.9 | 119.0 | Neg | – | – |
| 49 | Hesperetin | 36.7 | 301.0 | 136.0/286.0 | Neg | – | – |
| 50 | Luteolin | 36.7 | 284.8 | 151.0/175.0 | Neg | – | – |
| 51 | Genistein | 36.9 | 269.0 | 135.0 | Neg | – | – |
| 52 | Kaempferol | 37.9 | 285.0 | 239.0 | Neg | – | – |
| 53 | Apigenin | 38.2 | 268.8 | 151.0/149.0 | Neg | – | – |
| 54 | Amentoflavone | 39.7 | 537.0 | 417.0 | Neg | 0.006 ± 0.000 | 0.004 ± 0.000 |
| 55 | Chrysin | 40.5 | 252.8 | 145.0/119.0 | Neg | – | – |
| 56 | Acacetin | 40.7 | 283.0 | 239.0 | Neg | – | – |
Various studies have been performed to estimate the phenolic compounds in Musa species. The phenolic compounds in the methanol extract of Musa acuminata bracts and flowers were determined by using an ultra performance liquid chromatography-high resolution electrospray ionization-mass spectrometry (UPLC-HRESI-MS) technique, and quinic acid was found in the extracts (Sandjo et al., 2019). The methanol extract of Musa ABB flowers was rich in terms of quinic acid, as shown in one study (Begum and Deka, 2019). In another study, a water extract of the peel and pulp of Musa ABB was analyzed via HPLC that identified caffeic acid (21.54–45.67 ppm), apigenin (67.98–97.26 ppm), chlorogenic acid (59.21–80.36 ppm), hydroquinone (42.33–60.32 ppm), ferulic acid (21.64 to 35.32 ppm), quinic acid (109.57–187.08 ppm) and p-coumaric acid (27.82–63.21 ppm) (Khawas and Deka, 2017).
In this study, aconitic acid, hesperidin and amentoflavone were also identified. This is the first time that these compounds have been reported in Musa cavendishii to the best of our knowledge. The methanol extract of Euphorbia macroclada was studied by LC–MS/MS and aconitic acid (0.16 mg/g) were identified (Ertas et al., 2014). Aconitic acid was detected by LC–MS in an investigation of the phenolics of Butia spp. by another study (Hoffmann et al., 2017). Li et al. (2019) reported a hesperidin content of 0.000537 mg/g dw for loquat fruit studied using UHPLC-QqQ-MS/MS. The phenolic content of methanol extract of Chamaerops humilis L. using LC–ESI–MS/MS and hesperidin (0.014 mg/g) was determined from Chamaerops humilis L. (Bouhafsoun et al., 2018).
Ibrahim et al. (2017) investigated the amentoflavone content of the methanol extract of Cupressus sempervirens L. collected from different locations in Egypt. They reported the amentoflavone content varied from 0.004 to 0.462 mg/g.
Given the context above, the outcome of this study is compatible with published literature. In addition, the results showed that the methanol extract obtained from the Anamur banana was more rich in phenolic compounds than other researchers, e.g. Ertas et al. (2014), Khawas and Deka (2017), Bouhafsoun et al. (2018), Li et al. (2019).
Total phenolic content and antioxidant activity
Evidence from epidemiological studies indicate that long-term consumption of diets rich in plant polyphenols exert a protective effect against certain diseases and disorders. Their structure varies from a very simple phenolic molecule to complex polymers of high-molecular weight (Shahidi and Ambigaipalan, 2015). The efficiency of the extraction of phenolic compounds is affected by the chemical properties of phenolic compounds, the particle size of the sample, the extraction method used and the extraction conditions (Boeing et al., 2014).
The total phenolic contents of Anamur banana extracts were estimated spectrophotometrically, and the outcomes are shown in Table 2. The total phenolic contents of the extracts ranged from 0.33 to 2.12 mg GAE/g of extract. The methanol extract had the higher amount of phenols, which is similar to the results reported by other researchers, e.g., Ilokiassanga et al. (2015) and Nguyena et al. (2015). Shian et al. (2012) used 50%, 70% and 100% methanol to extract phenolic compounds from different Musa species, and they observed a total phenolic content ranging from 0.3 to 1.14 mg GAE/g of extract. Hexane was not useful in extracting phenolics as evidenced by their low total phenolic content compared to the other solvents used.
