Abstract
The main aim of the study is to evaluate the efficacy and safety profile of ocrelizumab (OCR), rituximab (RTX), and cladribine (CLA), employed as natalizumab (NTZ) exit strategies in relapsing–remitting multiple sclerosis (RRMS) patients at high-risk for progressive multifocal leukoencephalopathy (PML). This is a multicentre, retrospective, real-world study on consecutive RRMS patients from eleven tertiary Italian MS centres, who switched from NTZ to OCR, RTX, and CLA from January 1st, 2019, to December 31st, 2019. The primary study outcomes were the annualized relapse rate (ARR) and magnetic resonance imaging (MRI) outcome. Treatment effects were estimated by the inverse probability treatment weighting (IPTW), based on propensity-score (PS) approach. Additional endpoint included confirmed disability progression (CDP) as measured by Expanded Disability Status Scale and adverse events (AEs). Patients satisfying predefined inclusion and exclusion criteria were 120; 64 switched to OCR, 36 to RTX, and 20 to CLA. Patients from the 3 groups did not show differences for baseline characteristics, also after post hoc analysis. The IPTW PS-adjusted models revealed that patients on OCR had a lower risk for ARR than patients on CLA (ExpBOCR 0.485, CI 95% 0.264–0.893, p = 0.020). This result was confirmed also for 12-month MRI activity (ExpBOCR 0.248 CI 95% 0.065–0.948, p = 0.042). No differences were found in other pairwise comparisons (OCR vs RTX and RTX vs CLA) for the investigated outcomes. AEs were similar among the 3 groups. Anti-CD20 drugs were revealed to be effective and safe options as NTZ exit strategies. All investigated DMTs showed a good safety profile.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01037-2.
Key Words: Natalizumab, Ocrelizumab, Rituximab, Cladribine, Exit strategy, Disease activity
Introduction
Natalizumab (NTZ) has improved the possibilities to treat highly active relapsing–remitting forms of multiple sclerosis (RRMS) patients (1).
However, a long exposure to NTZ treatment in anti-JC virus (JCV)–seropositive patients expose them to a higher risk to develop progressive multifocal leukoencephalopathy (PML), a serious and potentially lethal opportunistic brain infection [2–5]. To manage PML risk, a magnetic resonance imaging (MRI) monitoring every 3–4 months has been recommended for JCV–seropositive patients on NTZ treatment for more than 18 months [5]. Furthermore, although several retrospective studies have investigated the effect of extended interval dose (EID) on reducing PML risk, the reliability of their conclusions is limited by the nonrandomized designs, and the extreme variable definitions of EID (ranging from 5 to 8 weeks) [5].
All above considered, a therapeutic switch in patients who respond to NTZ but are exposed to a high PML risk represents an important and increasingly frequent therapeutic challenge in MS clinical practice. Since highly effective drugs have been licensed for the treatment of highly active RRMS, there is an urgent need of clinical-MRI data to develop guidelines addressing/regarding exit strategies to follow in JCV-positive NTZ-treated RRMS patients at high risk of PML [6–8].
The decision to switch to another drug is always shared between neurologist and patient, and it should derive from the balance of several factors, including the risk of side effects, the maintenance of a good clinical-MRI response, and the occurrence of a clinical and radiological rebound that has been frequently observed early after NTZ discontinuation [3, 9–17].
About therapeutic options after NTZ withdrawal, the drugs targeting CD20 + B cells (rituximab (RTX) and ocrelizumab (OCR)) have proven to be very effective in suppressing inflammatory activity in RRMS [18], although not associated with a significant PML risk [19].
More recently, cladribine (CLA) tablets have been approved for highly active RRMS, but scarce data are available on the use of CLA after NTZ [7, 20].
On this background, the aim of the present multicentre real-world study was to compare the effectiveness, tolerability, and safety of a therapeutic switch to OCR, RTX, or CLA in RRMS patients treated with NTZ who were considered responder to such treatment that needed or required to stop NTZ for the high risk of PML.
Methods
Setting and Participants
In this retrospective observational study, we collected prospective clinical and MRI data from RRMS patients followed at 11 tertiary Italian MS centres. In detail, we identified adult RRMS patients who switched from NTZ to OCR, RTX, and CLA from January 1st, 2019, to December 31st, 2019.
Inclusion criteria were: 1) ≥ 18 years of age, 2) a diagnosis of RRMS according to McDonald revised diagnostic criteria [21]; 3) no evidence of clinical and radiological activity in the last 12 months of NTZ treatment (with a standard or EID regimen); and 4) washout period from NTZ treatment not longer than 12 weeks.