Table 2.
Total phenolics and antioxidant activities of Anamur banana extracts
| Solvent | Total phenolics (mg GAE /g extract) | DPPH (mM TE/g extract) | FRAP (mM TE/g extract) |
|---|---|---|---|
| Methanol | 2.12 ± 0.06a | 4.84 ± 0.00a | 6.32 ± 0.09a |
| Ethanol | 1.91 ± 0.01b | 2.05 ± 0.03b | 4.58 ± 0.11b |
| Hexane | 0.33 ± 0.01c | 0.64 ± 0.03c | 0.98 ± 0.01c |
Mean values with different letters (a,b,c) within the same column are statistically different at p < 0.005
Many disorders such as arteriosclerosis, arthritis, inflammation, diabetes, age-related macular degeneration, Alzheimer’s disease, certain types of cancer and genotoxicity are related to reactive nitrogen species (RNS) and reactive oxygen species (ROS), for example, nitric oxide radicals, hydrogen peroxide, singlet oxygen, superoxide ions and hydroxyl radicals (Sidhu and Zafar, 2018). The antioxidant activities of the samples were determined by the DPPH and FRAP methods. The results are presented in Table 2. The DPPH assay is based on the reduction of DPPH radicals (DPPH·) by accepting an electron or proton radical from the antioxidant to form the reduced DPPH (DPPH-H). The FRAP assay measures the reduction of ferric ion (Fe3+)–ligand complex to the intensely blue-colored ferrous (Fe2+) complex by antioxidants in an acidic medium (Gülçin, 2012).
All extracts showed antioxidant activity (Table 2). The methanol extract had the highest antioxidant capacity as determined by the FRAP (6.32 ± 0.09 mM TE/g of extract) and DPPH (4.84 ± 0.00 mM TE/g of extract) methods. The hexane extract had the lowest antioxidant activity in both methods. Boeing et al. (2014), studied the effect of solvents on the extraction of phenolic compounds and antioxidant capacities from berries, and they found that methanol was the most efficient solvent for extraction of antioxidant compounds, followed by water and ethanol. The finding is similar to the results of this study.
Anti-α-glucosidase and anti-α-amylase activity
Dietary carbohydrates are rapidly absorbed with the help of glycoside hydrolases (α-glucosidase, α-amylase) that convert them into elementary monosaccharide units. This results in elevated blood glucose levels, which is known as postprandial hyperglycaemia. This phenomenon is regarded as the initial symptom of diabetes. The use of glycoside inhibitors is broadly regarded as an efficient method in preventing postprandial hyperglycaemia by inhibiting the release of free glucose units and facilitating an even glucose profile. Therefore, these enzymes are a therapeutic target to regulate blood glucose level (Wulan et al., 2015). The inhibitory activities of methanol, ethanol and hexane extracts on both α-glucosidase and α-amylase were examined. Table 3 shows the outcomes. Both methanol and ethanol extracts inhibited α-amylase and α-glucosidase. The hexane extract, on the other hand, showed no inhibitory effects on α- amylase or α-glucosidase.
Table 3.
Antidiabetic activities of Anamur banana extracts
| Solvent | IC50 (mg/mL) α-glucosidase ınhibition | IC50 (mg/mL) α-amylase ınhibition |
|---|---|---|
| Methanol | 5.45 ± 0.39b | 9.70 ± 0.29b |
| Ethanol | 7.45 ± 0.40a | 13.2 ± 0.32a |
| Hexane | 0.0 ± 0.0d | 0.0 ± 0.0d |
| Acarbose | 4.34 ± 0.29c | 6.37 ± 0.35c |
Mean values with different letters (a,b,c,d) within the same column are statistically different at p < 0.005
The inhibitory effects of methanol extracts on α-glucosidase (IC50:5.45 ± 0.39 mg/mL) and α-amylase (IC50:9.70 ± 0.29 mg/mL) were found to be higher than the ethanol extract (IC50:7.45 ± 0.40 mg/mL for α-glucosidase and IC50:13.2 ± 0.32 mg/mL for α-amylase). Acarbose was used as the standard, and it showed higher activity than the Anamur banana extracts for α-glucosidase (4.34 ± 0.29 mg/mL) and α-amylase (6.37 ± 0.35 mg/mL).
It has been reported that polyphenols have insulin-like effects in glucose utilization and work as good inhibitors of key enzymes such as α-glucosidase and α-amylase that are associated with lipid peroxidation in tissues and type II diabetes. In addition, it has been reported that the polyphenol-rich compound, quinic acid, has significant antihyperglycaemic activity (Kotha et al., 2017).
The LC–MS/MS analysis showed that quinic acid is the most abundant phenolic acid present in the extracts, which contain plenty of quinic acid as phenolic acid. For this reason, the methanol and ethanol extracts provided good inhibitory activity on α glucosidase and α-amylase enzymes. Furthermore, the methanol extract was found to be more effective compared to the other two extracts. Many studies have reported that phenolic compounds, mainly quinic acid, aconitic acid, hesperidin and amentoflavone, demonstrate α-glucosidase and α-amylase inhibitory activity (Vinayagam and Xu, 2015; Kotha et al., 2017).
Anamur banana extracts are abundant with polyphenols as mentioned earlier and the LC–MS/MS analysis outcomes of the banana cultivars studied confirmed their antidiabetic activities. This includes anti-α-glucosidase and anti-α-amylase inhibitory activity as well. The results of the study emphasized that the methanol extracts of Anamur bananas possess in vitro antidiabetic properties.
Type II diabetes mellitus is one of the rapidly growing diseases worldwide and disabling micro and macrovascular complications (Vinayagam and Xu, 2015). In addition, it has been predicted that by 2035 approximately 592 million adults will become diabetic patients because of increased urbanization, high population growth, aging, the abundance of calorie rich, fatty and fast foods, improvements in living standards and a high prevalence of obesity (Fowler et al., 2015). This study showed that Anamur bananas have good enzyme inhibition activities and might be considered as a potential agent for the topical treatments of patients suffering from diabetes. Various effective food formulations using Anamur banana extracts can be developed to treat and control diabetes.
Antibacterial activity
The antibacterial activity of the extracts obtained was assessed against S. aureus and E. coli by the disc diffusion method. The antibacterial activity presented in Table 4 was expressed as an inhibitory zone diameter. As seen in this table, the antibacterial activity of the extracts varied among the solvents used, with methanol being the most effective against both microorganisms tested. The hexane extract showed no antibacterial effects on E. coli and S. aureus, whereas the antibacterial activity of the methanol extract achieved an inhibitory zone diameter of 12.73 ± 0.35 mm against E. coli and 3.87 ± 0.25 mm against S. aureus. The antibacterial activity of the ethanol extract achieved an inhibitory zone diameter of 9.87 ± 0.30 mm and 2.43 ± 0.21 mm against E. coli and S. aureus, respectively. Ampicillin was used as the standard compound. This compound exhibited higher antibacterial activity than the Anamur banana extracts with 17.7 ± 0.35 mm for E. coli and 19.4 ± 0.2 mm for S. aureus. Fadhilah et al. (2014) reported in their study that inhibition zones for the methanol extract of different Musa species were 7.0 mm and 8 mm for E. coli and no antibacterial activity was reported against S. aureus.
Table 4.
Antibacterial activities of Anamur banana extracts
| E. coli | S. aureus | |
|---|---|---|
| Zone diameter (mm) | Zone diameter (mm) | |
| Methanol | 12.73 ± 0.35b | 3.87 ± 0.25b |
| Ethanol | 9.87 ± 0.30c | 2.43 ± 0.21c |
| Hexane | 0.0 ± 0.0d | 0.0 ± 0.0d |
| Ampicillin | 17.7 ± 0.35a | 19.4 ± 0.2a |
Values expressed are means ± SEM of three parallel measurements
Significance compared to control at p < 0.005
Mean values with different letters (a,b,c,d) within the same column are statistically different at p < 0.005
The phenomenon of the emergence and increase of antimicrobial-resistant bacteria, which are resistant to most of the conventional antimicrobial drugs, has posed a significant threat to public health. This dictates the importance of alternative treatments against diseases which are related to these microorganisms. Previous studies showed that polyphenols have antibacterial activity (Rezende et al., 2015). Quinic acid is a cyclic polyol present in the domain Eukarya. Derivatives of quinic acid already provide in vitro antibacterial activity against various bacterial strains (Ozcelik et al., 2011). Furthermore, quinic acid derivatives, such as amides, were proved to be promising compounds in therapeutics. In addition to in vitro and in vivo anti‐inflammatory activity, it has been reported that they are stable at room temperature, are water soluble and exhibit no cytotoxicity. In addition, enzymes produced by gut bacteria that are available for absorption into the circulation do not degrade these compounds (Zeng et al., 2009; Yates et al., 2009). In this study, the dominant phenolic compound was quinic acid and thus, extracts of Anamur banana demonstrated a strong inhibitory effect on E. coli. But these extracts demonstrated lower inhibitor activity on S. aureus than E. coli.