We considered for the analysis only RRMS patients who switched form NTZ for the following safety reasons:
a JCV index ≥ 1.5 and more than 24 NTZ infusions
patient’s decision to discontinue NTZ for the fear of an increased risk of PML, even if the positive JCV index was < 1.5.
The decision to stop NTZ was reached after a consultation between the neurologist and the patient, during which the risks and benefits of continuing or interrupting NTZ were clearly explained and discussed.
Procedures and Outcomes
Patients were treated in accordance with treatment procedures and guidelines approved by European and Italian Medicines Agencies.
In detail, OCR is administered at the dosage of 600 mg/intravenous, and the first 2 infusions—each of 300 mg—are given 2 weeks apart and subsequent 600-mg infusions are given every 6 months [22].
RTX is used as off-label treatment in highly active RRMS patients [23]. It is administered as an intravenous infusion in doses of 1000 mg [24]. Subsequent doses and timing of administration are usually every 6–12 months, but no consensus guidelines exist. Among our RRMS patients, the interval between the first and second infusions was on average 7 months (range 6–9).
CLA tablets are administered in 2 treatment courses ~1 year apart [7]. The recommended cumulative dosage is 3.5 mg/kg body weight administered orally and divided into 2 yearly treatment courses (1.75 mg/kg per treatment course). Each treatment course is divided into 2 treatment cycles [7]. The first treatment course (year 1) is structured as follows: a first cycle (month 1) that starts at any given time and a second cycle (month 2) which starts 23–27 days after the last dose (~1 month after beginning first cycle).
Data were recorded retrospectively (including data until 12 months before NTZ starting, time on NTZ, and the washout period) and prospectively (until the last available visit of follow-up) from the beginning of 1 the 3 investigated drugs (the index date).
The data entry portal was iMed© software's (iMed, Merck Serono SA - Geneva, Switzerland). Data were extracted on September 30th, 2020.
Disability was assessed by Expanded Disability Status Scale (EDSS) by a neurostatus-certified MS specialist. MRI data were acquired on 1.5-T scanners (the same at each centre from baseline to the end of the follow-up) and included T2- and pre- and postcontrast T1-weighted sequences [25]. Postcontrast T1-weighted sequences were acquired after intravenous injection of gadolinium contrast agent (0.1 mmol/kg). A cerebral MRI acquired within 30 days before the treatment start (during the washout period) was considered the baseline MRI, and the number of brain T2-, pre-, and postcontrast T1 lesions was recorded. Follow-up MRIs to assess disease activity were acquired at 6 and 12 months after the start of post-NTZ treatment.
Study Endpoints
The primary study outcome was the annualized relapse rate (ARR) on investigated drugs. Additional endpoints included MRI activity after 12 months and confirmed disability progression (CDP) as measured by EDSS until the last follow-up.
Safety profile of the investigated DMTs was also investigated and reported.
A relapse was defined as the development of new symptoms or exacerbation of existing symptoms that persisted for ≥ 24 h, in the absence of concurrent illness or fever, and occurred ≥ 30 days after a previous relapse. ARR was defined as total number of relapses divided by patient–months on therapy.
CDP was defined as an increase in EDSS by ≥ 1.5 points for those with a baseline EDSS score of 0, by 1 or more points for a baseline score of ≤ 5.5, or by 0.5 points for a baseline score of > 5.5, which was sustained for 12 weeks or longer. EDSS recorded within 30 days after the onset of a relapse were excluded.
MRI activity was considered new T1-gadolinium enhancing brain lesion and/or a new or newly enlarging T2 brain lesion [26].
We defined EID if the mean interval between doses were ⩾5 weeks and standard interval dose (SID) if the mean interval between doses were < 5 weeks. The 5-week cut-off was defined a priori being the midpoint between SID (4 weeks) and EID (6–8 weeks) [27]. All patients received SID NTZ for at least 1 year, and after that, some were switched to an EID regimen due to the risk of PML.
We collected data on the safety and tolerability, reporting the frequency of Adverse Events (AEs) in accordance to EMA definition [28]. Registered AEs were severe infections requiring medication, except for uncomplicated lower urinary tract infections; AEs causing discontinuation of therapy; and AEs related to each infusion of OCR/RTX or the first dosing of CLA cycle (both reported separately).
Protocol Approval Standard, Registrations, and Patient Consents
The study protocol was approved by the local ethics committee (Comitato Etico Catania 1 no. 140/2020/PO) of the coordinating centre (Policlinico Vittorio Emanuele, Catania, Italy), and patients provided written informed consent. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and with the appropriate national regulations.
Statistical Analysis
All patient characteristics summary statistics are reported in terms of frequencies (%) for categorical variables, mean standard deviation (S.D.), or median with interquartile range (IQR) for continuous variables. The Kolmogorov test was used to verify data distribution. According to this latter, parametric or nonparametric test was employed. The Bonferroni test was used for post hoc analysis.
According to the Akaike information criterion, we selected the model with the best statistical inferential properties. All the models were estimated using the Breslow’s tie correction.
To consider the imbalance of the 2 groups, a propensity score (PS) was calculated as the following.
A logistic regression was performed to score all patients according to the treatment (OCR = 1 vs CLA = 0, OCR = 1 vs RTX = 0 and RTX = 1 vs CLA = 0) used as independent variable and the following covariates at baseline: age, sex, EDSS in the year prior to switch to new DMT, number of NTZ infusions, and EID during the NTZ treatment as covariates.
Inverse probability of treatment weight (IPTW) and the stabilized inverse probability of treatment weight (SIPTW) were also calculated. HRs and CI 95% were calculated.
Two generalized regression models IPTW PS-adjusted were performed to evaluate relationship between: I) ARR and treatment groups and II) MRI activity after 12 months and treatment groups. The generalized equation models employed were adapted according to the nature of variables, respectively, linear (for ARR expressed as a continuous variable) and logistic binary (for MRI activities, expressed as dichotomic).
CDP, as measured by EDSS, was compared using a contingency table.
SPSS version 21.0 was used for all analyses (IBM SPSS Statistics 21, IBM©, Armonk, NY, USA).
Results
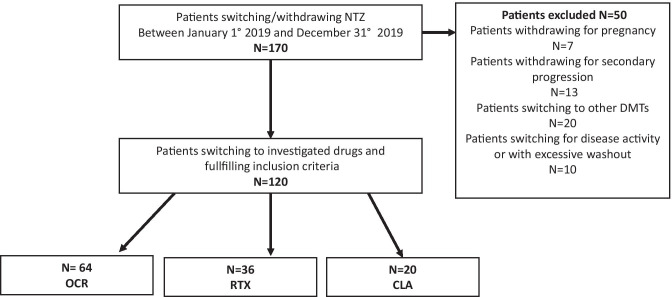
From a total cohort of 980 RRMS patients treated with NTZ in the enrolled centres, 170 stopped NTZ for any reasons during the index window, and 120 fulfilled the required criteria. Out of them, 64 switched to OCR, 36 switched to RTX, and 20 switched to CLA (Fig. 1). Demographical and clinical characteristics of the whole cohort and groups are reported in Table 1. Overall, the entire cohort did not show differences for baseline characteristics (Table 1). After post hoc test, the results were confirmed.
Fig. 1.
Patients’ selection flow chart. CLA = cladribine; NTZ = natalizumab; OCR = ocrelizumab; RTX = rituximab
Table 1.
Baseline characteristic among the 3 groups
| Variables° | OCR (64) | RTX (36) | CLA (20) | p* |
|---|---|---|---|---|
| Female n, (%) | 42, (65.6) | 26, (72.2) | 13, (65) | ns |
| Age at disease onset (± mean, S.D.) (year) | 24.4 ± 9.5 | 23.7 ± 9.9 | 26.5 ± 10.2 | ns |
| Number of DMTs before NTZ | 1.3 ± 1.1 | 1.7 ± 1.1 | 1.1 ± 0.8 | ns |
| Number of relapses 12 m before NTZ start | 1.6 ± 1.1 | 1.4 ± 1.2 | 1.6 ± 0.9 | ns |
| Number of T2-weighted brain lesions 12 m before NTZ start | 28.2 ± 16.1 | 23.8 ± 12.5 | 21.6 ± 13.1 | ns |
| Number of T1-Gd + brain lesions 12 m before NTZ start | 1.6 ± 2.2 | 1.5 ± 1.7 | 1.0 ± 1.2 | ns |
| EDSS at NTZ start, median (interquartile range) | 3.0 (2.0–4.5) | 2.5 (2.0–4.0) | 2.0 (1.0–3.0) | ns |
| Number of NTZ infusions | 35.1 ± 26.9 | 40.3 ± 24.9 | 26.7 ± 15.8 | ns |
| EID (n, %) | 25 (39.1) | 11 (30.6) | 6 (30) | ns |
| EID duration (weeks) | 16 ± 3.2 | 15 ± 4.7 | 14 ± 5.1 | ns |
| Washout period (weeks) | 8 ± 4.2 | 7 ± 3.9 | 6 ± 2.9 | ns |
Data are expressed as mean ± S.D. when otherwise specified
DMT disease modifying therapy; EDSS Expanded Disability Status Scale; EID extended interval dose; NTZ natalizumab
*via χ2, Fisher exact test or ANOVA according to the nature of variables
Table 2 shows the main clinical and radiological findings after switch among the 3 groups.