Various plants have been considered a fundamental source of potent antidiabetic and antibacterial formulations for centuries. At present, plants with medicinal properties are recommended for the treatment of diseases, including diabetes and bacterial diseases. These plants may show antidiabetic activities since they contain various phytoconstituents such as saponins, carotenoids, glycosides, alkaloids, terpenoids and phenolics. Many plants have attracted special interest and have been used from ancient times, especially for their antidiabetic and antibacterial effects, and for topical applications. This caused new impetus to study different extracts of Anamur bananas by screening them for α-glucosidase, α-amylase, E. coli and S. aureus inhibition to evaluate their antidiabetic and antibacterial properties.
Methanol and ethanol extracts of Anamur banana provided good enzyme and bacterial inhibition activities, with the methanol extract chiefly found to be more active than the ethanol extract. An estimation of the chemical components of the extracts in question were performed by LC–MS/MS analysis. Both extracts were good sources of phenolic compounds according to the study results. The antidiabetic and antibacterial potential of these two extracts may be a consequence of them being good sources of phenolic constituents, which include quinic acid, acotinic acid, hesperidin and amentoflavone. This study showed that the methanol extract has a high potential to be utilized as active compounds in functional ingredients and foods that might be used to prevent diabetic and bacterial disorders.
Acknowledgements
This study was supported by the Commission for the Scientific Research Projects of University of Adana Alparslan Turkes Science and Technology, Turkey.
Author contributions
Aysun Sener Geduk: Project administration, Investigation, Methodology, Formal analysis, Resources, Writing—review & editing. Fatma Zengin: Investigation, formal analysis, methodology, writing.
Declarations
Conflict of interest
The authors declare no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aysun Şener Gedük, Email: asener00@gmail.com, Email: asener@atu.edu.tr.
Fatma Zengin, Email: fatmazen2014@gmail.com.
References
- Aurore G, Parfait B, Fahrasmane L. Bananas, raw materials for making processed food products. Trends in Food Science & Technology. 2009;20:78–91. doi: 10.1016/j.tifs.2008.10.003. [DOI] [Google Scholar]
- Ayoola IO, Gueye B, Sonibare MA, Abberton MT. Antioxidant activity and acetylcholinesterase inhibition of field and in vitro grown Musa L. species. Journal of Food Measurement and Characterization. 11: 488-499 (2017)
- Begum YA, Deka SC. Chemical profiling and functional properties of dietary fibre rich inner and outer bracts of culinary banana flower. Journal of Food Science and Technology. 2019;56(12):5298–5308. doi: 10.1007/s13197-019-04000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeing JS, Barizao ÉO, Silva BC, Montanher PF, de Cinque AV, Visentainer JV. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis. Chemistry Central Journal. 2014;8(48):1–9. doi: 10.1186/s13065-014-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhafsoun A, Yilmaz MA, Boukeloua A, Temel H, Kaidharche M Simultaneous quantification of phenolic acids and flavonoids in Chamaerops humilis L. using LC–ESI-MS/MS. Food Science and Technology (Campinas). 38: 242-247 (2018)
- Ertas A, Yilmaz MA, Firat M. Chemical profile by LC–MS/MS, GC/MS and antioxidant activities of the essential oils and crude extracts of two Euphorbia species. Natural Product Research. 2014;29:1–6. doi: 10.1080/14786419.2014.954113. [DOI] [PubMed] [Google Scholar]
- Fadhilah F, Jalani M, Mohamad S, Nazatul W, Shahidan S. Antibacterial effects of banana pulp extracts based on different extraction methods against selected microorganisms. Asian Journal of Biomedical and Pharmaceutical Sciences. 2014;4(36):14–19. [Google Scholar]