Table 2.
Clinical and radiological findings among the 3 groups after switch
| Variables° | OCR (64) | RTX (36) | CLA (20) | p* |
|---|---|---|---|---|
| Patients relapsing during treatment n (%) | 5 (7.8) | 5 (13.9) | 4 (20) | ns |
| Patients with more than 1 relapse during treatment n (%) | 0 | 3 (8.3) | 3 (15) | 0.017 |
| Median time to first relapse median (q1–q3) (months) | 3 (2–3) | 6 (3–8) | 3 (2.7–3.7) | ns |
| EDSS at baseline median (q1–q3) | 3.0 (2.0–4.5) | 4.0 (2.0–4.5) | 2.0 (1.0–3.0) | ns |
| EDSS after 6 months median (q1–q3) | 3.0 (2.0–4.5) | 4.0 (2.0–4.5) | 2.0 (1.0–4.0) | ns |
| EDSS after 12 months median (q1–q3) | 3.0 (2.0–4.5) | 3.5 (1.5–4.0) | 3.0 (1.5–5.0) | ns |
| EDSS after 18 months median (q1–q3) | 3.0 (2.0–4.5) | 3.5 (1.5–4.0) | 3.0 (1.5–5.0) | ns |
| Patients with CDP at last follow-up n (%) | 5 (7.8) | 3 (8.3) | 2 (10) | ns |
| Patients with increased lesions load on T2-weighted or T1 Gad + weighted brain MRI lesions after 6 months n (%) | 5 (7.8) | 3 (8.3) | 4 (20) | ns |
| Patients with increased lesions load on T2-weighted or T1 Gad + weighted brain MRI lesions after 12 months n (%)** | 6 (9.3) | 6 (16.7) | 4 (20) | ns |
| Follow-up in months median (q1–q3) | 18 (15–19) | 17 (14–20) | 16 (13–18) | ns |
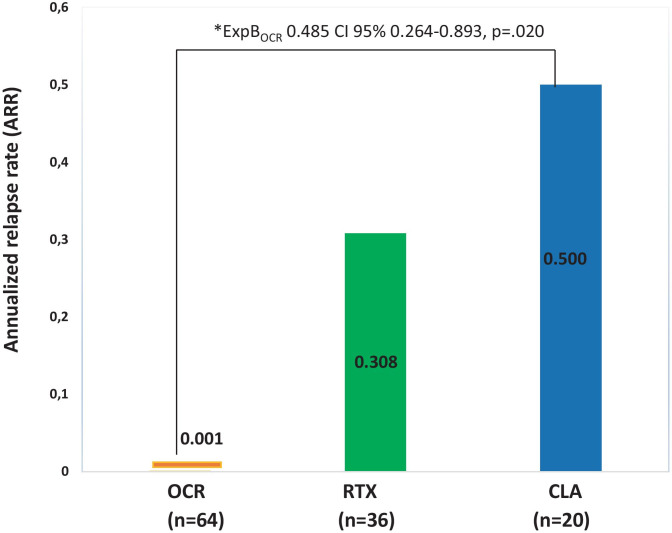
The estimated means for ARR showed a trend of significativity among the 3 groups, with value of 0.001 for patients on OCR, 0.308 for patients on RTX, and 0.500 for patients on CLA (p = 0.053).
The generalized regression model IPTW PS-adjusted revealed that patients on OCR had a lower risk for ARR than patients on CLA (ExpBOCR 0.485 CI 95% 0.264–0.893, p = 0.020).
No differences were found for the investigated outcome between OCR and RTX (ExpBOCR 0.875 CI 95% 0.749–1.021, p = 0.089) and between RTX and CLA (ExpBRTX 0.858 CI 95% 0.640–1.149 p = 0.304) (Fig. 2).
Fig. 2.
ARR endpoint (asterisk). The treatment effects were explored by a propensity-score adjustment in quintiles for age, sex, and EDSS in the year prior to switch to new DMT, number of NTZ infusions, and EID during the NTZ treatment. ARR = annualized relapse rate; CI = confidence interval; CLA = cladribine; NTZ = natalizumab; OCR = ocrelizumab; RTX = rituximab
The generalized regression model IPTW PS-adjusted revealed that patients on OCR had a lower risk for MRI activity than patients on CLA (ExpBOCR 0.248 CI 95% 0.065–0.948, p = 0.042).
No differences were found for the investigated outcome between OCR and RTX (ExpBOCR 1.247 CI 95% 0.573–2.717, p = 0.578) and between RTX and CLA (ExpBRTX 1.240 CI 95% 0.263–5.851 p = 0.786) (Fig. 2).
The CDP at the last follow-up did not differ among the 3 groups (p = 0.953).
No patient received a diagnosis of PML. Fourteen patients reported AEs within the first 12 months of treatment. Out of them, severe infections were reported in 3 patients on OCR, 1 on RTX, and 1 on CLA (Table 3).
Table 3.
Adverse events among the 3 groups
| OCR (n = 64) |
RTX (n = 36) |
CLA (n = 20) |
|
|---|---|---|---|
| AE, within 12 months | |||
| Patients with AEs |
3 (2 urinary infections, 1 gastrointestinal infection) |
1 (urinary infection) | 1 (1 genitourinary infection) |
| First-dosing AEs | |||
| Patients with first-dosing AEs | 5 (headache, flushing, articular pain) | 3 (headache, flushing, articular pain) | 1 (seborrheic dermatitis of the scalp) |
| AEs resulting in DMT discontinuation, within 12 months | |||
| Patients discontinuing for safety concerns | 0 | 0 | 0 |
AEs adverse events; OCR ocrelizumab; RTX rituximab; CLA cladribine; DMT disease modifying therapy
First-dosing AEs were reported in 5 on OCR and in 3 patients on RTX. One patient on CLA after the first cycle reported seborrheic dermatitis of the scalp. None of the AEs reported lead to DMT discontinuation.
Discussion
Our study revealed a lower risk of experiencing relapses and new MRI activity for patients that switched from NTZ to OCR than CLA. Contrariwise, no differences were found between those switching to RTX and CLA.
Regarding CDP, no differences were found, at the end of the follow-up, between the 3-switching group.
Overall, all DMTs revealed a good safety profile with no cases of PML.
The increased risk of PML in NTZ long-treated patients who show JCV antibodies positivity represents a matter of great concern in clinical practice. Different schemes of NTZ monitoring and/or administration, as EID regimen, have been proposed and evaluated, but they do not cancel PML risk and its consequences [5, 29, 30].
We could speculate that our results reflect the different mechanisms of action and pharmacodynamics of the investigated DMTs.
Little is known about the reasons of clinical and radiological rebound after NTZ discontinuation. It was considered the role of increased percentage of activated T cells producing cytokines in the peripheral circulation during NTZ treatment [31].
Real-world observational data about OCR as NTZ exit strategy are recent, whilst efficacy and safety of RTX have been highlighted since 2016 [9, 32–35].
A recent study investigated 42 RRMS patients who switched to OCR from NTZ after EID (5–8 weeks) and who were followed up for 6 months, clinical relapses occurred during the first 3 months of observation in 5 patients, and the EDSS remained stable in 38 (90%) patients. No serious AEs were described [34].
The most relevant observational study compared patients switching from NTZ to RTX (n = 114) to patients switching to fingolimod (n = 142) with an average follow-up of 1.5 years. Here, relapses occurred in 1.8% of RTX-treated patients compared with 17.6% of those who switched to fingolimod. The rates of AEs (5.3% vs 21.1%) and treatment discontinuation (1.8% vs 28.2%) were also lower in RTX groups. These results have been confirmed by a number of recent case series [19, 32, 33].
In our cohort, OCR was associated to lower relapse and lower MRI activity than CLA.
The comparative efficacy of CLA versus other DMTs in naïve patients has been analysed through meta-regression and matching-adjusted indirect treatment comparison approaches [36]. In detail, for the outcome ARR, CLA tablets were predicted to be less efficacious than OCR (relative risk 1.06, CI 95% 0.78–1.45) [36].
CLA is considered an immune reconstitution therapy (IRT) [37, 38]. Characteristics of IRTs include transient reductions of B and T lymphocyte counts and/or select lymphocyte subtypes, followed by a recovery period in which the B and T populations gradually recover, and immune function is restored. The reconstituted lymphocyte population usually begins within weeks after each treatment course in the first and second years and stabilized on return to baseline [39–42]. Such mechanism of action could explain why the first relapse in CLA group happened between the first and second trimesters from the therapeutic switch. Such timing could coincide to partial reconstitution of different T cells subtypes as pooled data from clinical trials showed [38, 42–45].