- Faostat. Statistical database of the food and agriculture organization of the united nations. http://faostat.fao.org/ (2017)
- Fernandez-Agullo A, Pereira E, Freire MS, Valentao P, Andrade PB, Gonzalez Álvarez J, Pereira JA. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Industrial Crops and Products. 2013;42:126–132. doi: 10.1016/j.indcrop.2012.05.021. [DOI] [Google Scholar]
- Fowler F, Voyer B, Marino M, Finzel J, Veltri M, Wachter NM. Rapid screening and quantification of synthetic cannabinoids in herbal products with NMR spectroscopic methods. Analytical Methods. 2015;7(18):7907–7916. doi: 10.1039/C5AY01754H. [DOI] [Google Scholar]
- Gulçin I. Antioxidant activity of food constituents: An overview. Archives of Toxicology. 2012;86:345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann JF, Carvalho IR, Barbieri RL, Rombaldi CV. Chaves FC Butia spp. (Arecaceae) LC-MS-based metabolomics for species and geographical origin discrimination. Journal of Agricultural and Food Chemistry. 2017;65:523–532. doi: 10.1021/acs.jafc.6b03203. [DOI] [PubMed] [Google Scholar]
- Ibrahim EA, Desoukey SY, Hadad GM, Salam RA, Ibrahim A, Ahmed SA, Radwan MM, Wanas AS, ElSohly MA. Analysis of cupressuflavone and amentoflavone from Cupressus sempervirens L. and its tissue cultured callus using HPLC-dad method. Pharmacy Pharmacology International Journal. 2017;5:174–180. [Google Scholar]
- Ilokiassanga SB, Lewislujan LM, Laraespinoza CL, Gilsalido AA, Fernandezangulo D, Rubiopino JL, David DH. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Research Notes. 2015;8(396):1–14. doi: 10.1186/s13104-015-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber H, Baydoun E, El-Zein O, Kreydiyyeh SI. Anti-hyperglycemic effect of the aqueous extract of banana infructescence stalks in streptozotocin-induced diabetic rats. Plant Foods for Human Nutrition. 2013;68:83–89. doi: 10.1007/s11130-013-0341-5. [DOI] [PubMed] [Google Scholar]
- Kalita H, Boruah DC, Deori M, Hazarika A, Sarma R, Kumari S, Kandimalla R, Kotoky J, Devi R. Antidiabetic and antilipidemic effect of musa balbisiana root extract: a potent agent for glucose homeostasis in streptozotocin-ınduced diabetic rat. Frontiers in Pharmacology. 2016;7(102):1–11. doi: 10.3389/fphar.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam A, Li KM, Razmovski-Naumovski V, Nammi S, Chan K, Li GQ. A comparative study on the inhibitory effects of different parts and chemical constituents of pomegranate on α-amylase and α-glucosidase. Phytotherapy Research. 2013;27:1614–1620. doi: 10.1002/ptr.4913. [DOI] [PubMed] [Google Scholar]
- Khawas P, Deka SC. Encapsulation of natural antioxidant compounds from culınary banana by cocrystallization. Journal of Food Processing and Preservation. 2017;41:1–13. [Google Scholar]
- Kotha P, Badrib KR, Nagalapurama R, Allagaddaa R, Chippadaa AR. Anti-diabetic potential of the leaves of Anisomeles malabarica in streptozotocin induced diabetic rats. Cellular Physiology and Biochemistry. 2017;43:1689–1702. doi: 10.1159/000484030. [DOI] [PubMed] [Google Scholar]
- Lehmann U, Jacobasch G, Schmiedl D. Characterization of resistant starch type III from banana (Musa acuminata) Journal of Agricultural and Food Chemistry. 2002;50:5236–5240. doi: 10.1021/jf0203390. [DOI] [PubMed] [Google Scholar]
- Li W, Wang X, Zhang J, Zhao X, Wu Y, Tan S, Gao X. Multivariate analysis illuminates the effects of vacuum drying on the extractable and nonextractable polyphenols profile of loquat fruit. Journal of Food Science. 2019;84:726–737. doi: 10.1111/1750-3841.14500. [DOI] [PubMed] [Google Scholar]
- Linde K. St. John’s wort–an overview. Complementary Medicine Research. 2009;16(3):146–155. doi: 10.1159/000209290. [DOI] [PubMed] [Google Scholar]