A previous short report by Mohon et al. [20] analysed 17 patients switching from NTZ to CLA with a median follow-up of 9.7 months (range 1.5–15 months) [20]. No patients presented a clinical relapse during the observation period, and only 2 patients showed new T2 lesions on brain MRI. [20]. However, the absence of comparisons or inferential statistical models represent limits of this study.
Our observational study firstly compared 3 high-efficacy DMTs employing a generalized model IPTW PS-adjusted for baseline characteristics to mitigate unbalance among groups, and this latter certainly constitutes an element of strength of the study.
Although IPTW has not been deeply evaluated in the context of small sample sizes, simulation studies revealed that, even in case of small study samples or low prevalence of treatment, both neighbour matching and IPTW PS can yield unbiased estimations of treatment effect [46, 47].
However, our study has some limits.
As this is a retrospective study, not all participants have the same follow-up, and the characteristics of sample size warrant cautious interpretation of the data. Moreover, we did not report lymphocytic count from investigated patients, and it could have added further data about the type and timing of lymphocytic subset repopulation.
In conclusion, prospective/longitudinal studies are needed to better clarify if switching to OCR is the choice with the best risk/benefit ratio as exit strategy after NTZ interruption because of unacceptable high risk of PML.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
Open access funding provided by Università degli Studi di Catania within the CRUI-CARE Agreement.
Declarations
Protocol Approval Standard, Registrations, and Patient Consents
The study protocol was approved by the local ethics committee (Comitato Etico Catania 1 no. 140/2020/PO) of the coordinating centre (Policlinico Vittorio Emanuele, Catania, Italy), and patients provided written informed consent. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and with the appropriate national regulations.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yin Y, Wang J, Wang X, Gu L, Pei H, Kuai S, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: a meta-analysis. Clinics (Sao Paulo, Brazil). 2015;70:524–530. doi: 10.6061/clinics/2015(07)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico E, Zanghì A, Leone C, Tumani H, Patti F. Treatment-related progressive multifocal leukoencephalopathy in multiple sclerosis: a comprehensive review of current evidence and future needs. Drug Safety. 2016;39:1163–1174. doi: 10.1007/s40264-016-0461-6. [DOI] [PubMed] [Google Scholar]
- 4.Fragoso YD, Arruda NM, Arruda WO, Brooks JB, Correa EC, Damasceno A, et al. We know how to prescribe natalizumab for multiple sclerosis, but do we know how to withdraw it? Expert review of neurotherapeutics. 2014;14:127–130. doi: 10.1586/14737175.2014.874947. [DOI] [PubMed] [Google Scholar]
- 5.Ryerson LZ, Foley J, Chang I, Kister I, Cutter G, Metzger RR, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology. 2019;93:e1452–e1462. doi: 10.1212/WNL.0000000000008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sellner J, Rommer PS. A review of the evidence for a natalizumab exit strategy for patients with multiple sclerosis. Autoimmun Rev. 2019;18:255–261. doi: 10.1016/j.autrev.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Zhao W, Beers DR, Hooten KG, Sieglaff DH, Zhang A, Kalyana-Sundaram S, et al. Characterization of gene expression phenotype in amyotrophic lateral sclerosis monocytes. JAMA neurology. 2017;74:677–685. doi: 10.1001/jamaneurol.2017.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plavina T, Subramanyam M, Bloomgren G, Richman S, Pace A, Lee S, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Annals of neurology. 2014;76:802–812. doi: 10.1002/ana.24286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alping P, Frisell T, Novakova L, Islam-Jakobsson P, Salzer J, Björck A, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Annals of neurology. 2016;79:950–958. doi: 10.1002/ana.24651. [DOI] [PubMed] [Google Scholar]
- 10.Prosperini L, Kinkel RP, Miravalle AA, Iaffaldano P, Fantaccini S. Post-natalizumab disease reactivation in multiple sclerosis: systematic review and meta-analysis. Ther Adv Neurol Disord. 2019;12:1756286419837809. doi: 10.1177/1756286419837809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhovtis Ryerson L, Frohman TC, Foley J, Kister I, Weinstock-Guttman B, Tornatore C, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87:885–889. doi: 10.1136/jnnp-2015-312940. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese M, Pitteri M, Farina G, Bajrami A, Castellaro M, Magliozzi R, et al. Dimethyl fumarate: a possible exit strategy from natalizumab treatment in patients with multiple sclerosis at risk for severe adverse events. J Neurol Neurosurg Psychiatry. 2017;88:1073. doi: 10.1136/jnnp-2017-316236. [DOI] [PubMed] [Google Scholar]
- 13.Jokubaitis VG, Li V, Kalincik T, Izquierdo G, Hodgkinson S, Alroughani R, et al. Fingolimod after natalizumab and the risk of short-term relapse. Neurology. 2014;82:1204–1211. doi: 10.1212/WNL.0000000000000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasenack M, Derfuss T. Disease activity return after natalizumab cessation in multiple sclerosis. Expert Rev Neurotherapeutics. 2016;16:587–594. doi: 10.1586/14737175.2016.1168295. [DOI] [PubMed] [Google Scholar]
- 15. D’Amico E, Patti F, Zanghì A, Zappia M. A personalized approach in progressive multiple sclerosis: the current status of disease modifying therapies (DMTs) and future perspectives. Int J Mol Sci 2016;17. [DOI] [PMC free article] [PubMed]
- 16.D'Amico E, Leone C, Zanghì A, Fermo SL, Patti F. Lateral and escalation therapy in relapsing-remitting multiple sclerosis: a comparative study. J Neurol. 2016;263:1802–1809. doi: 10.1007/s00415-016-8207-z. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico E, Leone C, Caserta C, Patti F. Oral drugs in multiple sclerosis therapy: an overview and a critical appraisal. Expert Rev Neurotherapeutics. 2015;15:803–824. doi: 10.1586/14737175.2015.1058162. [DOI] [PubMed] [Google Scholar]
- 18.D'Amico E, Zanghì A, Gastaldi M, Patti F, Zappia M, Franciotta D. Placing CD20-targeted B cell depletion in multiple sclerosis therapeutic scenario: Present and future perspectives. Autoimmun Rev. 2019;18:665–672. doi: 10.1016/j.autrev.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Mancinelli CR, Scarpazza C, Santuccio G, De Rossi N, Capra R. Dealing with highly active multiple sclerosis after natalizumab-associated PML: could rituximab be of help? Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2018;39:965–966. doi: 10.1007/s10072-017-3228-7. [DOI] [PubMed] [Google Scholar]
- 20. Möhn N, Skripuletz T, Sühs KW, Menck S, Voß E, Stangel M. Therapy with cladribine is efficient and safe in patients previously treated with natalizumab. Ther Adv Neurol Disord 2019;12:1756286419887596. [DOI] [PMC free article] [PubMed]
- 21.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. The Lancet Neurology. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 22.Broce I, Karch CM, Wen N, Fan CC, Wang Y, Tan CH, et al. Immune-related genetic enrichment in frontotemporal dementia: an analysis of genome-wide association studies. PLoS medicine. 2018;15:e1002487. doi: 10.1371/journal.pmed.1002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.https://www.ema.europa.eu/en/documents/product-information/mabthera-epar-product-information_it.pdf.
- 24.Ellwardt E, Ellwardt L, Bittner S, Zipp F. Monitoring B-cell repopulation after depletion therapy in neurologic patients. Neurology - Neuroimmunology Neuroinflammation. 2018;5:e463. doi: 10.1212/NXI.0000000000000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreau C, Devos D, Brunaud-Danel V, Defebvre L, Perez T, Destée A, et al. Elevated IL-6 and TNF-alpha levels in patients with ALS: inflammation or hypoxia? Neurology. 2005;65(12):1958–1960. doi: 10.1212/01.wnl.0000188907.97339.76. [DOI] [PubMed] [Google Scholar]
- 26.Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Multiple sclerosis and related disorders. 2015;4:329–333. doi: 10.1016/j.msard.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Clerico M, De Mercanti SF, Signori A, Iudicello M, Cordioli C, Signoriello E, et al. Extending the interval of natalizumab dosing: is efficacy preserved? Neurotherapeutics: J Amer Soc Exper NeuroTherapeutics. 2020;17:200–207. doi: 10.1007/s13311-019-00776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auger J, Sermondade N, Eustache F. Semen quality of 4480 young cancer and systemic disease patients: baseline data and clinical considerations. Basic Clin Androl. 2016;26:3. doi: 10.1186/s12610-016-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarpazza C, Signori A, Prosperini L, Sormani MP, Cosottini M, Capra R, et al. Early diagnosis of progressive multifocal leucoencephalopathy: longitudinal lesion evolution. J Neurol Neurosurg Psychiatry. 2019;90:261. doi: 10.1136/jnnp-2018-319208. [DOI] [PubMed] [Google Scholar]