- Liu S, Li D, Huang B, Chen Y, Lu X, Wang Y. Inhibition of pancreatic lipase, α-glucosidase, α-amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. Journal of Ethnopharmacology. 2013;149(1):263–269. doi: 10.1016/j.jep.2013.06.034. [DOI] [PubMed] [Google Scholar]
- Nguyena VT, Bowyer MC, Vuonga QV, Altenaa IA, Scarlett CS. Phytochemicals and antioxidant capacity of Xao tam phan (Paramignya trimera) root as affected by various solvents and extraction methods. Industrial Crops and Products. 2015;67:192–200. doi: 10.1016/j.indcrop.2015.01.051. [DOI] [Google Scholar]
- Ough CS, Amerine MA. Methods for analysis of musts and wines. New York: Wiley; 1988. p. 377. [Google Scholar]
- Ozcelik B, Kartal M, Orhan I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharmaceutical Biology. 2011;49:396–402. doi: 10.3109/13880209.2010.519390. [DOI] [PubMed] [Google Scholar]
- Rezende CO, Oliveira LA, Oliveira BA, Almeida CG, Ferreira BS, Hyaric M, Carvalho GSL, Lourenço MCS, Batista M, Marchini FK, Silva VL, Diniz CG, Almeida MV. Synthesis and antibacterial activity of alkylated diamines and amphiphilic amides of quinic acid derivatives. Chemical Biology & Drug Design. 2015;86:344–350. doi: 10.1111/cbdd.12498. [DOI] [PubMed] [Google Scholar]
- Sandjo LP, Marcus VP, dos Santos N, Moraes MH, Rodrigues LM, Dalmarco EM, Biavatti MW, Steindel M. NOx-, IL-1-, TNF-α-, and IL-6-inhibiting effects and trypanocidal activity of banana (Musa acuminata) bracts and flowers: UPLC-HRESI-MS detection of phenylpropanoid sucrose esters. Molecules. 2019;24:4564. doi: 10.3390/molecules24244564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects - A review. Journal of Functional Foods. 2015;18:820–897. doi: 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- Shian T, Abdullah A, Musa KH, Ghani MM. Antioxidant properties of three banana cultivars (Musa acuminata ‘Berangan’, ‘Mas’ and ‘Raja’) extracts. Sains Malaysiana. 2012;41(3):319–324. [Google Scholar]
- Sidhu JS, Zafar TA. Bioactive compounds in banana fruits and their health benefits. Food Safe Quality Food. 2018;2:183–188. doi: 10.1093/fqsafe/fyy019. [DOI] [Google Scholar]
- Silva AR, Cerdeira CD, Brito AR, Salles BCC, Ravazi GF, Moraes GDI, Rufino LRA, de Oliveira RBS, Santos GB. Green banana pasta diet prevents oxidative damage in liver and kidney and improves biochemical parameters in type 1 diabetic rats. Archives of Endocrinology Metabolism. 2016;60:355–366. doi: 10.1590/2359-3997000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Singh JP, Kaur A, Singh N. Bioactive compounds in banana and their associated health benefits—a review. Food Chemistry. 2016;206:1–11. doi: 10.1016/j.foodchem.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- Vinayagam R, Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutrition and Metabolis. 2015;12(60):1–20. doi: 10.1186/s12986-015-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulan DR, Utomo EP, Mahdi C. Antidiabetic activity of Ruellia tuberosa L., role of a-amylase inhibitor: in silico, in vitro, and in vivo approaches. Biochemistry Research International. 2015;6:1–9. doi: 10.1155/2015/349261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates CR, Zeng K, Miller DD, Thompson KE. Anti-inflammatory quinic acid derivatives for oral administration. Patent Application Publication. 2009;17:1–12. [Google Scholar]
- Yilmaz MA. Simultaneous quantitative screening of 53 phytochemicals in 33 species of medicinal and aromatic plants: A detailed, robust and comprehensive LC–MS/MS method validation. Industrial Crops and Products. 2020;149:112347. doi: 10.1016/j.indcrop.2020.112347. [DOI] [Google Scholar]
- Zeng K, Thompson KE, Yates CR, Miller DD. Synthesis and biological evaluation of quinic acid derivatives as anti-inflammatory agents. Bioorganic & Medicinal Chemistry Letters. 2009;19:5458–5460. doi: 10.1016/j.bmcl.2009.07.096. [DOI] [PubMed] [Google Scholar]