- 30.Hodel J, Outteryck O, Dubron C, Dutouquet B, Benadjaoud MA, Duhin E, et al. Asymptomatic progressive multifocal leukoencephalopathy associated with natalizumab: diagnostic precision with MR imaging. Radiology. 2016;278:863–872. doi: 10.1148/radiol.2015150673. [DOI] [PubMed] [Google Scholar]
- 31.Kivisäkk P, Healy BC, Viglietta V, Quintana FJ, Hootstein MA, Weiner HL, et al. Natalizumab treatment is associated with peripheral sequestration of proinflammatory T cells. Neurology. 2009;72:1922–1930. doi: 10.1212/WNL.0b013e3181a8266f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Amico E, Zanghì A, Chisari CG, Fermo SL, Toscano S, Arena S, et al. Effectiveness and safety of rituximab in demyelinating diseases spectrum: an Italian experience. Multi Scler Relat Dis. 2019;27:324–326. doi: 10.1016/j.msard.2018.09.041. [DOI] [PubMed] [Google Scholar]
- 33.Malucchi S, Capobianco M, di Sapio A, Re ML, Cavalla P, Bertolotto A. Rituximab suppresses disease activity after natalizumab withdrawal: an exploratory study. Multi Scler Demyelinating Dis. 2016;1:11. doi: 10.1186/s40893-016-0013-z. [DOI] [Google Scholar]
- 34.Mancinelli CR, Scarpazza C, Cordioli C, et al. Switching to ocrelizumab in RRMS patients at risk of PML previously treated with extended interval dosing of natalizumab. Multi Scler J 2020:1352458520946017. [DOI] [PubMed]
- 35.Ng HS, Rosenbult CL, Tremlett H. Safety profile of ocrelizumab for the treatment of multiple sclerosis: a systematic review. Expert Opin Drug Saf. 2020;19:1069–1094. doi: 10.1080/14740338.2020.1807002. [DOI] [PubMed] [Google Scholar]
- 36.Berardi A, Siddiqui MK, Treharne C, Harty G, Wong SL. Estimating the comparative efficacy of cladribine tablets versus alternative disease modifying treatments in active relapsing-remitting multiple sclerosis: adjusting for patient characteristics using meta-regression and matching-adjusted indirect treatment comparison approaches. Curr Med Res Opin. 2019;35:1371–1378. doi: 10.1080/03007995.2019.1585779. [DOI] [PubMed] [Google Scholar]
- 37.Boyko AN, Boyko OV. Cladribine tablets' potential role as a key example of selective immune reconstitution therapy in multiple sclerosis. Degener Neurol Neuromuscul Dis. 2018;8:35–44. doi: 10.2147/DNND.S161450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Comi G, Cook S, Giovannoni G, Rieckmann P, Sørensen PS, Vermersch P, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Multi Scler Rel Dis. 2019;29:168–174. doi: 10.1016/j.msard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 39.Ceronie B, Jacobs BM, Baker D, Dubuisson N, Mao Z, Ammoscato F, et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J Neurol. 2018;265:1199–1209. doi: 10.1007/s00415-018-8830-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiendl H. Cladribine - an old newcomer for pulsed immune reconstitution in MS. Nat Rev Neurol. 2017;13:573–574. doi: 10.1038/nrneurol.2017.119. [DOI] [PubMed] [Google Scholar]
- 41.Comi G, Cook S, Rammohan K, Soelberg Sorensen P, Vermersch P, Adeniji AK, et al. Long-term effects of cladribine tablets on MRI activity outcomes in patients with relapsing-remitting multiple sclerosis: the CLARITY Extension study. Ther Adv Neurol Disord. 2018;11:1756285617753365. doi: 10.1177/1756285617753365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giovannoni G, Soelberg Sorensen P, Cook S, Rammohan K, Rieckmann P, Comi G, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Multiple sclerosis (Houndmills, Basingstoke, England). 2018;24:1594–1604. doi: 10.1177/1352458517727603. [DOI] [PubMed] [Google Scholar]
- 43.Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Annals of neurology. 2010;67:452–461. doi: 10.1002/ana.21939. [DOI] [PubMed] [Google Scholar]
- 44. Palanichamy A, Jahn S, Nickles D, et al. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J Immunol (Baltimore, Md : 1950) 2014;193:580–586. [DOI] [PMC free article] [PubMed]
- 45. Schuh E, Berer K, Mulazzani M, et al. Features of human CD3+CD20+ T cells. J Immunol (Baltimore, Md : 1950) 2016;197:1111–1117. [DOI] [PubMed]
- 46.Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996;52:249–264. doi: 10.2307/2533160. [DOI] [PubMed] [Google Scholar]
- 47.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Weaknesses of goodness-of-fit tests for evaluating propensity score models: the case of the omitted confounder. Pharmacoepidemiology Drug Saf. 2005;14:227–238. doi: 10.1002/pds.986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.